Abstract
An individual's dietary and supplement strategies can influence markedly their physical performance. Personalized nutrition in athletic populations aims to optimize health, body composition, and exercise performance by targeting dietary recommendations to an individual's genetic profile. Sport dietitians and nutritionists have long been adept at placing additional scrutiny on the one-size-fits-all general population dietary guidelines to accommodate various sporting populations. However, generic “one-size-fits-all” recommendations still remain. Genetic differences are known to impact absorption, metabolism, uptake, utilization and excretion of nutrients and food bioactives, which ultimately affects a number of metabolic pathways. Nutrigenomics and nutrigenetics are experimental approaches that use genomic information and genetic testing technologies to examine the role of individual genetic differences in modifying an athlete's response to nutrients and other food components. Although there have been few randomized, controlled trials examining the effects of genetic variation on performance in response to an ergogenic aid, there is a growing foundation of research linking gene-diet interactions on biomarkers of nutritional status, which impact exercise and sport performance. This foundation forms the basis from which the field of sport nutrigenomics continues to develop. We review the science of genetic modifiers of various dietary factors that impact an athlete's nutritional status, body composition and, ultimately athletic performance.
Keywords: nutrigenomics, nutrigenetics, personalized nutrition, athletic performance, genetic testing, sports nutrition, caffeine, ergogenic aids
Introduction
Sport and exercise performance are significantly influenced by nutrition, yet individuals respond differently to the same foods, nutrients and supplements consumed. This holds true for a variety of ages, ethnicities, and level of skill, and whether the goal is optimizing physical activity for health and fitness or for high performance sport. The importance of a personalized sports nutrition plan was highlighted in the recent “Nutrition and Athletic Performance” Joint Position Statement by the American College of Sports Medicine, the Academy of Nutrition and Dietetics and the Dietitians of Canada, which states that “Nutrition plans need to be personalized to the individual athlete… and take into account specificity and uniqueness of responses to various strategies” (1). These strategies encompass overall dietary patterns, macronutrient ratios, micronutrient requirements, eating behaviors (e.g., nutrient timing), and the judicious use of supplements and ergogenic aids.
The paradigm shift, away from the one-size-fits-all group approach and toward personalization for the individual, is moving nutrigenomics research from basic science into practice. While it has long been recognized that genetics play an influential role in determining how an athlete responds to foods and nutrients, the surge in research into gene-diet interactions over the past decade has provided a scientific basis for this hypothesis through various research initiatives and the corresponding increase in published studies. Genetic variants affect the way we absorb, metabolize, utilize and excrete nutrients, and gene-diet interactions that affect metabolic pathways relevant to health and performance are now widely recognized (2). Personal genetic testing can provide information that will guide recommendations for dietary choices that are more effective at the individual level than current dietary advice, which has been set by government agencies and other health and sport organizations. Disclosure of genetic information has also been shown to enhance motivation and behavior change and strengthen adherence to the dietary recommendations provided (2–6). Although athletes tend to exhibit higher levels of motivation in general (7), nutrition professionals still encounter significant barriers to behavior change when counseling athletes on the adoption of beneficial sports nutrition practices (8, 9). A recent systematic review found that when genetic information included actionable advice, individuals were more likely to change health behaviors, including their dietary choices and intakes (10).
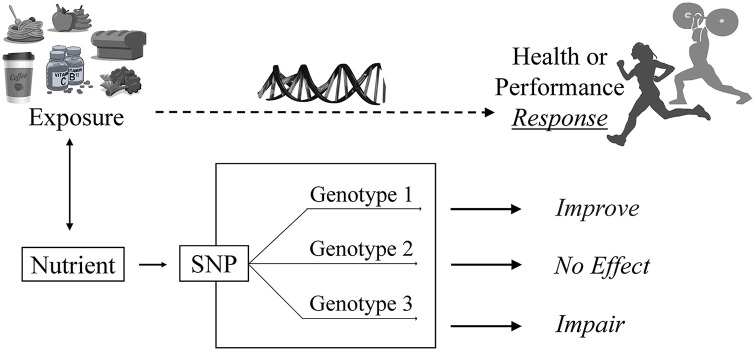
The practical application of the scientific knowledge gained from research on health and performance is to enable athletes to utilize genetic test results for personalized nutrition in an actionable manner. The demand for genetic testing for personalized nutrition and associated performance outcomes by athletes and active individuals is growing, and there is an increased need for dietitian-nutritionists, fitness professionals, coaches, and other sports medicine practitioners to understand the current evidence in this developing field (11–14). The sport environment is dynamic, progressive, innovative, and extremely competitive. Providing athletes with individually tailored dietary and other performance-related information based on their DNA could yield a competitive edge. The growing body of science in nutrition and genetics is the foundational building block by which practitioners can help athletes reach their genetic potential through implementation of dietary and supplement strategies that are aligned to their genetic makeup (Figure 1). Scientific advancements along with increased interest in genetic testing have resulted in a necessary growth for professional support, where tools for proficient and knowledgeable nutrition counseling based on genetics are now more widely available. For example, the Dietitians of Canada now offer a course on “Nutrigenomics: Genetic testing for personalized nutrition” as part of their online Learning-on-Demand portal.
Figure 1.
The nutrigenomics approach to sport nutrition. An athlete is exposed to a food, beverage, nutrient or bioactive. A genetic variant such as a single nucleotide polymorphism (SNP) associated with that exposure modifiers the individual's requirement for or response to that exposure. Their unique response depends on their version of the gene or “genotype.” For example, in the CYP1A2 rs726551 SNP, individuals with the AA genotype (fast metabolizers) experience a positive or “improved” response (i.e., performance) to caffeine. Individuals with the CYP1A2 AC or CC genotype experience no effect or impaired performance, respectively, from caffeine use (19).
Personalized nutrition, based on an individual's genotype, is not a novel concept, and there are several examples of rare (e.g., phenylketonuria) and common (e.g., lactose intolerance) genetic variants that require specific dietary strategies to manage (15). Although genetic testing is well-established in the clinical setting, there is a growth in opportunities to improve health, wellness and sport performance in athletes through nutrition-focused genetic testing. In the ongoing battles against dangerous supplements (16) and unprecedented numbers of doping violations (17, 18), the sport science community is seeking novel, yet evidence-based, approaches for athletes to gain a competitive edge which are safe, effective and legal. Personalized nutrition is not limited to the identification of genetic variants. Genotype is one aspect of personal information that can be used to individualize dietary advice. An individual's genetic profile as it relates to diet should be used combination with other relevant information such as sex, age, anthropometrics, health status, family history, and socioeconomic status along with dietary preferences and the presence of food intolerances or allergies. Accompanying blood work is also useful to evaluate current nutrition status and for ongoing monitoring.
Personalized dietary and supplement advice derived from genetic testing should be based on clear and defensible interpretations of relevant research studies. Traditional genome-wide association studies (GWAS) can be used to identify associations between genotypes and outcomes of interest such as blood levels of a micronutrient. However, the utility of such markers in providing actionable information on dietary advice is limited because it is not known what dietary intakes are required to counter effects of the genetic variant(s). For example, although a genetic variant that has been associated with low serum values of a vitamin is identified, a specific recommendation for intakes to prevent the risk of deficiency or to alleviate low levels of this micronutrient may remain undetermined. Such studies require the appropriate design that demonstrates how a genetic variant modifies the response to dietary intake on the outcome trait of interest and perhaps identify responders and non-responders. Genetic markers related to a performance trait, such as aerobic capacity or power, also provide little information on what factors could be used to improve the trait of interest.
With the exception of investigations exploring genetic variation and supplemental caffeine, which have been shown to modify endurance exercise outcomes (19, 20), there are few performance studies that have examined the role of genetics and other dietary factors on athletic outcomes. A gene-diet interaction may not be associated directly with a quantifiable performance outcome, such as increased aerobic capacity, speed or strength, but rather with intermediate biomarkers or phenotypes, such as body composition or circulating vitamin D levels, which are independent determinants of athletic performance, injury-risk and post-training recovery (1, 21–24). For example, it is well-known that low iron stores impact hemoglobin production which in turn decreases the oxygen carrying capacity of the blood, leading to a lack of oxygen to working muscles and resulting in impaired muscle contraction and aerobic endurance (21). As such, genetic markers that impact iron stores in response to intake can indirectly affect performance through the oxygen carrying capacity of hemoglobin (25, 26).
Sport Nutrigenomics Vs. Talent Identification and Exercise Prescription
In an effort to achieve specific sport goals, there is generally considerable overlap in the development of complementary training and dietary plans for athletes (27–29). However, it is essential to underscore the distinction between the strength of evidence supporting DNA-based advice for personalized nutrition vs. that for fitness programming. Despite the fervent interest and ubiquity of commercial genetic testing to assess and improve exercise or sport performance (30–32), it should be noted that there is a lack of evidence encompassing exercise prescription and talent identification, such as the ability to predict the likelihood for the next generation of Olympians (33, 34). Similarly, at this time there is insufficient evidence to recommended training protocols (strength or endurance) based on genotype or polygenic scores, that target specific fitness, weight loss or sport goals (35–38). The practical and ethical considerations of genetic testing for sports performance have also been described (39).
Some commercial genetic tests claim to use proprietary algorithmic approaches to prescribe training protocols based on evidence reported in peer-reviewed research (35). Although this may provide some initial supportive documentation for differing responses to training based on genotype, much larger sample sizes and improved methodologies are required, and should be pursued (36). The approach by which individuals are categorized as having an “endurance” or “power” advantage by genotype or being “responders” and “non-responders” to different training protocols, requires transparency and standardization across the field to avoid potential bias and to allow other researchers to replicate a study's methodology (37). Attempts to replicate studies to test training outcomes based on genotype require the use of the identical scoring systems and it appears that essential details of methods for grading of the strength of scientific evidence used in these scoring systems are not reported (35).
There is a considerable amount of ongoing research investigating individual variation in response to exercise training, however, sport and exercise genomics is still in its early stages and clinical or sport utility is lacking (36, 40–44). Mainstream testing for personalized training or exercise prescription based on genotype is not currently supported as a scientifically-sound approach, although it is likely to be a common and viably employed coaching tool within the next decade (35–37, 43, 44).
Genes Associated With Sport Nutrition
The objective of this review is to examine the scientific evidence on specific nutrients and food bioactives whereby genetic variants appear to modify individual responses related to athlete health and athletic performance. Although many studies reviewed herein have not been studied in athletes exclusively, they have been carried out in healthy individuals. Accordingly, several studies outlined reflect optimal health, body composition and nutritional status, which for athletes, provides the foundation for athletic success. Genetic variation impacting response to various micro- and macronutrients, as well as bioactives such as caffeine, on performance-related traits will be reviewed (Table 1).
Table 1.
Summary of Genetic Variants that modify the association between various dietary factors and performance-related outcomes.
| Gene (rs number) | Function | Dietary factor | Dietary sources | Performance-related outcome |
|---|---|---|---|---|
| CYP1A2 (rs762551) | Encodes CYP1A2 liver enzyme: metabolizes caffeine; identifies individuals as fast or slow metabolizers | Caffeine | Coffee, tea, soda, energy drinks, caffeine supplements | Cardiovascular health, endurance (21, 22, 57, 58) |
| ADORA2A (rs5751876) | Regulates myocardial oxygen demand; increases coronary circulation via vasodilation | Caffeine | Coffee, tea, soda, energy drinks, caffeine supplements | Vigilance when fatigued, sleep quality (49, 51–53) |
| BCMO1 (rs11645428) | Converts provitamin A carotenoids to Vitamin A | Vitamin A | Bluefin tuna, hard goat cheese, eggs, mackerel, carrots, sweet potato | Visuomotor skills and immunity (93, 95, 98–101) |
| MTHFR (rs1801133) | Produces the enzyme methylenetetrahydrofolate reductase, which is involved in the conversion of folic acid and folate into their biologically active form, L-methylfolate | Folate | Edamame, chicken liver, lentils, asparagus, black beans, kale, avocado | Megaloblastic anemia and hyperhomocysteinemia risk (112, 116–118) |
| HFE (rs1800562 and rs1799945) | Regulates intestinal iron uptake | Iron | Beef, chicken, fish, organ meats (heme iron); almonds, parsley, spinach (non-heme iron) | Hereditary hemochromatosis (130–132) |
| TMPRSS6 (rs4820268), TFR2 (rs7385804), TF (rs3811647) | Regulate the peptide hormone, hepcidin, which controls iron absorption | Iron | Beef, chicken, fish, organ meats (heme iron); almonds, parsley, spinach (non-heme iron) | Iron-deficiency anemia risk (24, 27, 120, 123–125) |
| FUT2 (rs602662) | Involved in vitamin B12 cell transport and absorption | Vitamin B12 | Clams, oysters, herring, nutritional yeast, beef, salmon | Megaloblastic anemia and hyperhomocysteinemia (142) |
| GSTT1 (Ins/Del) | Plays a role in vitamin C utilization via glutathione S-transferase enzymes | Vitamin C | Red peppers, strawberries, pineapple, oranges, broccoli | Circulating ascorbic acid levels Mitigate exercise-induced ROS production (153, 155) |
| GC (rs2282679) and CYP2R1 (rs10741657) | GC encodes vitamin D-binding protein, involved in binding and transporting vitamin D to tissues; CYP2R1 encodes the enzyme vitamin D 25-hydroxylase involved in vitamin D activation | Vitamin D | Salmon, white fish, rainbow trout, halibut, milk | Circulating 25(OH)D levels impacting immunity, bone health, inflammation, strength training and recovery (1, 162, 164, 166, 168) |
| GC (rs7041 and rs4588) | GC encodes vitamin D-binding protein, involved in binding and transporting vitamin D to tissues; Vitamin D is required for calcium absorption | Calcium | Yogurt, milk, cheese, firm tofu, canned salmon (with bones), edamame | Bone/stress fracture risk Muscle contraction, nerve conduction, blood clotting (162, 164, 166, 168) |
| PEMT (rs12325817) | Involved in endogenous choline synthesis via the hepatic phosphatidylethanolamine N-methyltransferase pathway | Choline | Eggs, beef, poultry, fish, shrimp, broccoli, salmon | Muscle or liver damage, reduced neurotransmitters (174, 175, 185, 186) |
| MTHFD1 (rs2236225) | Encodes protein involved in trifunctional enzyme activities related to metabolic handling of choline and folate | Folate/Choline | Folate: Edamame, chicken liver, lentils, asparagus, blck beans, kale, avocado Choline: Eggs, beef, poultry, fish, shrimp, broccoli, salmon |
Muscle or liver damage, reduced neurotransmitters (185, 186) |
| FTO (rs1558902/rs9939609) | Precise function undetermined; plays a role in metabolism and has been consistently linked to weight, BMI and body composition | Protein/SFA:PUFA | Protein: chicken, beef, tofu, salmon, cottage cheese, lentils, milk, Greek yogurt SFA: cheese, butter, red meat, baked goods PUFA: flaxseed oil, grape seed oil, sunflower oil |
Optimizing body composition (190, 191) |
| TCF7L2 (rs7903146) | Involved in expression of body fat | Fat | Nuts/seeds, butter, oils, cheese, red meat, high-fat dairy | Optimizing body composition (192, 193) |
| PPARγ2 (rs1801282) | Regulates adipocyte differentiation | MUFA | Macadamia nuts, almond butter, peanut butter, olive oil, canola oil, sesame oil | Optimizing body composition (194) |
Caffeine
Caffeine, found naturally occurring in several plant species including coffee, tea, cocoa, and guarana, is widely used in sport as a performance enhancer or ergogenic aid often in the form of caffeinated tablets, gels or chews.
In the field of nutrigenomics, caffeine is the most widely researched compound with several randomized controlled trials investigating the modifying effects of genetic variation on athletic performance (19, 20, 45). Numerous studies have investigated the effect of supplemental caffeine on exercise performance, but there is considerable inter-individual variability in the magnitude of these effects (46–48), or in the lack of an effect (49, 50) when compared to placebo. These inter-individual differences appear to be partly due, to variation in genes such as CYP1A2 and possibly ADORA2, which are associated with caffeine metabolism, sensitivity and response (51).
Over 95% of caffeine is metabolized by the CYP1A2 enzyme, which is encoded by the CYP1A2 gene (52). The−163A>C (rs762551) single nucleotide polymorphism (SNP) has been shown to alter CYP1A2 enzyme activity (53–55), and has been used to identify individuals as “fast” or “slow” metabolizers of caffeine. Individuals who are considered slow metabolizers, that is with the AC or CC genotype, have an elevated risk of myocardial infarction (56), hypertension and elevated blood pressure (57, 58), and pre-diabetes (59), with increasing caffeinated coffee consumption, whereas those with the AA genotype (fast metabolizers) do not appear to carry these risks.
The largest caffeine and exercise study to date (19), examined the effects of caffeine and CYP1A2 genotype, on 10-km cycling time trial performance in competitive male athletes after ingestion of caffeine at 0 mg, 2 mg (low dose) or 4 mg (moderate dose) per kg body mass. There was a 3% improvement in cycling time in the moderate dose in all subjects, which is consistent with previous cycling time trial studies using similar doses (46, 60). However, there was a significant caffeine-gene interaction where improvements in performance were seen at both caffeine doses, but only in those with the AA genotype who are “fast metabolizers” of caffeine. In that group, a 6.8% improvement in cycling time was observed at 4 mg/kg, which is >2–4% mean improvement seen in several other cycling time trial studies, using similar doses (46, 60–65). Among those with the CC genotype, 4 mg/kg caffeine impaired performance by 13.7%, and in those with the AC genotype there was no effect of either caffeine dose (19). The findings are consistent with a previous study (20), which observed a caffeine-gene interaction and improved time trial cycling performance with caffeine only in those with the AA genotype.
Some previous endurance-type studies either did not observe any impact of the CYP1A2 gene on caffeine-exercise studies (66, 67), or reported benefits only in slow metabolizers (45). There are several reasons that may explain discrepancies in study outcomes including smaller sample sizes (<20 subjects) that cause very low numbers and/or no subjects with the CC genotype (45, 67, 68), and shorter distance or different type (power vs. endurance) of performance test (45), compared to those that reported improved endurance after caffeine ingestion in those with the AA genotype of CYP1A2 (19, 20). The effects of genotype on performance appear to be most prominent during exercise of longer duration or an accumulation of fatigue (aerobic or muscular endurance) (69, 70). Fast metabolizers may quickly metabolize caffeine and achieve the benefits of caffeine metabolites as exercise progresses, or override the short duration of negative impacts (the initial stages of exercise), whereas the adverse effects of restricted blood flow and/or other impacts of adenosine blockage in slow metabolizers are likely to remain for a longer duration (71, 72). Indeed, in a study of basketball performance in elite players, caffeine improved repeated jumps (muscular endurance; an accumulation of fatigue), but only in those with the AA genotype, however, there was no genotype effect in the other two performance components of the basketball simulation (73). Similarly, a cross-over design of 30 resistance-trained men found that caffeine ingestion resulted in a higher number of repetitions in repeated sets of three different exercises, and for total repetitions in all resistance exercises combined, which resulted in a greater volume of work compared to placebo conditions, but only in those with the CYP1A2 AA genotype (74). Taken together, the weight of the evidence supports the role of CYP1A2 in modifying the effects of caffeine ingestion on aerobic or muscular endurance-type exercise.
The ADORA2A gene is another potential genetic modifier of the effects of caffeine on performance. The adenosine A2A receptor, encoded by the ADORA2A gene, has been shown to regulate myocardial oxygen demand and increase coronary circulation by vasodilation (71, 72). The A2A receptor is also expressed in the brain, where it regulates glutamate and dopamine release, with associated effects on insomnia and pain (75, 76). The antagonism of adenosine receptors by caffeine could differ by ADORA2A genotype, resulting in altered dopamine signaling (51). Dopamine has been associated with motivation and effort in exercising individuals, and this may be a mechanism by which differences in response to caffeine are manifested (77–79).
One small pilot study has examined the effect of ADORA2A genotype (rs5751876) on the ergogenic effects of caffeine under exercise conditions (80). Twelve female subjects underwent a double-blinded, crossover trial comprising two 10-min cycling time trials following caffeine ingestion or placebo. Caffeine benefitted all six subjects with the TT genotype but only one of the six C allele carriers. Further studies are needed to confirm these preliminary findings and include a larger sample to distinguish any effects between the different C allele carriers (i.e., CT vs. CC genotypes).
Sleep is recognized as an essential component of physiological and psychological recovery from, and preparation for, high-intensity training in athletes (81, 82). The ADORA2A rs5751876 genotype has also been implicated, by both objective and subjective measures, in various parameters of sleep quality after caffeine ingestion in several studies (83–86). Adenosine promotes sleep by binding to its receptors in the brain, mainly A1 and A2A receptors, and caffeine reverses these effects by blocking the adenosine receptor, which promotes wakefulness (83). This action, as well as the potency of caffeine to restore performance (cognitive or physical) in ecological situations, such as highway-driving during the night (87), support the notion that the adenosine neuromodulator/receptor system plays a major role in sleep–wake regulation. This action of caffeine may also serve athletes well under conditions of jetlag, and irregular or early training or competition schedules. Psychomotor speed relies on the ability to respond, rapidly and reliably, to randomly occurring stimuli which is a critical component of most sports (88). Genetic variation in ADORA2A has been shown to be a relevant determinant of psychomotor vigilance in the rested and sleep-deprived state and modulates individual responses to caffeine after sleep deprivation (85). In support of this notion, individuals who had the TT genotype for ADORA2A rs5751876 consistently had faster response times (in seconds) than C allele carriers after ingesting 400 mg caffeine during a sustained vigilant attention task after sleep loss (85).
Consistent with the “adenosine hypothesis” of sleep where the accumulation of adenosine in the brain promotes sleep, caffeine prolongs the time to fall asleep, decreases the deep stages of non-rapid-eye movement (nonREM) sleep, reduces sleep efficiency, and alters the waking and sleep electroencephalogram (EEG) frequencies, which reliably reflect the need for sleep (89–91). Although additional research in this area is warranted, genetic variation appears to contribute to subjective and objective responses to caffeine on sleep. Carriers of the ADORA2A (rs5751876) C allele have greater sensitivity toward caffeine-induced sleep disturbance compared to those with the TT genotype (84). Taken together, it appears that individuals with the TT genotype for the rs5751876 SNP in the ADORA2A gene may have better performance outcomes, faster response times and less sleep disturbance following caffeine ingestion.
Vitamin A
No studies have examined the role of genetic modifiers of vitamin A status directly on athletic performance, however, there are several important functions of this micronutrient that are associated with optimal health, immunity and performance in athletes.
Vitamin A is a fat-soluble vitamin, which plays a key role in both vision (92) and immunity (93) in its biologically active forms (retinal and retinoic acid). Vitamin A has diverse immune modulatory roles; hence, vitamin A deficiency has been associated with both immune dysfunctions in the gut, and several systemic immune disorders (93). Vitamin A is also a powerful antioxidant, protecting eyes from ocular diseases and helping to maintain vision (92).
High-performance athletes appear to have superior visual abilities based on their capacity to access distinct visual skills, such as contrast sensitivity, dynamic acuity, stereoacuity, and ocular judgment, needed to accomplish interceptive actions (e.g., hand-eye coordination) and resolve fine spatial detail, which is required by many sports (94, 95). In addition, slow visuomotor reaction time (VMRT) has been associated with musculoskeletal injury risk in sporting situations where there are greater challenges to visual stimulus detection and motor response execution (96). These visuomotor skills are key contributors to enhanced sport performance, and accordingly, require exceptional eye health.
Deficiencies of certain micronutrients such as vitamin A decrease immune defense against invading pathogens and can cause the athlete to be more susceptible to infection. Low energy availability (dieting), poor food choices, jetlag, physical and psychological stress, and exposure to pollution and foreign pathogens in air, food and water while traveling can result in a deterioration in immune function and increased susceptibility to illness (97). Athletes following high volume, high intensity training and competition schedules are also known to have more frequent upper respiratory tract infections (URTI) compared to both sedentary and moderately exercising populations (97).
Upon absorption, provitamin A carotenoids are readily converted to vitamin A by the BCMO1 enzyme expressed in enterocytes of the intestinal mucosa (98). β-Carotene is the most abundant provitamin A carotenoid in the diet and the conversion of beta-carotene to retinal or retinoic acid is necessary for vitamin A to exert its biological functions. The rs11645428 variant in the BCMO1 gene affects circulating plasma carotenoid levels by impacting the conversion of dietary provitamin A carotenoids to active forms of vitamin A in the small intestine (99). Individuals with the GG genotype are inefficient at this conversion, and may be at higher risk for vitamin A deficiency (100). These individuals are considered low responders to dietary β-carotene so consuming enough dietary pre-formed vitamin A (or supplements for vegans), can help to ensure that circulating levels of active vitamin A are adequate to support vision, immunity and normal growth and development.
Anemia-Related Micronutrients: Iron, Folate, and Vitamin B12
There is an abundance of research demonstrating the adverse effects of low iron storage and anemia on athletic performance (23, 101–103). The estimated prevalence of anemias and low levels of iron, folate, and vitamin B12 appear to be higher in elite-level athletes than in the general population, and these deficiencies can have significant negative impacts on performance (22, 23, 104–107). The most common symptoms of this disorder are fatigue, weakness and, in extreme cases, shortness of breath or palpitations (103).
The importance of iron to athletes is established through its biological role in supporting the function of proteins and enzymes essential for maintaining physical and cognitive performance (108). Iron is incorporated into hemoglobin and myoglobin, proteins responsible for the transport and storage of oxygen. Iron-deficiency anemia is the most common type of anemia among athletes, who have higher iron requirements due to increased erythropoietic drive through higher intensities and volumes of training. The female athlete is at particular risk of iron deficiency due to menstruation and generally, a lower total energy or food intake compared to males (107, 109). Along with dietary intake, footstrike hemolysis, gastrointestinal bleeding, exercise-induced inflammation, non-steroidal autoinflammatory drug (NSAID) use and environmental factors such as hypoxia (altitude), may influence iron metabolism in athletes of both sexes (23). Macrocytic anemias, which occur when erythrocytes are larger than normal, are generally classified into megaloblastic or nonmegaloblastic anemia. Megaloblastic anemia is caused by deficiency or impaired utilization of vitamin B12 and/or folate, whereas non-megaloblastic macrocytic anemia is caused by various diseases, and will not be discussed here (110). Other factors that are associated with anemia risk include genetic variation, which can alter micronutrient metabolism, transport or absorption, and can be used to identify individuals at risk of inadequate levels of vitamin B12, folate and iron stores.
Performance improvements are usually seen with the treatment of anemia (23, 103, 104), which is related to improvements in symptoms such as general feelings of fatigue and weakness, difficulty exercising, and in more severe cases, dyspnea and palpitations (103). Hyperhomocysteinemia, which can result from low folate and/or vitamin B12 intake, may also increase the risk of skeletal muscle malfunction, including muscle weakness and muscle regeneration, and will be discussed further below (111).
Folate
Methylene tetrahydrofolate reductase (MTHFR) is the rate-limiting enzyme in the methyl cycle, and is encoded by the MTHFR gene (112). The C677T (rs1801133) polymorphism in the MTHFR gene has been associated with low serum and red blood cell folate as well as elevated plasma homocysteine levels, which is an independent risk factor for cardiovascular disease (CVD) (113, 114). Several studies in athletic and non-athletic populations have shown that individuals with the CT or TT genotype are at an increased risk of low circulating folate levels when their diet is low in folate (115–118).
Although there are no studies examining performance outcomes related to MTHFR genotypes or dietary folate intake, hyperhomocysteinemia has been shown to be associated with diminished muscle function (111). Several studies conducted in older adults have found a significant association between elevated plasma homocysteine concentrations and declined physical function (119–122), which may be mediated by a reduction in strength (120). Compared to those with the rs1801133 CC genotype, individuals with TT genotype and possibly the CT genotype may be at a greater risk for hyperhomocysteinemia, although this may not be causative for lower physical performance (111, 119, 120). However, soccer players and sedentary individuals with the CC genotype have been shown to have more favorable body composition and performance measures such as aerobic and anaerobic threshold rates, compared to carriers of the T allele (118).
Iron Overload
Genetic variation associated with serum iron levels involves several genes such as HFE, TMPRSS6, TFR2, and TF (25, 117, 123–128). The HFE gene is involved in the regulation of intestinal iron uptake (129), and variations in this gene, which are not very common, have been shown to increase the risk for hemochromatosis or iron overload (124, 130). Excess iron may be toxic to tissues and cells because highly reactive “free” iron reacts with reactive oxygen species (ROS) such as superoxide and hydrogen peroxides, or lipid peroxides to produce free radicals (131). In turn, these free radicals can cause cell and tissue damage (including muscle) and, ultimately, lead to cell death (132). Elevated biomarkers of iron such as ferritin and transferrin are more common in those who are genetically predisposed to iron overload based on the HFE gene variant (22, 124). Interestingly, athletes with the rare HFE (rs1800562) AA genotype, which is associated with an increased risk for hemochromatosis, may be at a genetic advantage to excel in sport if iron levels are at the high end of the normal range, but not excessive to cause tissue damage. Notably, several studies have found that certain variants of the HFE gene that increase risk of iron overload are more common in elite-level athletes compared to the general population, suggesting this may be beneficial for performance (133–135).
Two SNPs in the HFE gene (rs1800562 and rs1799945) can be used to predict risk of hereditary hemochromatosis. Based on the combination of variants from these two SNPs, individuals can be categorized as having a high, medium, or low risk for iron overload (124, 128). While genetic risk for iron overload may have a favorable impact on performance, it is necessary for athletes with a medium or high risk to avoid iron supplementation as this could lead to adverse health outcomes (124) and diminished performance.
Low Iron Status
Three main SNPs: TMPRSS6 (rs4820268), TFR2 (rs7385804), TF (rs3811647) can be used to assess genetic risk for low iron status, primarily due to their involvement in regulating the expression of hepcidin, which is a peptide hormone that controls iron absorption (25, 123, 127). Iron-deficiency anemia impairs performance by reducing oxygen-carrying capacity, but a number of reports indicate that iron deficiency without anemia may affect physiological performance and work capacity as well (21), particularly in women who experience iron deficiency more frequently (22, 23).
There is a fine balance in achieving and maintaining adequate, but not excessive, iron levels for optimal performance. Individuals with the GG genotype in the TMPRSS6 gene have an increased risk of low transferrin saturation and hemoglobin, compared to those who are carriers of the A allele (25, 26, 123, 136). In the TF gene, individuals have a greater risk for low ferritin and elevated transferrin when they possess the AA genotype (25, 123, 136). Variation in the TFR2 gene can impact hematocrit, mean corpuscular volume, and red blood cell count where individuals with the CC genotype have an increased risk of low serum levels (25). Utilizing algorithms to assess various genotype combinations, these genes can help to determine an individual's overall risk for low iron status, which can lead to iron-deficiency anemia, and can be used to target dietary iron intake. Although iron supplementation is common and frequently prescribed in athletes, many individuals are at risk of taking iron supplements in excess (22, 137, 138). Although iron supplements are commonly “prescribed” by healthcare professionals and nutritionists (139, 140), excess iron stored in skeletal muscle may not only be dangerous to the health of the athlete (124, 141), but also can lead to oxidative stress and the formation of free radicals, and reduced athletic performance (135, 142, 143).
Vitamin B12
Vitamin B12 is also associated with RBC formation and aerobic capacity. Megaloblastic anemia results from vitamin B12 deficiency and is associated with elevated homocysteine, and results in general feelings of fatigue and weakness. Megaloblastic anemia limits the blood's oxygen carrying capacity, thus reducing its availability to cells (144). Variation in the FUT2 gene (rs602662) has a significant impact on serum B12 levels where individuals with GG or GA genotypes possess the greatest risk for low serum vitamin B12 levels, but only when the diet is low in bioavailable sources of vitamin B12 (145). This is consistent with previous genome-wide association studies, which found that individuals with the AA genotype had significantly higher concentrations of serum vitamin B12 compared to carriers of the G allele (145).
Vitamin C
Vitamin C is a water-soluble antioxidant that aids in the reduction of exercise-induced free-radical production (146). The production of potentially harmful ROS (147–149) in athletes is greater than in non-athletes due to the massive increases (up to 200-fold at the level of skeletal muscle) in oxygen consumption during strenuous exercise (146, 150). Vitamin C supplementation was once thought to mitigate this risk; however, studies have shown that excess vitamin C supplementation during endurance training can blunt beneficial training-induced physiological adaptations, such as muscle oxidative capacity and mitochondrial biogenesis and may actually diminish performance (148, 149, 151, 152). Dietary consumption of vitamin C, up to 250 mg daily from fruits and vegetables, is likely sufficient to reduce oxidative stress without having a negative effect on performance (151, 153). Additionally, collagen is a key constituent of connective tissue such as tendons and ligaments, and vitamin C is necessary for collagen production. This suggests that vitamin C may play a role in muscle growth and repair (154, 155). Indeed, a recent landmark study examining collagen synthesis in athletes, reported that adding a gelatin and vitamin C supplement to an intermittent exercise protocol improves collagen synthesis and could play a beneficial role in injury prevention and accelerate musculoskeletal, ligament, and/or tendon tissue repair (155).
The relationship between dietary vitamin C and circulating levels of ascorbic acid depend on an individual's GSTT1 genotype (156). Individuals who do not meet the Recommended Dietary Allowance (RDA) for vitamin C are significantly more likely to be vitamin C deficient (as assessed by serum ascorbic acid levels) than those who meet the RDA, but this effect is much greater in individuals with the GSTT1 Del/Del genotype than those with the Ins allele (156).
Genetic testing can help to identify athletes who may be at the greatest risk of low circulating vitamin C (ascorbic acid) levels in response to intake. These low circulating ascorbic acid levels may, in turn, diminish performance through an increased risk of high ROS and diminished muscle or connective tissue repair. Although studies have identified associations between circulating ascorbic acid concentrations and vitamin C transporters, SVCT1 and SVCT2, which are encoded by SLC23A1 and SLC23A2 (157), there is no evidence that response to vitamin C intake differs by genotype (158). As such, the use of variants in SLC23A1 and SLC23A2 to make personalized dietary recommendations is not supported by the studies to date.
Vitamin D
There are no studies that link genetic modifiers of vitamin D status on athletic performance outcomes; however, there are several functions of this vitamin that are associated with bone health, immunity, recovery from training and various performance variables. Genetic determinants of circulating 25-hydroxyvitamin D (25(OH)D) can influence each of these factors thereby influencing performance.
Vitamin D is essential to calcium metabolism, increasing calcium absorption for optimal bone health (1), which is relevant to all athletes, but particularly those participating in sports with a high risk of stress fracture (159–161). Research comparing individuals with sufficient levels to insufficient or deficient levels of 25(OH)D has shown that it helps to prevent injury (159–161), promote larger type II muscle fiber size (24), reduce inflammation (162), reduce risk of acute respiratory illness (159, 160) enhance functional rehabilitation (162), thereby optimizing recovery and acute adaptive responses to intense training through reduced inflammation and increased blood flow (163, 164).
Two genes that have been shown to impact vitamin D status are the GC gene and the CYP2R1 gene (165, 166). Variations in the GC and CYP2R1 genes are associated with a greater risk for low serum 25(OH)D. In one study (165), where 50% of participants took vitamin D supplements, only 22% of the participants had sufficient serum 25(OH)D levels. In the remaining 78% who had insufficient levels, also only about half (47%) took vitamin D supplements. Within this population, vitamin D supplementation only explained 18% of the variation, compared to 30% from genetics, suggesting that genetics may play a greater role than supplementation in determining risk for low 25(OH)D levels (165). Out of the four genotypes analyzed, only CYP2R1 (rs10741657) and GC (rs2282679) were significantly associated with vitamin D status. Specifically, participants with the GG or GA genotype of CYP2R1 (rs10741657) were nearly four times more likely to have insufficient vitamin D levels. Those with the GG genotype of the GC gene (rs2282679) were significantly more likely to have low vitamin D levels compared to those with the TT genotype (165). These results were consistent with findings from previous studies, including the Study of Underlying Genetic Determinants of Vitamin D and Highly Related Traits (SUNLIGHT), which found significance on a genome-wide basis in 15 cohorts with over 30,000 participants between three genetic variants including CYP2R1 (rs10741657) and GC (rs2282679) on vitamin D status. Not surprisingly, the number of risk variants that the participants possessed was directly related to their risk for vitamin D insufficiency (166). These findings demonstrate that genetic variation may be more impactful than supplementation intakes and behaviors on determining risk for vitamin D insufficiency.
Calcium
Although studies linking calcium intake, genetics and bone fracture has not been conducted in athletes specifically, genetic variation as it relates to risk of calcium deficiency and fracture risk have been studied in a large cohort of individuals, described below (167). Calcium is necessary for growth, maintenance and repair of bone tissue and impacts maintenance of blood calcium levels, regulation of muscle contraction, nerve conduction, and normal blood clotting (168). In order to absorb calcium, adequate vitamin D intake is also necessary. Inadequate dietary calcium and vitamin D increases the risk of low bone mineral density (BMD) and stress fractures. Low energy intakes, and menstrual dysfunction in female athletes, along with low vitamin D and calcium intakes further increase the risk of stress fractures in both males and females (169–171), and stress fractures are common and serious injuries in athletes (172).
Some individuals do not utilize dietary calcium as efficiently as others and this may depend on variations in the GC gene. In one study (167), subjects (n = 6,181) were genotyped for two SNPs in the GC gene, rs7041 and rs4588, and calcium intake was assessed in relation to the participants' risk for bone fracture (167). In the entire sample of participants, only a small increased risk of bone fracture was observed for individuals homozygous for the G allele of GC (rs7041) and the C allele of GC (rs4588). However, in participants with low dietary calcium intake (<1.09 g/day) and who were homozygous for the G allele of rs7041 and the C allele of rs4588, there was a 42% increased risk of fracture compared to other genotypes. No differences between genotypes were found in participants with high dietary calcium intakes (167). These findings suggest that calcium intake recommendations could be based on GC genotype in athletes to help prevent stress fracture.
Choline
Choline was officially recognized as an essential nutrient by the Institute of Medicine (IOM) in 1998 (173). Choline plays a central role in many physiological pathways including neurotransmitter synthesis (acetylcholine), cell-membrane signaling (phospholipids), bile and lipid transport (lipoproteins), and methyl-group metabolism (homocysteine reduction) (174). Human requirements for choline are dependent on gender, age and physical activity level as well as genetics. Choline is produced in the body in small amounts, however, de novo synthesis of choline alone is not sufficient to meet human requirements for optimal health (174, 175). The liver and muscles are the major organs for methyl group metabolism, and choline deficiency has been shown to cause both liver and muscle damage (176, 177). Signs of choline deficiency are identified through elevated serum creatine phosphokinase (CPK), a marker of muscle damage (178, 179), and abnormal deposition of fat in the liver, which may result in non-alcoholic fatty liver disease (NAFLD) (176, 180). Reductions in plasma choline associated with strenuous exercise such as triathlons and marathon running have been reported (181, 182). Acetylcholine, a neurotransmitter involved in learning, memory, and attention, depends on adequate choline and a reduction in the release of this neurotransmitter may contribute to the development of fatigue and exercise performance impairment (181–183). Choline supplementation may also improve lipid metabolism, as it has been associated with more favorable body composition (184) and the ability to aid rapid body mass reduction in weight class sports (185).
Common genetic variants in choline (PEMT gene) and a folate pathway enzyme (MTHFD1) have been shown to impact the metabolic handling of choline and the risk of choline deficiency across differing nutrient intakes (178, 186, 187). The relationship between genetic variants in folate metabolism and choline requirement may arise from the overlapping roles of folate and choline in methionine and phosphatidylcholine (PC) biosynthesis. PC is critical for the structural integrity of cell membranes and cell survival, and methionine is an essential amino acid that plays a critical role in human metabolism and health (187, 188). The MTHFD1 rs2236225 SNP, which is associated with folate metabolism, has been shown to increase the demands for choline as a methyl-group donor, thereby increasing dietary requirements for this nutrient (188). Individuals that are A allele carriers of the MTHFD1 gene have been shown to develop signs of choline deficiency and organ (liver and muscle) dysfunction compared to those with the GG genotype (186, 188, 189).
While humans can make choline endogenously via the hepatic phosphatidylethanolamine N-methyltransferase (PEMT) pathway, a SNP in the PEMT gene (rs12325817) has been shown to influence the risk of choline deficiency and the partitioning of more dietary choline toward PC biosynthesis at the expense of betaine synthesis (used a methyl donor) (186). Individuals who are C allele carriers of the PEMT gene have been shown to develop signs of choline deficiency and organ (liver and muscle) dysfunction compared to those with the GG genotype (178).
Athletes by nature experience muscle damage through high volume and high intensity training (195). A deficient or suboptimal status of choline may place additional stressors on an athlete's ability to recover, repair and adapt to their given training stimulus.
Macronutrients and Body Composition
Several aspects of physique such as body size, shape and composition contribute to the success of an athlete, in most sports. In the athletic population, body composition is often the focus for change, as it can be easily manipulated through diet as both total energy intake and macronutrient composition (192, 196). Variations in macronutrient intake can significantly impact both body fat percentage and lean mass (29, 190, 193, 197–199), as well as performance, where macronutrient manipulation has long been used to partition calories to be used for specific goals across different sports (196).
Although research examining dietary factors and genetics has revealed that manipulation of dietary fat and protein intakes may have greater modifying effects on body composition than carbohydrates, all macronutrients serve a critical purpose. Carbohydrates provide a key fuel for the brain, CNS and working muscles, and the amount and timing of intake impacts sport performance over a large range of intensities (200, 201). Adequate dietary protein is essential for strength and lean body mass accretion, while also playing a relevant role in preserving lean body mass during caloric restriction and immune function (29, 202, 203). Dietary fat provides energy for aerobic activities and is required for the absorption of fat-soluble vitamins (204). Recent research shows that percent energy intake from protein and fat can be targeted to the individual based on genetic variation for optimizing body weight and composition (190, 193, 197–199). Percent energy from carbohydrates should be guided by fuel needs for training and competition while also considering targeted protein and fat intakes based on genetic variation.
Protein
The FTO gene is also known as the ‘fat mass and obesity-associated gene’ since it has been shown to impact weight management and body composition (194, 199, 205, 206). Dietary interventions may mitigate genetic predispositions associated with a higher body mass index (BMI) and body fat percentage, as determined by genetic variation in the FTO gene. Specifically, the Preventing Overweight Using Novel Dietary Strategies (POUNDS Lost) multicenter trial found that carrying an A allele of the FTO gene (rs1558902–a surrogate marker for rs9939609) and consuming a high protein diet was associated with a significantly lower fat mass at the 2-year follow up period compared to carrying two T alleles. Importantly, participants with the AA genotype (lesser effects in those with AT genotype) who were following the high protein diet protocol had significantly greater losses of total fat mass, total adipose tissue, visceral adipose tissue, lower total percent fat mass and percent trunk fat, compared to those following a lower protein diet protocol (199). Other studies have shown similar results where dietary protein intake was shown to be protective against the effect of the FTO risk variants on BMI and waist circumference (194). A randomized controlled trial (RCT) in 195 individuals showed that a hypocaloric diet resulted in greater weight loss in rs9939609 A allele carriers than noncarriers in both higher and lower protein diets, although metabolic improvements improved in all genotypes in the higher protein diets (205). Athletes who possess the AA genotype of the FTO gene at rs1558902 would benefit the most in terms of consuming a moderate-to-high protein diet (at least 25% of energy from protein) to optimize body composition. Greater lean mass in athletes has been associated with improved performance in strength and power sports, as well as some endurance events, and a decreased risk for injuries (191, 207). For those athletes who do not possess the response variant (i.e., greater fat loss with higher protein intakes), following a diet with moderate protein intake (~15–20% energy), to achieve and maintain an ideal body composition is important to note, as excess protein calories may be counterproductive toward this goal. In this instance, dietary goals for optimal performance may be better met by substituting protein energy for other macronutrients such as carbohydrates for fuel, fiber, prebiotics and other micronutrients, or by increasing intakes of essential fats.
Dietary Fat
Dietary fat, an essential component of the human diet, provides energy for aerobic endurance exercise and is necessary for the absorption of the fat-soluble vitamins A, D, E, and K. Independent of total energy intake, the percentage of energy derived from fat in an athlete's diet can impact body composition, based on genetic variation (204). Individuals possessing the TT genotype of TCF7L2, transcription factor 7 like 2, at rs7903146 appear to benefit from consuming a lower percent of total energy from fat (20–25% of energy) to optimize body composition (198). Specifically, participants with the TT genotype lost more fat mass when they were consuming a low-fat diet, compared to a high-fat diet (40–45% of energy) (198). Moreover, individuals with the CC genotype in rs7903146 who consumed lower-fat diets actually lost significantly more lean mass, suggesting that these individuals should avoid low-fat nutrition interventions (197) in order to optimize body composition for athletic performance (191, 207). Body composition can, therefore, be optimized by targeting fat intake based on genetic variation in the TCF7L2 gene.
Monounsaturated Fat
Recommendations for fat intake can be further targeted to the different types of fats comprising total dietary fat. Athletes with the GG or GC genotype of the PPARγ2 gene at rs1801282 would benefit from a weight loss intervention that specifically targets body fat, while preserving lean body mass. Such individuals have been shown to demonstrate an enhanced weight loss response when consuming > 56% of total fat from monounsaturated fatty acids (MUFAs) compared to those with the GG or GC genotype who consume < 56% of total fat from MUFAs. These results have not been found in those with the CC genotype of PPARγ2 at rs1801282 (208).
MUFAs can be targeted in athletes who are aiming to decrease their body fat. It is well-known that a lower body fat percentage is associated with enhanced performance in most sports (191, 207), however, sport clinicians must be cautious about nutrition recommendations aimed at reducing body fat. Striving for very low levels of body fat is highly correlated with the Relative Energy Deficiency in Sport (RED-S) syndrome in both females and males, which refers to ‘impaired physiological functioning caused by relative energy deficiency and includes impairments of metabolic rate, menstrual function, bone health, immunity, protein synthesis and cardiovascular health (209).
Saturated Fat and Polyunsaturated Fat
A nested case-control study found that the ratio of dietary saturated fatty acids (SFA) to polyunsaturated fatty acids (PUFA) influenced the risk of obesity associated with the TA and AA variants of the FTO gene at rs9939609 (210). Specifically, participants possessing the A allele had a significantly higher BMI and waist circumference (WC) compared to TT homozygotes, but only when intakes of SFA were high and PUFAs were low. When participants with the A allele consumed < ~15% of energy from SFA and had a higher dietary PUFA:SFA ratio, there were no significant differences in WC and BMI between this group and participants with the TT genotype of rs9939609 (210). These findings have implications for nutrition counseling impacting body composition (abdominal fat specifically) and BMI. Athletes with the TA or AA genotype may have a greater risk for accumulating excessive abdominal fat. An athlete can mitigate this risk by aiming to consume <10% of energy from SFA (to also account for heart health) and > 4% of energy from PUFAs, resulting in a PUFA:SFA ratio of at least 0.4 (210).
Summary
This paper provided an overview of the current science linking genetic variation to nutritional or supplemental needs with a focus on sport performance. One of the ultimate goals in the field of personalized sport nutrition is the design of tailored nutritional recommendations to improve direct and indirect factors that influence athletic performance. More specifically, personalized nutrition pursuits aim to develop more comprehensive and dynamic nutritional and supplement recommendations based on shifting, interacting parameters in an athlete's internal and external (sport) environment throughout their athletic career and beyond.
Currently, there are few gene-diet interaction studies that have directly measured performance outcomes and been conducted in competitive athletes, so this should be a focus of future research. However, it has been established that serum levels and/or dietary intakes of several nutrients and food bioactives can impact overall health, body composition and in turn result in modest to sizable modifying effects in athletic performance. The strongest evidence to date appears to be for caffeine on endurance performance with several trials demonstrating the modifying effects of genetic variants with sports performance outcomes. Genetic testing for personalized nutrition may, therefore, be an additional tool that can be implemented into the practice of sport clinicians, nutritionists and coaches to guide nutritional counseling and meal planning with the aim of optimizing athletic performance.
Author Contributions
NG and AE-S conceived of the original idea for the review and NG wrote the first draft. All authors contributed to the writing and editing of the manuscript. AE-S secured funding.
Conflict of Interest Statement
AE-S is the Founder and holds shares in Nutrigenomix Inc. NG is on the Science Advisory Board of Nutrigenomix Inc. SV was a paid employee and remains a paid consultant with Nutrigenomix. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. Funding support for this review was provided by Mitacs and Nutrigenomix Inc.
References
- 1.Thomas DT, Erdman KA, Burke LM. American college of sports medicine joint position statement. nutrition and athletic performance. Med Sci Sports Exerc. (2016) 48:543–68. 10.1249/MSS.0000000000000852 [DOI] [PubMed] [Google Scholar]
- 2.Nielsen DE, El-Sohemy A. Disclosure of genetic information and change in dietary intake: a randomized controlled trial. PLoS ONE (2014) 9:e112665. 10.1371/journal.pone.0112665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hietaranta-Luoma HL, Tahvonen R, Iso-Touru T, Puolijoki H, Hopia A. An intervention study of individual, apoE genotype-based dietary and physical-activity advice: impact on health behavior. J Nutrigenet Nutrigenomics (2014) 7:161–74. 10.1159/000371743 [DOI] [PubMed] [Google Scholar]
- 4.Livingstone KM, Celis-Morales C, Navas-Carretero S, San-Cristobal R, Macready AL, Fallaize R, et al. Effect of an Internet-based, personalized nutrition randomized trial on dietary changes associated with the Mediterranean diet: the Food4Me Study. Am J Clin Nutr. (2016) 104:288–97. 10.3945/ajcn.115.129049 [DOI] [PubMed] [Google Scholar]
- 5.Celis-Morales C, Marsaux CF, Livingstone KM, Navas-Carretero S, San-Cristobal R, Fallaize R, et al. Can genetic-based advice help you lose weight? Findings from the Food4Me European randomized controlled trial. Am J Clin Nutr. (2017) 105:1204–1213. 10.3945/ajcn.116.145680 [DOI] [PubMed] [Google Scholar]
- 6.El-Sohemy A. Only DNA-based dietary advice improved adherence to the Mediterranean diet score. Am J Clin Nutr. (2017) 105:770. 10.3945/ajcn.116.149021 [DOI] [PubMed] [Google Scholar]
- 7.Keegana RJ, Harwood CG, Spray CM, Lavalleec D. A qualitative investigation of the motivational climate in elite sport. Psychol Sport Exerc. (2014) 15:97–107. 10.1016/j.psychsport.2013.10.006 [DOI] [Google Scholar]
- 8.Spronk I, Heaney SE, Prvan T, O'Connor HT. Relationship between general nutrition knowledge and dietary quality in elite athletes. Int J Sport Nutr Exerc Metab. (2015) 25:243–51. 10.1123/ijsnem.2014-0034 [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Roves PM, Garcia-Zapico P, Patterson AM, Iglesias-Gutierrez E. Nutrient intake and food habits of soccer players: analyzing the correlates of eating practice. Nutrients (2014) 6:2697–717. 10.3390/nu6072697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horne J, Madill J, O'Connor C, Shelley J, Gilliland J. A systematic review of genetic testing and lifestyle behaviour change: are we using high-quality genetic interventions and considering behaviour change theory? Lifestyle Genom. (2018) 11:49–63. 10.1159/000488086 [DOI] [PubMed] [Google Scholar]
- 11.Kicklighter JR, Dorner B, Hunter AM, Kyle M, Pflugh Prescott M, Roberts S, et al. Visioning report 2017: a preferred path forward for the nutrition and dietetics profession. J Acad Nutr Diet. (2017) 117:110–127. 10.1016/j.jand.2016.09.027 [DOI] [PubMed] [Google Scholar]
- 12.Collins J, Adamski MM, Twohig C, Murgia C. Opportunities for training for nutritional professionals in nutritional genomics: what is out there? Nutr Diet. (2018) 75:206–18. 10.1111/1747-0080.12398 [DOI] [PubMed] [Google Scholar]
- 13.Abrahams M, Frewer L, Bryant E, Stewart-Knox B. Factors determining the integration of nutritional genomics into clinical practice by registered dietitians. Trends Food Sci Technol. (2017) 59:139–47 10.1016/j.tifs.2016.11.005 [DOI] [Google Scholar]
- 14.Cormier H, Tremblay BL, Paradis AM, Garneau V, Desroches S, Robitaille J, et al. Nutrigenomics-perspectives from registered dietitians: a report from the Quebec-wide e-consultation on nutrigenomics among registered dietitians. J Hum Nutr Diet. (2014) 27:391–400. 10.1111/jhn.12194 [DOI] [PubMed] [Google Scholar]
- 15.Gorman U, Mathers JC, Grimaldi KA, Ahlgren J, Nordstrom K. Do we know enough? A scientific and ethical analysis of the basis for genetic-based personalized nutrition. Genes Nutr. (2013) 8:373–81. 10.1007/s12263-013-0338-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maughan RJ. Quality assurance issues in the use of dietary supplements, with special reference to protein supplements. J Nutr. (2013) 143:1843S−7S. 10.3945/jn.113.176651 [DOI] [PubMed] [Google Scholar]
- 17.Pitsiladis Y, Ferriani I, Geistlinger M, de Hon O, Bosch A, Pigozzi F. A holistic antidoping approach for a fairer future for sport. Curr Sports Med Rep. (2017) 16:222–4. 10.1249/JSR.0000000000000384 [DOI] [PubMed] [Google Scholar]
- 18.de Hon O, Kuipers H, van Bottenburg M. Prevalence of doping use in elite sports: a review of numbers and methods. Sports Med. (2015) 45:57–69. 10.1007/s40279-014-0247-x [DOI] [PubMed] [Google Scholar]
- 19.Guest N, Corey P, Vescovi J, El-Sohemy A. Caffeine, CYP1A2 genotype, and endurance performance in athletes. Med Sci Sports Exerc. (2018) 50:1570–8. 10.1249/MSS.0000000000001596 [DOI] [PubMed] [Google Scholar]
- 20.Womack CJ, Saunders MJ, Bechtel MK, Bolton DJ, Martin M, Luden ND, et al. The influence of a CYP1A2 polymorphism on the ergogenic effects of caffeine. J Int Soc Sports Nutr. (2012) 9:7. 10.1186/1550-2783-9-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haas JD, Brownlie TIV. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr. (2001) 131:676S−688S; discussion 688S-690S. 10.1093/jn/131.2.676S [DOI] [PubMed] [Google Scholar]
- 22.Clenin G, Cordes M, Huber A, Schumacher YO, Noack P, Scales J, et al. Iron deficiency in sports-definition, influence on performance and therapy. Swiss Med Wkly. (2015) 145:w14196. 10.4414/smw.2015.14196 [DOI] [PubMed] [Google Scholar]
- 23.DellaValle DM. Iron supplementation for female athletes: effects on iron status and performance outcomes. Curr Sports Med Rep. (2013) 12:234–9. 10.1249/JSR.0b013e31829a6f6b [DOI] [PubMed] [Google Scholar]
- 24.Cannell JJ, Hollis BW, Sorenson MB, Taft TN, Anderson JJ. Athletic performance and vitamin D. Med Sci Sports Exerc. (2009) 41:1102–10. 10.1249/MSS.0b013e3181930c2b [DOI] [PubMed] [Google Scholar]
- 25.Pichler I, Minelli C, Sanna S, Tanaka T, Schwienbacher C, Naitza S, et al. Identification of a common variant in the TFR2 gene implicated in the physiological regulation of serum iron levels. Hum Mol Genet. (2011) 20:1232–40. 10.1093/hmg/ddq552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du X, She E, Gelbart T, Truksa J, Lee P, Xia Y, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science (2008) 320:1088–92. 10.1126/science.1157121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia M, Martinez-Moreno JM, Reyes-Ortiz A, Suarez Moreno-Arrones L. Garcia AA, Garciacaballero M. Changes in body composition of high competition rugby players during the phases of a regular season; influence of diet and exercise load. Nutr Hosp. (2014) 29:913–21. 10.3305/nh.2014.29.4.7227 [DOI] [PubMed] [Google Scholar]
- 28.Pyne DB, Sharp RL. Physical and energy requirements of competitive swimming events. Int J Sport Nutr Exerc Metab. (2014) 24:351–9. 10.1123/ijsnem.2014-0047 [DOI] [PubMed] [Google Scholar]
- 29.Longland TM, Oikawa SY, Mitchell CJ, Devries MC, Phillips SM. Higher compared with lower dietary protein during an energy deficit combined with intense exercise promotes greater lean mass gain and fat mass loss: a randomized trial. Am J Clin Nutr. (2016) 103:738–46. 10.3945/ajcn.115.119339 [DOI] [PubMed] [Google Scholar]
- 30.Guth LM, Roth SM. Genetic influence on athletic performance. Curr Opin Pediatr. (2013) 25:653–8. 10.1097/MOP.0b013e3283659087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattsson CM, Wheeler MT, Waggott D, Caleshu C, Ashley EA. Sports genetics moving forward: lessons learned from medical research. Physiol Genomics (2016) 48:175–82. 10.1152/physiolgenomics.00109.2015 [DOI] [PubMed] [Google Scholar]
- 32.Varley I, Patel S, Williams AG, Hennis PJ. The current use, and opinions of elite athletes and support staff in relation to genetic testing in elite sport within the UK. Biol Sport. (2018) 35:13–9. 10.5114/biolsport.2018.70747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webborn N, Williams A, McNamee M, Bouchard C, Pitsiladis Y, Ahmetov I, et al. Direct-to-consumer genetic testing for predicting sports performance and talent identification: consensus statement. Br J Sports Med. (2015) 49:1486–91. 10.1136/bjsports-2015-095343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roth SM. Critical overview of applications of genetic testing in sport talent identification. Recent Pat DNA Gene Seq. (2012) 6:247–55. 10.2174/187221512802717402 [DOI] [PubMed] [Google Scholar]
- 35.Jones N, Kiely J, Suraci B, Collins DJ, de Lorenzo D, Pickering C, et al. A genetic-based algorithm for personalized resistance training. Biol Sport. (2016) 33:117–26. 10.5604/20831862.1198210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vlahovich N, Hughes DC, Griffiths LR, Wang G, Pitsiladis YP, Pigozzi F, et al. Genetic testing for exercise prescription and injury prevention: AIS-Athlome consortium-FIMS joint statement. BMC Genomics (2017) 18(Suppl. 8):818. 10.1186/s12864-017-4185-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karanikolou A, Wang G, Pitsiladis Y. Letter to the editor: a genetic-based algorithm for personalized resistance training. Biol Sport. (2017) 34:31–33. 10.5114/biolsport.2017.63385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vlahovich N, Fricker PA, Brown MA, Hughes D. Ethics of genetic testing and research in sport: a position statement from the Australian Institute of Sport. Br J Sports Med. (2017) 51:5–11. 10.1136/bjsports-2016-096661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams AG, Wackerhage H, Day SH. Genetic testing for sports performance, responses to training and injury risk: practical and ethical considerations. Med Sport Sci. (2016) 61:105–19. 10.1159/000445244 [DOI] [PubMed] [Google Scholar]
- 40.Mann TN, Lamberts RP, Lambert MI. High responders and low responders: factors associated with individual variation in response to standardized training. Sports Med. (2014) 44:1113–24. 10.1007/s40279-014-0197-3 [DOI] [PubMed] [Google Scholar]
- 41.Williams CJ, Williams MG, Eynon N, Ashton KJ, Little JP, Wisloff U, et al. Genes to predict VO2max trainability: a systematic review. BMC Genomics (2017) 18(Suppl. 8):831. 10.1186/s12864-017-4192-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan X, Eynon N, Papadimitriou ID, Kuang J, Munson F, Tirosh O, et al. The gene SMART study: method, study design, and preliminary findings. BMC Genomics (2017) 18(Suppl. 8):821. 10.1186/s12864-017-4186-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pitsiladis YP, Tanaka M, Eynon N, Bouchard C, North KN, Williams AG, et al. Athlome Project Consortium: a concerted effort to discover genomic and other “omic” markers of athletic performance. Physiol Genomics (2016) 48:183–90. 10.1152/physiolgenomics.00105.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahmetov II, Fedotovskaya ON. Current progress in sports genomics. Adv Clin Chem. (2015) 70:247–314. 10.1016/bs.acc.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 45.Pataky MW, Womack CJ, Saunders MJ, Goffe JL, D'Lugos AC, El-Sohemy A, et al. Caffeine and 3-km cycling performance: effects of mouth rinsing, genotype, and time of day. Scand J Med Sci Sports. (2016) 26:613–9. 10.1111/sms.12501 [DOI] [PubMed] [Google Scholar]
- 46.Ganio MS, Klau JF, Casa DJ, Armstrong LE, Maresh CM. Effect of caffeine on sport-specific endurance performance: a systematic review. J Strength Cond Res. (2009) 23:315–24. 10.1519/JSC.0b013e31818b979a [DOI] [PubMed] [Google Scholar]
- 47.Higgins S, Straight CR, Lewis RD. The effects of preexercise caffeinated coffee ingestion on endurance performance: an evidence-based review. Int J Sport Nutr Exerc Metab. (2016) 26:221–39. 10.1123/ijsnem.2015-0147 [DOI] [PubMed] [Google Scholar]
- 48.Graham TE, Spriet LL. Performance and metabolic responses to a high caffeine dose during prolonged exercise. J Appl Physiol. (1985). (1991) 71:2292–8. [DOI] [PubMed] [Google Scholar]
- 49.Hunter AM, St Clair Gibson A, Collins M, Lambert M, Noakes TD. Caffeine ingestion does not alter performance during a 100-km cycling time-trial performance. Int J Sport Nutr Exerc Metab. (2002) 12:438–52. 10.1123/ijsnem.12.4.438 [DOI] [PubMed] [Google Scholar]
- 50.Roelands B, Buyse L, Pauwels F, Delbeke F, Deventer K, Meeusen R. No effect of caffeine on exercise performance in high ambient temperature. Eur J Appl Physiol. (2011) 111:3089–95. 10.1007/s00421-011-1945-9 [DOI] [PubMed] [Google Scholar]
- 51.Yang A, Palmer AA, de Wit H. Genetics of caffeine consumption and responses to caffeine. Psychopharmacology (Berl). (2010) 211:245–57. 10.1007/s00213-010-1900-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Begas E, Kouvaras E, Tsakalof A, Papakosta S, Asprodini EK. In vivo evaluation of CYP1A2, CYP2A6, NAT-2 and xanthine oxidase activities in a Greek population sample by the RP-HPLC monitoring of caffeine metabolic ratios. Biomed Chromatogr. (2007) 21:190–200. 10.1002/bmc.736 [DOI] [PubMed] [Google Scholar]
- 53.Ghotbi R, Christensen M, Roh HK, Ingelman-Sundberg M, Aklillu E, Bertilsson L. Comparisons of CYP1A2 genetic polymorphisms, enzyme activity and the genotype-phenotype relationship in Swedes and Koreans. Eur J Clin Pharmacol. (2007) 63:537–46. 10.1007/s00228-007-0288-2 [DOI] [PubMed] [Google Scholar]
- 54.Djordjevic N, Ghotbi R, Bertilsson L, Jankovic S, Aklillu E. Induction of CYP1A2 by heavy coffee consumption in Serbs and Swedes. Eur J Clin Pharmacol. (2008) 64:381–5. 10.1007/s00228-007-0438-6 [DOI] [PubMed] [Google Scholar]
- 55.Koonrungsesomboon N, Khatsri R, Wongchompoo P, Teekachunhatean S. The impact of genetic polymorphisms on CYP1A2 activity in humans: a systematic review and meta-analysis. Pharmacogenomics J. (2017) 18:760–8. 10.1038/s41397-017-0011-3 [DOI] [PubMed] [Google Scholar]
- 56.Cornelis MC, El-Sohemy A, Kabagambe EK, Campos H. Coffee, CYP1A2 genotype, and risk of myocardial infarction. JAMA (2006) 295:1135–41. 10.1001/jama.295.10.1135 [DOI] [PubMed] [Google Scholar]
- 57.Palatini P, Ceolotto G, Ragazzo F, Dorigatti F, Saladini F, Papparella I, et al. CYP1A2 genotype modifies the association between coffee intake and the risk of hypertension. J Hypertens. (2009) 27:1594–601. 10.1097/HJH.0b013e32832ba850 [DOI] [PubMed] [Google Scholar]
- 58.Soares RN, Schneider A, Valle SC, Schenkel PC. The influence of CYP1A2 genotype in the blood pressure response to caffeine ingestion is affected by physical activity status and caffeine consumption level. Vascul Pharmacol. (2018) 106:67–73. 10.1016/j.vph.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 59.Palatini P, Benetti E, Mos L, Garavelli G, Mazzer A, Cozzio S, et al. Association of coffee consumption and CYP1A2 polymorphism with risk of impaired fasting glucose in hypertensive patients. Eur J Epidemiol. (2015) 30:209–17. 10.1007/s10654-015-9990-z [DOI] [PubMed] [Google Scholar]
- 60.Desbrow B, Biddulph C, Devlin B, Grant GD, Anoopkumar-Dukie S, Leveritt MD. The effects of different doses of caffeine on endurance cycling time trial performance. J Sports Sci. (2012) 30:115–20. 10.1080/02640414.2011.632431 [DOI] [PubMed] [Google Scholar]
- 61.Skinner TL, Jenkins DG, Taaffe DR, Leveritt MD, Coombes JS. Coinciding exercise with peak serum caffeine does not improve cycling performance. J Sci Med Sport. (2013) 16:54–9. 10.1016/j.jsams.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 62.Saunders B, de Oliveira LF, da Silva RP, de Salles Painelli V, Goncalves LS, Yamaguchi G, et al. Placebo in sports nutrition: a proof-of-principle study involving caffeine supplementation. Scand J Med Sci Sports. (2016) 27:1240–7. 10.1111/sms.12793 [DOI] [PubMed] [Google Scholar]
- 63.Jenkins NT, Trilk JL, Singhal A, O'Connor PJ, Cureton KJ. Ergogenic effects of low doses of caffeine on cycling performance. Int J Sport Nutr Exerc Metab. (2008) 18:328–42. 10.1123/ijsnem.18.3.328 [DOI] [PubMed] [Google Scholar]
- 64.Graham-Paulson T, Perret C, Goosey-Tolfrey V. Improvements in cycling but not handcycling 10 km time trial performance in habitual caffeine users. Nutrients (2016) 8:E393 10.3390/nu8070393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bortolotti H, Altimari LR, Vitor-Costa M, Cyrino ES. Performance during a 20-km cycling time-trial after caffeine ingestion. J Int Soc Sports Nutr. (2014) 11:45. 10.1186/s12970-014-0045-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Algrain HA, Thomas RM, Carrillo AE, Ryan EJ, Kim C-H, Lettan RBII, et al. The Effects of a Polymorphism in the cytochrome P450 CYP1A2 gene on performance enhancement with caffeine in recreational cyclists. J Caffeine Res. (2016) 6:34–39. 10.1089/jcr.2015.0029 [DOI] [Google Scholar]
- 67.Salinero JJ, Lara B, Ruiz-Vicente D, Areces F, Puente-Torres C, Gallo-Salazar C, et al. CYP1A2 Genotype variations do not modify the benefits and drawbacks of caffeine during exercise: a pilot study. Nutrients (2017) 9:E269 10.3390/nu9030269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Joy E, De Souza MJ, Nattiv A, Misra M, Williams NI, Mallinson RJ, et al. 2014 female athlete triad coalition consensus statement on treatment and return to play of the female athlete triad. Curr Sports Med Rep. (2014) 13:219–32. 10.1249/JSR.0000000000000077 [DOI] [PubMed] [Google Scholar]
- 69.Doherty M, Smith PM. Effects of caffeine ingestion on exercise testing: a meta-analysis. Int J Sport Nutr Exerc Metab. (2004) 14:626–46. 10.1123/ijsnem.14.6.626 [DOI] [PubMed] [Google Scholar]
- 70.Shen JG, Brooks MB, Cincotta J, Manjourides JD. Establishing a relationship between the effect of caffeine and duration of endurance athletic time trial events: a systematic review and meta-analysis. J Sci Med Sport. (2018) 22:232–8. 10.1016/j.jsams.2018.07.022 [DOI] [PubMed] [Google Scholar]
- 71.Higgins JP, Babu KM. Caffeine reduces myocardial blood flow during exercise. Am J Med. (2013) 126:730 e1–8. 10.1016/j.amjmed.2012.12.023 [DOI] [PubMed] [Google Scholar]
- 72.Namdar M, Schepis T, Koepfli P, Gaemperli O, Siegrist PT, Grathwohl R, et al. Caffeine impairs myocardial blood flow response to physical exercise in patients with coronary artery disease as well as in age-matched controls. PLoS ONE (2009) 4:e5665. 10.1371/journal.pone.0005665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Puente C, Abian-Vicen J, Del Coso J, Lara B, Salinero JJ. The CYP1A2−163C>A polymorphism does not alter the effects of caffeine on basketball performance. PLoS ONE (2018) 13:e0195943 10.1371/journal.pone.0195943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rahimi R. The effect of CYP1A2 genotype on the ergogenic properties of caffeine during resistance exercise: a randomized, double-blind, placebo-controlled, crossover study. Ir J Med Sci. (2018) 12:1–9. 10.1007/s11845-018-1780-7 [DOI] [PubMed] [Google Scholar]
- 75.Fried NT, Elliott MB, Oshinsky ML. The role of adenosine signaling in headache: a review. Brain Sci. (2017) 7:30. 10.3390/brainsci7030030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Urry E, Landolt HP. Adenosine, caffeine, and performance: from cognitive neuroscience of sleep to sleep pharmacogenetics. Curr Top Behav Neurosci. (2015) 25:331–66. 10.1007/7854_2014_274 [DOI] [PubMed] [Google Scholar]
- 77.Meeusen R, Roelands B, Spriet LL. Caffeine, exercise and the brain. Nestle Nutr Inst Workshop Ser. (2013) 76:1–12. 10.1159/000350223 [DOI] [PubMed] [Google Scholar]
- 78.Salamone JD, Farrar AM, Font L, Patel V, Schlar DE, Nunes EJ, et al. Differential actions of adenosine A1 and A2A antagonists on the effort-related effects of dopamine D2 antagonism. Behav Brain Res. (2009) 201:216–22. 10.1016/j.bbr.2009.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salamone JD, Correa M, Ferrigno S, Yang JH, Rotolo RA, Presby RE. The psychopharmacology of effort-related decision making: dopamine, adenosine, and insights into the neurochemistry of motivation. Pharmacol Rev. (2018) 70:747–62. 10.1124/pr.117.015107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Loy BD, O'Connor PJ, Lindheimer JB, Covert SF. Caffeine is ergogenic for adenosine A2A receptor gene (ADORA2A) T Allele Homozygotes: a pilot study. J Caffeine Res. (2015) 5:73–81. 10.1089/jcr.2014.0035 [DOI] [Google Scholar]
- 81.Reilly T, Edwards B. Altered sleep-wake cycles and physical performance in athletes. Physiol Behav. (2007) 90:274–84. 10.1016/j.physbeh.2006.09.017 [DOI] [PubMed] [Google Scholar]
- 82.Robson-Ansley PJ, Gleeson M, Ansley L. Fatigue management in the preparation of Olympic athletes. J Sports Sci. (2009) 27:1409–20. 10.1080/02640410802702186 [DOI] [PubMed] [Google Scholar]
- 83.Nunes RA, Mazzotti DR, Hirotsu C, Andersen ML, Tufik S, Bittencourt L. The association between caffeine consumption and objective sleep variables is dependent on ADORA2A c.1083T>C genotypes. Sleep Med. (2017) 30:210–5. 10.1016/j.sleep.2016.06.038 [DOI] [PubMed] [Google Scholar]
- 84.Retey JV, Adam M, Khatami R, Luhmann UF, Jung HH, Berger W, et al. A genetic variation in the adenosine A2A receptor gene (ADORA2A) contributes to individual sensitivity to caffeine effects on sleep. Clin Pharmacol Ther. (2007) 81:692–8. 10.1038/sj.clpt.6100102 [DOI] [PubMed] [Google Scholar]
- 85.Bodenmann S, Hohoff C, Freitag C, Deckert J, Retey JV, Bachmann V, et al. Polymorphisms of ADORA2A modulate psychomotor vigilance and the effects of caffeine on neurobehavioural performance and sleep EEG after sleep deprivation. Br J Pharmacol. (2012) 165:1904–13. 10.1111/j.1476-5381.2011.01689.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Byrne EM, Johnson J, McRae AF, Nyholt DR, Medland SE, Gehrman PR, et al. A genome-wide association study of caffeine-related sleep disturbance: confirmation of a role for a common variant in the adenosine receptor. Sleep (2012) 35:967–75. 10.5665/sleep.1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Philip P, Taillard J, Moore N, Delord S, Valtat C, Sagaspe P, et al. The effects of coffee and napping on nighttime highway driving: a randomized trial. Ann Intern Med. (2006) 144:785–91. 10.7326/0003-4819-144-11-200606060-00004 [DOI] [PubMed] [Google Scholar]
- 88.Drummond SP, Bischoff-Grethe A, Dinges DF, Ayalon L, Mednick SC, Meloy MJ. The neural basis of the psychomotor vigilance task. Sleep (2005) 28:1059–68. 10.1093/sleep/28.9.1059 [DOI] [PubMed] [Google Scholar]
- 89.Landolt HP, Retey JV, Tonz K, Gottselig JM, Khatami R, Buckelmuller I, et al. Caffeine attenuates waking and sleep electroencephalographic markers of sleep homeostasis in humans. Neuropsychopharmacology (2004) 29:1933–9. 10.1038/sj.npp.1300526 [DOI] [PubMed] [Google Scholar]
- 90.Landolt HP, Werth E, Borbely AA, Dijk DJ. Caffeine intake (200 mg) in the morning affects human sleep and EEG power spectra at night. Brain Res. (1995) 675: 67–74. 10.1016/0006-8993(95)00040-W [DOI] [PubMed] [Google Scholar]
- 91.Landolt HP, Dijk DJ, Gaus SE, Borbely AA. Caffeine reduces low-frequency delta activity in the human sleep EEG. Neuropsychopharmacology (1995) 12:229–38. [DOI] [PubMed] [Google Scholar]
- 92.Braakhuis A, Raman R, Vaghefi E. The association between dietary intake of antioxidants and ocular disease. Diseases (2017) 5. 10.3390/diseases5010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Czarnewski P, Das S, Parigi SM, Villablanca EJ. Retinoic acid and its role in modulating intestinal innate immunity. Nutrients (2017) 9. 10.3390/nu9010068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gao Y, Chen L, Yang SN, Wang H, Yao J, Dai Q, et al. Contributions of visuo-oculomotor abilities to interceptive skills in sports. Optom Vis Sci. (2015) 92:679–89. 10.1097/OPX.0000000000000599 [DOI] [PubMed] [Google Scholar]
- 95.Palidis DJ, Wyder-Hodge PA, Fooken J, Spering M. Distinct eye movement patterns enhance dynamic visual acuity. PLoS ONE (2017) 12:e0172061. 10.1371/journal.pone.0172061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wilkerson GB, Simpson KA, Clark RA. Assessment and training of visuomotor reaction time for football injury prevention. J Sport Rehabil. (2017) 26:26–34. 10.1123/jsr.2015-0068 [DOI] [PubMed] [Google Scholar]
- 97.Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, et al. Position statement. Part one: immune function and exercise. Exerc Immunol Rev. (2011) 17:6–63. [PubMed] [Google Scholar]
- 98.Lietz G, Lange J, Rimbach G. Molecular and dietary regulation of beta,beta-carotene 15,15'-monooxygenase 1 (BCMO1). Arch Biochem Biophys. (2010) 502:8–16. 10.1016/j.abb.2010.06.032 [DOI] [PubMed] [Google Scholar]
- 99.Ferrucci L, Perry JR, Matteini A, Perola M, Tanaka T, Silander K, et al. Common variation in the beta-carotene 15,15'-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am J Hum Genet. (2009) 84:123–33. 10.1016/j.ajhg.2008.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lietz G, Oxley A, Leung W, Hesketh J. Single nucleotide polymorphisms upstream from the beta-carotene 15,15'-monoxygenase gene influence provitamin A conversion efficiency in female volunteers. J Nutr. (2012) 142:161S–5S. 10.3945/jn.111.140756 [DOI] [PubMed] [Google Scholar]
- 101.Garvican LA, Saunders PU, Cardoso T, Macdougall IC, Lobigs LM, Fazakerley R, et al. Intravenous iron supplementation in distance runners with low or suboptimal ferritin. Med Sci Sports Exerc. (2014) 46:376–85. 10.1249/MSS.0b013e3182a53594 [DOI] [PubMed] [Google Scholar]
- 102.DellaValle DM, Haas JD. Iron supplementation improves energetic efficiency in iron-depleted female rowers. Med Sci Sports Exerc. (2014) 46:1204–15. 10.1249/MSS.0000000000000208 [DOI] [PubMed] [Google Scholar]
- 103.Sacirovic S, Asotic J, Maksimovic R, Radevic B, Muric B, Mekic H, et al. Monitoring and prevention of anemia relying on nutrition and environmental conditions in sports. Mater Sociomed. (2013) 25:136–9. 10.5455/msm.2013.25.136-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Myhre KE, Webber BJ, Cropper TL, Tchandja JN, Ahrendt DM, Dillon CA, et al. Prevalence and Impact of Anemia on Basic Trainees in the US Air Force. Sports Med Open (2015) 2:23. 10.1186/s40798-016-0047-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martinsson A, Andersson C, Andell P, Koul S, Engstrom G, Smith JG. Anemia in the general population: prevalence, clinical correlates and prognostic impact. Eur J Epidemiol. (2014) 29:489–98. 10.1007/s10654-014-9929-9 [DOI] [PubMed] [Google Scholar]
- 106.Latunde-Dada GO. Iron metabolism in athletes–achieving a gold standard. Eur J Haematol. (2013) 90:10–5. 10.1111/ejh.12026 [DOI] [PubMed] [Google Scholar]
- 107.Dubnov G, Foldes AJ, Mann G, Magazanik A, Siderer M, Constantini N. High prevalence of iron deficiency and anemia in female military recruits. Mil Med. (2006) 171:866–9. 10.7205/MILMED.171.9.866 [DOI] [PubMed] [Google Scholar]
- 108.McClung JP. Iron, zinc, and physical performance. Biol Trace Elem Res. (2018) 10.1007/s12011-018-1479-7. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 109.Pedlar CR, Brugnara C, Bruinvels G, Burden R. Iron balance and iron supplementation for the female athlete: a practical approach. Eur J Sport Sci. (2018) 18:295–305. 10.1080/17461391.2017.1416178 [DOI] [PubMed] [Google Scholar]
- 110.Nagao T, Hirokawa M. Diagnosis and treatment of macrocytic anemias in adults. J Gen Fam Med. (2017) 18:200–204. 10.1002/jgf2.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Veeranki S, Tyagi SC. Defective homocysteine metabolism: potential implications for skeletal muscle malfunction. Int J Mol Sci. (2013) 14:15074–91. 10.3390/ijms140715074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Goyette P, Sumner JS, Milos R, Duncan AM, Rosenblatt DS, Matthews RG, et al. Human methylenetetrahydrofolate reductase: isolation of cDNA mapping and mutation identification. Nat Genet. (1994) 7:551 10.1038/ng0694-195 [DOI] [PubMed] [Google Scholar]
- 113.Mennen LI, de Courcy GP, Guilland JC, Ducros V, Bertrais S, Nicolas JP, et al. Homocysteine, cardiovascular disease risk factors, and habitual diet in the French Supplementation with Antioxidant Vitamins and Minerals Study. Am J Clin Nutr. (2002) 76:1279–89. 10.1093/ajcn/76.6.1279 [DOI] [PubMed] [Google Scholar]
- 114.Rasmussen LB, Ovesen L, Bulow I, Knudsen N, Laurberg P, Perrild H. Folate intake, lifestyle factors, and homocysteine concentrations in younger and older women. Am J Clin Nutr. (2000) 72:1156–63. 10.1093/ajcn/72.5.1156 [DOI] [PubMed] [Google Scholar]
- 115.Solis C, Veenema K, Ivanov AA, Tran S, Li R, Wang W, et al. Folate intake at RDA levels is inadequate for Mexican American men with the methylenetetrahydrofolate reductase 677TT genotype. J Nutr. (2008) 138:67–72. 10.1093/jn/138.1.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Curro M, Di Mauro D, Bruschetta D, D'Amico F, Vecchio M, Trimarchi F, et al. Influence of MTHFR polymorphisms on cardiovascular risk markers in elite athletes. Clin Biochem. (2016) 49:183–5. 10.1016/j.clinbiochem.2015.08.014 [DOI] [PubMed] [Google Scholar]
- 117.Guinotte CL, Burns MG, Axume JA, Hata H, Urrutia TF, Alamilla A, et al. Methylenetetrahydrofolate reductase 677C–>T variant modulates folate status response to controlled folate intakes in young women. J Nutr. (2003) 133:1272–80. 10.1093/jn/133.5.1272 [DOI] [PubMed] [Google Scholar]
- 118.Dinc N, Yucel SB, Taneli F, Sayin MV. The effect of the MTHFR C677T mutation on athletic performance and the homocysteine level of soccer players and sedentary individuals. J Hum Kinet. (2016) 51:61–69. 10.1515/hukin-2015-0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Swart KM, Enneman AW, van Wijngaarden JP, van Dijk SC, Brouwer-Brolsma EM, Ham AC, et al. Homocysteine and the methylenetetrahydrofolate reductase 677C–>T polymorphism in relation to muscle mass and strength, physical performance and postural sway. Eur J Clin Nutr. (2013) 67:743–8. 10.1038/ejcn.2013.97 [DOI] [PubMed] [Google Scholar]
- 120.Vidoni ML, Pettee Gabriel K, Luo ST, Simonsick EM, Day RS. Relationship between homocysteine and muscle strength decline: the baltimore longitudinal study of aging. J Gerontol A Biol Sci Med Sci. (2018) 73:546–551. 10.1093/gerona/glx161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van Schoor NM, Swart KM, Pluijm SM, Visser M, Simsek S, Smulders Y, et al. Cross-sectional and longitudinal association between homocysteine, vitamin B12 and physical performance in older persons. Eur J Clin Nutr. (2012) 66:174–81. 10.1038/ejcn.2011.151 [DOI] [PubMed] [Google Scholar]
- 122.Rolita L, Holtzer R, Wang C, Lipton RB, Derby CA, Verghese J. Homocysteine and mobility in older adults. J Am Geriatr Soc. (2010) 58:545–50. 10.1111/j.1532-5415.2010.02718.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Benyamin B, Ferreira MA, Willemsen G, Gordon S, Middelberg RP, McEvoy BP, et al. Common variants in TMPRSS6 are associated with iron status and erythrocyte volume. Nat Genet. (2009) 41:1173–5. 10.1038/ng.456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Allen KJ, Gurrin LC, Constantine CC, Osborne NJ, Delatycki MB, Nicoll AJ, et al. Iron-overload-related disease in HFE hereditary hemochromatosis. N Engl J Med. (2008) 358:221–30. 10.1056/NEJMoa073286 [DOI] [PubMed] [Google Scholar]
- 125.Soranzo N, Spector TD, Mangino M, Kuhnel B, Rendon A, Teumer A, et al. A genome-wide meta-analysis identifies 22 loci associated with eight hematological parameters in the HaemGen consortium. Nat Genet. (2009) 41:1182–90. 10.1038/ng.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chambers JC, Zhang W, Li Y, Sehmi J, Wass MN, Zabaneh D, et al. Genome-wide association study identifies variants in TMPRSS6 associated with hemoglobin levels. Nat Genet. (2009) 41:1170–2. 10.1038/ng.462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Benyamin B, McRae AF, Zhu G, Gordon S, Henders AK, Palotie A, et al. Variants in TF and HFE explain approximately 40% of genetic variation in serum-transferrin levels. Am J Hum Genet. (2009) 84:60–5. 10.1016/j.ajhg.2008.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ganesh SK, Zakai NA, van Rooij FJ, Soranzo N, Smith AV, Nalls MA, et al. Multiple loci influence erythrocyte phenotypes in the CHARGE Consortium. Nat Genet. (2009) 41:1191–8. 10.1038/ng.466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Morgan EH, Oates PS. Mechanisms and regulation of intestinal iron absorption. Blood Cells Mol Dis. (2002) 29:384–99. 10.1006/bcmd.2002.0578 [DOI] [PubMed] [Google Scholar]
- 130.Marjot T CJ, Ryan JD. What is HFE haemochromatosis?. Br J Hosp Med. (2016) 77:C91–95. 10.12968/hmed.2016.77.6.C91 [DOI] [PubMed] [Google Scholar]
- 131.Pantopoulos K, Porwal SK, Tartakoff A, Devireddy L. Mechanisms of mammalian iron homeostasis. Biochemistry (2012) 51:5705–24. 10.1021/bi300752r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Recalcati S, Minotti G, Cairo G. Iron regulatory proteins: from molecular mechanisms to drug development. Antioxid Redox Signal. (2010) 13:1593–616. 10.1089/ars.2009.2983 [DOI] [PubMed] [Google Scholar]
- 133.Chicharro JL, Hoyos J, Gomez-Gallego F, Villa JG, Bandres F, Celaya P, et al. Mutations in the hereditary haemochromatosis gene HFE in professional endurance athletes. Br J Sports Med. (2004) 38:418–21. 10.1136/bjsm.2002.003921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Habte K, Adish A, Zerfu D, Kebede A, Moges T, Tesfaye B, et al. Iron, folate and vitamin B12 status of Ethiopian professional runners. Nutr Metab. (2015) 12:62. 10.1186/s12986-015-0056-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hermine ODG, Genty V, Marquet LA, Fumagalli G, Tafflet M, Guillem F, et al. Eighty percent of French sport winners in Olympic, World and Europeans competitions have mutations in the hemochromatosis HFE gene. Biochimie (2015) 119:1–5. 10.1016/j.biochi.2015.09.028 [DOI] [PubMed] [Google Scholar]
- 136.Tanaka T, Roy CN, Yao W, Matteini A, Semba RD, Arking D, et al. A genome-wide association analysis of serum iron concentrations. Blood (2010) 115:94–6. 10.1182/blood-2009-07-232496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Colombani PC MC. Energie- und Nährstoffaufnahme im Schweizer Spitzensport – eine erste Bestandsaufnahme zu Beginn des zweiten Jahrtausends. Sportmed Sporttraumatol. (2003) 51:7–16. [Google Scholar]
- 138.Mettler S, Zimmermann MB. Iron excess in recreational marathon runners. Eur J Clin Nutr. (2010) 64:490–4. 10.1038/ejcn.2010.16 [DOI] [PubMed] [Google Scholar]
- 139.Diehl K, Thiel A, Zipfel S, Mayer J, Schnell A, Schneider S. Elite adolescent athletes' use of dietary supplements: characteristics, opinions, and sources of supply and information. Int J Sport Nutr Exerc Metab. (2012) 22:165–74. 10.1123/ijsnem.22.3.165 [DOI] [PubMed] [Google Scholar]
- 140.Petroczi A, Naughton DP, Mazanov J, Holloway A, Bingham J. Performance enhancement with supplements: incongruence between rationale and practice. J Int Soc Sports Nutr. (2007) 4:19. 10.1186/1550-2783-4-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.De Matos LD, Azevedo LF, Vieira ML, Nomura CH, Hamerschlak N, Pinho JR, et al. The use of exogenous iron by professional cyclists pervades abdominal organs but not the heart. Int J Cardiol. (2013) 167:2341–3. 10.1016/j.ijcard.2012.11.041 [DOI] [PubMed] [Google Scholar]
- 142.Reardon TF, Allen DG. Iron injections in mice increase skeletal muscle iron content, induce oxidative stress and reduce exercise performance. Exp Physiol. (2009) 94:720–30. 10.1113/expphysiol.2008.046045 [DOI] [PubMed] [Google Scholar]
- 143.Messner DJ, Rhieu BH, Kowdley KV. Iron overload causes oxidative stress and impaired insulin signaling in AML-12 hepatocytes. Dig Dis Sci. (2013) 58:1899–908. 10.1007/s10620-013-2648-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Aslinia F, Mazza JJ, Yale SH. Megaloblastic anemia and other causes of macrocytosis. Clin Med Res. (2006) 4:236–41. 10.3121/cmr.4.3.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hazra A, Kraft P, Selhub J, Giovannucci EL, Thomas G, Hoover RN, et al. Common variants of FUT2 are associated with plasma vitamin B12 levels. Nat Genet. (2008) 40:1160–2. 10.1038/ng.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Powers SK, Radak Z, Ji LL. Exercise-induced oxidative stress: past, present and future. J Physiol. (2016) 594:5081–92. 10.1113/JP270646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Reid MB. Reactive oxygen species as agents of fatigue. Med Sci Sports Exerc. (2016) 48:2239–46. 10.1249/MSS.0000000000001006 [DOI] [PubMed] [Google Scholar]
- 148.Draeger CL, Naves A, Marques N, Baptistella AB, Carnauba RA, Paschoal V, et al. Controversies of antioxidant vitamins supplementation in exercise: ergogenic or ergolytic effects in humans? J Int Soc Sports Nutr. (2014) 11:4. 10.1186/1550-2783-11-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, et al. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. (2008) 87:142–9. 10.1093/ajcn/87.1.142 [DOI] [PubMed] [Google Scholar]
- 150.Taghiyar M, Darvishi L, Askari G, Feizi A, Hariri M, Mashhadi NS, et al. The effect of vitamin C and e supplementation on muscle damage and oxidative stress in female athletes: a clinical trial. Int J Prev Med. (2013) 4(Suppl. 1):S16–23. [PMC free article] [PubMed] [Google Scholar]
- 151.Peternelj TT, Coombes JS. Antioxidant supplementation during exercise training: beneficial or detrimental? Sports Med. (2011) 41:1043–69. 10.2165/11594400-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 152.Vidal K, Robinson N, Ives SJ. Exercise performance and physiological responses: the potential role of redox imbalance. Physiol Rep. (2017) 5:e13225. 10.14814/phy2.13225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Braakhuis AJ. Effect of vitamin C supplements on physical performance. Curr Sports Med Rep. (2012) 11:180–4. 10.1249/JSR.0b013e31825e19cd [DOI] [PubMed] [Google Scholar]
- 154.Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA (1999) 281:1415–23. 10.1001/jama.281.15.1415 [DOI] [PubMed] [Google Scholar]
- 155.Shaw G, Lee-Barthel A, Ross ML, Wang B, Baar K. Vitamin C-enriched gelatin supplementation before intermittent activity augments collagen synthesis. Am J Clin Nutr. (2017) 105:136–43. 10.3945/ajcn.116.138594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cahill LE, Fontaine-Bisson B, El-Sohemy A. Functional genetic variants of glutathione S-transferase protect against serum ascorbic acid deficiency. Am J Clin Nutr. (2009) 90:1411–7. 10.3945/ajcn.2009.28327 [DOI] [PubMed] [Google Scholar]
- 157.Eck P, Erichsen HC, Taylor JG, Yeager M, Hughes AL, Levine M, et al. Comparison of the genomic structure and variation in the two human sodium-dependent vitamin C transporters, SLC23A1 and SLC23A2. Hum Genet. (2004) 115:285–94. 10.1007/s00439-004-1167-x [DOI] [PubMed] [Google Scholar]
- 158.Cahill LE, El-Sohemy A. Vitamin C transporter gene polymorphisms, dietary vitamin C and serum ascorbic acid. J Nutrigenet Nutrigenomics (2009) 2:292–301. 10.1159/000314597 [DOI] [PubMed] [Google Scholar]
- 159.Angeline ME, Gee AO, Shindle M, Warren RF, Rodeo SA. The effects of vitamin D deficiency in athletes. Am J Sports Med. (2013) 41:461–4. 10.1177/0363546513475787 [DOI] [PubMed] [Google Scholar]
- 160.Halliday TM, Peterson NJ, Thomas JJ, Kleppinger K, Hollis BW, Larson-Meyer DE. Vitamin D status relative to diet, lifestyle, injury, and illness in college athletes. Med Sci Sports Exerc. (2011) 43:335–43. 10.1249/MSS.0b013e3181eb9d4d [DOI] [PubMed] [Google Scholar]
- 161.Ruohola JP, Laaksi I, Ylikomi T, Haataja R, Mattila VM, Sahi T, et al. Association between serum 25(OH)D concentrations and bone stress fractures in Finnish young men. J Bone Miner Res. (2006) 21:1483–8. 10.1359/jbmr.060607 [DOI] [PubMed] [Google Scholar]
- 162.Larson-Meyer DE, Willis KS. Vitamin D and athletes. Curr Sports Med Rep. (2010) 9:220–6. 10.1249/JSR.0b013e3181e7dd45 [DOI] [PubMed] [Google Scholar]
- 163.Barker T, Henriksen VT, Martins TB, Hill HR, Kjeldsberg CR, Schneider ED, et al. Higher serum 25-hydroxyvitamin D concentrations associate with a faster recovery of skeletal muscle strength after muscular injury. Nutrients (2013) 5:1253–75. 10.3390/nu5041253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Barker T, Schneider ED, Dixon BM, Henriksen VT, Weaver LK. Supplemental vitamin D enhances the recovery in peak isometric force shortly after intense exercise. Nutr Metab. (2013) 10:69. 10.1186/1743-7075-10-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Slater NA, Rager ML, Havrda DE, Harralson AF. Genetic Variation in CYP2R1 and GC Genes Associated With Vitamin D Deficiency Status. J Pharm Pract. (2015) 30:31–6. 10.1177/0897190015585876 [DOI] [PubMed] [Google Scholar]
- 166.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet (2010) 376:180–8. 10.1016/S0140-6736(10)60588-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fang Y, van Meurs JB, Arp P, van Leeuwen JP, Hofman A, Pols HA, et al. Vitamin D binding protein genotype and osteoporosis. Calcif Tissue Int. (2009) 85:85–93. 10.1007/s00223-009-9251-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yucha C. Guthrie D. Renal homeostasis of calcium. Nephrol Nurs J. (2003) 30:621–6; quiz 627–8. [PubMed] [Google Scholar]
- 169.Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin d supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. (2008) 23:741–9. 10.1359/jbmr.080102 [DOI] [PubMed] [Google Scholar]
- 170.Nieves JW, Melsop K, Curtis M, Kelsey JL, Bachrach LK, Greendale G, et al. Nutritional factors that influence change in bone density and stress fracture risk among young female cross-country runners. PM R (2010) 2:740–50; quiz 794. 10.1016/j.pmrj.2010.04.020 [DOI] [PubMed] [Google Scholar]
- 171.Patel DS, Roth M, Kapil N. Stress fractures: diagnosis, treatment, and prevention. Am Fam Physician. (2011) 83:39–46. [PubMed] [Google Scholar]
- 172.McInnis KC, Ramey LN. High-risk stress fractures: diagnosis and management. PM R (2016) 8(3 Suppl.):S113–24. 10.1016/j.pmrj.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 173.Food and Nutrition Board I.o.M. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC (1998). [PubMed] [Google Scholar]
- 174.Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. (2006) 26:229–50. 10.1146/annurev.nutr.26.061505.111156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zeisel SH, Da Costa KA, Franklin PD, Alexander EA, Lamont JT, Sheard NF, et al. Choline, an essential nutrient for humans. FASEB J. (1991) 5:2093–8. 10.1096/fasebj.5.7.2010061 [DOI] [PubMed] [Google Scholar]
- 176.Resseguie ME, da Costa KA, Galanko JA, Patel M, Davis IJ, Zeisel SH. Aberrant estrogen regulation of PEMT results in choline deficiency-associated liver dysfunction. J Biol Chem. (2011) 286:1649–58. 10.1074/jbc.M110.106922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.da Costa KA, Kozyreva OG, Song J, Galanko JA, Fischer LM, Zeisel SH. Common genetic polymorphisms affect the human requirement for the nutrient choline. FASEB J. (2006) 20:1336–44. 10.1096/fj.06-5734com [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.da Costa KA, Corbin KD, Niculescu MD, Galanko JA, Zeisel SH. Identification of new genetic polymorphisms that alter the dietary requirement for choline and vary in their distribution across ethnic and racial groups. FASEB J. (2014) 28:2970–8. 10.1096/fj.14-249557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.da Costa KA, Badea M, Fischer LM, Zeisel SH. Elevated serum creatine phosphokinase in choline-deficient humans: mechanistic studies in C2C12 mouse myoblasts. Am J Clin Nutr. (2004) 80:163–70. 10.1093/ajcn/80.1.163 [DOI] [PubMed] [Google Scholar]
- 180.Fischer LM, daCosta K.A., Kwock L, Stewart PW, Lu TS, Stabler SP. et al. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am J Clin Nutr. (2007) 85:1275–85. 10.1093/ajcn/85.5.1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Conlay LA, Sabounjian LA, Wurtman RJ. Exercise and neuromodulators: choline and acetylcholine in marathon runners. Int J Sports Med. (1992) 13(Suppl. 1):S141–2. 10.1055/s-2007-1024619 [DOI] [PubMed] [Google Scholar]
- 182.Jager R, Purpura M, Kingsley M. Phospholipids and sports performance. J Int Soc Sports Nutr. (2007) 4:5. 10.1186/1550-2783-4-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Meeusen R, Watson P, Hasegawa H, Roelands B, Piacentini MF. Central fatigue: the serotonin hypothesis and beyond. Sports Med. (2006) 36:881–909. 10.2165/00007256-200636100-00006 [DOI] [PubMed] [Google Scholar]
- 184.Gao X, Wang Y, Randell E, Pedram P, Yi Y, Gulliver W, et al. Higher dietary choline and betaine intakes are associated with better body composition in the adult population of newfoundland, Canada. PLoS ONE (2016) 11:e0155403. 10.1371/journal.pone.0155403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Elsawy G, Abdelrahman O, Hamza A. Effect of choline supplementation on rapid weight loss and biochemical variables among female taekwondo and judo athletes. J Hum Kinet. (2014) 40:77–82. 10.2478/hukin-2014-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kohlmeier M, da Costa KA, Fischer LM, Zeisel SH. Genetic variation of folate-mediated one-carbon transfer pathway predicts susceptibility to choline deficiency in humans. Proc Natl Acad Sci USA. (2005) 102:16025–30. 10.1073/pnas.0504285102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ganz AB, Klatt KC, Caudill MA. Common genetic variants alter metabolism and influence dietary choline requirements. Nutrients (2017) 9:E837. 10.3390/nu9080837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ganz AB, Shields K, Fomin VG, Lopez YS, Mohan S, Lovesky J, et al. Genetic impairments in folate enzymes increase dependence on dietary choline for phosphatidylcholine production at the expense of betaine synthesis. FASEB J. (2016) 30:3321–33. 10.1096/fj.201500138RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zeisel SH. Nutritional genomics: defining the dietary requirement and effects of choline. J Nutr. (2011) 141:531–4. 10.3945/jn.110.130369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Krieger JW, Sitren HS, Daniels MJ, Langkamp-Henken B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: a meta-regression 1. Am J Clin Nutr. (2006) 83:260–74. 10.1093/ajcn/83.2.260 [DOI] [PubMed] [Google Scholar]
- 191.Knapik JJ. The importance of physical fitness for injury prevention: part 2. J Spec Oper Med. (2015) 15:112–5. [DOI] [PubMed] [Google Scholar]
- 192.Nascimento M, Silva D, Ribeiro S, Nunes M, Almeida M, Mendes-Netto R. Effect of a nutritional intervention in Athlete's body composition, eating behaviour and nutritional knowledge: a comparison between adults and adolescents. Nutrients (2016) 8:E535. 10.3390/nu8090535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Goss AM, Goree LL, Ellis AC, Chandler-Laney PC, Casazza K, Lockhart ME, et al. Effects of diet macronutrient composition on body composition and fat distribution during weight maintenance and weight loss. Obesity (Silver Spring). (2013) 21:1139–42. 10.1002/oby.20191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Merritt DC, Jamnik J, El-Sohemy A. FTO genotype, dietary protein intake, and body weight in a multiethnic population of young adults: a cross-sectional study. Genes Nutr. (2018) 13:4. 10.1186/s12263-018-0593-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bartolomei S, Sadres E, Church DD, Arroyo E, Gordon JA, III, Varanoske AN, et al. Comparison of the recovery response from high-intensity and high-volume resistance exercise in trained men. Eur J Appl Physiol. (2017) 117:1287–98. 10.1007/s00421-017-3598-9 [DOI] [PubMed] [Google Scholar]
- 196.Aragon AA, Schoenfeld BJ, Wildman R, Kleiner S, VanDusseldorp T, Taylor L, et al. International society of sports nutrition position stand: diets and body composition. J Int Soc Sports Nutr. (2017) 14:16. 10.1186/s12970-017-0174-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mattei J, Qi Q, Hu FB, Sacks FM, Qi L. TCF7L2 genetic variants modulate the effect of dietary fat intake on changes in body composition during a weight-loss intervention. Am J Clin Nutr. (2012) 96:1129–36. 10.3945/ajcn.112.038125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Grau K, Cauchi S, Holst C, Astrup A, Martinez JA, Saris WH, et al. TCF7L2 rs7903146-macronutrient interaction in obese individuals' responses to a 10-wk randomized hypoenergetic diet. Am J Clin Nutr. (2010) 91:472–9. 10.3945/ajcn.2009.27947 [DOI] [PubMed] [Google Scholar]
- 199.Zhang X, Qi Q, Zhang C, Smith SR, Hu FB, Sacks FM, et al. FTO genotype and 2-year change in body composition and fat distribution in response to weight-loss diets: the POUNDS LOST Trial. Diabetes (2012) 61:3005–11. 10.2337/db11-1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Beelen M, Cermak NM, van Loon LJ. [Performance enhancement by carbohydrate intake during sport: effects of carbohydrates during and after high-intensity exercise]. Ned Tijdschr Geneeskd. (2015) 159:A7465. [PubMed] [Google Scholar]
- 201.Marquet LA, Brisswalter J, Louis J, Tiollier E, Burke LM, Hawley JA, et al. Enhanced Endurance Performance by Periodization of Carbohydrate Intake: “Sleep Low” Strategy. Med Sci Sports Exerc. (2016) 48:663–72. 10.1249/MSS.0000000000000823 [DOI] [PubMed] [Google Scholar]
- 202.Naclerio F, Larumbe-Zabala E. Effects of whey protein alone or as part of a multi-ingredient formulation on strength, fat-free mass, or lean body mass in resistance-trained individuals: a meta-analysis. Sports Med. (2016) 46:125–37. 10.1007/s40279-015-0403-y [DOI] [PubMed] [Google Scholar]
- 203.Wu G. Dietary protein intake and human health. Food Funct. (2016) 7:1251–65. 10.1039/C5FO01530H [DOI] [PubMed] [Google Scholar]
- 204.Noland RC. Exercise and regulation of lipid metabolism. Prog Mol Biol Transl Sci. (2015) 135:39–74. 10.1016/bs.pmbts.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 205.de Luis DA, Aller R, Izaola O, Primo D, Urdiales S, Romero E. Effects of a high-protein/low-carbohydrate diet versus a standard hypocaloric diet on weight and cardiovascular risk factors: Role of a Genetic Variation in the rs9939609 FTO Gene Variant. J Nutrigenet Nutrigenomics (2015) 8:128–36. 10.1159/000441142 [DOI] [PubMed] [Google Scholar]
- 206.Antonio J, Knafo S, Kapoor R, Tartar JL. A fat mass and obesity-associated gene polymorphism influences fat mass in exercise-trained individuals. J Int Soc Sports Nutr. (2018) 15:40. 10.1186/s12970-018-0246-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Beck B, Carstairs GL, Billing DC, Caldwell JN, Middleton KJ. Modifiable anthropometric characteristics are associated with unilateral and bilateral carry performance. J Strength Cond Res. (2016). 10.1519/JSC.0000000000001504 [DOI] [PubMed] [Google Scholar]
- 208.Garaulet M, Smith CE, Hernandez-Gonzalez T, Lee YC, Ordovas JM. PPARgamma Pro12Ala interacts with fat intake for obesity and weight loss in a behavioural treatment based on the Mediterranean diet. Mol Nutr Food Res. (2011) 55:1771–9. 10.1002/mnfr.201100437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mountjoy M, Sundgot-Borgen JK, Burke LM, Ackerman KE, Blauwet C, Constantini N, et al. IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Br J Sports Med. (2018) 52:687–97. 10.1136/bjsports-2018-099193 [DOI] [PubMed] [Google Scholar]
- 210.Phillips CM, Kesse-Guyot E, McManus R, Hercberg S, Lairon D, Planells R, et al. High dietary saturated fat intake accentuates obesity risk associated with the fat mass and obesity-associated gene in adults. J Nutr. (2012) 142:824–31. 10.3945/jn.111.153460 [DOI] [PubMed] [Google Scholar]