Abstract
The present study focuses on the influence of the tumor microenvironment on the expression of HLA-G in ovarian cancer and its impact on immune cells. We used carcinomatosis fluids (n = 16) collected from patients diagnosed with epithelial ovarian cancer, detected by an increase in CA125 levels. Our results indicate that HLA-G is expressed by 1) ascitic cell clusters, 2) stromal cells (hospicells) extracted from cancer cell clusters, and 3) cancer cell lines and tumor cells. The origin of HLA-G was linked to inflammatory cytokines present in the cancer microenvironment. In parallel, the ascitic fluid of patients with ovarian cancer contains soluble HLA-G (sHLA-G). The mesothelial cell layer and submesothelial tissues, as well as the immune cell infiltrate, do not secrete HLA-G. In contrast, sHLA-G is absorbed by peritoneal tissues along with mesothelial layers as well as immune cell infiltrates. We demonstrated that interleukin-1β along with TGF-β can be a major HLA-G–inducing factor that upregulates HLA-G expression through the NF-κB pathway. The level of HLA-G in ascites correlated positively with the expression of T regulatory (T-regs) cells, while it negatively correlated with the expression of natural killer and memory cells in tumor-infiltrating immune cells. In conclusion, the production of HLA-G is associated with the presence of inflammatory cytokines and is strongly correlated with microenvironment tolerant cells such as T-regs and diminution of NK and memory T cells.
Introduction
Immune cells are key players in the complex mechanisms involved in host defense [1], [2] and play a crucial role in cancer evolution and metastasis [3], [4], [5]. Failure of host antitumor immunity may be due to increased suppression of tumor-reactive lymphocytes, mediated by T regulatory (T-reg) cells [6]. Immunosuppression, particularly in ovarian cancer, is induced by intratumoral T-reg cells and is a major determinant in disease outcome [7], [8], [9]. Among the mechanisms set in motion by tumors to escape the immune system, programmed cell death protein-1 (PD-1) [10] and cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) [11] are already described. The contribution of HLA-G (human leukocyte antigen G “Gestation”; belonging to nonclassical MHC class I group) is considered to be one of the prime findings [12]. It operates by inducing tolerance to a variety of antitumor effector cells including T regulatory/suppressor cells [13], [14], [15]. HLA-G modulates the activation and effector role of key immune cells such as dendritic cells, macrophages, T lymphocytes, and NK cells through binding to ITIM (immunoreceptor tyrosine-based inhibitory motif) containing transmembrane receptors [16]. Structurally, HLA-G has seven isoforms. Four isoforms (HLA-G1 to HLA-G4) are membrane bound and three (HLA-G5 to HLA G-7) are soluble, which are generated by the alternative splicing of unique primary transcripts. Soluble HLA-G (sHLA-G) and membrane-bound HLA-G isoforms have similar functions [17]. In the normal healthy human, HLA-G is highly expressed in the placenta. However, low RNA expression is found in GIT and male reproductive tissues in the absence of protein expression [18]. In experimental animal models and patients with ovarian cancer, the expression of HLA-G is associated with metastasis and poor survival rate [19], [20], [21].
The present study looks into the origin of HLA-G expression in ovarian carcinomatosis and its impact on immune cells.
Material and methods
Peritoneal fluid
Ascitic effusions (n = 16) were obtained from patients above the age of 18 years with ovarian cancer (confirmed by histological analysis) without treatment and in advanced state (Department of Oncology, Hôtel-Dieu Hospital). All the patients had double the levels of CA125 when compared to normal patients according to Gynecological Cancer Intergroup criteria. As ascitic fluid extraction is a part of the routine management of patients, only oral consents were obtained from them. The peritoneal fluids obtained were divided into two categories: one for collecting floating cell clusters and the other for obtaining cell-free supernatants after a short spin at 1000 rpm for 5 minutes. The floating cell clusters were used for studies as described below. The supernatants were aliquoted and stored for further use at −30°C.
HLA-G Expression by Clusters
A small volume of ascites was diluted with 0.9% NaCl and observed microscopically for the presence of clusters (magnification ×40). Floating cell clusters were picked up using a pipette from the diluted samples. A volume of 200 μl containing cells, diluted in PBS, was deposited on glass slides and centrifuged using a Shandon CytoSpin 3. Cells were fixed for 10 minutes in ethanol–acetic acid (10/90). The slides were then rinsed, and the cells were rehydrated with PBS (1×). After rehydration, cells were incubated in serum albumin (10% BSA) and 90% PBS to saturate nonspecific sites. After washing with PBS (1×) containing BSA 0.1%, the cells were incubated overnight at room temperature with the primary antibodies anti-HLA-G1 and -G5 (MEMG9; 10 μg/ml). After a second wash, the cells were further incubated with secondary antimouse IgG (dilution 1 μg/100 μl). Finally, cells were incubated for a further 45 minutes with streptavidin coupled to fluorescein isothiocyanate (FITC) (diluted as 1 μg/100 μl). Negative controls were performed in the absence of primary antibodies. Fluorescence microscopy was performed at λ = 490 nm (excitation) and λ =525 nm (emission).
ELISA
sHLA-G concentrations were measured in ascitic samples collected from patients with ovarian cancer. Two distinct ELISAs were conducted. A 96-well plate (Corning Costar) was coated with PBS (pH 7.4) containing MEM-G/9 at 10 μg/ml. Plates were saturated with 250 μl of 2% BSA in PBS for 2 hours at 37°C. Samples were added to each well (100 μl) in triplicate. After an overnight incubation at 4°C, anti–β2m-HRP (DakoCytomation) was added as detection Ab for 1 hour at 37°C. The chromogenic substrate (tetramethylbenzidine; Sigma-Aldrich) was added for 30 minutes in the dark. Finally, the reaction was stopped using HCl (1 N). On the other hand, a similar test was performed using 5A6G7 mAb at 5 μg/ml as capture Ab and W6/32-biotin (Interchim) plus streptavidin-HRP as a detection antibody (Amersham). This 5A6G7/W6/32 combination can only detect HLA-G5 but not HLA-G6 because of the inability of W6/32 to bind HLA-G6. Optical densities were measured at 450 nm. Standard curves were generated using serial dilutions of purified soluble recombinant HLA-G5 protein. The detection limit of both ELISAs was 5 ng/ml.
Immunohistochemistry
The tissue sections were obtained from anatomopathological department from patients with and without cancer to evaluate the expression of HLA-G and sHLA-g in the peritoneal membrane. These tissue sections were obtained from patients different from the ones used in the study for ascites. The tissue sections were stained using antibodies directed against HLA-G (clone 5A6G7; CliniSciences, Nanterre, France), sHLA-G (clone 4H84; Santa Cruz Biotechnology, USA), CD16 (DAKO), CD20 (DAKO), CD8 (DAKO), CD56 (Leica Biosystems), CD3 (Fisher Scientific, France), and CD4 (Ventana). The images were then obtained using EVOS FL Auto Imaging System (Life Technologies, Waltham, USA).
Cell Lines
The human cancer cell lines used were ovarian (OVCAR; ATCC), breast (MDA-MB231; ATCC), lung (A549; ATCC), colorectal (HT-29, HCT-8R; ATCC), and a leukemic cell line (HL60; ATCC). Cells were cultured in DMEM (for MDA-MB231, A549, HT-29m HCT-8R, and HL60) or RPMI 1640 medium (for HL60) containing 10% fetal calf serum, penicillin (50 U/ml), and streptomycin (50 μg/ml). The human mesothelial cell lines were purchased from ZenBio, Inc., and cultured in mesothelium-specific culture medium obtained from ZenBio, Inc. All cell lines were incubated in a humidified atmosphere containing 5% CO2 at 37°C, as recommended by the supplier (PAA Laboratories, Inc., Etobicoke, ON, Canada).
HLA-G mRNA Expression
Total RNA was extracted using RNA/DNA (NucleoSpin RNA) kit. Cells were incubated for 15 minutes in lysis buffer. After centrifugation, the pellets were suspended and precipitated with 70% ethanol. After centrifugation, the resulting pellet was washed thrice, dried, and dissolved in RNase-free sterile water (Invitrogen). An aliquot of RNA was taken, to which random primers (Random Hexam) were added along with dNTP and RT buffer. The samples were centrifuged and heated at 65°C. Then, reverse transcriptase (M-MLV-RT, 200 U/μl) was added to each tube. After incubation at 42°C for 30 minutes, the reaction was stopped by heating at 72°C for 3 minutes. Finally, a volume of DNase-free water was added to each tube, which was then frozen at −20°C until further analysis.
The cDNAs were amplified by PCR using specific oligonucleotide primers. HLA-G primers used were G.257F (exon 2; 5′-GGAAGAGGAGACACGGAACA) and G.1004R (exon 5 and exon 6 junction; 5′-CCTTTTCAATCTGAGCTCTTCTTT). PCR cycle conditions were 1 minute at 94°C, 1 minute 30 seconds at 61°C, and 2 minutes at 72°C. The amplification products along with the size marker (770-bp DNA ladder) were separated by agarose gel electrophoresis in TBE 1× (Invitrogen) and then visualized under UV light (Vilber Lourmat) after the addition of ethidium bromide.
For quantitative RT-PCR of mesothelial cells, cDNA was amplified using SYBR green mix (ROCHE) with ROCHE LightCycler 96 System. The beta-actin gene was used as the housekeeping gene. Primer sequences used were HLA-G (sense: 5′-GCG GCT ACT ACA ACC AGA GC; antisense: 5′-GAG GTA ATC CTT GCC ATC GTA G) and beta-actin (sense: 5′-AGA GCT ACG AGC TGC CTG AC; antisense: 5′-AGC ACT GTG TTG GCG TAC AG).
Ascitic Mononuclear Cell Characterization
Cluster cells were dissociated by accutase (PAA) before cytometry analysis to characterize the different cell populations present in these clusters. Mononuclear cells were labeled using appropriate antibodies linked to different fluorescent agents. Antibodies bound to cells were identified and semiquantified through flow cytometry. Results obtained were expressed as percentage of cells in each sample. Antibodies used were CD8 FITC, CD56 PE, CD14 FITC, CD25 PE, CD45RO FITC, and CD127 FITC (all from Becton Dickinson); CD45 RPECy5, CD45 APC, CD3 RPECy, and CD4 APC (all from DAKO); and AF750-anti-CD16 (Beckman Coulter). The controls were performed using corresponding isotype antibodies. The results were expressed as percentage of cells in each sample. The LSRII cytometer was used as an analyzer with nine colors and four lasers.
Isolation and Purification of Stromal Cells
Stromal cells were purified from clusters picked up from ovarian cancer patients' ascites. Clusters, taken directly from the ascitic fluid, were disaggregated using accutase (PAA, France) and cultured in vitro. Hospicells are cancer-associated mesenchymal stem cells and first derived from ascites of patients with ovarian cancer [22], which facilitate the tumorigenicity by promoting angiogenesis [23] and help the cancer evade the immune response through T-cell inhibition [24]. Characteristic hospicells were identified in these cultures by their ability to aggregate HL60 (leukemia cell line) and were then isolated for the present study using the method published by Rafii [22], [25], [26].
FACS for the Expression of HLA-G
The immunolabeling of hospicells was performed on cells in suspension. The expression of HLA-G membrane form was analyzed (no permeabilization of cells) using the antibody MEM-G/9-FITC. Briefly, each immunolabeling was done using 2100 cells/chamber. Receptors for the Fc fragment of Ig (to avoid nonspecific markings) were blocked by incubating with blocker buffer reagent at 4°C in dark. After washing in 1% BSA/PBS, cells were fixed at 3.7% PFA in PBS before flow cytometry analysis (Epics, XL4, and Beckman Coulter).
Regulation of HLA-G Expression by Hospicells
Hospicells were incubated with complete RPMI 1640 (PAA) supplemented with 5% fetal calf serum (PAA). For induction of HLA-G, IL-1β (10 ng/ml), IL-6 (10 ng/ml), TGF-β (10 ng/ml), IGF (200 ng/ml), VEGF (50 ng/ml), FGF (10 ng/ml), IL-13 (100 ng/ml), IL-2 (100 ng/ml), and EGF (10 ng/ml) were used (all from Miltenyi Biotec, France). For inhibition of HLA-G, Janus kinase inhibitor (JAK inhibitor I; Calbiochem, France), STAT3 inhibitor (Calbiochem, France), NF-κB inhibitor (Merck-Chemicals, France), and mitogen-activated protein kinases (MAP-kinases; MPK) inhibitor were applied (Sigma, France) at 1 μM.
Cytokine Antibody Array
Samples were analyzed for cytokines using RayBio Human Cytokine Antibody Array 6 as previously described [27]. Membranes were exposed to an X-ray film (Kodak X-OMAT AR film) within 30 minutes of exposure to the substrate. Signal intensities were quantified with a Bio-Imaging System MF-ChemiBIS 4.2 (FSVT, Courbevoie, France) and analyzed using Multi Gauge V3.2 software (Fujifilm). For each spot, the net optical density level was determined by subtracting the background optical level from the total raw optical density level.
Statistical Analysis
Statistical analysis reported was performed using GraphPad Prism 6 (GraphPad Software, Inc., San Diego, CA). Difference between the expression level of HLA-G5 (5A6G7 Ab) and that of sHLA-G1 + HLA-G5 (MEMG9) was compared using the Mann-Whitney test. The correlation (rs values) of HLA-G with different immune cell markers in the ascites and blood was calculated using Spearman correlation coefficient.
Results
Presence of Immune Cells in Cancer Cell Clusters
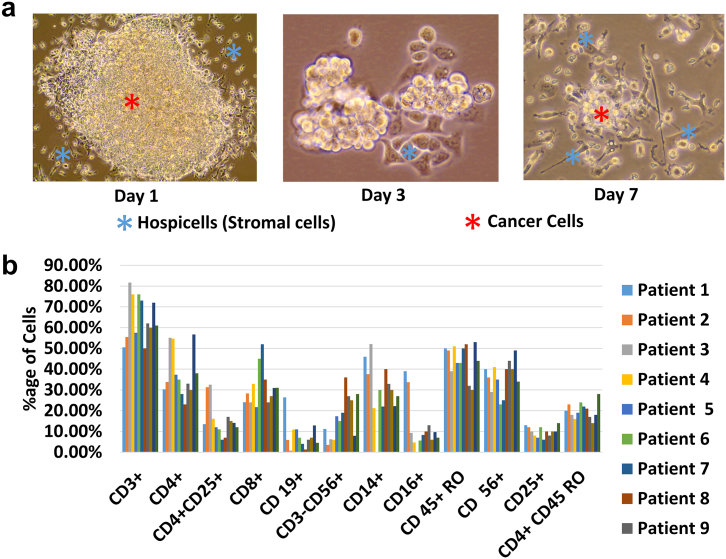
Fluid accumulation in the abdomen or ascites is a characteristic of the peritoneal carcinomatosis. The ascites provides microenvironment for the growth of cancer [28]. This ascites contains several types of cell, i.e., stromal cells (hospicells), cancer cells, and immune cells. We collected ascites from the patient and cultured it as 25% in DMEM supplemented with 10% fetal calf serum, 1% penicillin–streptomycin, and 1% L-glutamine. As shown in Figure 1A, colonies of cells were found, which can support attachment of nonadherent cells. These hospicells, as part of the peritoneum, can provide a niche for the cancer cells to attach and form a cancer nodule. Further, we performed flow cytometry (using antibodies against immune markers), and we found that the majority of the immune cell phenotypes were present in all the ascites collected from different patients (Figure 1B). The absence of immune response in cancer even in the abundance of immune cells is quite persuasive to study cancer ascites in the context of immune suppressive protein like HLA-G.
Figure 1.
Presence of hospicells, cancer cells, and immune cells in ascitic cell clusters.
(A) Cancer ascites contain hospicells that provide support to floating cancer cells, shown in vitro when ascites was cultured in DMEM. Stromal cells attach to the plastic earlier than the other types of cells and can be seen adherent starting from day 1 after in vitro culture.
(B) Several kinds of immune cells were found in different ascites collected from nine ovarian cancer patients, suggesting strong presence of the immune system in the cancer microenvironment, especially the presence of monocytes-macrophages.
HLA-G Expression by Ascitic Cell Clusters
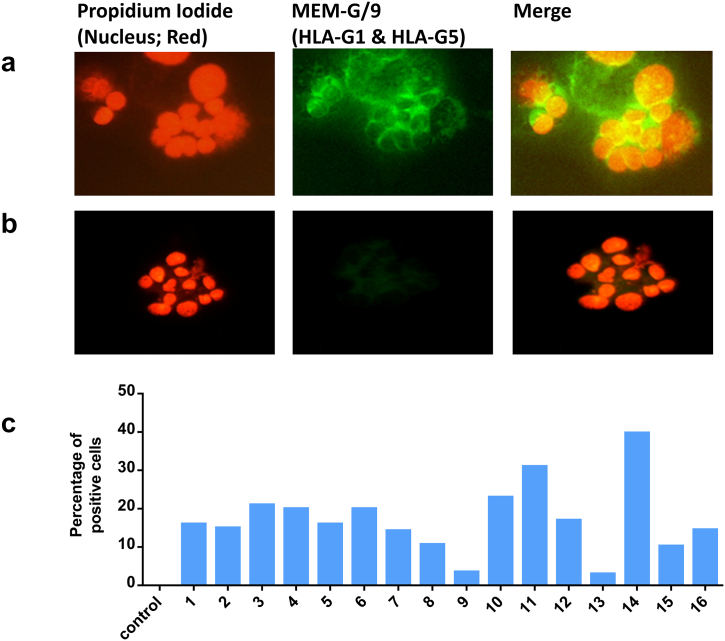
To assess the expression of HLA-G by cell cluster, the cell clusters were collected from the ovarian ascites, and the expression of HLA-G by immunofluorescence staining from the cluster cells is shown in Figure 2. The figure shows that cell clusters of ovarian carcinomatosis expressed HLA-G (green) in the cell cytoplasm and also on the cell membranes (Figure 2A). Negative controls were performed in the absence of primary antibodies (Figure 2B).
Figure 2.
HLA-G expression in cancer cell clusters. Immunofluorescence staining of HLA-G in cell clusters derived from ascites of patients with ovarian cancer.
(A) Cytospin cell clusters (which were taken directly from a patient specimen) were labeled with HLA-G1– and HLA-G5–specific mAb (MEM-G/9; primary) conjugated using antimouse IgG-FITC secondary antibody (green). DNA was labeled with propidium iodide (red). The merged pictures show that HLA-G1 and HLA-G5 are expressed in ovarian cancer in these cell clusters, and their expression was localized both in the cytoplasm and on the membrane.
(B) Negative controls were performed in the absence of primary antibodies.
(C) Percentage of HLA-G–positive cells in the cell cluster. Data show the heterogeneity in the expression of HLA-G in patients.
The mean ratio between the number of HLA-G positive cells and the total number of nucleated cells in the samples, for each ascites (n = 16), was calculated and is presented in Figure 2C. Almost all the cell clusters in different ascites expressed HLA-G ranging from 2% to 40% of the total number of cells.
Detection of sHLA-G in Ascitic Fluids of Patients with Ovarian Cancer
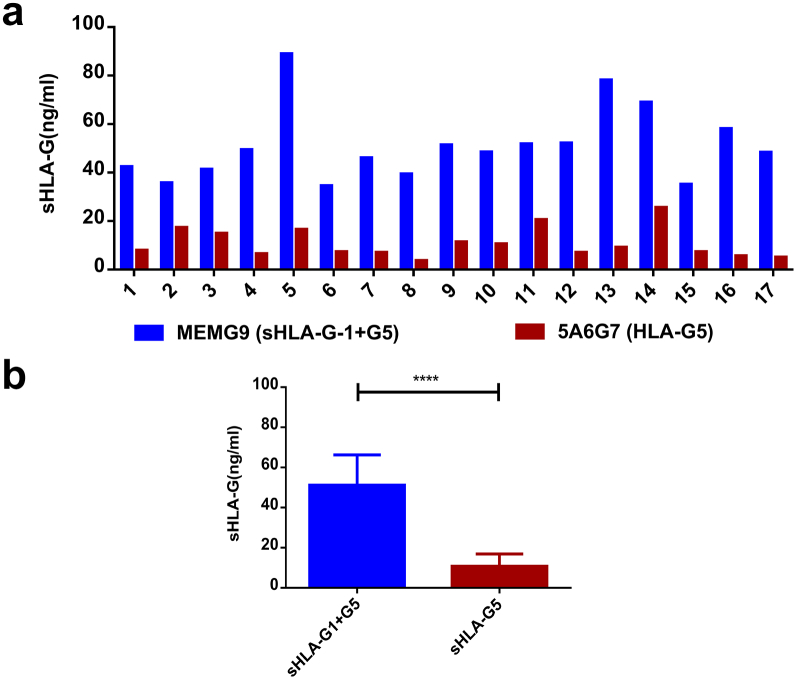
Further, to assess the presence of HLA-G in ascites, sHLA-G concentrations were measured in ascitic fluid samples from patients with ovarian cancer (Figure 3). ELISAs using MEM-G/9 antibody, which detects both sHLA-G1 and HLA-G5, and using 5A6G7 antibody, which detects only HLA-G5, were performed. As presented in Figure 3A, the level of sHLA-G1 + G5 was higher in all samples, and the level of HLA-G5 isoform was specifically higher in some of the ascites such as in samples 2, 3, 5, 9, 10, 11, and 14. These results confirm that HLA-G is not only expressed by the cells but also released into the microenvironment, hence making the microenvironment of cancer immunosuppressive.
Figure 3.
Detection of sHLA-G1 and G5 in ascitic fluids of patients with ovarian cancer.
(A) HLA-G concentrations were measured in ascitic fluid samples collected from patients with ovarian cancer. ELISA was performed using MEM-G/9 antibody, which detects both HLA-G1 shedding and HLA-G5, and 5A6G7 antibody, which detects only HLA-G5. The level of sHLA-G1 + G5 was high in all samples, and the level of HLA-G5 isoform was particularly higher in some of them such as in ascitic samples 2, 3, 5, 9, 10, 11, and 14.
(B) Levels of HLA-G1 + G5 (using MEM-G9 antibody) and sHLA-G5 (5A6G7) determined through ELISA showed significant difference in ascitic samples collected from 17 patients, with sHLA-G1 + G5 levels being higher. This indicates the presence of both soluble isoforms of HLA-G, i.e., HLA-G1 and HLA-G5 (Mann-Whitney test ****P < .0001).
HLA-G Expression by Mesothelial Cells in the Peritoneum Wall
Expression of sHLA-G in the Absence of Membrane-Bound HLA-G
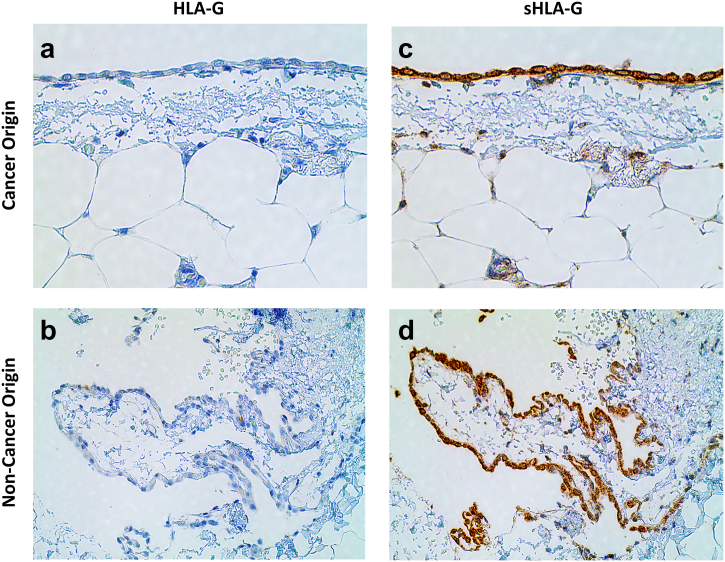
The peritoneal membrane tissue sections were obtained from patients with and without cancer (but with inflammation). We found no expression of HLA-G in histological stains of the mesothelial layer obtained from either cancer (Figure 4A) or noncancer origin (Figure 4B). The cells in the mesothelial layer were detected positive for the sHLA-G in the tissue from cancer (Figure 4C) and noncancer origin (Figure 4D). The presence of sHLA-G in the noncancerous tissue does exhibit that the sHLA-G expression may be due to inflammatory cytokines as well as cancer microenvironment. Although the mesothelial cells do not produce HLA-G on their own, presented by the absence of membrane-bound HLA-G, sHLA-G can be absorbed by these mesothelial cells from cancer ascites.
Figure 4.
Expression of HLA-G and sHLA-G by mesothelial cells in the peritoneal membrane tissue section of cancer and noncancer origin.
The membrane-bound HLA-g was found negative in the tissues of both cancer (A) and noncancer origin (C). However, we detected the presence of sHLA-G in both the tissues (B and D). sHLA-G expression may be attributed to the presence of inflammatory cytokines.
In Vitro Retaining of sHLA-G by Mesothelial Cells
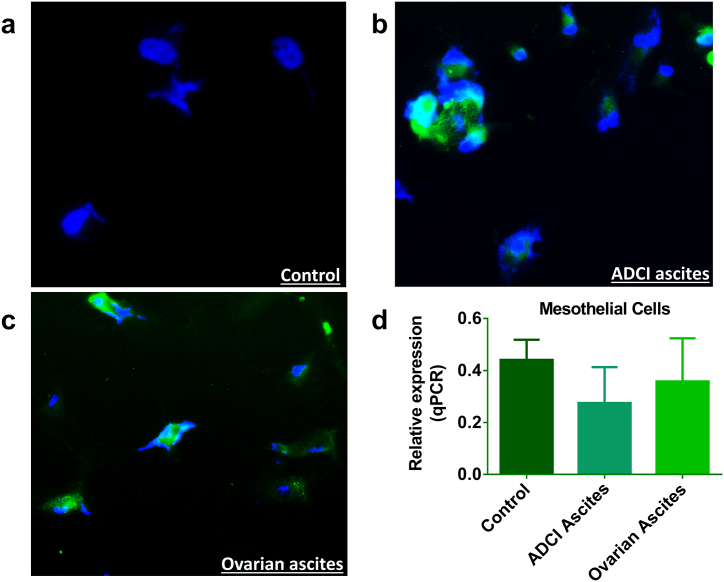
Further, although the above findings were confirmed when mesothelial cells were cultured in different ascites obtained from ADCI and patients with ovarian cancer, we did not find any increase in HLA-G expression (qPCR; Figure 5D). However, immunofluorescence with polyclonal HLA-G antibody showed that the cells were detected positive for HLA-G after being cultured with 25% ascites in the culture medium (Figure 5, A-C).
Figure 5.
Expression of HLA-G in human adult mesothelial cell line. (A-C) Immunofluorescence using polyclonal anti–HLA-G antibody (green for HLA-G and blue for nucleus). (D) Relative expression of HLA-G (qPCR).
The strong expression of HLA-G was found in the majority of cells after being cultured in a medium containing 25% ascites supernatant for 6 days.
(A) Control: Mesothelial cells cultured in the classic culture medium from ZenBio, Inc., without bovine calf serum. There was no expression of HLA-G.
(B) ADCI ascites: Human adult mesothelial cells grown in 25% ADCI ascites supernatant. HLA-G expression was found positive after 6 days.
(C) Ovarian ascites: Human adult mesothelial cells grown in 25% ovarian ascites. HLA-G expression was found positive after 6 days.
(D) There was no significant difference in the qPCR analysis with regard to the relative expression of HLA-G in mesothelial cells when cultured in a medium with 25% ascites (ADCI ascites and ovarian ascites).
The expression of HLA-G through immunofluorescence despite no difference in mRNA expression shows that the mesothelial cells retain HLA-G from ascites in the soluble form, and this sHLA-G is not produced intrinsically. Moreover, the above results confirm that even if the cells do not secrete HLA-G, it can be absorbed from the ascites. This presence of sHLA-G results in providing an immunosuppressive microenvironment for the cancer cells.
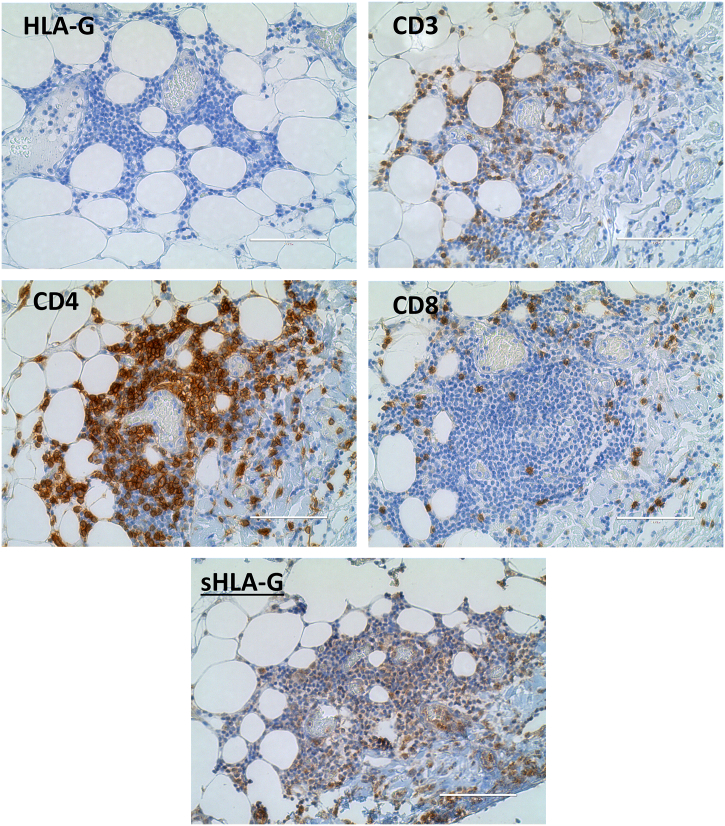
In Situ Immune Infiltration
In addition, we found that the immune cell infiltrate containing CD3, CD4, and CD8 cells did not express HLA-G in the periphery of the cancer nodule (Figure 6). The immune cell infiltrate was found to express the CD8, CD3, and CD4 phenotype of immune cells. Although the cells were negative with the HLA-G expression, the presence of sHLA-G along with immune phenotypes was found positive.
Figure 6.
Expression of HLA-G and immune cell markers in immune cell infiltrate (in situ).
The immune cell infiltrate in the periphery of cancer cells expresses CD3+-, CD4+-, and CD8+-phenotype immune cells. No expression of HLA-G was found for the immune cell infiltrate. However, sHLA-G was detected positive.
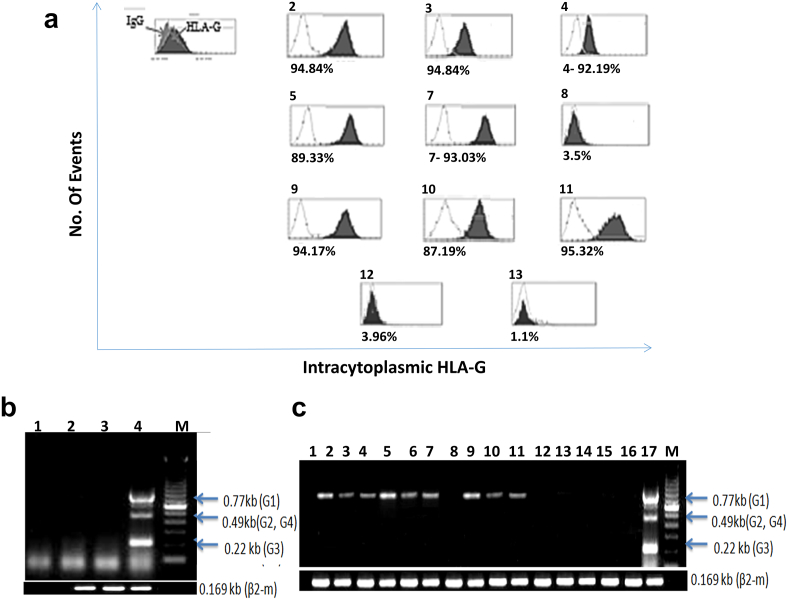
HLA-G Protein Expression by Hospicells
The hospicells separated from ascites cell cluster as previously described [22], [25], [26] were cultured for 24 hours in complete medium with or without 30% ascites supernatant and analyzed by flow cytometry. Cells were permeabilized and stained either by FITC-conjugated anti-HLA-G Ab (clone MEM-G/9, which recognizes both HLA-G1 and HLA-G5 isoforms; red histograms) or by FITC-conjugated irrelevant Ab control (transparent histograms).
As presented in Figure 7A, the HLA-G protein was found to be induced in hospicells by a majority of ascitic fluids (as compared to the control test). The level of induced expression, however, varied according to the patients from whom ascites was collected (Figure 7A-2, 3, 4, 5, 7, 9, 10, 11), while some of them were devoid of HLA-G–inducing factors (Figure 7A-8, 12, 13). These results were confirmed by immunocytochemistry using HLA-G1– and HLA-G5–specific mAb (MEM-G/9) (results not shown). Results indicated that the level of induction was dictated by the components present in the ascitic fluids.
Figure 7.
HLA-G expression by hospicells:
(A) The hospicells were cultured for 24 hours in complete medium with 30% of ascites supernatant and analyzed by flow cytometry. Cells were permeabilized and stained either by FITC-conjugated anti–HLA-G Ab (clone MEM-G/9, which recognizes both HLA-G1 and HLA-G5 isoforms; filled histograms) or by FITC-conjugated irrelevant Ab control (transparent histograms).
(B) RT-PCR analysis of the mRNA HLA-G in hospicells. Lanes 2 and 3 are the cells before treatment with ascitic fluid, lane 1 is the negative control with H2O, lane 4 is the positive control, and lane M is the size marker.
(C) Hospicells were incubated for 6 hours with 30% ascites. Lane1 is the negative control, lanes 2-13 correspond to cells treated with a medium containing bovine calf serum and different ascitic fluid supernatants, lanes 14-16 correspond to the cells treated with a medium without bovine calf serum and ascitic fluid supernatants, lane 17 is a positive control, and lane M is a size marker. All ascites except 8, 12, and 13 induced HLA-G expression.
The above results were reconfirmed by RT-PCR analysis, as presented in Figure 2, B and C. The mRNA corresponding to HLA-G in hospicells was undetectable before treatment with ascitic fluids (Figure 7B, lanes 2 and 3); lane 1 is the negative control with H2O, lane 4 is the positive control, and lane M is the size marker. On the other hand, when hospicells were incubated for 6 hours with 30% ascites supernatants of complete culture medium, it was observed that the hospicells expressed a significant amount of HLA-G-1 mRNA. These results are presented in Figure 7C (lane 1 is the negative control, lanes 2-13 correspond to cells treated with a medium containing ascitic fluid supernatants, lanes 14-16 correspond to the cells treated with a medium without bovine calf serum and ascitic fluid supernatants, lane 17 is a positive control, and lane M is a size marker).
These results demonstrate the importance of bovine calf serum in HLA-G induction (lanes 14, 15, and 16 in Figure 7C) and also underline the role of ascites supernatants on the expression of HLA-G by hospicells.
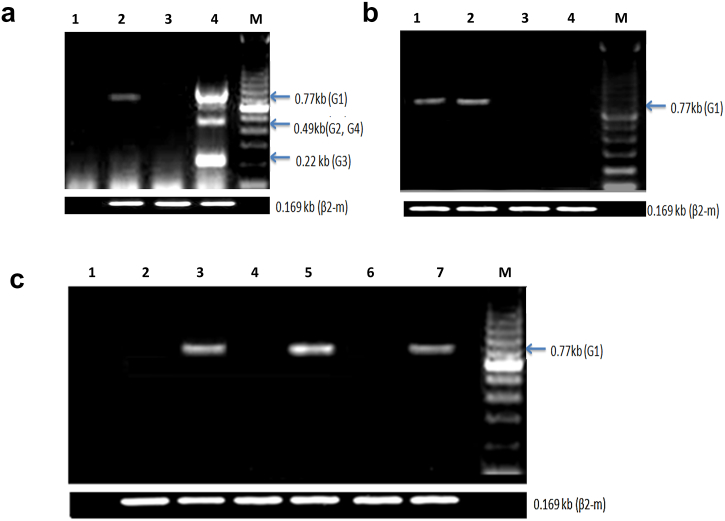
HLA-G Expression by Cancer Cell Lines
The expression of HLA-G by human cancer cell lines (ovarian, breast, lung, colon, myeloblastic leukemia, and chronic B cell leukemia) is presented in Figure 8. OVCAR-3 (Figure 8A; lane 2), MDA-MB231 (Figure 8A; lane 3), HL60 (Figure 8B; lanes 1 and 2), MEC-1 (Figure 8B; lanes 3 and 4), A549 (Figure 8C; lane 2), HCT29 (Figure 8C; lane 3), and HCT-8R (Figure 8C; lane 5) were analyzed by RT-PCR using specific primers as presented. Each cell line was treated with 30% ascitic supernatants and 70% culture medium with 10% of bovine calf serum, or only with RPMI and 10% of bovine calf serum. The results presented in Figure 8 show that, except for MDA-MB231 (Figure 8A; lane 3) and MEC-1 (Figure 8B; lane 4), the expression of HLA-G, more specifically the HLA-G1 isoform, was induced by the presence of added ascitic fluids at 30% concentrations. Cell lines such as HL-60 and OVCAR-3 expressed HLA-G without ascites when cultured in a complete medium containing 10% bovine calf serum (Ovcar-3; results not shown). We found that different cancer cell lines express different levels of HLA-G, and this ability of ascites to induce HLA-G is also dependent on the type of cancer cells.
Figure 8.
HLA-G expression by cancer cell lines before and after culture with ascites supernatant or serum.
(A0 Human ovarian cancer (OVCAR-3) and human breast cancer (MDA-MB231) cell lines incubated with the supernatant of ascites: lane 1: H2O, lane 2: OVCAR after incubation for 6 hours with 30% supernatant of ascites and 70% complete RPMI medium with serum, lane 3: MDA-MB231 after incubation for 6 hours with 30% supernatant of ascites and 70% complete RPMI medium with serum, lane 4: JEG-3, and lane M: size marker.
(B) Human promyelocytic leukemia (HL60) and human chronic B cell leukemia (MEC-1) cell line: lane 1: HL60 after incubation for 6 hours with 30% supernatant of ascites and 70% of complete RPMI medium with serum, lane 2: HL60 after incubation for 6 hours with complete medium with serum, lane 3: MEC1 after incubation for 6 hours with 30% supernatant of ascites and 70% of complete RPMI medium with serum, lane 4: MEC-1 after incubation for 6 hours with complete medium with serum, and lane M: size marker.
(C) Human lung cancer (A549) and human colon cancer (HCT-8R, HCT29) cell line: lane 1: H2O, lane 2: A549 after incubation for 6 hours with complete medium containing serum, lane 3: A549 after incubation for 6 hours with 30% supernatant of ascites and 70% complete RPMI medium with serum, lane 4: HCT-8R after incubation for 6 hours with complete medium with serum, lane 5: HCT-8R after incubation for 6 hours with 30% supernatant of ascites and 70% complete RPMI medium with serum, lane 6: HCT-29 after incubation for 6 hours with complete medium with serum, and lane 7: HCT-29 after incubation for 6 hours with 30% supernatant of ascites and 70% complete RPMI medium with serum.
Correlation between HLA-G Levels and Composition of Immune Cell Population in Ascitic Clusters
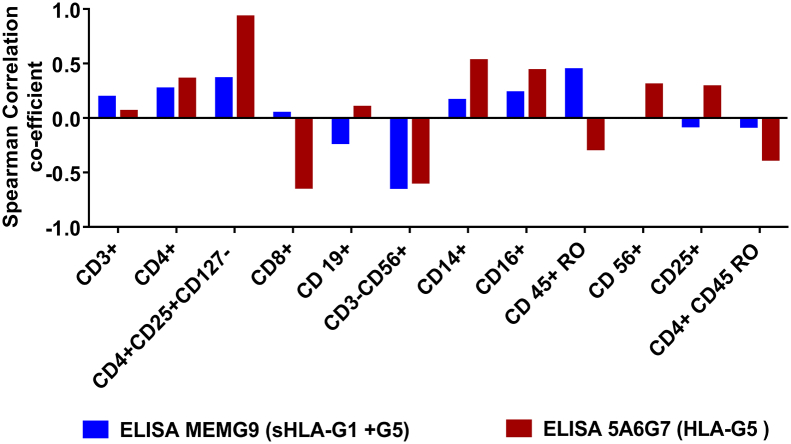
After quantification of sHLA-G in ascitic fluids, the correlation between the level of sHLA-G (HLA-G1/G5 and HLA-G5 only) and percentage of immune cells in clusters from different patients was evaluated. As presented in Table 1 A, T-reg cell content was quite different from one patient to the other. There was a positive correlation between the quantity of HLA-G5 and the percentage of regulatory T cells (Table 1 B). A comparative study between the level of sHLA-G and the content of T-reg cells in the ascites of patients with ovarian cancer shows a strong positive correlation between the number of CD4+CD25+CD127− T cells and sHLA-G1 + G5 (rs = 0.361) or between these T-reg cells and HLA-G5 (rs = 0.93). The results presented in Figure 9 show mild positive correlation of sHLA-G1 + G5 detected only with CD3+ (rs = 0.19), CD4+ (rs = 0.26), and CD4/CD25+CD127− (rs = 0.36) subpopulations. The negative coefficient of correlation of sHLA-G1 + G5 was only detected with CD3−/CD56+ (rs = −0.61) subpopulation. Similar results were observed with another antibody (5A6G7) used to detect HLA-G5 in ascites. We observed a positive correlation between HLA-G5 with CD3+ (rs = 0.06), CD4+ (rs = +0.35), CD4/CD25+CD127− (rs = 0.93), and CD14+ (rs = 0.52), whereas a negative correlation was observed for CD3−/CD56+ (rs = −0.59). These results suggest that sHLA-G in ascites is associated with the increase of T-reg and decrease of NK cells in ovarian cancer.
Table 1.
Correlation between HLA-G Levels and Composition of Immune Cell Population in Ascitic Clusters
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | Patient 11 | Patient 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Table 1A | ||||||||||||
| CD3+ | 50.50% | 55.50% | 81.70% | 76.00% | 57.50% | 76.00% | 73.00% | 50.00% | 62.00% | 60.00% | 72.00% | 61.00% |
| CD4+ | 30.30% | 33.80% | 55.10% | 54.70% | 37.30% | 35.00% | 28.00% | 23.00% | 33.00% | 30.00% | 56.70% | 38.00% |
| CD4+CD25+CD127− | 13.50% | 31.30% | 32.50% | 16.10% | 12.00% | 11.00% | 6.00% | 7.00% | 17.00% | 15.00% | 14.10% | 12.00% |
| CD8+ | 24.10% | 28.30% | 24.00% | 32.90% | 21.70% | 45.00% | 52.00% | 35.00% | 24.00% | 27.00% | 30.90% | 31.00% |
| CD19+ | 26.40% | 5.90% | 0.80% | 10.90% | 11.00% | 7.00% | 4.00% | 1.30% | 6.00% | 7.00% | 12.90% | 4.50% |
| CD3−CD56+ | 11.20% | 3.50% | 6.20% | 5.90% | 17.30% | 15.00% | 19.00% | 36.00% | 27.00% | 25.00% | 7.90% | 28.00% |
| CD14+ | 46.00% | 37.60% | 52.10% | 21.20% | 0.00% | 30.00% | 22.00% | 40.00% | 32.90% | 30.00% | 22.20% | 27.00% |
| CD16+ | 39.00% | 33.70% | 9.30% | 4.70% | 0.00% | 5.60% | 8.30% | 10.00% | 13.00% | 6.00% | 9.70% | 7.00% |
| CD45+ RO | 50.00% | 49.00% | 39.00% | 51.00% | 43.00% | 43.00% | 50.00% | 52.00% | 32.00% | 30.00% | 53.00% | 44.00% |
| CD56+ | 40.00% | 36.00% | 29.00% | 41.00% | 35.00% | 23.00% | 25.00% | 40.00% | 44.00% | 40.00% | 49.00% | 34.00% |
| CD25+ | 13.00% | 12.00% | 10.00% | 8.00% | 7.00% | 12.00% | 6.00% | 10.00% | 8.00% | 10.00% | 10.00% | 14.00% |
| CD4+ CD45 RO | 20.00% | 23.00% | 18.00% | 16.00% | 19.00% | 24.00% | 22.00% | 21.00% | 17.00% | 14.00% | 18.00% | 28.00% |
| ELISA MEMG9 (sHLA-G1 + G5) ng/ml | 51.75 | 68.99 | 100.51 | 88.96 | 42.41 | 35.09 | 58.08 | 49.4 | 35.73 | 41.25 | 51.37 | 48.48 |
| ELISA 5A6G7 (HLA-G5) ng/ml | 20.5 | 25.54 | 125.02 | 16.52 | 7.85 | 7.25 | 5.65 | 6.48 | 17.25 | 14.89 | 11.36 | 10.5 |
| Table 1B | ||||||||||||
| CD4+CD25+high CD127−% (age of cells) | 13.50 | 31.30 | 32.50 | 16.10 | 12.00 | 11.00 | 6.00 | 7.00 | 17.00 | 15.00 | 14.10 | 12.00 |
| ELISA MEMG9 (sHLA-G1 + G5) ng/ml | 51.75 | 68.99 | 100.51 | 88.96 | 42.41 | 35.09 | 58.08 | 49.4 | 35.73 | 41.25 | 51.37 | 48.48 rs = 0.361 |
| ELISA 5A6G7 (HLA-G5) ng/ml | 20.5 | 25.54 | 125.02 | 16.52 | 7.85 | 7.25 | 5.65 | 6.48 | 17.25 | 14.89 | 11.36 | 10.5 rs = 0.928 |
(A) Representative percentage for each type of immune cells obtained through FACS and levels of HLA-G1 + G5 (using MEM-G9 antibody) and sHLA-G5 (5A6G7) obtained through ELISA in patients' ascites are presented in the table.
(B) Comparative results using Spearman's correlation (value given as rs) between the sHLA-G level and the content of Treg cells in the ascites of patients with ovarian cancer showed a positive correlation (rs = 0.36) between the amount of CD4+CD25+CD127− T cells and HLA-G1+G5 and a very strong positive correlation (rs = 0.93) between these Treg cells and sHLA-G5.
Figure 9.
Correlation between HLA-G levels and composition of immune cell population in ascitic clusters.
A positive correlation coefficient of HLA-G1 + G5 was detected only with CD45+RO (rs = 0.44), CD4+CD25+CD127− (rs = 0.36), and CD4+ (rs = 0.26) subpopulations, whereas a negative coefficient of correlation of HLA-G1 + G5 was detected for CD3−CD56+ (rs = −0.63) subpopulation. We used another antibody (5A6G7) to detect sHLA-G5 in ascites. A strong negative correlation was found for sHLA-G5 with CD8+ (rs = −0.63), CD3−CD56+ (rs = −0.58), and CD4+CD45RO (rs = −0.37), whereas a positive correlation was found with CD4+ (rs = 0.35) and CD4+CD25+CD127− (rs = 0.93).
IL-1β and TGF-β as HLA-G–Inducing Factors
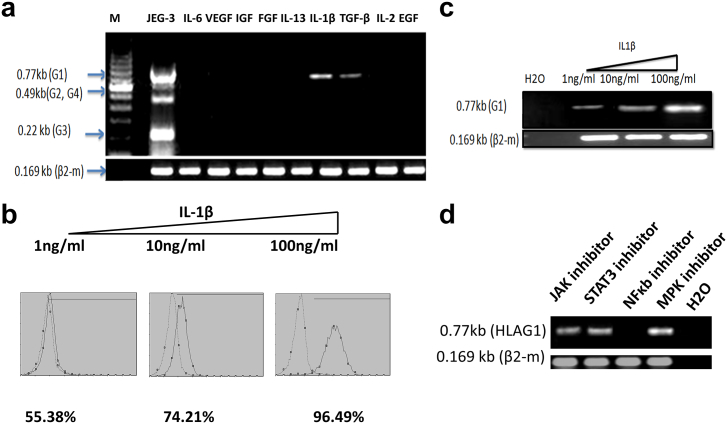
For the identification of principal HLA-G–inducing factor(s) in ascitic fluids, six ascites from ovarian carcinomatosis patients were chosen. Three of them that did not induce HLA-G mRNA expression were compared with other ascites that induced HLA-G mRNA expression by cells. After comparative analysis of 174 cytokines in peritoneal fluids (results not shown), we chose, among the candidate bioactive proteins, IL-1β, IL-6, IL-13, IL-2, VEGF, IGF, FGF, TGF-β, and EGF, which were higher in ascites that induced HLA-G expression than the ones that did not produce HLA-G expression in hospicells as shown in Figure 7. We tested these proteins, for induction potential in hospicells, individually to narrow down and identify the actual inducer protein(s). Hospicells, cultured for 6 hours, were tested for HLA-G induction by using RT-PCR and flow cytometry. The results are presented in Figure 10A. The results of these experiments revealed the presence of two inducer proteins in the ascitic fluids, which were TGF-β and IL-1β. TGF-β has been already reported by other authors as an inducer of HLA-G [29].The inducer effect of IL-1β was dose dependent (Figure 10, C and D). We found that IL-1β was also an inducer of HLA-G, and it was more potent than TGF-β at concentrations as low as 1 ng/ml. To define the signaling pathway of IL-1β implicated in the induction of HLA-G, the signaling pathway inhibitors (JAK1, STAT3, NF-κB, and MPK) were cultured with hospicells in a medium containing bovine calf serum. Total RNA was extracted after 6 hours of treatment, and RT-PCR was carried out. As shown in Figure 10D, the NF-κB signaling pathway seems to be involved in the induction of mRNA of HLA-G by IL-1β.
Figure 10.
HLA-G–inducing factors in ascitic fluids.
Hospicells, treated for 6 hours with different cytokines, were tested for sHLA-G induction by RT-PCR and flow cytometry:
(A) IL-1β and TGF-β were only found as inducers of sHLA-G.
(B, C). IL-1β was found to be more potent than TGF-β at concentrations as low as 1 ng/ml. The inducer effect of IL-1β was dose dependent, measured by FACS and RT-PCR.
(D) Signaling pathway inhibitors (JAK1, STA3, NF-κB, and MPkk) were used on hospicells in culture using a medium containing bovine calf serum. Total RNA was extracted after 6 hours of treatment, and RT-PCR was carried out. HLA-G by IL-1β was found to be induced through the NF-κB pathway.
Discussion
Epithelial ovarian carcinoma is the sixth most common malignancy in woman and the leading cause of death from gynecological cancer in the world [30]. The patients have a predisposition to metastatic involvement of the peritoneal cavity [31], [32] and are characterized by increased CA125 levels [33]. Late stage is characterized by widespread peritoneal dissemination, presence of ascites, and a high rate of mortality, with an overall survival ranging from 20% to 30% at 5 years after surgery [34].
Despite the presence of tumor-infiltrating T cells (Figure 1), ovarian cancer is the most lethal gynecological malignancy due to frequent peritoneal metastasis and development of ascites. A tumoral microenvironment contains immunoregulatory cells and immunosuppressor mediators. The tumor stroma generally plays an essential role in tumor cell survival and growth through the production of cytokines, growth factors, and metalloproteinases. Direct interaction between stromal cells and cancer cells prevents apoptosis through the upregulation of antiapoptotic proteins [35] or chemokines [26] or through oncologic trogocytosis between hospicells and tumor cells [22]. In ovarian cancer, hospicells inhibit T cell proliferation and cytokine productions [36]. Stroma-derived hospicells provide the adequate microenvironment for the development and survival of newly forming tumors, and the presence of HLA-G contributes to ensure safe conduct.
HLA-G complex (with its isoforms) modulates the activation and effector functions of key immune cells. Expression of HLA-G as an immune suppressor in mesenchymal cells has already been reported [14], [37]. Hospicells can attract cancer cells and provide a premetastatic niche. The presence of hospicells within the patient's tumor might be predictive for chemoresistance [22]. Hospicells in the microenvironment of tumors may also mediate the immunosuppression of T cells [36]. We found several phenotypes of immune cells such as CD3+, CD4+, CD8+, CD19+, CD4+CD25+, CD14+, CD16+, and CD56+ being present in the cancer cell cluster. However, the immune response by these cells is not efficient enough to stop the metastasis of cancer. This suggests that the immune system in ovarian cancer is compromised, and cancer cells exhibit a phenomenon to escape the immune surveillance. The expression of HLA-G by hospicells may therefore allow ovarian cancers to evade immune surveillance. We found that the diverse percentage of cells from cancer cell cluster express HLA-G in various patients (Figure 2). In addition, we measured the sHLA-G concentrations in ascitic fluid samples from patients with ovarian cancer. Analysis of ascitic fluids allowed detection of both HLA-G1 shedding and HLA-G5 in all patients. The level of sHLA-G1 + G5 was higher than that of the HLA-G5 isoform, suggesting the presence of two isoforms shedding HLA-G1 and sHLA-G5 in patients' ascites (Figure 3). This expression of shedding HLA-G1 could have resulted from the release of membrane-bound HLA-G isoforms (HLA-G1). sHLA-G, particularly HLA-G5, may affect antitumor immune response both in situ and in circulation [38].
To find the origin of HLA-G, we performed immunohistochemistry using the tissue specimen. We found that mesothelial cells in the tissue did not secrete HLA-G. However, sHLA-G was detected in these mesothelial cells, suggesting that the HLA-G is not produced by mesothelial cells in the peritoneal membrane but rather acquired from the microenvironment. This sHLA-G further provides the immunosuppressive environment to cancer cells, which may support carcinomatosis and anchoring of cancer cells on the peritoneal membrane to form a cancer nodule. We observed a similar expression of sHLA-G in the noncancerous tissue with inflammatory conditions (Figure 4). This suggests that the inflammatory cytokines play an important role in the expression of HLA-G. Further, the results were confirmed in vitro when the mesothelial cells stained positive for HLA-G after being cultured in a medium containing 25% ascites, while no expression of HLA-G was found in cells cultured with a normal medium. As there was no increase in the mRNA expression of HLA-G, the expression of HLA-G is not the intrinsic production of HLA-G; rather, it is absorbed from the ascites (Figure 5). Moreover, when we analyzed the immune infiltrate in the peritoneal membrane, we found the expression of sHLA-G on CD3+, CD4+, and CD8+ immune cell phenotypes (Figure 6). Therefore, the sHLA-G affects the overall immune system and results in the suppression of immune cell activity.
The expression of HLA-G by stromal and cancer cells can be considered as an in situ immunosuppressor cofactor. Stromal cells, cancer cells, and immune cells are the basic elements of cell clusters present in ascitic fluids. The quantity of HLA-G can increase in ascitic fluids on account of shedding of HLA-G from cell clusters. The components of ascitic fluid in ovarian cancers have a crucial role in the expression of HLA-G by stromal cells. When we incubated the hospicells in the presence of ascites, we found an overall increase in the expression of HLA-G (Figure 7). However, ascites from three patients were not able to induce the expression of HLA-G. This proves the importance of various cytokines in ascites for inducing the expression of HLA-G. Already, several HLA-G–inducing factors such as IL-10 [39], interferon [40], and TGF or glucocorticoid [41] in different cell models, especially in monocyte and trophoblasts, have been reported. In parallel, fetal calf serum and plasma rich in platelet induce the expression of HLG on the cells [37].
In parallel, we show that the mRNA expression of HLA-G, especially the HLA-G1 isoform, by human ovarian cancer (OVCAR-3), human lung cancer (A549), human colon cancer (HCT29 and HCT-8R), and human myeloblastic leukemia (HL-60) occurs after incubation with cancer ascites. However, some cancer cell lines such as human breast cancer (MDA-MB231) and human chronic B cell leukemia (MEC-1) seem not to express HLA-G mRNA (Figure 8) even in the presence of ascites. These results again suggest the crucial role of tumor microenvironment in increased expression of HLA-G for the majority of cell lines. Increased HLA-G expression in situ was observed in nearly 30 types of different malignancies [42]. HLA-G was preferentially expressed in tumor cells in situ and rarely detected in adjacent tissues. Elevated expression of HLA-G was found associated with malignant, invasive, or metastatic status and poor prognosis [12], [17], [19].
Further, we found that increased expression of HLA-G in ascites is associated with the increase in regulatory cells such as B cells and T-helper cells while decrease in effector cells like NK cells (Figure 9). In our study, we first analyzed the content of immune cells in ascitic cell clusters. In all patients, we found a large amount of immune cells such as T cells, B cells, macrophage-dendritic cells and NK cells present in all patients (Figure 9). A comparative study between the level of sHLA-G in ascitic fluids and immune cells from ascitic cell clusters (Figure 9) showed that the intensity of T-reg cells has a positive correlation with the amount of CD4+CD25+ high CD127− and sHLA-G1/G5 (rs = 0.361) or between these T-reg cells and HLA-G5 (rs = 0.928). A positive correlation of sHLA-G1 + G5 was detected only with CD3+ (rs = 0.19), CD4+ (rs = 0.27), and CD4+CD25+ high CD127− (rs = 0.36) subpopulations, and negative coefficient of correlation of sHLA-G1 /G5 was detected for CD3−/CD56+ (rs = −0.63) subpopulation and CD4+CD45RO+ memory cells (rs = −0.37). The large quantity of evidence that has accumulated concerning the ascitic fluid and cell clusters points out to the important role of sHLA-G in the downregulation of immune cell efficiency in tumor microenvironments. This may be through the upregulation of T-reg cells and downregulation of NK cells.
When probing of ascites protein array for their property as an inductor of HLA-G or not (results not shown), we found that IL-1β and TGFβ are the two most potent inductors of HLA-G proteins in hospicells. We demonstrated the role of IL-1β and its pathway in the upregulation of HLA-G (Figure 10). We demonstrated that the induction of HLA-G by IL-1β required the nuclear factor-kappa B (NF-κB) pathway. IL-1β is reported to induce the expression of HLA-G in an HIF-1α–dependent manner in glioblastoma cell lines through TLR4 signaling [43]. These results suggest that HLA-G, in addition to SMAD signaling pathway for TGFβ [29], [44], JAK-STAT for interferon, and IL-10 [45], can be regulated by a multitude of cell signaling pathways.
Conclusion
The expression of HLA-G is enhanced not only by the cytokines present in the tumor microenvironment but also due to the absorbed sHLA-G present in the ascites produced by cancer cells. This absorbance of sHLA-G by mesothelial cells in the peritoneal membrane helps in providing an immunosuppressive environment to cancer cells to anchor to the peritoneal membrane, which can further help in metastasis. Further, the expression of sHLA-G in ascites was found to be in positive correlation with T-reg cells and negative correlation with NK cells, resulting in increased immunotolerance for cancer cells. For the first time, we present here the role of IL-1β from the microenvironment in the expression of sHLA-G. Moreover, we found that the expression of sHLA-G is modulated through the NF-κB pathway.
References
- 1.Spurrell EL, Lockley M. Adaptive immunity in cancer immunology and therapeutics. Ecancermedicalscience. 2014;8 doi: 10.3332/ecancer.2014.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Etzioni A. Immune deficiency and autoimmunity. Autoimmun Rev. 2003;2:364–369. doi: 10.1016/s1568-9972(03)00052-1. [DOI] [PubMed] [Google Scholar]
- 3.Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HS. vol. 35. 2015. Immune evasion in cancer: mechanistic basis and therapeutic strategies; pp. S185–S198. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia A, Kumar Y. Cellular and molecular mechanisms in cancer immune escape: a comprehensive review. Expert Rev Clin Immunol. 2014;10:41–62. doi: 10.1586/1744666X.2014.865519. [DOI] [PubMed] [Google Scholar]
- 5.Kandalaft LE, Motz GT, Duraiswamy J, Coukos G. Vol. 30. 2011. Tumor immune surveillance and ovarian cancer and Metastasis Reviews; pp. 141–151. [DOI] [PubMed] [Google Scholar]
- 6.Własiuk P, Putowski M, Giannopoulos K. PD1/PD1L pathway, HLA-G and T regulatory cells as new markers of immunosuppression in cancers. Postepy Hig Med Dosw (Online) 2016;70:1044–1058. doi: 10.5604/17322693.1220994. [DOI] [PubMed] [Google Scholar]
- 7.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Medicine. 2004;10:942. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 8.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf D, Wolf AM, Rumpold H, Fiegl H, Zeimet AG, Muller-Holzner E, Deibl M, Gastl G, Gunsilius E, Marth C. The expression of the regulatory T cell–specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res. 2005;11:8326–8331. doi: 10.1158/1078-0432.CCR-05-1244. [DOI] [PubMed] [Google Scholar]
- 10.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 11.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 12.S-m Yie, Hu Z. Human leukocyte antigen-G (HLA-G) as a marker for diagnosis, prognosis and tumor immune escape in human malignancies. Histol Histopathol. 2011;26:409. doi: 10.14670/HH-26.409. [DOI] [PubMed] [Google Scholar]
- 13.Rouas-Freiss N, GONCalves RM-B, Menier C, Dausset J, Carosella ED. Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc Natl Acad Sci U S A. 1997;94:11520–11525. doi: 10.1073/pnas.94.21.11520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selmani Z, Naji A, Gaiffe E, Obert L, Tiberghien P, Rouas-Freiss N, Carosella ED, Deschaseaux F. HLA-G is a crucial immunosuppressive molecule secreted by adult human mesenchymal stem cells. Transplantation. 2009;87:S62–S66. doi: 10.1097/TP.0b013e3181a2a4b3. [DOI] [PubMed] [Google Scholar]
- 15.Carosella ED, Gregori S, LeMaoult J. The tolerogenic interplay (s) among HLA-G, myeloid APCs, and regulatory cells. Blood. 2011;118:6499–6505. doi: 10.1182/blood-2011-07-370742. [DOI] [PubMed] [Google Scholar]
- 16.Naji A, Menier C, Morandi F, Agaugué S, Maki G, Ferretti E, Bruel S, Pistoia V, Carosella ED, Rouas-Freiss N. Binding of HLA-G to ITIM-bearing Ig-like transcript 2 receptor suppresses B cell responses. J Immunol. 2014;192:1536–1546. doi: 10.4049/jimmunol.1300438. [DOI] [PubMed] [Google Scholar]
- 17.Carosella ED, Favier B, Rouas-Freiss N, Moreau P, LeMaoult J. Beyond the increasing complexity of the immunomodulatory HLA-G molecule. Blood. 2008;111:4862–4870. doi: 10.1182/blood-2007-12-127662. [DOI] [PubMed] [Google Scholar]
- 18.The Human Protein Atlas, Vol. 2018. [Google Scholar]
- 19.Lin A, Zhang X, Xu HH, Xu DP, Ruan YY, Yan WH. HLA-G expression is associated with metastasis and poor survival in the Balb/c nu/nu murine tumor model with ovarian cancer. Int J Cancer. 2012;131:150–157. doi: 10.1002/ijc.26375. [DOI] [PubMed] [Google Scholar]
- 20.Jung YW, Kim YT, Kim SW, Kim S, Kim JH, Cho NH, Kim JW. Correlation of human leukocyte antigen-G (HLA-G) expression and disease progression in epithelial ovarian cancer. Reprod Sci. 2009;16:1103–1111. doi: 10.1177/1933719109342131. [DOI] [PubMed] [Google Scholar]
- 21.Sheu JJ-C, Shih I-M. vol. 17. 2007. Clinical and biological significance of HLA-G expression in ovarian cancer; pp. 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rafii A, Mirshahi P, Poupot M, Faussat A-M, Simon A, Ducros E, Mery E, Couderc B, Lis R, Capdet J. Oncologic trogocytosis of an original stromal cells induces chemoresistance of ovarian tumours. PLoS One. 2008;3 doi: 10.1371/journal.pone.0003894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castells M, Thibault B, Mery E, Golzio M, Pasquet M, Hennebelle I, Bourin P, Mirshahi M, Delord JP, Querleu D. Ovarian ascites-derived Hospicells promote angiogenesis via activation of macrophages. Cancer Lett. 2012;326:59–68. doi: 10.1016/j.canlet.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 24.Martinet L, Poupot R, Mirshahi P, Rafii A, Fournié JJ, Mirshahi M, Poupot M. Hospicells derived from ovarian cancer stroma inhibit T-cell immune responses. Int J Cancer. 2010;126:2143–2152. doi: 10.1002/ijc.24881. [DOI] [PubMed] [Google Scholar]
- 25.Pasquet M, Golzio M, Mery E, Rafii A, Benabbou N, Mirshahi P, Hennebelle I, Bourin P, Allal B, Teissie J. Hospicells (ascites-derived stromal cells) promote tumorigenicity and angiogenesis. Int J Cancer. 2010;126:2090–2101. doi: 10.1002/ijc.24886. [DOI] [PubMed] [Google Scholar]
- 26.Lis R, Touboul C, Raynaud CM, Malek JA, Suhre K, Mirshahi M, Rafii A. Mesenchymal cell interaction with ovarian cancer cells triggers pro-metastatic properties. PLoS One. 2012;7 doi: 10.1371/journal.pone.0038340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berthaut A, Mirshahi P, Benabbou N, Ducros E, Agra A, Therwath A, Legeais JM, Mirshahi M. Insulin growth factor promotes human corneal fibroblast network formation in vitro. Invest Ophthalmol Vis Sci. 2011;52:7647–7653. doi: 10.1167/iovs.10-5625. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed N, Stenvers K. Getting to know ovarian cancer ascites: opportunities for targeted therapy-based translational research. Front Oncol. 2013;3:256. doi: 10.3389/fonc.2013.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan Z, Song B, Liu F, Sun D, Wang K, Qu H. TGF-β induces HLA-G expression through inhibiting miR-152 in gastric cancer cells. J Biomed Sci. 2015;22:107. doi: 10.1186/s12929-015-0177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 31.Bhoola S, Hoskins WJ. Diagnosis and management of epithelial ovarian cancer. Obstet Gynecol. 2006;107:1399–1410. doi: 10.1097/01.AOG.0000220516.34053.48. [DOI] [PubMed] [Google Scholar]
- 32.Eisenkop SM, Spirtos NM, Lin W-CM. “Optimal” cytoreduction for advanced epithelial ovarian cancer: a commentary. Gynecol Oncol. 2006;103:329–335. doi: 10.1016/j.ygyno.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Selle F, Sevin E, Ray-Coquard I, Mari V, Berton-Rigaud D, Favier L, Fabbro M, Lesoin A, Lortholary A, Pujade-Lauraine E. A phase II study of lenalidomide in platinum-sensitive recurrent ovarian carcinoma. Ann Oncol. 2014;25:2191–2196. doi: 10.1093/annonc/mdu392. [DOI] [PubMed] [Google Scholar]
- 34.Pfisterer J, Ledermann JA. vol. 33. 2006. Management of platinum-sensitive recurrent ovarian cancer; pp. 12–16. [DOI] [PubMed] [Google Scholar]
- 35.Konopleva M, Konoplev S, Hu W, Zaritskey A, Afanasiev B, Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16:1713. doi: 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
- 36.Martinet L, Jean C, Dietrich G, Fournié J-J, Poupot R. PGE2 inhibits natural killer and γδ T cell cytotoxicity triggered by NKR and TCR through a cAMP-mediated PKA type I-dependent signaling. Biochem Pharmacol. 2010;80:838–845. doi: 10.1016/j.bcp.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Nasef A, Chapel A, Mazurier C, Bouchet S, Lopez M, Mathieu N, Sensebé L, Zhang Y, Gorin N-C, Thierry D. Identification of IL-10 and TGF-β transcripts involved in the inhibition of T-lymphocyte proliferation during cell contact with human mesenchymal stem cells. Gene Expr. 2006;13:217–226. doi: 10.3727/000000006780666957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rebmann V, Busemann A, Lindemann M, Grosse-Wilde H. Detection of HLA-G5 secreting cells. Hum Immunol. 2003;64:1017–1024. doi: 10.1016/j.humimm.2003.08.354. [DOI] [PubMed] [Google Scholar]
- 39.Moreau P, Adrian-Cabestre F, Menier C, Guiard V, Gourand L, Dausset J, Carosella ED, Paul P. IL-10 selectively induces HLA-G expression in human trophoblasts and monocytes. Int Immunol. 1999;11:803–811. doi: 10.1093/intimm/11.5.803. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y, Geraghty DE, Hunt JS. Cytokine regulation of HLA-G expression in human trophoblast cell lines. J Reprod Immunol. 1995;29:179–195. doi: 10.1016/0165-0378(95)00942-e. [DOI] [PubMed] [Google Scholar]
- 41.Moreau P, Faure O, Lefebvre S, Ibrahim E, O'Brien M, Gourand L, Dausset J, Carosella E, Paul P. vol. 33. 2001. Glucocorticoid hormones upregulate levels of HLA-G transcripts in trophoblasts; pp. 2277–2280. [DOI] [PubMed] [Google Scholar]
- 42.Lin A, Chen HX, Zhu CC, Zhang X, Xu HH, Zhang JG, Wang Q, Zhou WJ, Yan WH. Aberrant human leucocyte antigen-G expression and its clinical relevance in hepatocellular carcinoma. J Cell Mol Med. 2010;14:2162–2171. doi: 10.1111/j.1582-4934.2009.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta P, Ghosh S, Nagarajan A, Mehta VS, Sen E. β-defensin-3 negatively regulates TLR4–HMGB1 axis mediated HLA-G expression in IL-1β treated glioma cells. Cell Signal. 2013;25:682–689. doi: 10.1016/j.cellsig.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Zhu Y, Zhao S, Zhao H, Yao Y. Gene expression profiles of HLA-G1 overexpressed in hES cells. Biochem Genet. 2012;50:809–821. doi: 10.1007/s10528-012-9522-4. [DOI] [PubMed] [Google Scholar]
- 45.Castelli EC, Veiga-Castelli LC, Yaghi L, Moreau P, Donadi EA. Transcriptional and posttranscriptional regulations of the HLA-G gene. J Immunol Res. 2014:2014. doi: 10.1155/2014/734068. [DOI] [PMC free article] [PubMed] [Google Scholar]