Abstract
Background
Operative fixation for femoral metastatic bone disease is based on the principles of reducing pain and restoring function. Recent literature has proposed a number of prognostic models for appendicular metastatic bone disease. The aim of this study was to compare the accuracy of proposed soring systems in the setting of femoral metastatic bone disease in order to provide surgeons with information to determine the most appropriate scoring system in this setting.
Methods
A retrospective cohort analysis of patients who underwent surgical management of femoral metastatic bone disease at a single institution were included. A pre-operative predicted survival for all 114 patients was retrospectively calculated utilising the revised Katagiri model, PathFx model, SSG score, Janssen nomogram, OPTModel and SPRING 13 nomogram. Univariate and multivariate Cox regression proportional hazard models were constructed to assess the role of prognostic variables in the patient group. Area under the receiver characteristics and Brier scores were calculated for each prognostic model from comparison of predicted survival and actual survival of patients to quantify the accuracy of each model.
Results
For the femoral metastatic bone disease patients treated with surgical fixation, multivariate analysis demonstrated a number of pre-operative factors associated with survival in femoral metastatic bone disease, consistent with established literature. The OPTIModel demonstrated the highest accuracy at predicting 12-month (Area Under the Curve [AUC] = 0.79) and 24-month (AUC = 0.77) survival after surgical management. PathFx model was the most accurate at predicting 3-month survival (AUC = 0.70) and 6-month (AUC = 0.70) survival. The PathFx model was successfully externally validated in the femoral patient dataset for all time periods.
Conclusions
Among six prognostic models assessed in the setting of femoral metastatic bone disease, the present study observed the most accurate model for 3-month, 6-month, 12-month and 24-month survival. The results of this study may be utilised by the treating surgical team to determine the most accurate model for the required time period and therefore improve decision-making in the care of patients with femoral metastatic bone disease.
Keywords: Metastatic bone disease, Femur, Prognosis, Scoring system, Nomogram, Surgery
Abbreviations: MBD, metastatic bone disease; AUC, Area Under the Curve; SSG, Scandinavian Sarcoma Group; ASA, American Society of Anaesthesiologists score; ECOG, Eastern Cooperation Oncology Group; ALP, alkaline phosphatase; ROC, Receiver Operating Characteristic; CCI, Charlson Comorbidity Index
1. Introduction
Metastatic bone disease (MBD) is common. In the USA, it was estimated that almost 300,000 patients in 2008 were living with MBD [1]. The femur is the most commonly affected bone in the appendicular skeleton in MBD with 1/3 of skeletal metastases occurring in the proximal femur [2], [3]. Femoral MBD causes significant pain, decreased quality of life and eventual pathological fracture resulting in immobility [4].
The majority of all MBD pathological fractures that require surgical management, occur in the femur [3], [5]. Surgical treatment for metastatic disease of the femur is palliative and not curative. The goals of surgical management in this context are to achieve structural stability, restore function, reduce pain, improve quality of life and decrease the risk of revision surgery [6], [7].
Surgical management of femoral MBD achieves both pain relief and maintains function in almost 90% of patients [8]. Surgical options for MBD of the femur include intramedullary fixation and endoprosthetic replacement surgery. Both have similar functional scores in femoral MBD patients [7]. Endoprosthetic replacement is associated with a decreased mechanical failure rate after 1-year when compared with intramedullary fixation [3], [7], [9]. Therefore, patient prognosis is an important consideration with regard to fixation type in femoral MBD. Patients with shorter expected survival may benefit from rigid intramedullary fixation and adjunct radiotherapy, a less aggressive and less morbid treatment. Patients with a prolonged life expectancy should be considered for endoprosthetic replacement [6], [10], [11], [12].
Expected survival is the most important factor to determine the treatment modality. In order to assess the survival time after occurrence of appendicular metastases, pre-operative prognosis models have been devised by Katagiri et al., Forsberg et al. (referred to as the PathFx model), Ratasvuori et al. (Scandinavian Sarcoma Group – SSG), Janssen et al., Willeumier et al. (referred to as the OPTIModel model) and Sorensen et al. (referred to as the SPRING model) [3], [13], [14], [15], [16], [17]. Currently, there is no consensus regarding which prognostic model is the most accurate. The patients in the development datasets for these prognostic models included all appendicular and, in some models, axial MBD. Therefore, there is a need to assess and evaluate the accuracy and reliability of the prognostic models in patients for the most commonly surgically managed location of MBD, the femur. The aim of this study was to compare the performance of these models in the setting of femoral MBD, enabling treating surgical teams to select the most appropriate prognostic model for their clinical setting.
2. Material and methods
2.1. Study design
Ethics approval was obtained from the local area health ethics committee and a retrospective review of patient records was performed. All patients who underwent operative fixation for femoral MBD at a single institution between 2003 and 2014 were reviewed. The inclusion criteria were: patient age greater than 18 at the time of surgery; complete medical records including clinical presentation, past medical history, pre-operative bloods and operative information; and known patient survival or documented most recent follow-up. Patients for whom the date of death was missing due to lost to follow-up were censored at the last time they were known to be alive. No funding was required for the support of this study.
As a retrospective study, there was no set guideline for surgical management regarding patient selection and fixation type. The decision to operate and fixation type was made by the treating surgical team in consultation with radiation oncology and medical oncology based on expected survival, type of fracture, comorbidities, patient function and pain profile, primary tumour type and progression of the neoplastic disease.
2.2. Prognostic models
All patients were assessed using six scoring systems for appendicular MBD. The prognostic models included the revised Katagiri model, PathFx model, Ratasvuori SSG scale, Janssen nomogram, OPTIModel and SPRING 13 nomogram [3], [13], [14], [15], [16], [17]. These estimated prognoses were calculated using the retrospective data for each patient at the time of operative management. Only objective inputs were utilised. The PathFx model has an optional subjective variable which was omitted from the model for this analysis [14]. Patient data was complete for all prognostic models at the time of operative management.
2.3. Statistical analysis
Basic demographic data were summarised as categorical variables and mean with range, standard error (SE) and 95% confidence interval (CI). Univariate analysis was performed on all variables collected to assess significance (p < 0.05). Categorical variables were established as growth primary tumour type, duration of time from diagnosis to operation less than 2.5 years, presence of visceral metastases, presence of multiple skeletal metastases, femoral neck location, history of systemic treatment, history of radiation therapy, presence of pathological fracture, required pre-operative blood transfusion, American Society of Anaesthesiologists (ASA) score less than three, Eastern Cooperation Oncology Group (ECOG) performance status score greater than two, pre-operative haemoglobulin less than 12 g/dL, platelets less than 10 g/dL, alkaline phosphatase (ALP) greater than 129 U/L, serum calcium greater than 2.6 mmol/L, and albumin less than 3 g/dL. All of the variables in univariate analysis with p values <0.20 were constructed into a Cox proportional hazard multivariate model with bidirectional elimination.
All patients were categorised into different prognostic groups defined by the primary tumour type as outlined by Katagiri et al. [13] and Bollen et al. [18]. The variables between the test dataset and prognostic model development cohorts were compared with the Fisher exact test and the Mann–Whitney U test.
Receiver Operating Characteristic (ROC) analysis and Brier score calculation was performed for 3-, 6-, 12-, and 24-month predicted survival periods. The predictive accuracy for the prognostic models was assessed using the Area Under the Curve (AUC) as a quantifiable measure. An AUC of 1.0 indicates perfect accuracy whereas an AUC of 0.5 indicates no relationship or predictive accuracy. It is considered an AUC of greater than 0.7 to indicate satisfactory predictive value [19]. The Brier score measures the accuracy of a probabilistic model summing the difference between expected survival and actual survival period, scores vary between 0 and 1 with a lower score indicating better predictions for the model. Confidence intervals (CI) for the AUC values and Brier scores were calculated using bootstrap standard errors (1000 replications). AUC values were compared with the Delong–Delong test [20]. Level of significance was set at 5% (p < 0.05) and confidence intervals were reported as 95% interval. All statistical analyses were performed using PythonTM 2.7 with the Numpy, Pandas and Lifelines script libraries.
3. Results
3.1. Patient demographics and survival
Upon retrospective review of patient medical records for patients surgically treated for femoral metastatic bone disease, 125 patients were initially observed. 11 patients did not have complete medical records and were excluded. The remaining 114 patients met the inclusion criteria. Table 1 summarises the characteristic of the 114 patients studied. The mean age of the patients at the time of operative management was 70 years (SD ± 11.7, 95% CI 67.8–72.2). There were 46 female and 68 male patients. At presentation, the mean and median of the BMI were 26 and 25 respectively (SD ± 4.8, range 17–44). The median Charlson Comorbidity Index (CCI) for the patients pre-operatively was 9 (SD ± 2.1, range 6–17). The most common primary tumour types were prostate (24.6%), lung (20.2%), breast (17.5%) and renal (9.6%). Compared to the prognostic model development patient cohorts, the femoral patient dataset was characterised by male sex, pathological fracture of the femur on presentation, high proportion of moderate growth primary tumour type and poorer patient pre-op level of function (Table 2).
Table 1.
Patient characteristics for the 114 patients with femoral MBD.
| Mean | Median | |
|---|---|---|
| Demographics | ||
| Age (years) | 68.9 | 70 |
| BMI (kg/m2) | 26 | 25 |
| Modified CCI | 9.0614 | 9 |
| Pre-op Serum Markers | ||
| Haemoglobulin (g/L) | 113.5 | 113.0 |
| Platelets (× 109/L) | 275.2 | 264.0 |
| White celll count (× 109/L) | 8.9 | 8.3 |
| Neutrophil count (× 109/L) | 6.9 | 6.2 |
| Alkaline phosphotase (U/L) | 333.3 | 151.0 |
| Creatinine (umol/L) | 97.7 | 84.5 |
| Calcium (mmol/L) | 2.3 | 2.3 |
| Operative Details | ||
| ASA Score | 3.2 | 3.0 |
| Operative time (mins) | 102.1 | 98.0 |
| Comorbidities | ||
| ECOG score | 2.1 | 2.0 |
| Demographics | Number | % |
| Sex (M/F) | 68 / 47 | 60% / 40% |
| Primary Cancer | ||
| Slow growth | 31 | 27.2 |
| Breast (Hormone dependent) | 6 | 5.3 |
| Prostate (Hormone dependent) | 15 | 13.2 |
| Multiple myeloma | 7 | 6.1 |
| Lymphoma | 3 | 2.6 |
| Thyroid | 1 | 0.9 |
| Moderate growth | 45 | 39.5 |
| Breast (Hormone independent) | 14 | 12.3 |
| Prostate (Hormone independent) | 13 | 11.4 |
| Renal | 11 | 9.6 |
| Ovarian | 1 | 0.9 |
| Adrenal | 1 | 0.9 |
| Fast Growth | 38 | 33.3 |
| Lung | 23 | 20.2 |
| Head and neck | 5 | 4.4 |
| Melanoma | 4 | 3.5 |
| Colorectal | 4 | 3.5 |
| Bladder | 3 | 2.6 |
| Unknown | 2 | 1.8 |
| Oesophageal | 1 | 0.9 |
| Staging / Treatment | ||
| Multiple skeletal metasases | 91 | 79.8 |
| Visceral metastases | 44 | 38.6 |
| Brain metastases | 8 | 7.0 |
| Previous chemotherapy | 47 | 41.2 |
| Previous hormonal therapy | 21 | 18.4 |
| Previous radiotherapy to femoral site | 30 | 26.3 |
| Previous radiotherapy elsewhere | 62 | 54.4 |
| Comorbidities | ||
| Diabetes | 23 | 20.2 |
| Smoker | 18 | 15.8 |
| Pleural effusion | 17 | 14.9 |
| Operative Details | ||
| Impending fracture | 37 | 32.5 |
| Pathological fracture | 77 | 67.5 |
| Location | ||
| Head/Neck | 50 | 43.9 |
| Intertroc | 14 | 12.3 |
| Subtroc | 23 | 20.2 |
| Diaphyseal | 21 | 18.4 |
| Distal | 6 | 5.3 |
| Pre-Op transfusion | 20 | 17.5 |
BMI, Body Mass Index; CCI, Charlson Comorbidity Index; ASA, American Society of Anesthesiologists score; ECOG, Eastern Cooperative Oncology Group.
Table 2.
Baseline characteristics and differences between the femoral MBD dataset and the prognostic model development datasets.
| Femur Patients Dataset | Katagiri Development Cohort |
P-Value | Janssen Development Dataset |
P-Value | Willeumier Development Cohort | P-Value | SSG Dataset | P-Value | PathFx Dataset | P-Value | SPRING 2013 Dataset | P-Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | 114 | 808 | 927 | 1520 | 651 | 189 | 270 | ||||||
| Age (mean) | 62 | 65 | 62.4 | ||||||||||
| BMI (mean) | 27 | ||||||||||||
| Men | 60.0% | 55% | 0.000 | 43% | 0.001 | 46% | 0.003 | 45.0% | 0.013 | ||||
| Pathological Fracture | 67.54% | 32% | 56% | 0.026 | 76.0% | 0.060 | 44.2% | 0.000 | 73.33% | 0.264 | |||
| Slow | 27% | 16% | 0.005 | 35% | 0.102 | 55.3% | 0.000 | 27.3% | 1.000 | 42.96% | 0.004 | ||
| Moderate | 39% | 28% | 0.015 | 28% | 0.013 | 18.2% | 0.000 | 25.93% | 0.010 | ||||
| Fast | 33% | 57% | 0.000 | 38% | 0.367 | 54.5% | 0.000 | 31.11% | 0.719 | ||||
| Breast | 17.5% | 23% | 0.233 | ||||||||||
| Lung | 20.2% | 23% | 0.555 | ||||||||||
| Myeloma | 6.1% | 16% | 0.003 | ||||||||||
| Kidney | 9.6% | 9% | 0.732 | ||||||||||
| Prostate | 24.6% | 5% | 0.000 | ||||||||||
| Multiple bone metastases | 78% | 75% | 0.561 | 20% | 0.000 | 77.70% | 1.000 | 77.6% | 1.000 | 71.0% | 0.226 | 66.30% | 0.028 |
| Lung and/or liver metastases | 39% | 29% | 0.039 | 38.70% | 1.000 | 41.0% | 0.680 | 60.3% | 0.000 | 38.89% | 1.000 | ||
| Brain metastases | 7% | 16% | 0.012 | 5.60% | 0.527 | ||||||||
| Previous systemic treatment | 60% | 62% | 0.683 | ||||||||||
| Previous chemo | 41% | 56% | 0.004 | ||||||||||
| Previous radiotherapy to affected long bone | 26% | 18% | 0.041 | ||||||||||
| ECOG <2 | 23% | 42.60% | 0.000 | 51.9% | 0.000 | 63.33% | 0.000 | ||||||
| Abnormal laboratory | 83% | 57% | 0.000 | ||||||||||
| Critical laboratory | 10% | 19% | 0.013 | ||||||||||
| Survival: | |||||||||||||
| 3 month | 67% | 73% | 0.220 | 68.3% | 0.800 | ||||||||
| 6 month | 51% | 57% | 0.190 | 58.0% | 0.150 | ||||||||
| 12 month | 31% | 36% | 0.248 | 42% | 0.015 | 41.0% | 0.037 | 41.8% | 0.049 | ||||
| 24 month | 17% | 23% | 0.183 | ||||||||||
| 36 month | 13% | 16% | 0.405 |
BMI, Body Mass Index; ECOG, Eastern Cooperative Oncology Group. Bold indicates significance (two tailed P-value < 0.05).
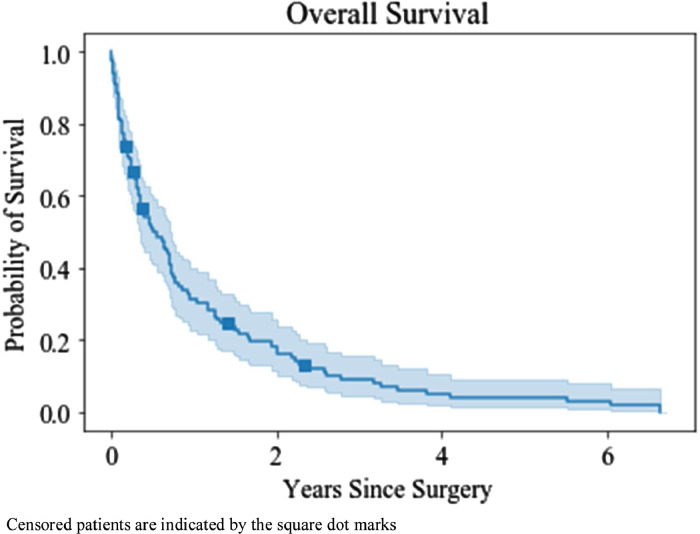
The median duration of survival to death or last follow-up was 5.6 months (SD ± 15.6, 95% CI 2.7–8.5 months) with a range of 1 day–79.8 months. Fig. 1 is the overall survival for all patients after operative fixation. The survival rate was 49.3% at 6 months (95% CI 39.6–58.3), 28.7% at 12 months (95% CI 20.5–37.4), 14.6% at 24 months (95% CI 8.7–22.0) and 1.1% at 5 years (95% CI 0.0–5.2).
Fig. 1.
Kaplan–Meier estimate with 95% CI of overall survival post-operative fixation (n = 114). Median survival post-operative fixation was 5.6 months (95% CI 2.7–8.5 months).
3.2. Factors associated with survival
Univariate Cox regression was performed on the femoral patient dataset (Table 3). Slow growth primary tumour type (Hazard Ratio [HR] 0.47 [95% CI 0.29–0.74], p = 0.001), rapid growth primary tumour type (HR 2.69 [95% CI 1.75–4.13], p < 0.001), presence of visceral metastases (HR 1.76 [95% CI 1.17–2.64], p = 0.006), metastatic lesion involving the femoral neck (HR 1.52 [95% CI 1.03–2.24], p = 0.036), ASA score greater then three (HR 2.15 [95% CI 1.42–3.26], p < 0.001), ECOG score equal or greater than three (HR 1.55 [95% CI 1.03–2.33], p = 0.037), haemoglobulin (≤12 g/dL, HR 1.49 [95% CI 1.00–2.22], p = 0.048), platelets (≤100 × 109/L, HR 2.98 [95% CI 1.27–6.97], p = 0.012), ALP (>129 U/L, HR 1.69 [95% CI 1.15–2.50], p = 0.008) and albumin (<30 g/L, HR 2.13 [95% CI 1.42–3.21], p < 0.001).
Table 3.
Univariate Cox regression analysis on femoral MBD dataset.
| Explanatory variables | β Regression Coefficient | Hazard Ratio (HR) | 95% CI | P-value |
|---|---|---|---|---|
| Primary cancer growth type | ||||
| Slow | −0.763 ± 0.234 | 0.47 | 0.29–0.74 | 0.001 |
| Moderate | −0.070 ± 0.199 | 0.93 | 0.63–1.38 | 0.725 |
| Rapid | 0.988 ± 0.220 | 2.69 | 1.75–4.13 | 0.000 |
| Staging | ||||
| Operation at time of diagnosis | 0.474 ± 0.278 | 0.62 | 0.35–1.12 | 0.112 |
| Diagnosis time to femur fracture > 2.5 years | 0.325 ± 0.199 | 1.27 | 0.87–1.87 | 0.217 |
| Visceral metastases | 0.565 ± 0.206 | 1.76 | 1.17–2.64 | 0.006 |
| Multiple skeletal metastases | 0.175 ± 0.243 | 1.19 | 0.74–1.92 | 0.473 |
| Spine metastases | −0.028 ± 0.203 | 0.97 | 0.65–1.45 | 0.889 |
| Femoral neck/head lesion | 0.416 ± 0.198 | 1.52 | 1.03–2.24 | 0.036 |
| Pathological fracture | 0.230 ± 0.209 | 1.26 | 0.84–1.90 | 0.271 |
| Previous radiotherapy to femur | 0.111 ± 0.221 | 1.12 | 0.73–1.72 | 0.614 |
| Previous systemic treatment | −0.161 ± 0.202 | 0.85 | 0.57–1.27 | 0.427 |
| Operative factors | ||||
| ASA > 3 | 0.765 ± 0.212 | 2.15 | 1.42–3.26 | 0.000 |
| Perioperative transfusion | 0.326 ± 0.265 | 1.38 | 0.82–2.33 | 0.219 |
| Comorbidities and function | ||||
| ECOG ≥ 3 | 0.437 ± 0.210 | 1.55 | 1.03–2.33 | 0.037 |
| Pre-operative pleural effusion | 0.594 ± 0.276 | 1.81 | 1.05–3.11 | 0.032 |
| CCI score > 8 | 0.339 ± 0.203 | 1.40 | 0.94–2.09 | 0.095 |
| Serum markers | ||||
| Haemoglobulin < 120 g/L | 0.401 ± 0.203 | 1.49 | 1.00–2.22 | 0.048 |
| PMN < 5.68 × 109/L | −0.121 ± 0.201 | 0.89 | 0.60–1.31 | 0.547 |
| Platelets < 100 × 109/L | 1.091 ± 0.434 | 2.98 | 1.27–6.97 | 0.012 |
| ALP > 129 U/L | 0.527 ± 0.199 | 1.69 | 1.15–2.50 | 0.008 |
| Albumin < 30 g/L | 0.758 ± 0.207 | 2.13 | 1.42–3.21 | 0.000 |
| Calcium > 2.6 mmol/L | 0.431 ± 0.351 | 1.54 | 0.77–3.06 | 0.220 |
ASA, American Society of Anesthesiologists score; ECOG, Eastern Cooperative Oncology Group; CCI, Charlson Comorbidity Index; PMN, polymorphonuclear leukocytes; ALP, alkaline phosphatase.
Slow growth type cancer included hormone-dependent breast and prostate, thyroid, multiple myeloma and lymphoma. Moderate growth included lung cancer treated with molecularly targeted drugs, hormone-independent breast and prostate, renal, endometrial and ovarian. Rapid growth included lung cancer without molecularly targeted drugs, colorectal, gastric, pancreatic, head and neck, eosophageal, other urological, melanoma, hepatocellular, gall bladder, cervical and cancers of unknown origin.
These values are given as the β coefficient and standard error.
These values are given as the Hazard Ratio (HR) with the following column the corresponding 95% confidence interval (CI).
These p-values were significant and had a two tailed p-value <0.05.
Following this, a multivariate analysis was performed (Table 4). The following variables had a significant and independent association with patient survival after controlling for relevant confounding variables: slow growth primary tumour type (HR 0.47 [95% CI 0.29–0.77], p = 0.003), presence of visceral metastases (HR 1.91 [95% CI 1.18–3.09], p = 0.008), lesion location within the femoral neck or head (HR 1.78 [1.16–2.72], p = 0.008), ASA score of greater than three (HR 1.74 [95% CI 1.12–2.69], p = 0.014), serum ALP greater than 129 U/L (HR 1.89 [95% CI 1.24–2.88], p = 0.003) and serum albumin less than 30 g/L (HR 1.75 [95% CI 1.13–2.72], p = 0.013).
Table 4.
Multivariate Cox regression analysis on femoral MBD dataset.
| Explanatory variables | β Regression Coefficient | Hazard Ratio (HR) | 95% CI | P-value |
|---|---|---|---|---|
| Slow growth cancer type | −0.753 ± 0.251 | 0.47 | 0.29–0.77 | 0.003 |
| Visceral metastases | 0.648 ± 0.245 | 1.91 | 1.18–3.09 | 0.008 |
| Femoral neck lesion | 0.576 ± 0.217 | 1.78 | 1.16–2.72 | 0.008 |
| ASA > 3 | 0.551 ± 0.224 | 1.74 | 1.12–2.69 | 0.014 |
| ALP > 129 U/L | 0.638 ± 0.214 | 1.89 | 1.24–2.88 | 0.003 |
| Albumin < 30 g/L | 0.560 ± 0.225 | 1.75 | 1.13–2.72 | 0.013 |
ASA, American Society of Anesthesiologists score; ALP, alkaline phosphatase.
Slow growth type cancer included hormone-dependent breast and prostate, thyroid, multiple myeloma and lymphoma.
Variables found to be non-significant in multivariate analysis are not shown. These include: Operation at time of diagnosis, ECOG>3, pre-operative pleural effusion, CCI > 8, Haemoglobulin <120g/L and Platelets <100 × 109/L).
Both ‘Rapid growth cancer type’ and ‘Slow growth cancer type’ variables are representative of the underlying tumour type and upon including the ‘Rapid growth cancer type’ variable we observed that ‘Slow growth cancer type’ was no longer significant. We opted to omit ‘Rapid growth cancer type’ and include ‘Slow growth cancer type’ as a variable in the multivariate analysis in order to aid the interpretability of the model.
These values are given as the β coefficient and standard error.
These values are given as the Hazard Ratio (HR) with the following column the corresponding 95% confidence interval (CI).
These p-values were significant and had a two tailed p-value <0.05.
3.3. Comparison of prognostic models
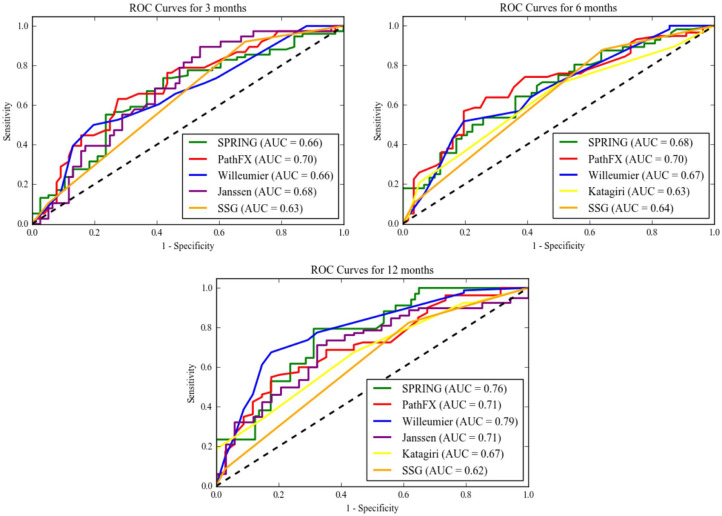
Among all patients surgically managed for femoral metastatic bone disease, the PathFx model demonstrated the highest accuracy at predicting 3-month and 6-month survival (AUC 0.70 and AUC 0.71 respectively) and was the only model to be sufficiently accurate at predicting 3-month and 6-month survival. PathFx yielded a Brier score of 0.233 (95% CI 0.23–0.234) at 3-months and 0.266 (95% CI 0.224–0.228) at 6-months (Table 5 and Fig. 2).
Table 5.
Area Under the Curve (AUC) from Receiver Operating Characteristics (ROC) and brier scores for all patients at different time periods after surgical management for femoral MBD.
| Prediction Period | Revised Katagiri score return AUC (95% CI) | Revised Katagiri Brier score (95% CI) |
Janssen nomogram return AUC (95% CI) | Janssen nomogram Brier score (95% CI) | OPTIModel return AUC (95% CI) | OPTIModel Brier score (95% CI) | SSG return AUC (95% CI) | SSG Brier score (95% CI) | PathFx return AUC (95% CI) | PathFx Brier score (95% CI) | SPRING 13 return AUC (95% CI) | SPRING 13 Brier score (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 month | 0.68 | 0.21 | 0.66 | 0.206 | 0.63 | 0.221 | 0.70 (0.69–0.70) | 0.233 (0.230–0.234) | 0.66 | 0.245 | ||
| 6 month | 0.63 | 0.392 | 0.67 | 0.237 | 0.64 | 0.244 | 0.70 (0.69–0.70) | 0.226 (0.224–0.228) | 0.68 | 0.259 | ||
| 12 month | 0.67 | 0.374 | 0.71 (0.70–0.71) | 0.220 (0.218 - 0.221) | 0.79 (0.78–0.79) | 0.160 (0.158–0.161) | 0.62 | 0.214 | 0.71 (0.70–0.71) | 0.190 (0.186–0.192) | 0.76 (0.75–0.76) | 0.186 |
| 24 month | 0.69 | 0.280 | 0.77 (0.77–0.77) | 0.107 (0.107–0.110) | 0.75 (0.74–0.75) | 0.114 (0.114–0.118) |
Scoring systems that were sufficiently accurate (AUC > 0.70) appear in bold.
Values are given as the AUC (95% confidence interval [CI]) for values achieving sufficient accuracy.
Brier scores and corresponding CI are only given for models achieving sufficient accuracy (AUC > 0.70).
Fig. 2.
Receiver Operating Characteristic (ROC) curves for 3-months, 6-months and 12-months.
OPTIModel (AUC = 0.79) was the most accurate at predicting 12-month survival with a Brier score of 0.16 (95% CI 0.158–0.161). Additionally, the Janssen nomogram (AUC 0.71), the PathFx model (AUC = 0.71) and SPRING nomogram (AUC = 0.76) all achieved sufficient predictive accuracy for 12-month survival. OPTIModel (AUC = 0.79) and PathFx (AUC = 0.75) were satisfactorily accurate for predicting 24-month survival with respective Brier scores of 0.107 (95% CI 0.107–0.11) and 0.114 (95% CI 0.114–0.118) (Table 5 and Fig. 2).
4. Discussion
To our knowledge, no previous studies have been conducted before comparing more than two prognostic systems for patients in the setting appendicular metastases. Shimada et al. compared the Ratasvouri SSG scale with the original Katagiri score for 145 patients with both axial and appendicular metastatic disease in 2015 [21]. Of all six prognostic models analysed in the femoral MBD patient cohort, the PathFx score was consistently the most reliable for sufficient accuracy in survival estimation for all time periods (Table 5).
Nathan et al. measured the accuracy of predicting survival pre-operatively in MBD patients undergoing operative fixation by the treating surgeon to be only accurate in 18% of cases [22]. Additionally, Chow et al. showed that physician's estimates of survival in cancer were inaccurate [23]. Endoprosthetic reconstruction and intramedullary fixation remain the mainstays of treatment [6], [10], [24], [25]. Unfortunately, there is currently no Level I evidence assessing the recommended survival period that patients require for endoprosthetic reconstruction. The literature is mixed with regard to 6- or 12-month patient survival as necessary life expectancy for endoprosthetic reconstruction [6], [7], [10], [24]. Thus, highlighting the need for more formalised and systematic approaches to prognostication of survival in patients with MBD.
There are a number of limitations with this single centre, retrospective cohort study. Treatment decisions were made by the treating surgical team and not based on standardised protocols. Patients were not randomly allocated to treatment and this introduces selection bias. This will range from referral to the orthopaedic team by the oncology service to the decision by the surgical and anaesthetic team to perform operative fixation as there was no set criteria for operative management. Additionally, applying the prognostic models to a potentially homogenous femoral MBD patient group may introduce bias to the analysis. Including more institutions and increasing the population size in future studies will increase heterogeneity in the patient cohort and reduce selection bias. However, the PathFx model performed well on this patient dataset despite significant differences with the PathFx development dataset (Table 2). The use of billing codes to identify potential patients may have missed a number of eligible patients. We do expect this number to be low and not influence the results. Patient data was complete and the patient group is large enough compare prognostic models [26]. A minimum follow-up period was not defined or utilised, however this should be accounted for by the use of Cox proportional-hazard regression that factors in loss to follow-up in the analysis.
In the investigation of femoral metastatic bone disease survival, univariate and multivariate analysis observed a number of investigated factors previously identified were significantly associated with decreased survival (see Table 3 and 4). The variables classifying growth type were representative of the underlying tumour type. As expected, there is a degree of collinearity present between these variables. Rapid growth primary type was omitted and only slow growth primary type included to enhance model interpretability. Of note, elevated ALP and hypoalbumaemia were found to be independent negative pre-operative prognostic markers for survival in our patient population (Table 4). This observation has validated Sorenson et al.’s ALP cut off value in the setting of MBD [27]. ALP is expressed by bone and the liver and previous studies have shown that ALP was a biomarker for the presence of skeletal metastases before detectable on skeletal scintigraphy [28]. Serum albumin provides a simple method of assessing protein function which is impaired in malnutrition and inflammatory states [29]. Hypoalbuminaemia has been observed to be prognostic in various cancer types [30]. To the author's knowledge, this is the first-time pre-operative albumin has been observed to be an independent prognostic factor in a MBD patient cohort. These variables remained independent prognostic factors when adjusting for the presence of visceral metastases in our dataset.
The presence of a pre-operative pathological fracture was not associated with survival in our independent femoral patient cohort. The SPRING nomogram and PathFx model include the presence of a pre-operative pathological fracture in their assessment of survival for the patient yet the other models do not utilise it for survival estimation [14], [16]. Further work and a meta-analysis may be required to investigate the value of this prognostic variable. If pathological fracture were an independent negative prognostic variable, this would strengthen the argument to lower the threshold to prophylactically surgically manage femoral MBD [31].
In the current study, the PathFx model was the accurate predictive system for 3- and 6- month survival. Additionally, the PathFx model was the most consistently reliable prognostic system, proving sufficiently accurate for all time periods thus exhibiting statistical validity and clinical usefulness. The PathFx was sufficiently accurate at 6-months survival estimation with the lowest Brier score (AUC = 0.70, Brier score 0.266) of the prognostic models. The PathFx model recommends endoprosthetic reconstruction for patients with an expected survival of greater than 12 months [14]. Seen here in the femoral MBD group, the model remained sufficiently reliable with a modest Brier score for 12-month (AUC = 0.71, Brier score 0.190) survival estimation, therefore strengthening the usefulness of the PathFx model for the surgical team in determining operative fixation options.
The PathFx model is a machine learned Bayesian belief network applicable to both axial and appendicular MBD. The model includes both objective quantifiable variables (age, sex, primary type, ECOG, presence of visceral metastases, presence of multiple skeletal metastases, pathological fracture, haemoglobulin and lymphocyte count) and subjective variables (surgeon's estimate of survival). It can function without this subjective variable and has been shown here to remain accurate without this variable and in other studies [32]. The PathFx model has been externally validated in a number of different patient populations and has been further validated in the femoral MBD population from this analysis [32], [33], [34].
It remains controversial whether to include multiple myeloma in a prognostic model for metastatic bone disease as it is a primary haematological cancer. However, all prognostic models except OPTIModel included multiple myeloma and lymphoma it the assessment of patient survival. These haematological malignancies cause pathological fractures of the femur requiring orthopaedic management [3], [13], [14], [15], [16], [35], [36]. This analysis included multiple myeloma and lymphoma primary cancer types in the femoral MBD patient dataset. Interestingly, OPTIModel was the most accurate model at predicting survival at 12-month (AUC = 0.79, Brier score =0.160) and 24-month (AUC = 0.77, Bier score = 0.107) time periods. OPTIModel is a recently developed prognostic model developed from a large dataset of appendicular MBD including patients managed non-operatively and operatively. Our results confirm the impact and simplicity of the OPTIModel algorithm. It is dependent on only three variables: primary tumour type; Karnofsky performance score; and the presence of visceral or brain metastases. Patients are then classified into four prognostic groups, with a median survival of 15.5, 6.2, 4.9 and 2.3 months in our femoral MBD dataset.
The revised Katagiri model and SSG scale were not sufficiently accurate in their application to the femoral MBD dataset (Table 5). The revised Katagiri model's poor performance may be reflective of the low number of surgically managed patients in the RKS development dataset [13]. The SSG scale reduces the impact of the primary type to just a single categorical variable capturing breast, kidney, thyroid cancer, myeloma and lymphoma patients. This classification of primary type is not supported by other studies highlighting the significance of classifying cancer types into growth / favourable types [13], [18], [37]. The age of both models may also be a factor in their accuracy, as it has been observed that modern data is more effective than larger older datasets when producing prediction models [38].
The Janssen nomogram and SPRING-13 nomograms were only sufficiently accurate at the 12-month time period (AUC = 0.71, Brier score 0.220 and AUC 0.76, Brier score 0.186 respectively). They both lacked reliability at shorter time periods in estimating patient survival. There were significant differences between the femoral MBD dataset and Janssen and SPRING development datasets (Table 2). It is suspected that a less implicity biased dataset may lead to an improvement in prognostic model accuracy. This highlights the need to approach these results with caution and the necessity of multicentre studies with large population numbers to assess prognostic models.
5. Conclusions
Operative fixation in femoral MBD is a palliative procedure that is aimed at alleviating pain and maintaining the patient's function. Accurate pre-operative estimated patient prognosis is paramount to allow informed operative fixation decision making. It must be stressed that the use of a prognostic model and the clinical acumen of the clinician will outperform either of these two options alone [38]. PathFx was the most consistently reliable model across all time periods and the OPTIModel the most accurate at 12-month survival prognosis. To date, this is the only study comparing the accuracy of multiple prognostic models in the setting of femoral MBD. Clinicians may use the results in this study to determine the most accurate model for the required time period and therefore improve decision-making in the care of patients with femoral MBD.
Acknowledgments
Funding
Disclosures: There was no funding source for this study.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Li S., Peng Y., Weinhandl E.D., Blaes A.H., Cetin K., Chia V.M. Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clin. Epidemiol. 2012;4:87–93. doi: 10.2147/CLEP.S28339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hage W.D., Aboulafia A.J., Aboulafia D.M. Incidence, location and diagnostic evaluation of metastatic bone disease. Orthop. Clin. North Am. 2000;31(4):515–528. doi: 10.1016/s0030-5898(05)70171-1. [DOI] [PubMed] [Google Scholar]
- 3.Ratasvuori M., Wedin R., Keller J., Nottrott M., Zaikova O., Bergh P. Insight opinion to surgically treated metastatic bone disease: scandinavian sarcoma group skeletal metastasis registry report of 1195 operated skeletal metastasis. Surg. Oncol. 2013;22(2):132–138. doi: 10.1016/j.suronc.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Coleman R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006;12(20):6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 5.Harrington K.D. Orthopedic surgical management of skeletal complications of malignancy. Cancer. 1997 Oct 15;80(8 Suppl):1614–1627. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1614::aid-cncr12>3.3.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Bickels J., Dadia S., Lidar Z. Surgical management of metastatic bone disease. J. Bone Joint Surg. Am. vol. 2009;91(6):1503–1516. doi: 10.2106/JBJS.H.00175. [DOI] [PubMed] [Google Scholar]
- 7.Harvey N., Ahlmann E.R., Allison D.C., Wang L., Menendez L.R. Endoprostheses last longer than intramedullary devices in proximal femur metastases. Clin. Orthop. Relat. Res. 2012;470(3):684–691. doi: 10.1007/s11999-011-2038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood T.J., Racano A., Yeung H., Farrokhyar F., Ghert M., Deheshi B.M. Surgical management of bone metastases: quality of evidence and systematic review. Ann. Surg. Oncol. 2014;21(13):4081–4089. doi: 10.1245/s10434-014-4002-1. [DOI] [PubMed] [Google Scholar]
- 9.Wedin R., Bauer H.C. Surgical treatment of skeletal metastatic lesions of the proximal femur: endoprosthesis or reconstruction nail? J. Bone Joint Surg. Br. 2005;87(12):1653–1657. doi: 10.1302/0301-620X.87B12.16629. [DOI] [PubMed] [Google Scholar]
- 10.Issack P.S., Barker J., Baker M., Kotwal S.Y., Lane J.M. Surgical management of metastatic disease of the proximal part of the femur. J. Bone Joint Surg. Am. Vol. 2014;96(24):2091–2098. doi: 10.2106/JBJS.N.00083. [DOI] [PubMed] [Google Scholar]
- 11.Janssen S.J., Teunis T., Hornicek F.J., van Dijk C.N., Bramer J.A., Schwab J.H. Outcome after fixation of metastatic proximal femoral fractures: a systematic review of 40 studies. J. Surg. Oncol. 2016;114(4):507–519. doi: 10.1002/jso.24345. [DOI] [PubMed] [Google Scholar]
- 12.Ruggieri P., Mavrogenis A.F., Casadei R., Errani C., Angelini A., Calabro T. Protocol of surgical treatment of long bone pathological fractures. Injury. 2010;41(11):1161–1167. doi: 10.1016/j.injury.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Katagiri H., Okada R., Takagi T., Takahashi M., Murata H., Harada H. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med. 2014;3(5):1359–1367. doi: 10.1002/cam4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forsberg J.A., Eberhardt J., Boland P.J., Wedin R., Healey J.H. Estimating survival in patients with operable skeletal metastases: an application of a Bayesian belief network. PLoS One. 2011;6(5):e19956. doi: 10.1371/journal.pone.0019956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janssen S.J., van der Heijden A.S., van Dijke M., Ready J.E., Raskin K.A., Ferrone M.L. 2015 Marshall urist young investigator award: prognostication in patients with long bone metastases: does a boosting algorithm improve survival estimates? Clin. Orthop. Relat. Res. 2015;473(10):3112–3121. doi: 10.1007/s11999-015-4446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorensen M.S., Gerds T.A., Hindso K., Petersen M.M. External validation and optimization of the SPRING model for prediction of survival after surgical treatment of bone metastases of the extremities. Clin. Orthop. Relat. Res. 2018;476(8):1591–1599. doi: 10.1097/01.blo.0000534678.44152.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willeumier J.J., van der Linden Y.M., van der Wal C., Jutte P.C., van der Velden J.M., Smolle M.A. An easy-to-use prognostic model for survival estimation for patients with symptomatic long bone metastases. J. Bone Joint Surg. Am. Vol. 2018;100(3):196–204. doi: 10.2106/JBJS.16.01514. [DOI] [PubMed] [Google Scholar]
- 18.Bollen L., van der Linden Y.M., Pondaag W., Fiocco M., Pattynama B.P., Marijnen C.A. Prognostic factors associated with survival in patients with symptomatic spinal bone metastases: a retrospective cohort study of 1043 patients. Neuro Oncol. 2014;16(7):991–998. doi: 10.1093/neuonc/not318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrell Frank E., Lee Kerry L., Mark Daniel B. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1998;15(4):361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 20.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 21.Shimada H., Setoguchi T., Nakamura S., Yokouchi M., Ishidou Y., Tominaga H. Evaluation of prognostic scoring systems for bone metastases using single-center data. Mol. Clin. Oncol. 2015;3(6):1361–1370. doi: 10.3892/mco.2015.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nathan S.S., Healey J.H., Mellano D., Hoang B., Lewis I., Morris C.D. Survival in patients operated on for pathologic fracture: implications for end-of-life orthopedic care. J. Clin. Oncol. 2005;23(25):6072–6082. doi: 10.1200/JCO.2005.08.104. [DOI] [PubMed] [Google Scholar]
- 23.Chow E., Harth T., Hruby G., Finkelstein J., Wu J., Danjoux C. How accurate are physicians' clinical predictions of survival and the available prognostic tools in estimating survival times in terminally ill cancer patients? A systematic review. Clin. Oncol. (R. Coll. Radiol.) 2001;13(3):209–218. doi: 10.1053/clon.2001.9256. [DOI] [PubMed] [Google Scholar]
- 24.Errani C., Mavrogenis A.F., Cevolani L., Spinelli S., Piccioli A., Maccauro G. Treatment for long bone metastases based on a systematic literature review. Eur. j. orthop. surg. traumatol. orthop. traumatol. 2017;27(2):205–211. doi: 10.1007/s00590-016-1857-9. [DOI] [PubMed] [Google Scholar]
- 25.Kistler B.J., Damron T.A. Latest developments in surgical and minimally invasive treatment of metastatic bone disease. Curr. Surg. Rep. 2014;2(4):49. [Google Scholar]
- 26.Collins G.S., Ogundimu E.O., Altman D.G. Sample size considerations for the external validation of a multivariable prognostic model: a resampling study. Stat. Med. 2016;35(2):214–226. doi: 10.1002/sim.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorensen M.S., Hovgaard T.B., Hindso K., Petersen M.M. Prognostic value of biochemical variables for survival after surgery for metastatic bone disease of the extremities. J. Surg. Oncol. 2017;115(4):442–448. doi: 10.1002/jso.24537. [DOI] [PubMed] [Google Scholar]
- 28.Lim S.M., Kim Y.N., Park K.H., Kang B., Chon H.J., Kim C. Bone alkaline phosphatase as a surrogate marker of bone metastasis in gastric cancer patients. BMC Cancer. 2016;16:385. doi: 10.1186/s12885-016-2415-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeun J.Y., Kaysen G.A. Factors influencing serum albumin in dialysis patients. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 1998;32(6 Suppl 4):S118–S125. doi: 10.1016/s0272-6386(98)70174-x. [DOI] [PubMed] [Google Scholar]
- 30.Gupta D., Lis C.G. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr. J. 2010;9:69. doi: 10.1186/1475-2891-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ward W.G., Holsenbeck S., Dorey F.J., Spang J., Howe D. Metastatic disease of the femur: surgical treatment. Clin. Orthop. Relat. Res. 2003;415(Suppl):S230–S244. doi: 10.1097/01.blo.0000093849.72468.82. [DOI] [PubMed] [Google Scholar]
- 32.Piccioli A., Spinelli M.S., Forsberg J.A., Wedin R., Healey J.H., Ippolito V. How do we estimate survival? External validation of a tool for survival estimation in patients with metastatic bone disease-decision analysis and comparison of three international patient populations. BMC Cancer. 2015;15:424. doi: 10.1186/s12885-015-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forsberg J.A., Wedin R., Bauer H.C.F., Hansen B.H., Laitinen M., Trovik C.S. External validation of the Bayesian estimated tools for survival (BETS) models in patients with surgically treated skeletal metastases. BMC Cancer. 2012;12:493. doi: 10.1186/1471-2407-12-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogura K., Gokita T., Shinoda Y., Kawano H., Takagi T., Ae K. Can a multivariate model for survival estimation in skeletal metastases (PATHFx) be externally validated using japanese patients? Clin. Orthop. Relat. Res. 2017;475(9):2263–2270. doi: 10.1007/s11999-017-5389-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsuda Y., Yasunaga H., Horiguchi H., Fushimi K., Kawano H., Tanaka S. Complications and postoperative mortality rate after surgery for pathological femur fracture related to bone metastasis: analysis of a nationwide database. Ann. Surg. Oncol. 2016;23(3):801–810. doi: 10.1245/s10434-015-4881-9. [DOI] [PubMed] [Google Scholar]
- 36.Peterson J.R., Decilveo A.P., O'Connor I.T., Golub I., Wittig J.C. What are the functional results and complications with long stem hemiarthroplasty in patients with metastases to the proximal femur? Clin. Orthop. Relat. Res. 2017;475(3):745–756. doi: 10.1007/s11999-016-4810-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomita K., Kawahara N., Kobayashi T., Yoshida A., Murakami H., Akamaru T. Surgical strategy for spinal metastases. Spine. 2001;26(3):298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 38.Chen H.-C., Kodell R.L., Cheng K.F., Chen J.J. Assessment of performance of survival prediction models for cancer prognosis. BMC Med. Res. Method. 2012;12(1):102. doi: 10.1186/1471-2288-12-102. [DOI] [PMC free article] [PubMed] [Google Scholar]