Abstract
The activation of NADPH oxidase contributes to dopaminergic neurodegeneration and motor deficits in Parkinson's disease (PD). However, whether NADPH oxidase is involved in non-motor symptoms, especially cognitive dysfunction in PD remains unknown. This study is undertaken to characterize the effects of inhibition of NADPH oxidase by a widely used NADPH oxidase inhibitor apocynin on learning and memory deficits in paraquat and maneb-induced mouse PD model. Results showed that mice injected with paraquat and maneb displayed impairments of spatial learning and memory, which was associated with reduced tyrosine hydroxylase expression as well as increased neurodegeneration, synaptic loss, α-synuclein expression and Ser129-phosphorylation in the hippocampus. Interestingly, apocynin treatment significantly ameliorated learning and memory deficits as well as hippocampal neurodegeneration and α-synuclein pathology in mice treated with these two pesticides. Mechanistically, we found that apocynin mitigated paraquat and maneb-induced NADPH oxidase activation and related oxidative stress. Furthermore, reduced microglial activation and M1 polarization were observed in apocynin and paraquat and maneb co-treated mice compared with paraquat and maneb alone group. Finally, apocynin inhibited the activation of signal transducers and activators of transcription 1 (STAT1) and nuclear factor kappa B (NF-κB) pathways, two key regulatory factors for microglial M1 inflammatory responses, in paraquat and maneb-treated mice. Altogether, our findings implied that NADPH oxidase mediates learning and memory deficits in PD, and inhibition of NADPH oxidase by apocynin blocks impairments of learning and memory via the suppression of oxidative stress and neuroinflammation.
Abbreviations: AD, Alzheimer disease; Iba-1, ionized calcium binding adaptor molecule-1; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; MDA, malondialdehyde; MPO, myeloperoxidase; MWM, morris maze test; NF-κB, nuclear factor-κB; PD, Parkinson's disease; P + M, paraquat and maneb; ROS, reactive oxygen species; SNpc, substantia nigra pars compacta; SOD, superoxide dismutase; STAT1, signal transducers and activators of transcription 1; TH, tyrosine hydroxylase; TNFα, tumor necrosis factor α
Keywords: Parkinson's disease, Nonmotor symptoms, Cognitive deficits, NADPH oxidase, Neuroinflammation
1. Introduction
Parkinson's disease (PD) is traditionally considered as a movement disorder, which is attributed to the degeneration of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc) and resulted dopamine deletion in the striatum [1]. Aside from classical motor deficits, PD patients also experience various nonmotor symptoms, including cognitive deficits [2]. Strong evidence revealed that mild cognitive impairments are extremely common in the early stage of PD, and dementia is a major disability particularly later in the course of disease [3]. Cognitive disturbance not only reduces the life quality of PD patients but also contributes to distress amongst the care givers and problems in treatment [4], [5]. However, at present, motor dysfunction is still the focus in clinic and cognitive deficits are often neglected and left untreated. Thus, exploring the mechanisms and novel therapeutic strategies behind the cognitive disturbance is urgently needed.
Microglia-mediated neuroinflammation is gradually recognized to be involved in the pathogenesis of PD, whereby cytotoxic factors released from activated microglia have been shown to damage DA neurons in the SNpc and therefore resulting in motor deficits [6]. Interestingly, more and more evidence revealed that neuroinflammation also contributes to non-motor symptoms [7]. PET study and post-mortem analysis in patients with PD showed microglial activation in the hippocampus [8], [9]. Furthermore, the levels of inflammatory cytokines in serum [10], plasma [11] and cerebrospinal fluid [12] of PD patients have been found to correlate with multiple non-motor symptoms, including cognitive difficulties. Moreover, directly stimulating neuroinflammation by inflammogen lipopolysaccharide (LPS) induces spatial learning and memory impairments in experimental animals [13], [14].
NADPH oxidase, a superoxide-producing enzyme, is highly expressed in microglia [15], [16]. The activation of NADPH oxidase produces both extracellular and intracellular reactive oxygen species, which play important roles in mediating chronic neuroinflammatory responses and related neuronal damage [17], [18], [19]. Previous study showed that the expression and activation of NADPH oxidase are greatly up-regulated in the brain of PD patients [20]. Moreover, pharmacological inhibition or genetic deletion of NADPH oxidase mitigated microglial activation and degeneration of DA neurons in multiple PD models [19]. However, whether NADPH oxidase is involved in non-motor symptoms, especially cognitive dysfunction remains to be investigated.
This study is therefore undertaken to characterize the effects of inhibition of NADPH oxidase on learning and memory deficits in a mouse PD model induced by paraquat and maneb (referred to subsequently as P + M). Apocynin, a plant-derived antioxidant originally isolated from the medicinal plant Picrorhiza kurroa, has been widely used as an NADPH oxidase inhibitor in experimental PD models [21], [22]. Apocynin is well-known to acquire its selective inhibitory capacity on NADPH oxidase activation via metabolic activation by myeloperoxidase (MPO) [23]. We recently reported that apocynin can also reduce membrane translocation of NADPH oxidase cytosolic subunits, leading to inactivation of NADPH oxidase [24]. The present study showed that paraquat and maneb injection resulted in impairments of spatial learning and memory in mice, which were significantly mitigated by apocynin. Inhibition of oxidative stress and neuroinflammation via signal transducers and activators of transcription 1 (STAT1) and nuclear factor kappa B (NF-κB) pathways contributed to apocynin-afforded protection.
2. Materials and methods
2.1. Animal dosing
Three-month old male C57BL/6 J mice were randomly divided into 2 groups, i.e. control and P + M group (n = 45 in each group). Mice in P + M group were administrated (i.p., 5 μl/g body weight) with combined paraquat (10 mg/kg) and maneb (30 mg/kg) for consecutive 6 weeks (twice per week) according to our previous report [24]. Mice in control group received an equal volume of 0.9% saline. After 2, 4 and 6 weeks of initial P + M treatment, the morris water maze (MWM) test was performed in these mice (n = 8–15 in each group for each time point). After MWM test, mice were euthanized and brains were collected. Housing and breeding of animals were performed strictly with Dalian Medical University's Guide for the Care and Use of Laboratory Animals. All animal procedures and their care were carried out in accordance the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Dalian Medical University.
2.2. Apocynin treatment
Apocynin was administrated to mice 2 days before P + M or saline treatment for consecutive 6 weeks. The concentration of apocynin in drinking water was 1 mg/ml. The conversion to mg/kg was estimated based on the average daily water intake of approximately 4 ml/mouse [25], giving rise to dosing of approximately 150 mg/kg/d. Apocynin at this concentration has been found no significant effect on water consumption of mice [26]. Water was changed every 5 days, a time point that no detectable decay of apocynin was detected [26].
2.3. MWM test
Mice were tested for their spatial learning and memory abilities using the MWM test. The device is geographically divided into four quadrants (N, S, E, and W) with equal size. The test included the navigation test and the spatial probe test [27]. Briefly, in spatial navigation test, a circular escape platform hidden approximately 1 cm below the water surface, was placed in the middle of one quadrant and remained unmoved during the spatial navigation test. The spatial navigation test consisted of four trials per day for each mouse and lasted for four training days. Mice were released into water with their heads pointing towards the pool wall randomly at the four starting positions of N, E, S and W. They were required to escape from the water and arrive onto the platform hidden beneath the water surface. The swim paths of mice were recorded through the camera and analyzed on the computer by the tracking software. Different parameters describing the spatial learning function could be calculated, such as escape latency (time to locate the hidden platform), distance travelled (path length to reach the platform) and swimming speed. In one trial, mice were allowed to swim until they found the platform or for 90 s at the most. Mice that did not find the platform were gently guided onto it and their latencies were recorded as 90 s. All mice were given a break of 5 mins on the platform between trials.
On the fifth day, mice were given a spatial probe test, in which the platform was taken away, and each mouse was allowed to navigate freely in the pool for 60 s. The percentage of time spent in target quadrant and the latency for first time crossing the location where the platform was previously located were recorded. The swimming time and movement paths of mice were recorded and analyzed using a smart video tracing system (NoldusEtho Vision system, version 5, Everett, WA, USA).
2.4. Immunohistochemistry
Brains samples were fixed in 4% paraformaldehyde and processed for immune-staining as described previously [19], [28]. We used the following primary antibodies for immunohistochemistry: antibodies against Neu-N, PSD-95 (EMD Millipore, Temecula, CA, USA), ionized calcium binding adaptor molecule-1 (Iba-1, Wako Chemicals, Richmond, VA, USA) and CD11b (Dako, Santa Clara, CA, USA). Immuno-staining was visualized by using 3,3′-diaminobenzidine (DAB) according to manufacturer's instruction. The densities of PSD-95, Iba-1 and CD11b immunostaining were measured using ImageJ software (National Institutes of Health) [29]. Quantification was performed from four adjacent brain sections, spaced 120 µm apart, and was subsequently averaged for each animal.
2.5. Automated counting assessment of neurodegeneration
Free-floating 35 µm coronal sections containing the hippocampus and cortex were cut on a horizontal sliding microtome. Neu-N+ neurons in the hippocampus were enumerated by automated counting in ImageJ using mono-colored immunographs [30]. Briefly, immunographs images were converted to gray scale and threshold adjusted to optimize to distinguish individual neurons. The binary watershed filter was used to separate clustered groups of neurons (by adjusting target particles size in attempts to reduce false-positive “noise” particles) and the total cell numbers were analyzed. Threshold and size parameters were consistently maintained among the images of all subjects.
2.6. Superoxide detection
The production of superoxide was determined by measuring the superoxide dismutase (SOD)-inhibitable reduction of WST-1 as described previously [31], [32]. Briefly, membrane fractions were prepared from the hippocampus of P + M-injected mice with or without apocynin pre-treatment. Equal amount of membrane fractions among groups was added to a 96-well plate with and without SOD (50 U/ml). Then, 50 μl WST-1 (1 mM) in HBSS buffer was added to each well and the absorbance at 450 nm was read with a SpectraMax Plus microplate spectrophotometer (Molecular Devices). The difference between the absorbance in the presence and absence of SOD was considered to be the amount of produced superoxide.
2.7. In situ visualization of superoxide and superoxide-derived oxidant production
In situ visualization of oxidative stress was assessed by dihydroethidium (DHE) according to a previous report [19]. Briefly, mice in each group (n = 3) were administered single injections (i.p.) of DHE (20 mg/kg). Eighteen hours later, mice were perfused transcardially with PBS, and coronal hippocampal sections were examined for the DHE oxidation using fluorescence microscopy (excitation 534 nm; emission 580 nm).
2.8. BV2 microglial cells
The mouse microglia BV2 cell line was maintained as described previously. Briefly, BV2 microglial cells were maintained at 37 °C in DMEM supplemented with 10% fetal bovine serum, 50 U/ml penicillin and 50 μg/ml streptomycin in a humidified incubator with 5% CO2 and 95% air. The cells were split or harvested every 3–5 days.
2.9. Membrane extraction
The membrane fractions of microglia and midbrain tissue were prepared using the membrane protein extraction kit (Beyotime, Jiangsu, China) as described previously [15]. Briefly, microglia and hippocampal tissues were lysed in lysis buffer A provided by the kit and then subjected to Dounce homogenization (20–25 St, tight pestle A). The lysates were centrifuged at 700 ×g for 10 mins; the supernatant was collected and centrifuged at 14,000 ×g for 30 mins. The pellets were suspended using extraction buffer B and incubated for 20 mins. After centrifugation at 14,000 ×g for 5 mins, the supernatant was used as membranous fraction.
2.10. Western blot analysis
Equal amounts of protein were separated by 4–12% Bis-Tris-polyacrylamide electrophoresis gel and transferred to polyvinylidene difluoride membranes. The membranes were incubated with primary antibody Neu-N, PSD-95, tyrosine hydroxylase (TH, EMD Millipore, Temecula, CA, USA), α-synuclein, signal transducers (Abcam, Cambridge, MA,USA) and activators of transcription 1 (STAT1), p-p65, p65, p-IκBα and IκBα (Cell Signaling Technology, Danvers, MA, USA) overnight at 4 °C and followed by horseradish peroxidase-linked anti-rabbit IgG (1:3000) for 2 h at 25 °C. ECL reagents (Biological Industries, Cromwell, CT, USA) were used as a detection system.
2.11. Malondialdehyde assay
The hippocampal tissues dissected from P + M-intoxicated mice with or without apocynin pre-treatment were homogenized and centrifuged at 10,000 ×g for 10 min at 4 °C. The levels of malondialdehyde (MDA) in the collected supernatant were determined spectrophotometrically with commercial kit (Beyotime, Shanghai, China) according to the manufacturer's instruction.
2.12. Lipid hydroperoxide assay
The contents of lipid hydroperoxide (LPO) were measured in hippocampus prepared from P + M-treated mice with or without apocynin pre-treatment using commercial LPO assay kit (Abcam, Cambridge, MA, USA).
2.13. Mitochondrial complex I assay
The activities of complex I were measured in P + M-treated mice with or without apocynin pre-treatment using commercial Complex I enzyme activity assay kits (Abcam, Cambridge, MA, USA).
2.14. Real-time PCR analysis
Total RNA was extracted by using RNAiso Plus and reverse transcribed with an oligodT primer according to our previous report [24], [33]. Real-time PCR amplification was performed using SYBR Premix Ex Taq™ II (Takara Bio Inc. Kusatsu, Shiga, Japan) and Takara Thermal Cycler Dice™ Real Time System according to manufacturer's protocols. The primers were listed in Table 1. The PCR conditions were 95 °C for 10 s, 55 °C for 30 s, and 72 °C for 30 s for 40 cycles. Relative mRNA gene levels were normalized to the GAPDH mRNA level and relative expressions were determined by the comparative Ct method.
Table 1.
Quantitative RT-PCR primer sequences.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| iNOS | CTGCCCCCCTGCTCACTC | TGGGAGGGGTCGTAATGTCC |
| TNFα | GACCCTCACACTCAGATCATCTTCT | CCTCCACTTGGTGGTTTGCT |
| IL-1β | CTGGTGTGTGACGTTCCCATTA | CCGACAGCACGAGGCTTT |
| Arg-1 | GAACACGGCAGTGGCTTTAAC | TGCTTAGCTCTGTCTGCTTTGC |
| Ym-1 | AGGAAGCCCTCCTAAGGACAAACA | ATGCCCATATGCTGGAAATCCCAC |
| CD206 | AAGGAAGGTTGGCATTTGT | CCTTTCAATCCTATGCAAGC |
| GAPDH | TTCAACGGCACAGTCAAGGC | GACTCCACGACATACTCAGCACC |
2.15. Statistical analysis
All values were expressed as mean ± SEM. Differences among means were analyzed using one-way or two-way ANOVA with treatment/time as the independent factors. When ANOVA showed significant differences, pair-wise comparisons between means were tested by Tukey's post hoc testing. In all analyses, the null hypothesis was rejected at the 0.05 level.
3. Results
3.1. P + M impair the spatial learning and memory abilities of mice
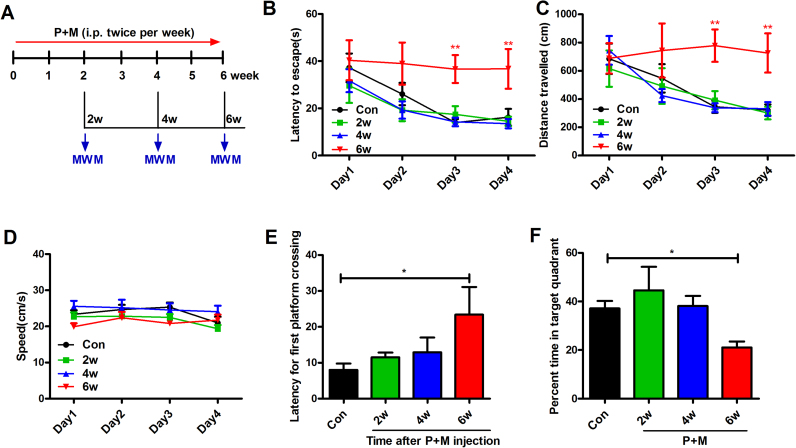
To investigate whether P + M-generated mouse PD model display cognitive dysfunction, the spatial learning and memory abilities of mice were detected after 2, 4 and 6 weeks of P + M treatment by using MWM test (Fig. 1A). As seen in Fig. 1B, control mice exhibited normal spatial learning function as indicated by their decreased escape latency associated with training. There was no statistically significant difference in escape latencies between P + M-treated mice and vehicle controls after 2 and 4 weeks of P + M injection. However, after 6 weeks of P + M exposure, mice spent more time than vehicle controls to locate the platform during the 4 days of acquisition trials. Consistently, P + M also increased the swimming distance of mice to locate the platform after 6 weeks exposure (Fig. 1C), indicating impairment of spatial learning ability. To ensure that the differences in MWM performance by P + M-treated mice were not confounded by locomotor deficit, we compared average swim speed among groups and saw no significant difference (Fig. 1D).
Fig. 1.
P + M progressively impair the spatial learning and memory capacity in mice. (A) Schematic presentation of the experimental paradigm. Mice were treated with P + M. After 2, 4, and 6 weeks of initial treatment, the MWM tests were performed to analyze the learning and memory ability of mice. (B) Escape latency, travelled distance (C) and swimming speed (D) to reach the platform were recorded in the navigation test. (E) The latency for first platform crossing and (F) percentage of time spent in the target quadrant in the probe test were recorded in the probe test. * p < 0.05, * * p < 0.01.
We next compared the performance of P + M-treated mice and vehicle controls in probe trials. At the 5th day of the probe trial, the platform was removed from the maze, and mice were allowed to swim freely for 90 s. The latency of first platform crossing and the percentage of time in the target quadrant were recorded to assess the retention of spatial memory. As shown in Fig. 1E, compared with control group, P + M exposure significantly increased the latency for first platform crossing in mice after 6 weeks of treatment. In agreement, mice treated with P + M for 6 weeks spent less time than vehicle controls in the target quadrant where the hidden platform was previously located (Fig. 1F), indicating impaired memory ability.
3.2. P + M exposure induces neurodegeneration in the hippocampus and cortex
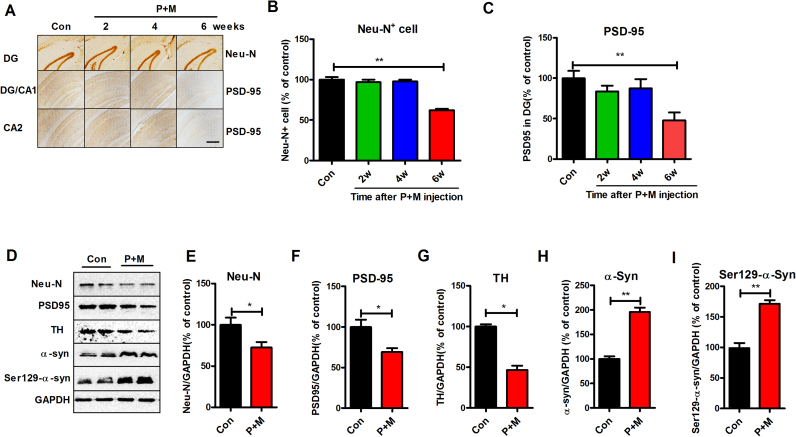
Previous studies indicated that neuronal damage and α-sunculein accumulation in the hippocampus and cortex are critical for dementia in PD patients [34], [35], [36]. We therefore investigated the effects of P + M on hippocampal and cortical neurons in mice. In agreement with impairments of learning and memory abilities, P + M exposure induced neurodegeneration in both hippocampus and cortex of mice. Neu-N is a specific neuronal nuclear antigen [37] and NeuN immunoreactivity has been widely used to identify neuron in brain sections [38]. Immunohistochemical staining with anti-Neu-N antibody revealed that compared with vehicle controls, the number of Neu-N+ cells in the hippocampal (dentate granular layer) and cortical regions of P + M-treated mice was significantly reduced after 6 weeks of P + M injection, although such neurodegeneration was not detected after 2 and 4 weeks of exposure (Fig. 2A, B and Supplementary Fig. S1). Western blot analysis further confirmed neuronal loss in the hippocampus of mice after 6 weeks of treatment by showing reduced levels of Neu-N in P + M group compared with vehicle controls (Fig. 2D, E and Supplementary Fig. S1).
Fig. 2.
P + M exposure induces progressive neurodegeneration and α-synuclein Ser129-phosphorylation in the hippocampus. (A) After 2, 4, and 6 weeks of initial P + M treatment, neuronal nuclei and synpase in the hippocampus were immunostained with antibodies against Neu-N and PSD-95, respectively, and the representative images were shown. (B) The number of Neu-N+ cells in the hippocampus was quantified by automated counting. (C) The density of PSD-95 immunostaining in the hippocampus was quantified. (D) The expressions of Neu-N, PSD-95, TH, total α-synuclein and Ser129-phosphorylated α-synuclein in the hippocampus after 6 weeks of P + M treatment were detected by using Western blot and the representative blots were shown. GAPDH was used as an internal control. (E-I) The band density of Neu-N (E), PSD-95 (F), TH (G), total α-synuclein (H) and Ser129-phosphorylated α-synuclein (I) blots was quantified. * p < 0.05, * * p < 0.01; Scale bar= 200 µm.
The decline in the cognitive functions is usually accompanied by a loss of synaptic markers such as PSD-95 [39], [40]. PSD-95 is a postsynaptic density protein that regulates synaptic maturation by interacting with glutamate receptors, cell adhesion molecules, and cytoskeletal elements [41]. Recently, there has been overwhelming evidence that associates PSD-95 disruption with cognitive and learning deficits observed in neurodegenerative diseases [42]. We thus investigated whether P + M exposure induces loss of synapses by examining the expression of PSD-95 in both hippocampus and cortex. As Fig. 2A and Supplementary Fig. S1 show, the expression of PSD-95 in the hippocampus and cortex after 2 and 4 weeks of P + M treatment revealed no significant difference compared with control group, while PSD-95 expression 6 weeks after P + M exposure was significantly decreased. Analysis of PSD-95 immunostaining density and PSD-95 protein levels by Western blot supported this conclusion (Fig. 2C, D, F, Supplementary Fig. S1 and S2).
Emerging data suggested that interactions between the dopaminergic systems and the hippocampus are also implicated in the cognitive dysfunction seen in some patients with PD [43]. TH is the rate-limiting enzyme in the synthesis of dopamine and the marker of dopaminergic neuron [44]. Western blot analysis showed that P + M treatment for 6 weeks decreased the expression of TH in the hippocampus of mice (Fig. 2D and G). Immunostaining using anti-TH antibody further revealed a reduction of TH density in the hippocampus (Supplementary Fig. S3), suggesting that damage of hippocampal dopaminergic system.
To detect whether P + M-induced neurodegeneration is associated with α-synuclein pathology, the expression of α-synuclein was initially examined in the hippocampus of mice after 6 weeks of treatment. Western blot analysis showed that compared with vehicle controls, P + M-treated mice showed high expressions of α-synuclein in the hippocampus (Fig. 2D and H). Since the phosphorylation of α-synuclein, especially at Ser129 site, is critical for its accumulation and toxicity [45], the Ser129-phosphorylation of α-synuclein was further examined. In agreement with total α-synuclein, P + M exposure also enhanced Ser129-phosphorylation of α-synuclein in the hippocampus of mice (Fig. 2D and I).
3.3. Apocynin attenuates P + M-induced deficits of spatial learning and memory and neurodegeneration in mice
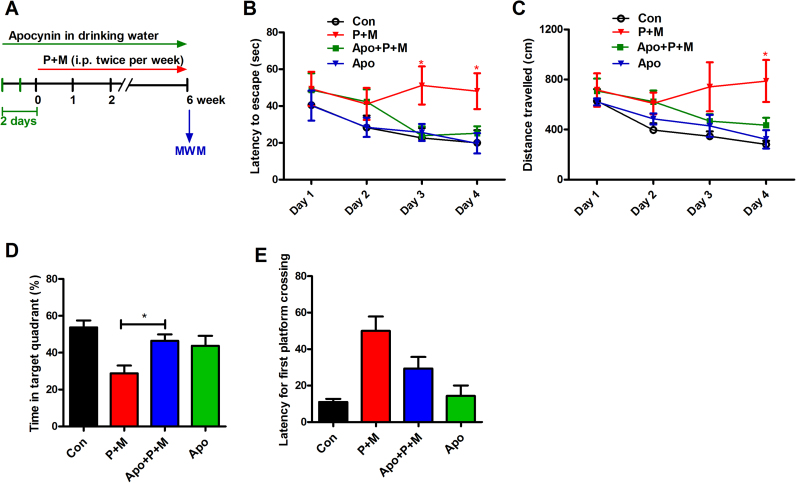
To determine whether NADPH oxidase is involved in learning and memory impairments induced by P + M, mice were administrated with NADPH oxidase inhibitor, apocynin prior to 2 days of initial P + M injection (Fig. 3A). We recently reported that apocynin at this concentration is capable of blocking P + M-induced activation of NADPH oxidase in vivo by reducing membrane translocation of cytosolic subunit, p47phox [24]. MWM test revealed that P + M induced deficits of spatial learning and memory were significantly ameliorated by apocynin as shown by reduced escape latency and travelled distance as well as recovered time percentage in target quadrant where the hidden platform was previously located in combined apocynin and P + M-treated mice compared with P + M alone group (Fig. 3B-D). Although apocynin treatment also reduced the latency for first platform crossing in mice, the difference of latency between combined apocynin and P + M-treated mice and P + M alone group did not reach statistical significance (Fig. 3E). Apocynin alone had no significant effects on spatial learning and memory performance in mice (Fig. 3B-E). The possibility that apocynin-afforded protection against P + M-induced cognitive deficits was due to recovered locomotor activity was excluded since no significant difference in average swimming speed was found among groups during the experiments.
Fig. 3.
Apocynin treatment ameliorates P + M-induced deficits of learning and memory in mice. (A) Schematic presentation of the experimental paradigm. Mice were administrated with apocynin 2 days prior to P + M treatment. After 6 weeks of initial P + M exposure, the MWM tests were performed to analyze the learning and memory ability of mice. (B) Escape latency and travelled distance (C) to reach the platform were recorded in the navigation test. (D) The percentage of time spent in the target quadrant and (E) latency for first platform crossing in the probe test were recorded in the probe test. * p < 0.05.
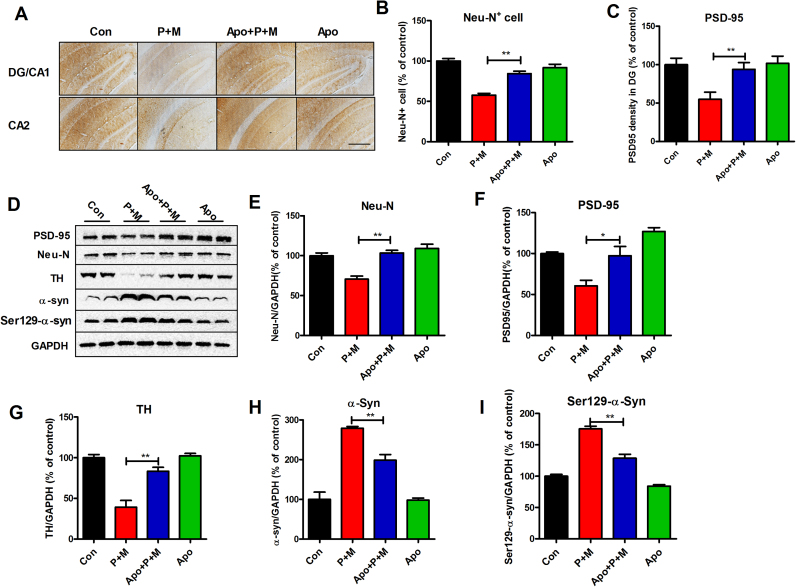
Next, we investigated the protective effects of NADPH oxidase inhibition against P + M-induced neurodegeneration in mice. Consistent with Fig. 2, the degeneration of hippocampal and cortical neurons in P + M-treated mice was observed. In contrast, compared with P + M alone group, mice treated with combined apocynin and P + M displayed reduced neuronal damage in both hippocampus and cortex. Apocynin treatment significantly recovered the number of Neu-N+ cells and expressions of PSD-95 in both hippocampus and cortex in P + M-treated mice (Fig. 4A–C and Supplementary Fig. S4). Consistently, Western blot analysis revealed a higher level of Neu-N and PSD-95 expression in the hippocampus of combined apocynin and P + M-treated mice than P + M alone group (Fig. 4D-F). P + M-induced reduction of TH expressions in the hippocampus was also recovered by apocynin (Fig. 4D and G), indicating neuroprotection of hippocampal dopaminergic system.
Fig. 4.
Apocynin mitigates P + M-induced hippocampal neurodegeneration and α-synuclein Ser129-phosphorylation in mice. (A) Mice were administrated with apocynin 2 days prior to P + M treatment. After 6 weeks of initial P + M exposure, neuronal synpase in the hippocampus were immunostained with anti-Neu-N and anti-PSD-95 antibodies, respectively, and the representative images were shown. (B) The number of Neu-N+ cells in the hippocampus was quantified by automated counting. (C) The density of PSD-95 immunostaining in the hippocampus was quantified. (D) The expressions of Neu-N, PSD-95, TH, total α-synuclein and Ser129-phosphorylated α-synuclein in the hippocampus were detected by using Western blot and the representative blots were shown. GAPDH was used as an internal control. (E-I) The band density of Neu-N (E), PSD-95 (F), TH (G), total α-synuclein (H) and Ser129-phosphorylated α-synuclein (I) blots was quantified. * p < 0.05, * * p < 0.01; Scale bar= 200 µm.
In agreement with neuroprotection, apocynin also mitigated the expression and Ser129-phosphorylation of α-synuclein in the hippocampus of P + M-treated mice (Fig. 4D, H and I). No significant difference of PSD-95, Neu-N, TH and α-synuclein was observed between apocynin alone and vehicle controls.
3.4. Apocynin abrogates P + M-induced oxidative stress in the hippocampus
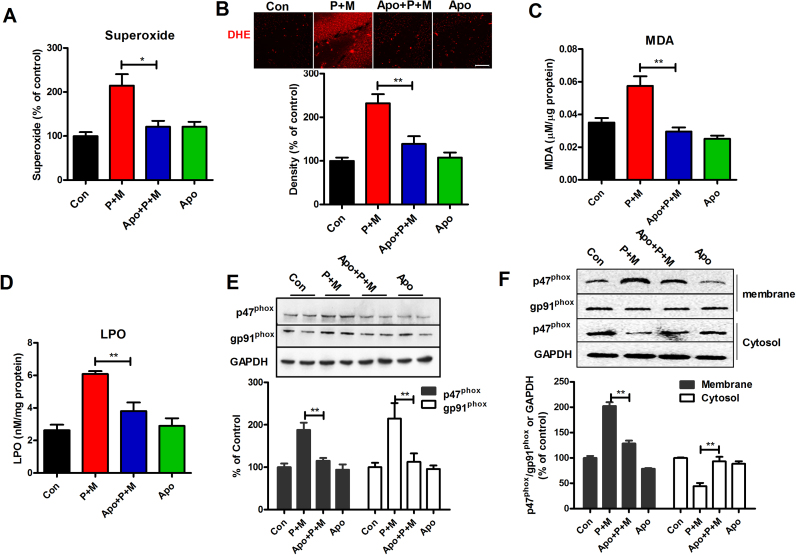
NADPH oxidase is a multi-complex superoxide-producing enzyme and is assembled in the membrane [46]. Previous study demonstrated that superoxide production induced by P + M is mainly due to activation of NADPH oxidase [24], [47]. We therefore initially investigated the effects of apocynin on P + M-induced superoxide production in the membrane prepared from hippocampus of mice. As illustrated in Fig. 5A, compared with vehicle controls, P + M exposure resulted in increase of superoxide production in the hippocampus of mice. Interestingly, apocynin significantly mitigated P + M-induced superoxide production (Fig. 5A). In situ visualization of reactive oxygen species production was further performed using DHE, a reactive oxygen species-sensitive dye. DHE can readily cross the blood–brain barrier and exhibits red fluorescence through interactions with superoxide and other free radicals in the brain [20], [48]. Consistently, compared with controls, the levels of red fluorescence of DHE oxidation in P + M-treated mice were increased, which was significantly blocked by apocynin (Fig. 5B and Supplementary Fig. S5). In agreement with increase of superoxide production, P + M exposure also resulted in increase of MDA and LPO in the hippocampus of mice, which were also mitigated by apocynin (Fig. 5C and D). These results suggested that apocynin attenuates P + M-induced oxidative stress.
Fig. 5.
Apocynin abrogates P + M-induced oxidative stress. (A) After 6 weeks of initial P + M exposure with or without apocyni pre-treatment, the production of ROS in membranes extracted from mice was measured by SOD-inhibitible reduction of WST-1. (B) The production of superoxide was assessed by DHE and the density of red fluorescence of DHE oxidation was quantified. (C) The level of MDA and LPO (D) in the hippocampus was determined by using commercial assay kits. (E) The expressions of p47phox and gp91phox in the hippocampus were detected by using Western blot and the density of blots were quantified. (F) The membrane translocation of NOX2 cytosolic subunit, p47phox was detected in BV2 microglia cells after 1 h of P + M stimulation with or without apocynin pre-treatment by using Western blot and the density of blots was quantified. Gp91phox and GAPDH were used as internal membrane and cytosolic control, respectively. * p < 0.05. * * p < 0.01; Scale bar= 50 µm.
To determine whether NADPH oxidase is involved in the inhibitory effects of apocynin on P + M-induced oxidative stress, the expressions of NADPH oxidase were determined. As seen in Fig. 5E, P + M intoxication elevated the expressions of p47phox and gp91phox, the cytosolic and catalytic subunit of NADPH oxidase, respectively, in the hippocampus of mice, which was significantly reduced by apocynin. The effects of apocynin on p47phox membrane translocation, a critical mechanism for NADPH oxidase activation [49], were further determined in P + M-treated BV2 microglial cells. Western blot analysis showed that the levels of p47phox were significantly decreased in the cytosolic fraction but increased in membrane fractions in P + M-treated microglia (Fig. 5F). P + M-induced p47phox membrane translocation was significantly attenuated by apocynin (Fig. 5F), indicating NADPH oxidase inactivation.
The source of superoxide production has recently become a subject of debate. Mitochondria have been traditionally considered a major source of superoxide production. To exclude the possibility that mitochondria are involved in the inhibitory effects of apocynin against P + M-induced superoxide production, we examined the activities of complex I, one important redox carrier in mitochondrial respiratory chain, in the hippocampus of P + M-treated mice with or without apocynin. As illustrated in supplementary Fig. S6, apocynin treatment failed to interfere with complex I activity in P + M-treated mice, although P + M exposure markedly reduced the activities of complex I. No significant difference of complex I activity was observed between apocynin alone and control groups. Similar results were also observed in P + M-treated BV2 microglial cells with or without apocynin pre-treatment (Supplementary Fig. S6).
3.5. Apocynin attenuates P + M-induced microglial activation and M1 polarization in the hippocampus
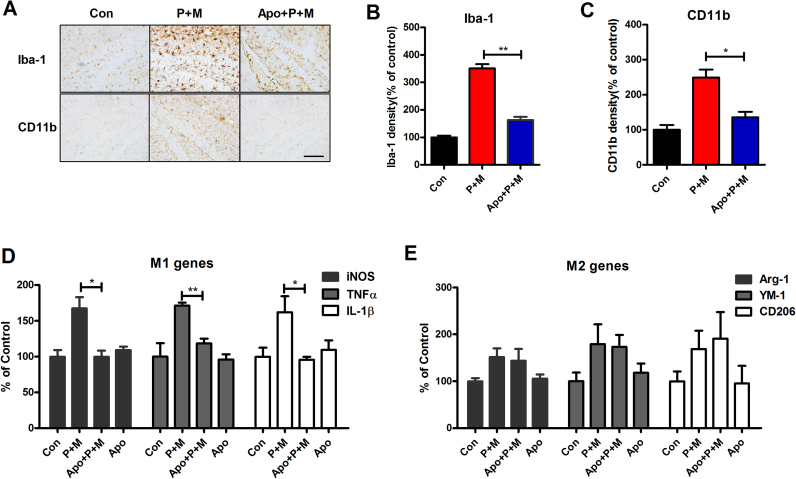
Considering the critical role of neuroinflammation in PD dementia, we subsequently determined the effects of apocynin on microglial activation in P + M-treated mice. Microglia in the hippocampus and cortex were immunostained with antibody against Iba-1 and CD11b, two markers for microglia. As seen in Fig. 6A, activated microglia characterized by hypertrophic morphology and increased Iba-1 and CD11b density were observed in the hippocampus and cortex of P + M-injected mice. However, such activated microglia were rarely detected in combined apocynin and P + M-treated mice (Fig. 6A). Quantitative analysis of Iba-1 and CD11b density supported the morphological observation (Fig. 6B and C).
Fig. 6.
Apocynin suppresses P + M-induced microglial activation and M1 polarization. (A) After 6 weeks of initial P + M exposure with or without apocyni pre-treatment, microglial cells in the hippocampus were immunostained with two microglial markers, Iba-1 and CD11b and the representative images were shown. Activated microglia are characterized by enlarged cell bodies and high staining density. (B, C) Microglial activation was quantified by calculating the density of expression of Iba-1 and CD11b in the hippocampus. (D and E) The mRNA levels of genes of M1 (iNOS, TNFα and IL-1β) and M2 (CD206, Arg-1 and YM-1) were determined in the hippocampus by using RT-PCR. * p < 0.05. * * p < 0.01; Scale bar= 50 µm.
Microglial activation can be polarized into two phenotypes, defined as “classical activation” (M1) and “alternative activation” (M2) that produce detrimental and beneficial effects, respectively [50]. To determine the mechanisms behind the inhibitory effects of apocynin on microglial activation, the phenotype of microglia were detected in P + M-treated mice with or without apocynin. The gene expressions of markers of M1 and M2 microglia were examined by RT-PCR. Compared with vehicle controls, the mRNA levels of iNOS, TNFα and IL-1β, which are generated by activated M1 microglia, were up-regulated in the hippocampus of P + M-treated mice. Interestingly, P + M-induced up-regulation of iNOS, TNFα and IL-1β mRNA were significantly reduced by apocynin (Fig. 6D). In contrast, apocynin treatment failed to interfere with the gene expressions of Arg-1, CD206 and YM-1, the markers for M2 microglia, although their mRNA levels were also elevated by P + M (Fig. 6E). These results suggested that apocynin mitigates P + M-induced M1 polarization.
3.6. Apocynin inhibits the activation of STAT1 and NF-κB pathways in P + M-treated mice
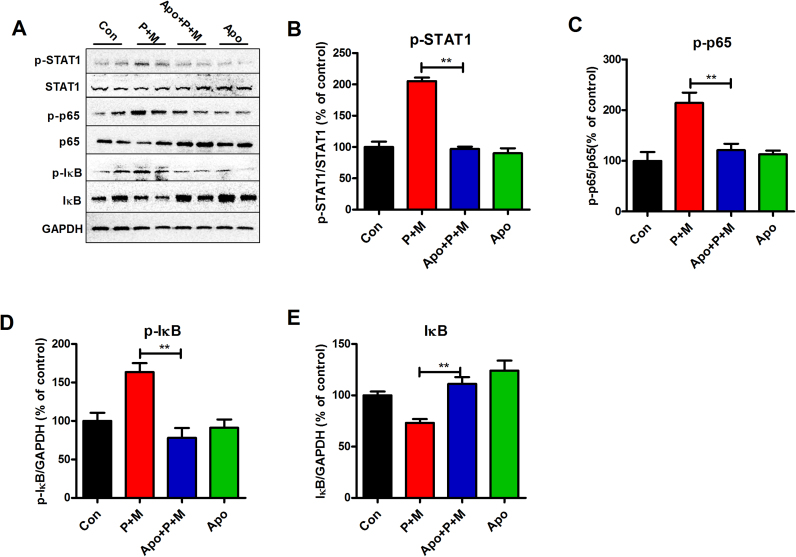
The STAT1 and nuclear factor-κB (NF-κB) signaling pathways are reported to be essential for regulating microglial phenotypes [50]. To determine whether STAT1 and NF-κB are involved in apocynin-inhibited microglial M1 polarization, the activation of these two signaling pathways was examined. As shown in Fig. 7A and B, P + M exposure elevated the phosphorylation of STAT1 in the hippocampus of mice, which was significantly reduced by apocynin, indicating reduced activation of STAT1 signaling pathway.
Fig. 7.
Apocynin inactivates P + M-induced activation of STAT1 and NF-κB pathways. (A) After 6 weeks of initial P + M exposure with or without apocyni pre-treatment, the levels of phosphorylated and nonphosphorylated STAT1, p65 and IκBα in the hippocampus of mice were determined by Western blot using specific antibodies and the representative blots were shown. (B-E) The phosphorylation of STAT1 (B), p65 (C) and p-IκBα (D) as well as total IκBα (E) were quantified by analysis of the blot density. * * p < 0.01.
We next determined the effects of apocynin on P + M-induced activation of NF-κB. P + M exposure resulted in activation of NF-κB pathway as shown by increased phosphorylation of p65 and IκBα and reduced expression of total IκBα compared with vehicle controls (Fig. 7A and C-E). In agreement with STAT1 signaling, P + M-induced activation of NF-κB pathway was also mitigated by apocynin. Fig. 7A and C-E depicted the reduced phosphorylation of p65 and IκBα as well as recovered IκBα expression in apocynin and P + M co-treated mice compared with P + M alone group.
4. Discussion
In the present study, we demonstrated that NADPH oxidase plays a critical role in driving learning and memory deficits in a two pesticide-induced mouse PD model. The salient features of our study are: 1) P + M-treated mice displayed progressive learning and memory impairments, which were significantly mitigated by NADPH oxidase inhibitor apocynin; 2) Apocynin ameliorated P + M-induced hippocampal neurodegeneration and synaptic loss as well as α-synuclein expression and Ser129 phosphorylation; 3) Apocynin abrogated P + M-induced oxidative stress as well as microglial activation and M1 polarization; 4) Apocynin suppressed activation of NF-κB and STAT1 signaling pathways.
Cognitive impairment is one of the most common and devastating non-motor symptoms of patients with PD. Moreover, the risk of cognitive impairment increases with PD progression. Pioneering studies have shown that mild cognitive deficits may be identified in 25~46.8% of newly diagnosed patients [51], [52], and dementia occurs in up to 80% of patients over the course of the disease [53]. However, the exact pathophysiology underlying the development of cognitive decline or dementia is less clear. It is now increasingly believed that dementia in PD is likely to be secondary to a wider neurodegeneration extending beyond dopaminergic systems [54], [55] since some aspects of cognition seem to respond to levodopa therapy whereas other aspects do not, or may even be worsened by levodopa [56]. Clinical studies revealed that hippocampal and cortical neurodegeneration and Lewy pathology are associated with cognitive impairments or dementia in PD patients [34], [54]. In the present study, mice treated with P + M displayed spatial learning and memory impairments as well as neurodegeneration, synaptic loss, α-synuclein expression and Ser129-phosphorylation in the hippocampur and/or cortex. The statistical significance was observed at 6 but not 2 and 4 weeks after P + M treatment. Interestingly, we found that inhibition of NADPH oxidase by apocynin ameliorated neurodegeneration, synaptic disruption and α-synuclein pathology in the hippocampus and/or cortex induced by P + M, which was associated with improved learning and memory performance in mice. Previous study indicated that dopaminergic system plays a critical role in hippocampus-dependent learning acquisition [43]. Apocynin treatment increased the expression of TH, the marker for dopaminergic neuron, in the hippocampus. Kumar et al. found that dopaminergic neurodegeneration in the SN of P + M-treated mice was also attenuated by apocynin [47]. These results suggested that dopaminergic system in the hippocampus is also involved in NADPH oxidase-regulated learning and memory. Consistent with our findings, Kan et al. reported that NADPH oxidase activation and related reactive oxygen species (ROS) production are involved in menopause‑associated learning and memory impairments [57]. Similarly, the activation of NADPH oxidase also contributes to long-term cognitive impairments in rats after repeated ketamine exposures [58]. Furthermore, in a rat model of Alzheimer's disease induced by Aβ1–42, inhibition of NADPH oxidase by electroacupuncture improves spatial learning and memory performance and hippocampal neuron survival [59]. These findings suggested that the activation of NADPH oxidase is critical for learning and memory impairments.
Oxidative stress and chronic neuroinflammation are common mechanisms shared by multiple neurodegenerative diseases [60]. There is increasing evidence that oxidative damage caused by excessive ROS production and microglial activation may correlate with cognitive impairments in Alzheimer disease (AD) patients or aged populations [61], [62], [63]. Although the exact mechanisms of how oxidative stress and neuroinflammation impair learning and memory capacity remain unclear, disruption or loss of synapse might be one of the reasons. It's reported that H2O2 induced by Aβ, the main component of plaque in AD, is capable of inducing reduction of synaptic proteins and loss of synapse, which is implicated in Aβ-induced cognitive deficits [64], [65]. Furthermore, in mouse AD models, inhibition of oxidative stress and neuroinflammation attenuates synaptic loss and cognitive dysfunction [66]. Similarly, in a diabetes-associated cognitive decline rat model, resveratrol ameliorates synaptic loss by preventing oxidative stress and inflammation, which is associated with improved learning and memory performance [67].
NADPH oxidase is a superoxide-producing enzyme in microglia. Accumulating evidence suggested that NADPH oxidase plays an important role in both oxidative stress and neuroinflammation in PD. Once activated, NADPH oxidase produces neurotoxic extracellular and intracellular ROS [68], resulting in oxidative damage [69], [70]. Additionally, intracellular ROS is thought to be important secondary messengers that regulate the activation of several downstream inflammatory signaling pathways, such as STATs and NF-κB [68], [71]. The up-regulated expression and activation of NADPH oxidase are observed in the brains of patients with PD and animal PD models [20], [33]. We recently reported that pharmacological inhibition or genetic deletion of NADPH oxidase 2 attenuates oxidative stress and microglia-mediated neuroinflammation in an inflammatory mouse PD model [19]. Consistently, in this study, inhibition of NADPH oxidase by apocynin also decreased P + M-induced oxidative stress, microglial activation and M1 polarization in the hippocampus.
The inhibitory effects of apocynin on microglial activation and M1 polarization might be related to inactivation of STAT1 and NF-κB pathways. Previous studies showed that the STAT, especially STAT1 and NF-κB signaling pathways play important roles in regulating microglial M1 proinflammatory responses [50]. LPS and IFN-γ, two classic stimulators for microglial M1 activation, has been shown to polarize microglia to M1 phenotype through the activation of NF-κB and STAT1 pathways [50]. In this study, the inhibitory effects of apocynin on P + M-induced activation of STAT1 and NF-κB signaling were also observed in the hippocampus. In agreement with our results, Sharma and Nehru found that apocyanin could also attenuate microglial activation and expressions of M1 markers, including iNOS, TNFα, IL-1β via NF-κB pathway in LPS-treated rats [22].
Notably, it's well known that NADPH oxidase is a family of enzymes that include NOX1, NOX2, NOX3, NOX4, NOX5, dual oxidase 1 (DUOX1), and DUOX2 [69]. Apocynin is capable of inhibiting the activation of NADPH oxidase. However, we cannot recognize which isoforms of NADPH oxidase is involved in P + M-induced learning and memory deficits in the present study. In addition, apocynin lacks specificity towards NADPH oxidase, although it is a widely used NADPH oxidase inhibitor [72]. Several other enzymes are also identified to be inhibited by apocynin, such as cytochrome P450 monooxygenase [73] and cyclooxygenase [74]. Therefore, in this study, we also cannot exclude the possibility that inhibition of other superoxide-producing enzymes but not NADPH oxidase could also contribute to the impairments of learning and memory in P + M-intoxicated mice.
In summary, this study showed that inhibition of NADPH oxidase by apocynin blocks impairments of learning and memory as well as hippocampal neurodegeneration and α-synuclein pathology via the suppression of oxidative stress and neuroinflammation in a mouse PD model, supporting the idea that NADPH oxidase is critical for driving the cognitive dysfunction in PD. Our findings reveal a novel mechanism for the pathogenesis of cognitive dysfunction and may offer a promising disease modifying therapeutic strategy for PD dementia.
Acknowledgements
This work was supported by “QiZhen” talent project of Dalian Medical University (No. 201122), Liaoning BaiQianWan Talents Program (No. [2017]90), Program for Liaoning Innovative Talents in University (LR2016008), National Natural Science Foundation of China (81630097, 81773718), CAMS Innovation Fund for Medical Sciences (No. 2016-I2M-3-011), National Major Scientific and Technological Special Project (2018ZX09711001-008-005, 2018ZX09711001-003-020).
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2019.101134.
Appendix A. Supplementary material
Supplementary material.
References
- 1.Hou L., Li Q., Jiang L., Qiu H., Geng C., Hong J.S., Li H., Wang Q. Hypertension and diagnosis of Parkinson's disease: a meta-analysis of cohort studies. Front. Neurol. 2018;9:162. doi: 10.3389/fneur.2018.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franke C., Storch A. Nonmotor fluctuations in Parkinson's disease. Int. Rev. Neurobiol. 2017;134:947–971. doi: 10.1016/bs.irn.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Bosboom J.L., Stoffers D., Wolters E. Cognitive dysfunction and dementia in Parkinson's disease. J. Neural Transm. (Vienna) 2004;111(10–11):1303–1315. doi: 10.1007/s00702-004-0168-1. [DOI] [PubMed] [Google Scholar]
- 4.Lawson R.A., Yarnall A.J., Duncan G.W., Khoo T.K., Breen D.P., Barker R.A., Collerton D., Taylor J.P., Burn D.J. Quality of life and mild cognitive impairment in early Parkinson's disease: does subtype matter? J. Park. Dis. 2014;4(3):331–336. doi: 10.3233/JPD-140390. [DOI] [PubMed] [Google Scholar]
- 5.Reginold W., Duff-Canning S., Meaney C., Armstrong M.J., Fox S., Rothberg B., Zadikoff C., Kennedy N., Gill D., Eslinger P., Marshall F., Mapstone M., Chou K.L., Persad C., Litvan I., Mast B., Tang-Wai D., Lang A.E., Marras C. Impact of mild cognitive impairment on health-related quality of life in Parkinson's disease. Dement Geriatr. Cogn. Disord. 2013;36(1–2):67–75. doi: 10.1159/000350032. [DOI] [PubMed] [Google Scholar]
- 6.Niranjan R. Recent advances in the mechanisms of neuroinflammation and their roles in neurodegeneration. Neurochem. Int. 2018;120:13–20. doi: 10.1016/j.neuint.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Barnum C.J., Tansey M.G. Modeling neuroinflammatory pathogenesis of Parkinson's disease. Prog. Brain Res. 2010;184:113–132. doi: 10.1016/S0079-6123(10)84006-3. [DOI] [PubMed] [Google Scholar]
- 8.Gerhard A., Pavese N., Hotton G., Turkheimer F., Es M., Hammers A., Eggert K., Oertel W., Banati R.B., Brooks D.J. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson's disease. Neurobiol. Dis. 2006;21(2):404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Imamura K., Hishikawa N., Sawada M., Nagatsu T., Yoshida M., Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson's disease brains. Acta Neuropathol. 2003;106(6):518–526. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- 10.Lindqvist D., Kaufman E., Brundin L., Hall S., Surova Y., Hansson O. Non-motor symptoms in patients with Parkinson's disease - correlations with inflammatory cytokines in serum. PLoS One. 2012;7(10):e47387. doi: 10.1371/journal.pone.0047387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menza M., Dobkin R.D., Marin H., Mark M.H., Gara M., Bienfait K., Dicke A., Kusnekov A. The role of inflammatory cytokines in cognition and other non-motor symptoms of Parkinson's disease. Psychosomatics. 2010;51(6):474–479. doi: 10.1176/appi.psy.51.6.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindqvist D., Hall S., Surova Y., Nielsen H.M., Janelidze S., Brundin L., Hansson O. Cerebrospinal fluid inflammatory markers in Parkinson's disease--associations with depression, fatigue, and cognitive impairment. Brain Behav. Immun. 2013;33:183–189. doi: 10.1016/j.bbi.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Gong Q.H., Wang Q., Pan L.L., Liu X.H., Xin H., Zhu Y.Z. S-propargyl-cysteine, a novel hydrogen sulfide-modulated agent, attenuates lipopolysaccharide-induced spatial learning and memory impairment: involvement of TNF signaling and NF-kappaB pathway in rats. Brain Behav. Immun. 2011;25(1):110–119. doi: 10.1016/j.bbi.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Hou Y., Xie G., Miao F., Ding L., Mou Y., Wang L., Su G., Chen G., Yang J., Wu C. Pterostilbene attenuates lipopolysaccharide-induced learning and memory impairment possibly via inhibiting microglia activation and protecting neuronal injury in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;54:92–102. doi: 10.1016/j.pnpbp.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Hou L., Wang K., Zhang C., Sun F., Che Y., Zhao X., Zhang D., Li H., Wang Q. Complement receptor 3 mediates NADPH oxidase activation and dopaminergic neurodegeneration through a Src-Erk-dependent pathway. Redox Biol. 2018;14:250–260. doi: 10.1016/j.redox.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou L., Bao X., Zang C., Yang H., Sun F., Che Y., Wu X., Li S., Zhang D., Wang Q. Integrin CD11b mediates alpha-synuclein-induced activation of NADPH oxidase through a Rho-dependent pathway. Redox Biol. 2018;14:600–608. doi: 10.1016/j.redox.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang C., Hou L., Yang J., Che Y., Sun F., Li H., Wang Q. 2,5-Hexanedione induces dopaminergic neurodegeneration through integrin alphaMbeta2/NADPH oxidase axis-mediated microglial activation. Cell Death Dis. 2018;9(2):60. doi: 10.1038/s41419-017-0091-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Che Y., Hou L., Sun F., Zhang C., Liu X., Piao F., Zhang D., Li H., Wang Q. Taurine protects dopaminergic neurons in a mouse Parkinson's disease model through inhibition of microglial M1 polarization. Cell Death Dis. 2018;9(4):435. doi: 10.1038/s41419-018-0468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q., Qian L., Chen S.H., Chu C.H., Wilson B., Oyarzabal E., Ali S., Robinson B., Rao D., Hong J.S. Post-treatment with an ultra-low dose of NADPH oxidase inhibitor diphenyleneiodonium attenuates disease progression in multiple Parkinson's disease models. Brain. 2015;138(Pt 5):1247–1262. doi: 10.1093/brain/awv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu D.C., Teismann P., Tieu K., Vila M., Jackson-Lewis V., Ischiropoulos H., Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. Proc. Natl. Acad. Sci. USA. 2003;100(10):6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simonyi A., Serfozo P., Lehmidi T.M., Cui J., Gu Z., Lubahn D.B., Sun A.Y., Sun G.Y. The neuroprotective effects of apocynin. Front. Biosci. 2012;4:2183–2193. doi: 10.2741/535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma N., Nehru B. Apocyanin, a microglial NADPH oxidase inhibitor prevents dopaminergic neuronal degeneration in lipopolysaccharide-induced Parkinson's disease model. Mol. Neurobiol. 2016;53(5):3326–3337. doi: 10.1007/s12035-015-9267-2. [DOI] [PubMed] [Google Scholar]
- 23.de Almeida A.C., Vilela M.M. Dos Santos, Condino-Neto A., Ximenes V.F. The importance of myeloperoxidase in apocynin-mediated NADPH oxidase inhibition. ISRN Inflamm. 2012;2012:260453. doi: 10.5402/2012/260453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou L., Zhang C., Wang K., Liu X., Wang H., Che Y., Sun F., Zhou X., Zhao X., Wang Q. Paraquat and maneb co-exposure induces noradrenergic locus coeruleus neurodegeneration through NADPH oxidase-mediated microglial activation. Toxicology. 2017;380:1–10. doi: 10.1016/j.tox.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Bachmanov A.A., Reed D.R., Beauchamp G.K., Tordoff M.G. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav. Genet. 2002;32(6):435–443. doi: 10.1023/a:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harraz M.M., Marden J.J., Zhou W., Zhang Y., Williams A., Sharov V.S., Nelson K., Luo M., Paulson H., Schoneich C., Engelhardt J.F. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J. Clin. Investig. 2008;118(2):659–670. doi: 10.1172/JCI34060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferretti V., Roullet P., Sargolini F., Rinaldi A., Perri V., Del Fabbro M., Costantini V.J., Annese V., Scesa G., De Stefano M.E., Oliverio A., Mele A. Ventral striatal plasticity and spatial memory. Proc. Natl. Acad. Sci. USA. 2010;107(17):7945–7950. doi: 10.1073/pnas.0911757107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou L., Zhou X., Zhang C., Wang K., Liu X., Che Y., Sun F., Li H., Wang Q., Zhang D., Hong J.S. NADPH oxidase-derived H2O2 mediates the regulatory effects of microglia on astrogliosis in experimental models of Parkinson's disease. Redox Biol. 2017;12:162–170. doi: 10.1016/j.redox.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Q., Shin E.J., Nguyen X.K., Li Q., Bach J.H., Bing G., Kim W.K., Kim H.C., Hong J.S. Endogenous dynorphin protects against neurotoxin-elicited nigrostriatal dopaminergic neuron damage and motor deficits in mice. J. Neuroinflamm. 2012;9:124. doi: 10.1186/1742-2094-9-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai Z., Chattopadhyay N., Liu W.J., Chan C., Pignol J.P., Reilly R.M. Optimized digital counting colonies of clonogenic assays using ImageJ software and customized macros: comparison with manual counting. Int. J. Radiat. Biol. 2011;87(11):1135–1146. doi: 10.3109/09553002.2011.622033. [DOI] [PubMed] [Google Scholar]
- 31.Wang Q., Chu C.H., Oyarzabal E., Jiang L., Chen S.H., Wilson B., Qian L., Hong J.S. Subpicomolar diphenyleneiodonium inhibits microglial NADPH oxidase with high specificity and shows great potential as a therapeutic agent for neurodegenerative diseases. Glia. 2014;62(12):2034–2043. doi: 10.1002/glia.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Q., Chu C.H., Qian L., Chen S.H., Wilson B., Oyarzabal E., Jiang L., Ali S., Robinson B., Kim H.C., Hong J.S. Substance P exacerbates dopaminergic neurodegeneration through neurokinin-1 receptor-independent activation of microglial NADPH oxidase. J. Neurosci. 2014;34(37):12490–12503. doi: 10.1523/JNEUROSCI.2238-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou L., Che Y., Sun F., Wang Q. Taurine protects noradrenergic locus coeruleus neurons in a mouse Parkinson's disease model by inhibiting microglial M1 polarization. Amino Acids. 2018;50(5):547–556. doi: 10.1007/s00726-018-2547-1. [DOI] [PubMed] [Google Scholar]
- 34.Hanganu A., Bedetti C., Degroot C., Mejia-Constain B., Lafontaine A.L., Soland V., Chouinard S., Bruneau M.A., Mellah S., Belleville S., Monchi O. Mild cognitive impairment is linked with faster rate of cortical thinning in patients with Parkinson's disease longitudinally. Brain. 2014;137(Pt 4):1120–1129. doi: 10.1093/brain/awu036. [DOI] [PubMed] [Google Scholar]
- 35.Gratwicke J., Jahanshahi M., Foltynie T. Parkinson's disease dementia: a neural networks perspective. Brain. 2015;138(Pt 6):1454–1476. doi: 10.1093/brain/awv104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cosgrove J., Alty J.E., Jamieson S. Cognitive impairment in Parkinson's disease. Postgrad. Med. J. 2015;91(1074):212–220. doi: 10.1136/postgradmedj-2015-133247. [DOI] [PubMed] [Google Scholar]
- 37.Keller-Peck C.R., Mullen R.J. Patterns of neuronal differentiation in neural tube mutant mice: curly tail and Pax3 splotch-delayed. J. Comp. Neurol. 1996;368(4):516–526. doi: 10.1002/(SICI)1096-9861(19960513)368:4<516::AID-CNE4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 38.Song S., Jiang L., Oyarzabal E.A., Wilson B., Li Z., Shih Y.I., Wang Q., Hong J.S. Loss of brain norepinephrine elicits neuroinflammation-mediated oxidative injury and selective Caudo-Rostral neurodegeneration. Mol. Neurobiol. 2018 doi: 10.1007/s12035-018-1235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marttinen M., Kurkinen K.M., Soininen H., Haapasalo A., Hiltunen M. Synaptic dysfunction and septin protein family members in neurodegenerative diseases. Mol. Neurodegener. 2015;10:16. doi: 10.1186/s13024-015-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sweet E.S., Saunier-Rebori B., Yue Z., Blitzer R.D. The Parkinson's disease-associated mutation LRRK2-G2019S impairs synaptic plasticity in mouse hippocampus. J. Neurosci. 2015;35(32):11190–11195. doi: 10.1523/JNEUROSCI.0040-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vallejo D., Codocedo J.F., Inestrosa N.C. Posttranslational modifications regulate the postsynaptic localization of PSD-95. Mol. Neurobiol. 2017;54(3):1759–1776. doi: 10.1007/s12035-016-9745-1. [DOI] [PubMed] [Google Scholar]
- 42.Coley A.A., Gao W.J. PSD95: a synaptic protein implicated in schizophrenia or autism? Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018;82:187–194. doi: 10.1016/j.pnpbp.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calabresi P., Castrioto A., Di Filippo M., Picconi B. New experimental and clinical links between the hippocampus and the dopaminergic system in Parkinson's disease. Lancet Neurol. 2013;12(8):811–821. doi: 10.1016/S1474-4422(13)70118-2. [DOI] [PubMed] [Google Scholar]
- 44.Nagatsu T., Nagatsu I. Tyrosine hydroxylase (TH), its cofactor tetrahydrobiopterin (BH4), other catecholamine-related enzymes, and their human genes in relation to the drug and gene therapies of Parkinson's disease (PD): historical overview and future prospects. J. Neural Transm. (Vienna) 2016;123(11):1255–1278. doi: 10.1007/s00702-016-1596-4. [DOI] [PubMed] [Google Scholar]
- 45.Arawaka S., Sato H., Sasaki A., Koyama S., Kato T. Mechanisms underlying extensive Ser129-phosphorylation in alpha-synuclein aggregates. Acta Neuropathol. Commun. 2017;5(1):48. doi: 10.1186/s40478-017-0452-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Block M.L., Hong J.S. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog. Neurobiol. 2005;76(2):77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Kumar A., Leinisch F., Kadiiska M.B., Corbett J., Mason R.P. Formation and implications of alpha-synuclein radical in maneb- and paraquat-induced models of Parkinson's disease. Mol. Neurobiol. 2016;53(5):2983–2994. doi: 10.1007/s12035-015-9179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zielonka J., Hardy M., Kalyanaraman B. HPLC study of oxidation products of hydroethidine in chemical and biological systems: ramifications in superoxide measurements. Free Radic. Biol. Med. 2009;46(3):329–338. doi: 10.1016/j.freeradbiomed.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Q., Zhou H., Gao H., Chen S.H., Chu C.H., Wilson B., Hong J.S. Naloxone inhibits immune cell function by suppressing superoxide production through a direct interaction with gp91phox subunit of NADPH oxidase. J. Neuroinflamm. 2012;9:32. doi: 10.1186/1742-2094-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Orihuela R., McPherson C.A., Harry G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016;173(4):649–665. doi: 10.1111/bph.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aarsland D., Bronnick K., Larsen J.P., Tysnes O.B., Alves G. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology. 2009;72(13):1121–1126. doi: 10.1212/01.wnl.0000338632.00552.cb. [DOI] [PubMed] [Google Scholar]
- 52.Yu R.L., Wu R.M., Tai C.H., Lin C.H., Cheng T.W., Hua M.S. Neuropsychological profile in patients with early stage of Parkinson's disease in Taiwan. Park. Relat. Disord. 2012;18(10):1067–1072. doi: 10.1016/j.parkreldis.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 53.Aarsland D., Beyer M.K., Kurz M.W. Dementia in Parkinson's disease. Curr. Opin. Neurol. 2008;21(6):676–682. doi: 10.1097/WCO.0b013e3283168df0. [DOI] [PubMed] [Google Scholar]
- 54.Hall H., Reyes S., Landeck N., Bye C., Leanza G., Double K., Thompson L., Halliday G., Kirik D. Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson's disease. Brain. 2014;137(Pt 9):2493–2508. doi: 10.1093/brain/awu193. [DOI] [PubMed] [Google Scholar]
- 55.Kehagia A.A., Barker R.A., Robbins T.W. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol. 2010;9(12):1200–1213. doi: 10.1016/S1474-4422(10)70212-X. [DOI] [PubMed] [Google Scholar]
- 56.Kehagia A.A., Barker R.A., Robbins T.W. Cognitive impairment in Parkinson's disease: the dual syndrome hypothesis. Neurodegener. Dis. 2013;11(2):79–92. doi: 10.1159/000341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kan H., Hu W., Wang Y., Wu W., Yin Y., Liang Y., Wang C., Huang D., Li W. NADPH oxidase-derived production of reactive oxygen species is involved in learning and memory impairments in 16-month-old female rats. Mol. Med. Rep. 2015;12(3):4546–4553. doi: 10.3892/mmr.2015.3894. [DOI] [PubMed] [Google Scholar]
- 58.Zhang H., Sun X.R., Wang J., Zhang Z.Z., Zhao H.T., Li H.H., Ji M.H., Li K.Y., Yang J.J. Reactive oxygen species-mediated loss of phenotype of parvalbumin interneurons contributes to long-term cognitive impairments after repeated neonatal ketamine exposures. Neurotox. Res. 2016;30(4):593–605. doi: 10.1007/s12640-016-9653-1. [DOI] [PubMed] [Google Scholar]
- 59.Wu G., Li L., Li H.M., Zeng Y., Wu W.C. Electroacupuncture ameliorates spatial learning and memory impairment via attenuating NOX2-related oxidative stress in a rat model of Alzheimer's disease induced by Abeta1-42. Cell Mol. Biol. 2017;63(4):38–45. doi: 10.14715/cmb/2017.63.4.7. [DOI] [PubMed] [Google Scholar]
- 60.Fischer R., Maier O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxid. Med. Cell. Longev. 2015;2015:610813. doi: 10.1155/2015/610813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Edison P., Archer H.A., Gerhard A., Hinz R., Pavese N., Turkheimer F.E., Hammers A., Tai Y.F., Fox N., Kennedy A., Rossor M., Brooks D.J. Microglia, amyloid, and cognition in Alzheimer's disease: an [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol. Dis. 2008;32(3):412–419. doi: 10.1016/j.nbd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 62.Cervellati C., Cremonini E., Bosi C., Magon S., Zurlo A., Bergamini C.M., Zuliani G. Systemic oxidative stress in older patients with mild cognitive impairment or late onset Alzheimer's disease. Curr. Alzheimer Res. 2013;10(4):365–372. doi: 10.2174/1567205011310040003. [DOI] [PubMed] [Google Scholar]
- 63.von Bernhardi R., Eugenin-vonBernhardi L., Eugenin J. Microglial cell dysregulation in brain aging and neurodegeneration. Front. Aging Neurosci. 2015;7:124. doi: 10.3389/fnagi.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Z., Zhong C. Oxidative stress in Alzheimer's disease. Neurosci. Bull. 2014;30(2):271–281. doi: 10.1007/s12264-013-1423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kuszczyk M.A., Sadowski M.J., Antkiewicz-Michaluk L., Lazarewicz J.W. 1MeTIQ provides protection against Abeta-induced reduction of surface expression of synaptic proteins and inhibits H(2)O(2)-induced oxidative stress in primary hippocampal neurons. Neurotox. Res. 2014;25(4):348–357. doi: 10.1007/s12640-013-9440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M., Herrup K., Frautschy S.A., Finsen B., Brown G.C., Verkhratsky A., Yamanaka K., Koistinaho J., Latz E., Halle A., Petzold G.C., Town T., Morgan D., Shinohara M.L., Perry V.H., Holmes C., Bazan N.G., Brooks D.J., Hunot S., Joseph B., Deigendesch N., Garaschuk O., Boddeke E., Dinarello C.A., Breitner J.C., Cole G.M., Golenbock D.T., Kummer M.P. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tian R., Hou Z., Hao S., Wu W., Mao X., Tao X., Lu T., Liu B. Hydrogen-rich water attenuates brain damage and inflammation after traumatic brain injury in rats. Brain Res. 1637;2016:1–13. doi: 10.1016/j.brainres.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 68.Lambeth J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4(3):181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 69.Ma M.W., Wang J., Zhang Q., Wang R., Dhandapani K.M., Vadlamudi R.K., Brann D.W. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener. 2017;12(1):7. doi: 10.1186/s13024-017-0150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hernandes M.S., Britto L.R. NADPH oxidase and neurodegeneration. Curr. Neuropharmacol. 2012;10(4):321–327. doi: 10.2174/157015912804143540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Block M.L., Zecca L., Hong J.S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007;8(1):57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 72.Aldieri E., Riganti C., Polimeni M., Gazzano E., Lussiana C., Campia I., Ghigo D. Classical inhibitors of NOX NAD(P)H oxidases are not specific. Curr. Drug Metab. 2008;9(8):686–696. doi: 10.2174/138920008786049285. [DOI] [PubMed] [Google Scholar]
- 73.Pietersma A., de Jong N., de Wit L.E., Kraak-Slee R.G., Koster J.F., Sluiter W. Evidence against the involvement of multiple radical generating sites in the expression of the vascular cell adhesion molecule-1. Free Radic. Res. 1998;28(2):137–150. doi: 10.3109/10715769809065800. [DOI] [PubMed] [Google Scholar]
- 74.Barbieri S.S., Cavalca V., Eligini S., Brambilla M., Caiani A., Tremoli E., Colli S. Apocynin prevents cyclooxygenase 2 expression in human monocytes through NADPH oxidase and glutathione redox-dependent mechanisms. Free Radic. Biol. Med. 2004;37(2):156–165. doi: 10.1016/j.freeradbiomed.2004.04.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.