Abstract
Objective
Cerebellar ataxia essentially includes deficient postural control. It remains unclear whether augmented sensory information might help cerebellar patients, as the cerebellum underlies processing of various sensory modalities for postural control. Here, we hypothesized that patients with cerebellar degeneration can still exploit audio‐biofeedback (ABF) of trunk acceleration as a real‐time assistive signal to compensate for deficient postural control.
Methods
Effects on postural sway during stance were assessed in an ABF intervention group versus a no‐ABF disease control group (23 vs. 17 cerebellar patients) in a clinico‐experimental study. A single‐session ABF paradigm of standing plus short exergaming under ABF was applied. Postural sway with eyes open and eyes closed was quantified prior to ABF, under ABF, and post ABF.
Results
Postural sway in the eyes closed condition was significantly reduced under ABF. Both benefit of ABF and benefit of vision correlated with the extent of postural sway at baseline, and both types of sensory benefits correlated with each other. Patients with strongest postural sway exhibited reduced postural sway also with eyes open, thus benefitting from both vision and ABF. No changes were observed in the no‐ABF control group.
Interpretation
Our findings provide proof‐of‐principle evidence that subjects with cerebellar degeneration are still able to integrate additional sensory modalities to compensate for deficient postural control: They can use auditory cues functionally similar to vision in the absence of vision, and additive to vision in the presence of vision (in case of pronounced postural sway). These findings might inform future assistive strategies for cerebellar ataxia.
Introduction
The use of augmented sensory modalities (e.g., auditory, vibro‐tactile, or electro‐tactile/lingual) has been shown to reduce postural sway in stance and gait in subjects with balance deficits due to aging, vestibular loss, Parkinson's disease, or Progressive Supranuclear Palsy1, 2, 3, 4, 5, 6, 7, 8, 9 (for reviews, see 10, 11). However, such approaches have not yet been systematically tested in patients with cerebellar dysfunction, e.g. degenerative cerebellar ataxia. The effects of augmented sensory information on improving postural control here seem questionable, as the cerebellum underlies processing of various sensory modalities for postural control, including vestibular,12 proprioceptive,13 and visual sources14 (for review, see 15). Moreover, the cerebellum is involved in multimodal sensory integration, for example, to provide estimates of body movement based on proprioceptive or vestibular information.16, 17 Such multisensory representations together with motor efferences are suggested to form internal forward models within the cerebellum, predicting the outcome of motor actions and subserving the calibration of motor actions including postural responses18 and the adaptation to changing environments.19, 20
In accordance with these hypotheses on the functional role of the cerebellum in postural control, patients with cerebellar dysfunctions show substantially increased postural sway in different posture conditions like normal stance, and stance with narrow feet position or on soft ground,21, 22, 23, 24, 25 which becomes particularly pronounced with closed eyes.21, 26 At the same time, these observations also already indicate that cerebellar patients might still be able use information from one sensory modality – here: vision – to partly compensate for deficits in other sensory modalities.15, 26 Further hints for the hypothesis that reweighting of different sensory modalities might still be partly preserved in cerebellar patients comes from a psychophysics study on the estimation of hand positions which indicates that these patients might still be able to perform sensory realignment and short‐term reweighting.27
Based on these first hints, we here hypothesized that cerebellar patients can still exploit auditory biofeedback (ABF) signals of trunk acceleration as an assistive signal to compensate for their deficient processing of proprioceptive and vestibular signals in postural control. This finding would provide proof‐of‐principle evidence for the notion that ‐ despite progressive cerebellar damage ‐ the brain is still able to act according to the principles of cue integration and sensory reweighting, namely to improve postural control by adding/increasing the weight of one additional sensory cue (here: auditory signals) and change the relative weight of the remaining sensory modalities.28, 29, 30, 31
Methods
Patients
40 consecutive patients with degenerative cerebellar ataxia were recruited from the Ataxia Clinic of the Center for Neurology, Tübingen, Germany, from February 2014 until May 2016. Patients were included based on following g inclusion criteria: (1) progressive degenerative cerebellar ataxia in the absence of any signs of secondary CNS disease; (2) age between 18 and 75 years; (3) SARA (Scale for the Assessment and Rating of Ataxia) total score >3, but SARA gait and stance subscores each <4 (i.e., walking and standing possible without support),32 thus ensuring sufficient capacity to benefit, but also to complete the tasks. The exclusion criteria were: (1) clinical signs or mutations known to cause afferent ataxia (e.g., Friedreich's ataxia) (2) severe visual or hearing disturbances, cognitive impairment, predominant nonataxia movement disorders, or orthopedic constraints. The experimental procedure was approved by the local ethics committee. All subjects gave their informed consent prior to participation.
The intervention group receiving ABF comprised of a consecutive series of n = 23 subjects with cerebellar ataxia (=ABF group). To control for the effects seen in the ABF group, we subsequently recruited a consecutive series of n = 17 subjects with cerebellar ataxia (same inclusion criteria as for the intervention group) who performed the same tasks as the ABF group, but without auditory feedback in any of the conditions (= CON group) (for group characteristics, see Table 1; for detailed patient descriptions, see Data S1 Patients Description). This block assignment of two strictly consecutive series of cerebellar ataxia patients into the ABF and then the CON group was geared to reduce selection bias. Subjects who received ABF were also assessed by quantitative vibration testing by a Rydel‐Seiffer tuning fork to determine the degree of possible vibration sense impairments and their relation to ABF effects.
Table 1.
Characteristics of subject groups
| Group | Number of subjects | Gender F/M | Age, y | Disease Duration, y | SARA |
|---|---|---|---|---|---|
| ABF | 23 | 8/15 | 51.2 (14.5) | 13 (9.2) | 11 (3.1) |
| CON | 17 | 7/10 | 54.5 (11.5) | 9.4 (6.3) | 9.9 (3.3) |
Given are mean values and standard deviations. ABF and CON did not differ in age (P = 0.58), disease duration (P = 0.33), or SARA score value (P = 0.25). ABF, feedback intervention group; CON, cerebellar ataxia control group, controlling for the ABF group. SARA, Scale for the Assessment and Rating of Ataxia.
Study design overview
The study was designed as a clinico‐experimental study aiming to deliver proof‐of‐principle evidence that cerebellar patients are able to perform short‐term multisensory integration and profit from ABF as a real‐time assistive signal. We designed a single‐session ABF paradigm, which provided the subjects of the ABF group with acoustic feedback of trunk acceleration during consecutive stance and exergaming conditions, allowing to test for improvements in postural control assessed at stance conditions. Effects were tested both within the intervention group (pre‐post within‐group control design) as well as between the intervention group and the control group (between‐group control design).
Audio biofeedback device
We used a wearable ABF system as established previously.33 It consists of two main components: (1) an inertial sensor node capturing trunk accelerations based on a 3D‐accelerometer, ‐gyroscope, and ‐magnetometer, and (2) a smartphone‐based application receiving trunk acceleration information via Bluetooth™ 2.1 connection. Audio signals are delivered via headphones (see Fig. 1 and Data S2 for technical details). Before the intervention, subjects familiarized with the ABF signal for 2 min.
Figure 1.
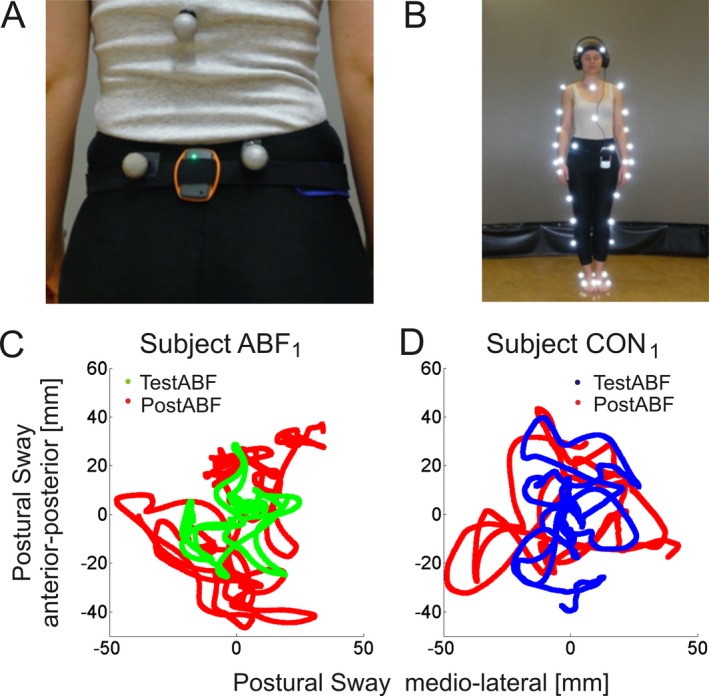
Experimental equipment for ABF (A + B). Subjects wore the sensor node (black sensor) mounted with a Velcro belt at L4/L5 (A). The sensor is linked to a smartphone tightly attached with the Velcro belt, which generated the ABF of sensor‐recorded trunk acceleration. The ABF is transmitted to the subject via headphones (B). In parallel, a VICON Motion Capture System was used to quantitatively assess trunk sway across the experimental trials, with reflective markers being attached to predefined body positions. Shown is an exemplary subject in stance position in the eyes closed condition. Postural sway in stance tasks (C+D). Shown are the paths of the centre of gravity (COG, projection of the center of mass on the floor) during stance tasks in anterior‐posterior and medio‐lateral direction from an exemplary subject of the ABF group (subject ABF 1, left) and of the CON group (subject CON 1, right). The ABF subject showed an improvement in postural sway with ABF in the TestABF phase (green) compared to the trial with no ABF in the PostABF phase (red), while the CON subject without ABF showed no difference in the corresponding trials.
Experimental Procedures
Subjects completed a sequence of quiet stance conditions, each of them lasting 30 seconds. During stance conditions subjects stood on a firm surface (=the floor) without footwear, arms loosely hanging down on the lateral sides of their body (Fig. 1). Feet were placed closely together. Two types of stance conditions were exploited: (1) standing with eyes open (EO) and (2) standing with eyes closed (EC).
The feedback intervention paradigm was structured into five consecutive phases: (1) PreABF, (2) Training I, (3) Training II, (4) TestABF, and (5) PostABF (for an overview of the experimental trial design, see Fig. 2). The stance conditions EO and EC were provided at the phases PreABF, Training I, TestABF, and PostABF. PreABF comprised of both stance conditions, each condition performed once, without ABF. These trials served to assess each subject's extent of trunk sway at baseline prior to training. In Training I, subjects completed each stance condition four times under the presence of ABF. The order of the two conditions was balanced between subjects, thus reducing possible order effects. Training II consisted of an exergaming period, exploring actively the ABF‐sensorimotor mapping. Here, subjects played a Microsoft Xbox Kinect® balance game (“Slip Slide”, Ice Age: Continental Drift, by Activision (R), Santa Monica, CA) for 10 min, controlling an avatar by quick eccentric trunk movements. This period of ABF served to provide subjects with the opportunity to exploit the acoustic signal during a full range of active trunk movements, facilitating the mapping of ABF signals to trunk movements. Such a period of active movements has been proposed to facilitate an auditory‐sensorimotor mapping processes compared to standing alone.34 TestABF was almost identical to Training I; that is, subjects performed both stance conditions with ABF, and in the same order as in Training I, but only two stance tasks per condition. TestABF served as the critical phase to test whether ABF has led to an effect on postural sway. PostABF comprised of both stance conditions, each performed once without ABF. This phase served as a within group control to rule out that possible effects seen in TestABF might just be due to unspecific effects, for example, due to prolonged standing, task repetition, or exergaming during the experimental phases.
Figure 2.
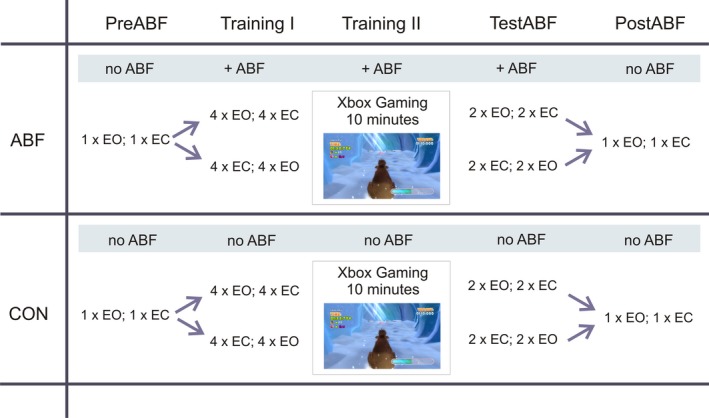
Experimental design: Combined between‐ and within‐group control design with five experimental phases. ABF: feedback intervention group; CON: control group. Between‐group control: Both groups executed the same protocol including stance trials as well as a 10 min exergame exploration period playing a postural controlled exergame. Only the ABF group received ABF (+ABF). The CON groups performed all the trials without ABF. Within‐group control: Effects of the ABF phases were also tested within the intervention group by comparing the TestABF phase with the PreABF as well as the PostABF phase. EO: stance task with eyes open; EC: stance task with eyes closed.
Quantitative movement analysis
Balance performance was evaluated by quantitative movement analysis using a VICON motion capture system (Oxford Metrics, UK). For a detailed description of the system, recording procedure, and analysis of stance, see 35, 36, 37. The extent of trunk sway was determined by the path length of the center of gravity (COG) during each stance trial in [mm/sec]. For exemplary subject results illustrating this measure, see Fig. 1C and D). For a comparison of this sway measure with the method of elliptical area fits38 see Data S4.
Statistical analysis
For comparison of both within‐group and between‐group differences in trunk sway, we pooled each (1) the trials 1–4 of Training I and (2) the two stance trials of TestABF to an average value. Averaging was performed for the EO and the EC condition separately. Before pooling, we controlled for significant differences within these trials using the nonparametric Friedman test (χ 2, P > 0.2) (for details of statistical methods and analyses without pooling see Data S3, confirming the results of the pooled data).
In order to examine whether in particular subjects with large body sway profit from ABF, we subclassified subjects according to their individual extent of postural sway at baseline. Subjects with an individual postural sway in the top tertile of the whole group in the EO condition at baseline, that is, with the highest postural sway (ABF>66% subgroup; postural sway >13.5 mm/sec, n = 8; CON>66% subgroup; postural sway >11.3 mm/sec, n = 6), were separated from subjects in the lower two tertiles, i.e. with less individual postural sway (ABF<66% subgroup n = 15, CON<66% subgroup n = 11, see Fig. 3H). Statistical analysis was performed using the software package MATLAB.
Figure 3.
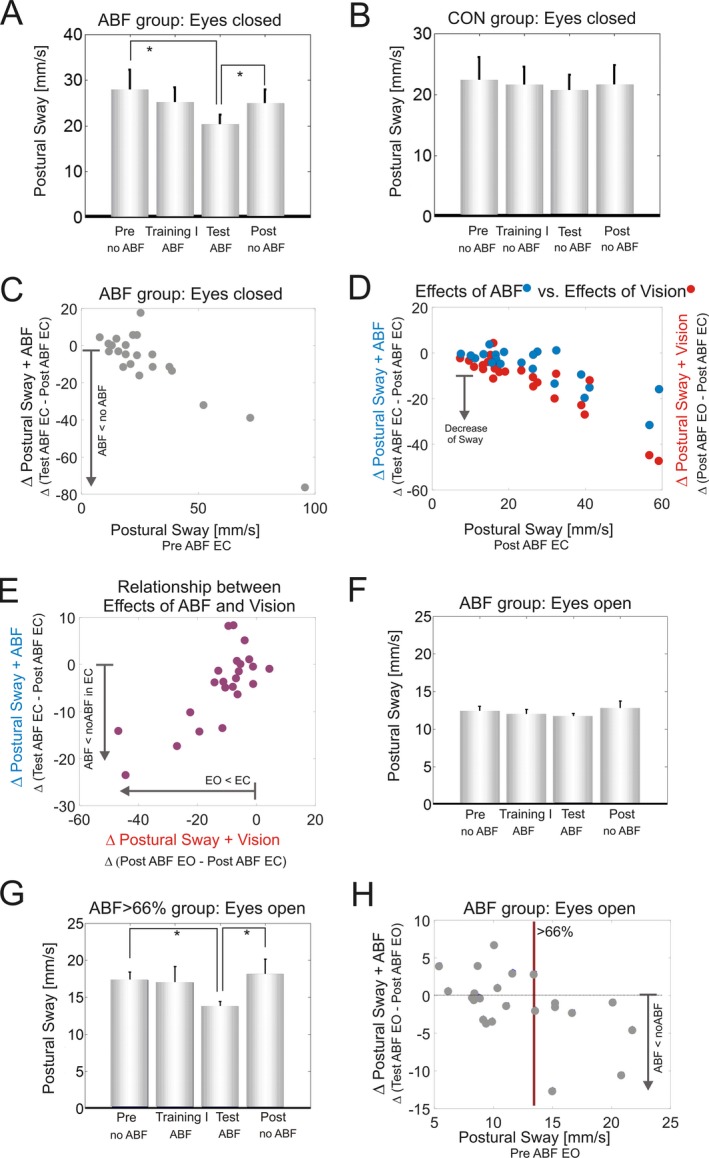
(A) Postural sway during Romberg stance in the ABF group in the eyes closed condition during the different experimental phases. The four bars indicate the consecutive experimental phases: PreABF, Training I, TestABF, and PostABF comparing trials with ABF (ABF) and without ABF (no ABF). (B) Postural sway during Romberg stance in the CON group in the eyes closed condition during the different experimental phases. (C) Relationship between baseline performance (x‐axis) and difference (Δ) of postural sway between the TestABF and the PostABF phase (y‐axis) for the ABF group in the eyes closed condition; (D) The effects of ABF in the closed eyes condition (in blue) compared to the effects of vision comparing the differences between conditions EO and EC (in red). (E) Difference in postural sway between eyes closed and eyes open without ABF (x‐axis) in relation to the improvement in postural sway under ABF (TestABF‐ PostABF) in the closed eye condition (y‐axis). Stars indicate significant differences (*P < 0.05) between different phases. (F) Postural sway during Romberg stance in the ABF group in the eyes open condition (EO); (G) Postural sway in the ABF >66% subgroup in the eyes open condition during the different experimental phases. (H) Relationship between the PreABF baseline performance (x‐axis) and difference (Δ) of postural sway between the TestABF and the PostABF phase (y‐axis) for the ABF group in the eyes open condition. The red vertical line demarcates the top tertile of postural sway at baseline (>13.5 mm/sec), categorizing a subgroup ABF >66% (n = 8) with increased postural sway.
Results
The disease control group CON did not differ from the ABF group in: (1) ataxia severity as determined by the SARA (ABF: 11 ± 3.13; CON: 9.85 ± 3.33; r = 0.22, P = 0.25), (2) age (ABF: 51.2 ± 14.5 years; CON: 54.5 ± 11.5 years; r = 0.11,P = 0.584), (3) disease duration (ABF: 12.7 ± 9.42 years; CON: 9.06 ± 6.33 years; r = 0.13, P = 0.328), or (4.) extent of postural sway at baseline in either of the two conditions (EC: ABF: 25.8 ± 20.8 mm/sec; CON: 22.2 ± 15.9 mm/sec; r = 0.24, P = 0.25; EO: ABF: 12.7 ± 5.5 mm/sec; CON: 11.1 ± 5.1 mm/sec; r = 0.22,P = 0.27). This demonstrates that the serial block assignment led to a good matching between the two groups.
Benefits of vision
Both groups ABF and CON revealed a significantly increased postural sway in the EC compared to the EO condition at baseline (PreABF; r > 0.87, P < 0.0001), at TestABF (r > 0.76, P < 0.0003) and at PostABF (r > 0.76, P < 0.0002), indicating a benefit of vision on postural control (Fig. 3A + F). In both groups, the amount of postural sway in the EC condition correlated with reduction of sway by vision in the EO condition: the larger the sway in EC, the larger the reduction of postural sway by visual information in EO (r > 0.59, P < 0.008, Fig. 3 D, red dots).
Benefits of ABF in the eyes closed condition
All subjects were able to complete the tasks and all subjects from the ABF group reported that interacting with the ABF system was well feasible. In the EC condition differences in postural sway for the ABF group were found across phases (Friedman‐test, X² = 79.6, P = 0.047, Fig. 3A). Post‐hoc analysis showed a significant reduction of postural sway in TestABF phase compared with PreABF phase (TestABF vs. PreABF: r = 041, P = 0.045). Comparison of Training I with PreABF, did not reveal any significant reduction in postural sway (Training I vs. PreABF: r = 0.12,P = 0.563), indicating that the Training I phase alone was not sufficient to yield a training effect.
After the exergaming period in Training phase II, comparison of phases with ABF (TestABF) versus without ABF (PostABF) revealed a significantly smaller postural sway in the ABF condition compared to the subsequent condition without ABF (TestABF vs. PostABF: r = 0.53, P = 0.011, Fig. 3A).
In contrast, the CON group did not show any differences in postural sway across stance phases for any of the two conditions (EO: X²=17.3, P = 0.63; EC: X²=21.5, P = 0.541, see Fig. 3B).
In the ABF group, the difference of postural sway between TestABF versus PostABF was highly correlated with the extent of postural sway at PreABF (r = 0.65, P = 0.001 see Fig. 3C). No such a correlation was observed in the CON group (r = 0.14, P = 0.65).
Comparing the effects of vision and acoustic feedback
We next analyzed the relationship between the effects of vision and of acoustic feedback. The effect of vision on postural control was determined by comparing PostABF EO versus PostABF EC; the effect of ABF by comparing TestABF versus PostABF in the EC condition. Both sensory modalities yielded a similar, functionally almost equivalent benefit on postural control, as shown by the large overlap in Figure 3D. This relationship was analyzed in more detail by a correlation analysis, confirming a positive correlation between adding vision and adding auditory feedback (Fig. 3E). That is, those subjects benefiting most from vision (i.e., with the most pronounced difference between eyes open vs. eyes closed) benefited to a similar extent from the ABF in the EC conditions (r = 0.53, P = 0.03). Neither baseline performance nor ABF or vision effects were related to tuning fork measures of vibration sense (see Data S1 and S5 for details).
Benefits of ABF in the eyes open condition
In the EO condition, subjects of the ABF group did not show a significant group difference in postural sway between trials with and without ABF (Friedman‐test, X²=12.8, P = 0.734, see Fig. 3F). However, again a significant correlation was observed between the extent of postural sway at baseline (PreABF) and the difference of postural sway between TestABF versus PostABF in the ABF group (r = 0.55, P = 0.007). This indicates that, also in the EO condition, subjects with more pronounced postural sway benefit from the augmented sensory signal. No such correlation was observed in the CON group (r = −0.17, P = 0.52).
To further explore this correlation we performed a subgroup analysis of the tertile of subjects with the most pronounced postural sway at baseline (ABF>66% subgroup, see Methods). This tertile showed a significant reduction in postural sway in the TestABF phase compared to both PreABF and PostABF (Friedman‐test, X²=6.75, P = 0.08, PreABF vs. TestABF: P = 0.023, TestABF vs. PostABF: P = 0.023, see Fig. 3G + H), indicating that these subjects profit from ABF also in the EO condition. No such change was seen in the CON group (neither overall CON group nor CON>66% subgroup, Friedman‐test, X²=0.2, P = 0.97).
Discussion
Here, we provide proof‐of‐principle evidence that cerebellar patients can still exploit augmented sensory information to partly compensate for their impairment in processing proprioceptive and vestibular signals in postural control. The reductions in postural sway were observed only in the ABF intervention group after ABF training, as shown by our combined between‐group and within‐group control design. This demonstrates that the improvements were induced by exploitation of the ABF and were not merely due to unspecific non‐ABF related factors, for example, exercise effects. Such a disease control group was missing in most other neurological conditions where bio‐feedback has been explored.1, 3, 6, 39, 40
ABF‐induced benefits are particularly pronounced in cerebellar patients with large postural sway
If it was indeed the deficient postural control which drives the integration of ABF, then in particular those patients with larger postural sway should show larger benefit by ABF. In line with this prediction, we observed that the larger the extent of body sway prior to ABF, the larger the ABF benefit (Fig. 3C). Such a correlation was seen not only in the EC condition (P = 0.001), but also in the EO condition (P = 0.007). Correspondingly, the subgroup of patients with the most pronounced sway showed a benefit of ABF on postural control also in the EO condition (P = 0.02, Fig. 3G).
In contrast, ABF might be of limited benefit for subjects with less postural sway with eyes open. If vision is available, these only mildly affected subjects do not need to rely on acoustic signals, but the use of visual signals suffices to maintain a sufficient level of postural stability.
Preserved sensory integration to compensate for deficient postural control: the use of vision and auditory feedback
The process of sensory reweighting in posture control has been characterized by changing the relative contribution of the sensory systems depending on their availability and reliability,28, 29, 41 thus allowing to constantly adjust sensory integration and subsequent postural control during the changing conditions of everyday living. According to this notion, those subjects who benefit most from vision for stabilizing postural control should rely most on the augmented sensory input (like auditory cues) ‐ when visual cues are less reliable or even absent.
Correspondingly, our results show a correlation between the benefit by ABF in EC and the benefit by vision in EO, and both types of benefits correlate with the amount of postural sway at baseline. This observation supports the hypothesis that, in the absence of vision, cerebellar patients can use auditory cues functionally similar to vision to compensate for deficient postural control. That is, the more severe the damage to processing of proprioceptive and vestibular signals, as indicated by an increased degree of postural sway, the more the patients integrate and reweight one of these two additional sensory modalities.
Thus, the correlation between the benefit of vision and auditory cues also indicates that a similar mechanism might underlie the integration of vision and auditory cues. This supports the hypothesis that indeed sensory reweighting might be the mechanism underlying the effects observed here (although other functional mechanisms might also add to the improvements observed here, e.g. cognitive alert mechanisms based on the auditory signal; for a discussion of sensor augmentation mechanisms see 10). Our results moreover show that cerebellar patients can use auditory cues and vision not only in substitution, but also in combination to yield a more stable postural control. In the EO condition, the ABF>66% subgroup showed a benefit from both types of sensory information, namely visual information (TestABF EO vs. TestABF EC:P = 0.03) and ABF (TestABF vs. PostABF in EO:P = 0.02, Fig. 3G). These results suggest that even these patients with pronounced impairments in postural control are capable to exploit the integration of both sensory signals.
These findings substantially extend the existing classical clinical observation that patients with sensory ataxia (e.g. Friedreich's Ataxia) ‐ and partly also with cerebellar ataxia ‐ profit from visual information in the Romberg test.21, 26, 42 Moreover, on the level of functional mechanisms, our results deliver additional pieces of evidence for the hypothesis that the process of sensory integration and reweighting is not necessarily dependent on the integrity of the cerebellum, thus corroborating findings from an earlier psychophysics study on the estimation of hand positions.27
Preserved sensory reweighting on a short time‐scale
Our protocol used a short‐term familiarization program of less than one hour, demonstrating that cerebellar patients are able to exploit sensory information and to perform sensor reweighting even on a rapid time scale. Such rapid reweighting might enable cerebellar patients to profit from ABF as a real‐time assistive signal in everyday life, for example, when walking in rooms with mixed light zones and poor visibility which is known to facilitate falls.43
Limitations of the Study
Our short‐term protocol does not allow to test for retention and carry‐over of effects after removing ABF as a potential rehabilitation device, which would require longer multisession protocols (e.g., see 8). In addition, although we used a short exergaming period for familiarization, the focus of this study was not to examine the facilitation of training effects by sensor augmentation (for review, see 44). These limitations point to interesting directions for further research.
Conclusion and outlook
Our findings provide proof‐of‐principle evidence that – despite intricate cerebellar damage – patients with degenerative cerebellar ataxia still have a preserved capacity to exploit ABF as a real‐time assistive signal to compensate for deficient postural control. In fact, they seem to be able to use auditory cues functionally similar to vision in the absence of vision, and additive to vision in case of pronounced postural sway. Future studies are warranted to transfer these proof‐of‐principle results to balance control also during walking and possibly also to other bio‐feedback signals being more suitable for daily application (e.g. vibro‐tactile feedback7 or bone conduction).
Finally, follow‐up studies testing the feasibility and effectiveness of sensory augmentation on walking and in longer clinical trials are required to confirm the clinical effectiveness of this translational work, ideally performed in a multicenter health‐care setting and utilizing additional patient reported and functional outcomes. These examinations might inform future assistive strategies for balance control in cerebellar patients.
Author Contributions
Mrs Fleszar: design and conceptualization of the study, acquisition of data, analysis of the data, drafting the manuscript. Dr. Mellone and Dr. Tacconi: design, development, and implementation of ABF application used in the study, interpretation of the data, revising the manuscript. Dr. Giese, Dr. Schöls and Dr. Becker: interpretation of data, revising the manuscript. Dr. Synofzik and Dr. Ilg: design, conceptualization and supervision of the study, acquisition of data, analysis of the data, drafting the manuscript.
Conflict of Interest
The authors declare that they have no competing interests.
Supporting information
Data S1. Detailed patient characteristics.
Data S2. Details of audio biofeedback device.
Data S3 Statistics.
Data S4 Details of movement analysis.
Data S5 Relationship of Vibration Sensing on posture control capabilities.
Acknowledgments
This study is funded by grants from the Forschungskolleg Geriatrie of the Robert‐Bosch‐Foundation Stuttgart, Germany (to M.S.) and the IZKF Promotionskolleg Tübingen (to Z.F.). Additional support has been provided by the project Cogimon EU ICT‐23‐2014.
Funding Information
This study is funded by grants from the Forschungskolleg Geriatrie of the Robert‐Bosch‐Foundation Stuttgart, Germany (to M.S.) and the IZKF Promotionskolleg Tübingen (to Z.F.) Mrs Fleszar and Dr. Synofzik received travel imbursements and honoraria, respectively, from Actelion Pharmaceuticals Ltd unrelated to this study. Dr. Giese, Dr Schöls, and Dr. Ilg report no disclosures. Dr. Carlo Tacconi is CEO of mHealth Technologies srl, Bologna, Italy. Dr. Sabato Mellone owns a share of mHealth Technologies srl, Bologna, Italy.
Funding Statement
This work was funded by Forschungskolleg Geriatrie grant ; IZKF Promotionskolleg Tübingen grant ; Actelion Pharmaceuticals Ltd grant .
Contributor Information
Matthis Synofzik, Email: matthis.synofzik@uni-tuebingen.de.
Winfried Ilg, Email: winfried.ilg@uni-tuebingen.de.
References
- 1. Dozza M, Chiari L, Horak FB. Audio‐biofeedback improves balance in patients with bilateral vestibular loss. Arch Phys Med Rehabil 2005;86:1401–1403. [DOI] [PubMed] [Google Scholar]
- 2. Nicolai S, Mirelman A, Herman T, et al. Improvement of balance after audio‐biofeedback. A 6‐week intervention study in patients with progressive supranuclear palsy. Z Gerontol Geriatr 2010;43:224–228. [DOI] [PubMed] [Google Scholar]
- 3. Mirelman A, Herman T, Nicolai S, et al. Audio‐biofeedback training for posture and balance in patients with Parkinson's disease. J Neuroeng Rehabil. 2011;8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dozza M, Chiari L, Horak FB. A portable audio‐biofeedback system to improve postural control. Conf Proc IEEE Eng Med Biol Soc 2004;7:4799–4802. [DOI] [PubMed] [Google Scholar]
- 5. Fleury A, Mourcou Q, Franco C, et al. Evaluation of a Smartphone‐based audio‐biofeedback system for improving balance in older adults–a pilot study. Conf Proc IEEE Eng Med Biol Soc 2013;2013:1198–1201. [DOI] [PubMed] [Google Scholar]
- 6. Franco C, Fleury A, Gumery PY, et al. iBalance‐ABF: a smartphone‐based audio‐biofeedback balance system. IEEE Trans Biomed Eng 2013;60:211–215. [DOI] [PubMed] [Google Scholar]
- 7. Horak FB, Dozza M, Peterka R, et al. Vibrotactile biofeedback improves tandem gait in patients with unilateral vestibular loss. Ann N Y Acad Sci 2009;1164:279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bao T, Carender WJ, Kinnaird C, et al. Effects of long‐term balance training with vibrotactile sensory augmentation among community‐dwelling healthy older adults: a randomized preliminary study. J Neuroeng Rehabil 2018;15:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sienko KH, Balkwill MD, Oddsson LI, Wall C III. The effect of vibrotactile feedback on postural sway during locomotor activities. J Neuroeng Rehabil 2013;10:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sienko KH, Whitney SL, Carender WJ, Wall C. The role of sensory augmentation for people with vestibular deficits: Real‐time balance aid and/or rehabilitation device? J Vestib Res 2017;27:63–76. [DOI] [PubMed] [Google Scholar]
- 11. Ma CZ, Wong DW, Lam WK, et al. Balance improvement effects of biofeedback systems with state‐of‐the‐art wearable sensors: a systematic review. Sensors 2016;16:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barmack NH. Central vestibular system: vestibular nuclei and posterior cerebellum. Brain Res Bull 2003;60:511–541. [DOI] [PubMed] [Google Scholar]
- 13. MacKay WA, Murphy JT. Cerebellar modulation of reflex gain. Prog Neurobiol 1979;13:361–417. [DOI] [PubMed] [Google Scholar]
- 14. Stein JF, Glickstein M. Role of the cerebellum in visual guidance of movement. Physiol Rev 1992;72:967–1017. [DOI] [PubMed] [Google Scholar]
- 15. Bunn LM, Marsden JF, Voyce DC, et al. Sensorimotor processing for balance in spinocerebellar ataxia type 6. Mov Disord 2015;30:1259–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brooks JX, Cullen KE. Multimodal integration in rostral fastigial nucleus provides an estimate of body movement. J Neurosci 2009;29:10499–10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brooks V, Thach WT. Cerebellar control of posture and movement In: Brooks V, ed. Handbook of physiology: motor control. Washington DC: American Physiology Society, 1981:877–946. [Google Scholar]
- 18. Macpherson JM, Horak F. PostureIn: ER Kandel, J Schwartz, TM Jessell, SA Siegelbaum, AJ Hudspeth, eds. Principles of neural science. pp. 935–958. 5th ed. New York: Elsevier, 2014. [Google Scholar]
- 19. Synofzik M, Lindner A, Thier P. The cerebellum updates predictions about the visual consequences of one's behavior. Curr Biol 2008;18:814–818. [DOI] [PubMed] [Google Scholar]
- 20. Therrien AS, Bastian AJ. Cerebellar damage impairs internal predictions for sensory and motor function. Curr Opin Neurobiol 2015;33:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diener HC, Dichgans J, Bacher M, Gompf B. Quantification of postural sway in normals and patients with cerebellar diseases. Electroencephalogr Clin Neurophysiol 1984;57:134–142. [DOI] [PubMed] [Google Scholar]
- 22. Van de Warrenburg BP, Bakker M, Kremer BP, et al. Trunk sway in patients with spinocerebellar ataxia. Mov Disord 2005;20:1006–1013. [DOI] [PubMed] [Google Scholar]
- 23. Brandt T, Dietrich M. Postural Imbalance in peripheral and central vestibular disorders In: Bronstein AM, Brandt T, Woollacott MH, Nutt JG, eds. Clinical disorders of balance, posture and gait, 2nd ed London: Arnold, 2004:147–162. [Google Scholar]
- 24. Horak FB, Diener HC. Cerebellar control of postural scaling and central set in stance. J Neurophysiol 1994;72:479–493. [DOI] [PubMed] [Google Scholar]
- 25. Bunn LM, Marsden JF, Giunti P, Day BL. Stance instability in spinocerebellar ataxia type 6. Mov Disord 2013;28:510–516. [DOI] [PubMed] [Google Scholar]
- 26. Bronstein AM, Hood JD, Gresty MA, Panagi C. Visual control of balance in cerebellar and parkinsonian syndromes. Brain 1990;113:767–779. [DOI] [PubMed] [Google Scholar]
- 27. Block HJ, Bastian AJ. Sensory weighting and realignment: independent compensatory processes. J Neurophysiol 2011;106:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Asslander L, Peterka RJ. Sensory reweighting dynamics following removal and addition of visual and proprioceptive cues. J Neurophysiol 2016;116:272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shumway‐Cook A, Woollacott MH. Motor control ‐ translating research into clinical practice 5th ed. p. 157. Philadelphia, PA: Lippincott Williams & Wilkins, 2017. [Google Scholar]
- 30. Synofzik M, Vosgerau G, Lindner A. Me or not me‐‐an optimal integration of agency cues? Conscious Cogn 2009;18:1065–1068. [DOI] [PubMed] [Google Scholar]
- 31. Synofzik M, Vosgerau G, Voss M. The experience of agency: an interplay between prediction and postdiction. Front Psychol 2013;4:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schmitz‐Hubsch T, du Montcel ST, Baliko L, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology 2006;66:1717–1720. [DOI] [PubMed] [Google Scholar]
- 33. Chiari L, Dozza M, Cappello A, et al. Audio‐biofeedback for balance improvement: an accelerometry‐based system. IEEE Trans Biomed Eng 2005;52:2108–2111. [DOI] [PubMed] [Google Scholar]
- 34. Dyer JF, Stapleton P, Rodger M. Mapping Sonification for Perception and Action in Motor Skill Learning. Front Neurosci 2017;11:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ilg W, Schatton C, Schicks J, et al. Video game‐based coordinative training improves ataxia in children with degenerative ataxia. Neurology 2012;79:2056–2060. [DOI] [PubMed] [Google Scholar]
- 36. Ilg W, Synofzik M, Brotz D, et al. Intensive coordinative training improves motor performance in degenerative cerebellar disease. Neurology 2009;1:1823–1830. [DOI] [PubMed] [Google Scholar]
- 37. Ilg W, Fleszar Z, Schatton C, et al. Individual changes in preclinical spinocerebellar ataxia identified via increased motor complexity. Mov Disord 2016;31:1891–1900. [DOI] [PubMed] [Google Scholar]
- 38. Schubert P, Kirchner M. Ellipse area calculations and their applicability in posturography. Gait Posture 2014;39:518–522. [DOI] [PubMed] [Google Scholar]
- 39. Dozza M, Horak FB, Chiari L. Auditory biofeedback substitutes for loss of sensory information in maintaining stance. Exp Brain Res 2007;178:37–48. [DOI] [PubMed] [Google Scholar]
- 40. Cakrt O, Vyhnalek M, Slaby K, et al. Balance rehabilitation therapy by tongue electrotactile biofeedback in patients with degenerative cerebellar disease. NeuroRehabilitation 2012;31:429–434. [DOI] [PubMed] [Google Scholar]
- 41. Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol 2002;88:1097–1118. [DOI] [PubMed] [Google Scholar]
- 42. Ilg W, Branscheidt M, Butala A, et al. Consensus paper: neurophysiological assessments of ataxias in daily practice. Cerebellum 2018;17:628–653. [DOI] [PubMed] [Google Scholar]
- 43. Schlick C, Rasoul A, Wuehr M, et al. Gait variability predicts a subset of falls in cerebellar gait disorders. J Neurol 2017;264:2322–2324. [DOI] [PubMed] [Google Scholar]
- 44. Gordt K, Gerhardy T, Najafi B, Schwenk M. Effects of wearable sensor‐based balance and gait training on balance, gait, and functional performance in healthy and patient populations: a systematic review and meta‐analysis of randomized controlled trials. Gerontology 2018;64:74–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Detailed patient characteristics.
Data S2. Details of audio biofeedback device.
Data S3 Statistics.
Data S4 Details of movement analysis.
Data S5 Relationship of Vibration Sensing on posture control capabilities.


