Abstract
Dyshormonogenic congenital hypothyroidism (CH) generally results from biallelic defects in thyroid hormone synthesis genes. Whole exome sequencing allows easier identification of multiple gene defects. Two Sudanese families with CH resulting from oligogenic defects identified by whole exome sequencing are presented. In family 1, the proposita with CH and goiter was heterozygous for three TPO, one TG, and one DUOX2 mutations, including three novel variants inherited from both parents. In family 2, two brothers with psychomotor delay and goiter were homozygous for digenic mutations in the DUOX2 and DUOX1 genes, while their asymptomatic parents were heterozygous. Accumulation of pathogenic mutations may contribute to CH.
Keywords: TPO gene, TG gene, DUOX2 gene, DUOX1 gene, oligogenic mutations, congenital hypothyroidism
Introduction
Until recently, the cause of dyshormonogenic congenital hypothyroidism (CH) was identified in individuals with biallelic mutations in single genes (1). Rarely, CH has been explained by digenic or oligogenic mutations independent of whole exome sequencing (WES) (2–4). Two Sudanese families with oligogenic variants resulting in CH are reported.
Patients
Family 1
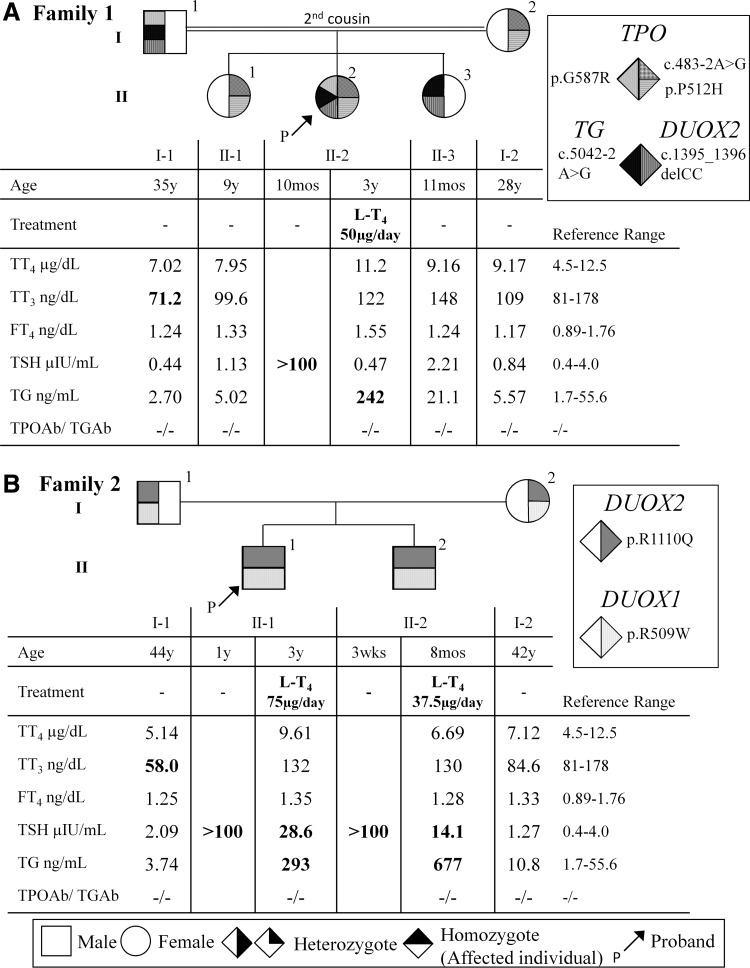
The proposita was a west Sudanese three-year-old female who presented with goiter and hypothyroidism (thyrotropin [TSH] >100 μIU/mL) at the age of 10 months. There is no neonatal screening for CH in the Sudan. She was started on levothyroxine (LT4), producing normal growth and development. There was no family history of thyroid disease, but her parents were second cousins. Thyroid function tests (TFTs) were performed using Immulite 1000® (Siemens, Munich, Germany). LT4 treatment normalized the TSH, but thyroglobulin (Tg) was high. Other family members had normal TFTs, except the father who had slightly low total triiodothyronine (T3; Fig. 1A).
FIG. 1.
Pedigree, thyroid function tests results, and mutations identified in family 1 (A) and family 2 (B). Generations are indicated with Roman numerals, and individuals with Arabic numbers above each symbol. Laboratory data are aligned below each symbol. Vertical shading of symbols indicates heterozygous for the mutation and horizontal shading of the symbols indicates homozygosity for the mutation. Abnormal values are in bold type. LT4, levothyroxine; TT4, total thyroxine; TT3, total triiodothyronine; fT4, free thyroxine; TSH, thyrotropin; Tg, thyroglobulin; TPOAb, thyroid peroxidase antibody; TgAb; thyroglobulin antibody. International System of Units: TT4, μg/dL = 12.87 nmol/L; TT3, ng/dL = 0.0154 nmol/L; fT4, ng/dL = 12.87 pmol/L; TBG, μg/mL = 0.0185 μmol/L.
Family 2
A north Sudanese three-year-old male was diagnosed with CH at one year of age when presenting with goiter and mild psychomotor delay. He had a serum TSH of >100 μIU/mL and a total thyroxine (TT4) of 7.3 nmol/L (reference range 66–181 nmol/L). Due to this history, a younger brother had TFTs evaluated at three weeks of age, which showed a serum TSH >100 μIU/mL and a free T4 of 2.46 pmol/L (reference range 12–22 pmol/L). Both brothers were placed on LT4. Their reportedly non-consanguineous parents had no history of thyroid disease. The affected siblings had high TSH and Tg on an inadequate dose of LT4. The father had low total T3 levels (Fig. 1B).
Molecular genetics
Studies were approved by The Human Subject Research Office of The University of Miami. Written informed consent was obtained from all adult subjects and parents of the children. WES was performed on each proband and mother. The data were analyzed for autosomal recessive inheritance pattern. Fifty-three genes related to thyroid disorders were evaluated (Supplementary Table S1). The proposita of family 1 had the following heterozygous abnormalities: (i) three mutations in the TPO gene, one splicing variant c.483-2A>G, and two missense mutations c.1535C>A, p.P512H and c.1759G>A, p.G587R; (ii) a splicing mutation, c.5042-2A>G, in the TG gene; and (iii) a frameshift deletion, c.1395_1396delCC, in the DUOX2 gene. Sanger sequencing confirmed inheritance of TPO G587R, TG, and DUOX2 gene mutations from her father, and TPO P512H and c.483-2A>G from the mother (Fig. 1A). The missense variants are predicted by an in silico algorithm to be deleterious (Supplementary Table S2). Protein modeling in silico was performed and all reported mutations result in significant protein conformational changes (Supplementary Fig. S1). They were previously reported in The Genome Aggregation Database (gnomAD). However, the clinical significance was unknown. The splicing and frameshift variants were not present in gnomAD, dbSNP, and the Exome Variant Server. Both splicing variants located in the acceptor splice site are predicted to affect splicing (Supplementary Tables S2–S4). The DUOX2 frameshift variant results in a premature stop at amino acid 514 (p.Q466fsX48).
WES of the propositus of family 2 identified a biallelic missense mutation resulting in DUOX2 c.3329G>A, p.R1110Q, inherited digenically with a homozygous missense mutation resulting in DUOX1 c.1525C>T, p.R509W. Sanger sequencing confirmed that affected siblings were homozygous, while their parents were heterozygous (Fig. 1B). The DUOX2 variant is a known pathogenic mutation producing CH (5). The DUOX1 mutation was reported in the gnomAD and predicted to be deleterious (Supplementary Table S2), but its clinical significance was unreported.
Discussion
The proposita of family 1 harbors three TPO and one mutation in each TG and DUOX2 genes. Interestingly, no hypothyroidism was found in other family members with two or three variants. This case supports that sporadic CH may result from combination of multiple gene mutations (2–4). Dissecting the contribution of each mutation to the phenotype would require complex functional studies. Regarding findings in family 2 with severe CH, digenic DUOX2 and DUOX1 mutations have been reported recently (6). A case from Japan with DUOX2 R1110Q had mild hypothyroidism (5) compared to the CH of the present case. However, DUOX1 mutations and environmental factors such as iodine intake that might contribute to clinical differences were not assessed. Even though expressed at a lower level (1), DUOX1 likely compensates for DUOX2 deficiency affecting the disease severity (6).
In conclusion, the accumulation of pathogenic mutations in several genes may contribute to the pathophysiology of CH. WES allows the identification of defects in multiple genes that, in combination, may be responsible for congenital dyshormonogenesis previously believed to be single gene recessive defects. Thus, digenic or oligogenic mutations may result in CH, a mechanism diverging from a simple autosomal recessive model.
Supplementary Material
Acknowledgments
This research was supported by funds from the Esformes Thyroid Research Fund, Estelle Rosenfield Family Thyroid Fund, and National Institutes of Health grant MD 010722 to R.W., DK 15070 to S.R., and DK 110322 to A.D.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Grasberger H, Refetoff S. 2011. Genetic causes of congenital hypothyroidism due to dyshormonogenesis. Curr Opin Pediatr 23:421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Filippis T, Gelmini G, Paraboschi E, Vigone MC, Di Frenna M, Marelli F, Bonomi M, Cassio A, Larizza D, Moro M, Radetti G, Salerno M, Ardissino D, Weber G, Gentilini D, Guizzardi F, Duga S, Persani L. 2017. A frequent oligogenic involvement in congenital hypothyroidism. Hum Mol Genet 26:2507–2514 [DOI] [PubMed] [Google Scholar]
- 3.Magne F, Ge B, Larrivee-Vanier S, Van Vliet G, Samuels ME, Pastinen T, Deladoey J. 2016. Demonstration of autosomal monoallelic expression in thyroid tissue assessed by whole-exome and bulk RNA sequencing. Thyroid 26:852–859 [DOI] [PubMed] [Google Scholar]
- 4.Pfarr N, Borck G, Turk A, Napiontek U, Keilmann A, Muller-Forell W, Kopp P, Pohlenz J. 2006. Goitrous congenital hypothyroidism and hearing impairment associated with mutations in the TPO and SLC26A4/PDS genes. J Clin Endocrinol Metab 91:2678–2681 [DOI] [PubMed] [Google Scholar]
- 5.Ohye H, Fukata S, Hishinuma A, Kudo T, Nishihara E, Ito M, Kubota S, Amino N, Ieiri T, Kuma K, Miyauchi A. 2008. A novel homozygous missense mutation of the dual oxidase 2 (DUOX2) gene in an adult patient with large goiter. Thyroid 18:561–566 [DOI] [PubMed] [Google Scholar]
- 6.Aycan Z, Cangul H, Muzza M, Bas VN, Fugazzola L, Chatterjee VK, Persani L, Schoenmakers N. 2017. Digenic DUOX1 and DUOX2 mutations in cases with congenital hypothyroidism. J Clin Endocrinol Metab 102:3085–3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.