Abstract
Ultrasound, which refers to frequencies above the audible limit of human hearing, is a candidate for inducing resistance to pathogens in plants. We revealed that aerial ultrasound of 40.5 kHz could induce disease resistance in tomatoes and rice when the plants were irradiated with ultrasound of ca. 100 dB for 2 weeks during nursery season and reduced the incidence of Fusarium wilt and blast diseases, respectively, when plants were inoculated with pathogen 0 or 1 week after terminating irradiation. Disease control efficacy was also observed with ultrasound at frequencies of 19.8 and 28.9 kHz. However, cabbage yellows and powdery mildew on lettuce were not suppressed by ultrasound irradiation. No significant positive or negative effect on growth was observed in tomato and rice plants. RT-qPCR showed that the expression of PR1a involved in the salicylic acid (SA) signaling pathway was upregulated in the ultrasound-irradiated tomato.
Keywords: ultrasound, physical control, SA signaling pathway, soilborne fusarium disease, blast, powdery mildew
Introduction
Plants are continuously challenged by a wide spectrum of environmental stimuli. These include extreme temperature, drought, wind, vibration, pests, and microbes. Plants can appropriately recognize these stimuli and respond to them.
Plants usually protect themselves from microbes by activating defense reactions after recognizing microbial stimuli. However, some microbes, designated pathogens, are able to weaken or invalidate the defense to invade plants. Therefore, activating the defense prior to the approach of pathogenic microbes seems to be a good way to control the diseases caused by the pathogens.
Interestingly, plants’ response pathways to abiotic and biotic stimuli partly overlap.1) Thus, when plants are exposed to abiotic stimuli, such as heat, wind, drought, or vibration, physiological changes caused by biotic stimuli can occur by chance.2) For example, chemical stimuli, such as probenazole (PBZ), validamycin A (VMA), acibenzolar-S-methyl (ASM), tiadinil (TDL), isotianil, and fosetyl, have been used as plant activators that do not have direct antipathogen activity but induce disease resistance in plants. All of these plant activators are reported to induce systemic acquired resistance (SAR) mediated by the salicylic acid (SA) signaling pathway (Fig. S1).3,4)
Light, one mechanical stimulus, is used to induce disease resistance in plants. For example, the irradiation of plants with a green LED of ca. 550 nm has been put to practical use under the name of “Midorikikuzou” (Shikoku Research Institute), which can suppress strawberry (Fragaria×ananassa) anthracnose caused by Colletotrichum acutatum.
There are several reports on using sound vibration, another mechanical stimulus, to generate physiological change in plants. For instance, “green music” which comprises natural sounds, such as songs of birds and insects, increased the seed germination rate in zucchini (Cucurbita pepo) and okra (Abelmoschus esculentus)5) and enhanced the uptake of polyamine and oxygen in Chinese cabbage (Brassica campestris) and cucumber (Cucumis sativus).6) Root elongation toward the 220 Hz sound source was observed in corn (Zea mays).7) Furthermore, indole acetic acid accumulated in Chrysanthemum spp. callus when irradiated with 1.4 kHz sound, while abscisic acid decreased.8) Recently, Choi et al. (2017)9) reported that 10 days (3 hr/day) of irradiation with 1 kHz sound primed SAR and increased resistance to Botrytis cinerea infection in Arabidopsis thaliana. However, few reports have described the induction of resistance to diseases, especially in edible plants by sound irradiation.
Despite a number of studies on ultrasound,10–13) no report has examined the physiological effects of ultrasound on plants. Ultrasound waves have frequencies higher than the upper audible limit (usually ca. 20 kHz) of human hearing. In this report, we found that ultrasound induces plant immunity against pathogens.
Materials and Methods
1. Plant materials and growth conditions
Tomato (Solanum lycopersicum cvs. Momotaro, Takii & Co., and Moneymaker, Baker Creek Heirloom Seeds), cabbage (Brassica oleracea var. capitata cv. Shikidori, Takii & Co.), and rice (Oryza sativa cvs. Aichi-asahi and Kinuhikari) were used. Plants were grown in a greenhouse (20–35°C) or in a growth chamber (25°C, light : dark=16 hr : 8 hr) in sterilized soil (JA Nippi Engei Baido, Nihon Hiryo).
2. Aerial ultrasound
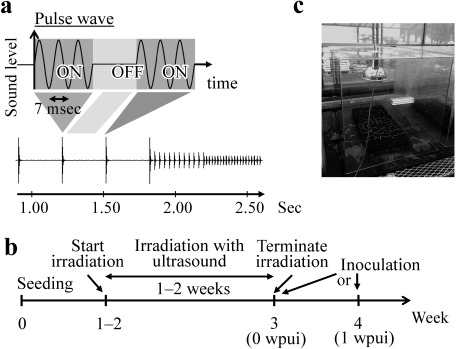
An aerial ultrasound oscillator with a frequency of 40.5 kHz was developed and used. The oscillation pattern was composed of intermittent pulse waves (Fig. 1a). The pulse width is 7 msec, and the pulse frequency repeatedly shifts. Pulse waves with the higher pulse frequency were oscillated with the lower sound pressure level. The sound pressure level inside a circle 50 cm in diameter was maintained at over 100 dB at a distance of 70 cm from the oscillator. The oscillator was set over the plants at a distance of 70 cm, and plants were irradiated with aerial ultrasound starting for 1- or 2-week-old plants and continuing for 1–2 weeks (24 hr a day), as shown in Fig. 1b, in a soundproof area covered with acrylic boards or PVC (polyvinyl chloride) sheets (Fig. 1c). Oscillators of 19.8 and 28.9 kHz ultrasound were also prepared to study the difference in effect depending on the frequencies.
Fig. 1. Aerial ultrasonic conditions and an experimental flow. Seedlings of each plant were irradiated with ultrasound of 40.5 kHz frequency with ca. 100 dB sound pressure level. The oscillation pattern was composed of intermittent pulse waves. The pulse width is 7 msec, and the pulse frequency repeatedly shifts. The intermittent pulse waves are illustrated (a). Irradiation with ultrasound started when plants were 1 week old, continued for 1–2 weeks (24 hr a day), and plants were inoculated with pathogens at 0 or 1 week after terminating ultrasound irradiation (b). Tomato seedlings irradiated with aerial ultrasound in a limited irradiation area (50 cm diameter at 70 cm from the oscillator) in a sound-proof area (c).
3. Fungal isolates, cultural conditions, and inoculation testing
Inoculation with pathogens was done 0 or 1 week after the termination of ultrasound irradiation.
The tomato wilt fungus Fusarium oxysporum f. sp. lycopersici JCM 12575 (Fol) and the cabbage yellows fungus F. oxysporum f. sp. conglutinans Cong:1-1 (Foc) were cultured for 3–5 days on a rotary shaker at 120 rpm and 25°C in potato sucrose broth (PSB) medium. Bud cells were collected by centrifugation (1630×g, 10 min), suspended (1.0×107 bud cells/mL for Fol or 1.0×105 bud cells/mL for Foc) in sterilized distilled water, and used as inocula. Each inoculum of Fol or Foc was poured (1 mL/100 g soil) into the soil for inoculation. To promote pathogen infection, plant roots were injured beforehand by inserting a plastic peg 5 times/plant into the soil. The severity of the disease of each tomato or cabbage plant was evaluated approximately 40 or 14 days after inoculation with Fol or Foc, respectively. Each plant’s symptoms were indexed following the precedent of Inami et al. (2014)14) or Kashiwa et al. (2013)15): 0, no symptoms; 1, lower leaves yellowing; 2, lower and upper leaves yellowing; 3, lower leaves yellowing and wilting, and upper leaves yellowing; 4, all leaves wilting and yellowing, or dead.
The tomato powdery mildew fungus, Oidium sp., was maintained on tomato plants in a greenhouse. Tomato plants presenting signs of powdery mildew were used as an inoculum by placing among the tested tomato seedlings. Two weeks after starting inoculation, the disease incidence of each plant was evaluated as ranging from 0 to 4, following the indexes: 0, no symptoms; 1, a foliar area of 0–5% indicating powdery mildew symptoms; 2, 6–30%; 3, 31–60%; and 4, 61–100%.
The rice blast fungus, Pyricularia oryzae Hoku 1 (Po), was cultured for 14 days at 25°C on an oatmeal agar medium plate. Aerial hyphae were removed by scraping with an autoclaved spatula, and the plate was irradiated with blue light of a blacklight (368 nm; FL20S·BL-B, Hitachi) for 3 days to stimulate conidial formation. The formed conidia were recovered using an autoclaved spatula and sterilized distilled water, and the conidial suspension was adjusted to 2.0×104 conidia/mL and used as an inoculum. The conidial suspension was sprayed onto rice leaves (ca. 0.2 mL/plant) using airbrush (PC-308, Olympos). After inoculation, plants were maintained in a humidified growth chamber at 27°C and 100% humidity in the dark for 24 hr and kept in a 25–28°C greenhouse for a week to promote infection and symptom development. The number of lesions on the upper three leaves of each rice plant was counted 1 week after inoculation with Po.16)
All statistical analyses were performed with R and EZR softwares.17)
4. RNA extraction and quantitative real-time PCR (RT-qPCR)
After the termination of 2 weeks of irradiation with ultrasound (40.5 kHz, ca. 100 dB and intermittent pulse wave), tomato (cv. Moneymaker) plants were inoculated with Fol as mentioned above. Total RNA was extracted from tomato leaves, stems, and roots, separately, at 0 and 24 hr post inoculation (hpi) using Trizol reagent in accordance with the manufacturer’s instructions (Invitrogen). Total RNA was treated with RQ1 DNase (Promega), and cDNA was generated using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). RT-qPCR was performed using GoTaq qPCR Master Mix (Promega) and Thermal Cycler Dice Real Time System II MRX [TP960] (Takara Bio Inc.). The relative expression levels of mRNA were determined by normalizing the PCR threshold cycle number of each gene with that of the Actin2 (Solyc11g005330) reference gene as the standard. Two or three biological replicates were used in each treatment. The primers used for RT-qPCR are shown in Table 1.18,19)
Table 1. List of primers used for RT-qPCR.
| Target gene | Accession number | Forward primer 5′→3′ | Reverse primer 5′→3′ | Amplification size | Reference |
|---|---|---|---|---|---|
| PAL1 | M83314 | TCCAGTGACTAACCATGTCCAAAG | AAGAGCCACGAGATAGGTTGATG | 136 bp | This study |
| NIM1 | AY640378 | GCTCCAGGCGGTAAGGAAA | GGCATCAAAACTCACCTCATACTC | 147 bp | This study |
| PR1a | M69247 | AGACTATCTTGCGGTTCACAACG | TCCCCAGCACCAGAATGAA | 144 bp | This study |
| LoxD | U37840 | GACTGGTCCAAGTTCACGATCC | ATGTGCTGCCAATATAAATGGTTCC | 178 bp | Uppalapati et al. 200518) |
| Actin2 | Solyc11g005330 | TTGCTGACCGTATGAGGAAG | GGACAATGGATGGACCAGAC | 186 bp | Du et al. 201719) |
* Functions of each gene are shown in Fig. S1.
Results and Discussion
1. Wilt was suppressed in tomato plants irradiated with 40.5 kHz aerial ultrasound before inoculation
We examined whether aerial ultrasound irradiation induces disease resistance in tomatoes and suppresses tomato wilt caused by Fol. We irradiated tomato seedlings with aerial ultrasound (40.5 kHz, ca. 100 dB, intermittent pulses; Fig. 1a) for 2 weeks before inoculation. As the ultrasound irradiation was terminated prior to inoculation, the pathogen was not directly affected by the ultrasound.
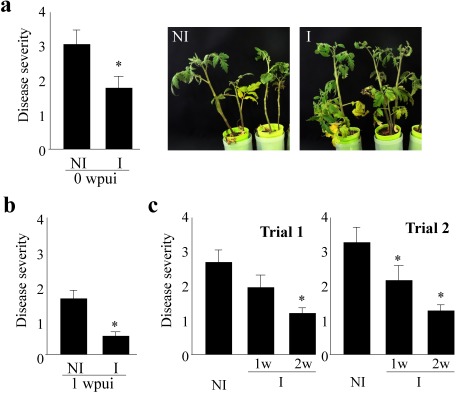
Wilt was significantly suppressed in tomato plants irradiated for 2 weeks with ultrasound as compared to non-irradiated plants when they were inoculated with Fol just after the termination of ultrasound irradiation (0 weeks after the termination of ultrasound irradiation, 0 wpui; Fig. 2a). The disease was also suppressed in tomato plants irradiated for 2 weeks with ultrasound when they were inoculated with Fol 1 week after the termination of ultrasound irradiation (1 wpui; Fig. 2b). As the disease prevention effect of ultrasound irradiation lasted longer than 1 week after irradiation was terminated, the ultrasound irradiation seemed to induce disease resistance in tomatoes.
Fig. 2. Wilt was suppressed in tomato plants irradiated by aerial ultrasound before inoculation. One-week-old tomato seedlings were irradiated with 40.5 kHz ultrasound for 1 or 2 weeks (I) or without irradiation (NI) and inoculated with the tomato wilt pathogen F. oxysporum f. sp. lycopersici (Fol) 0 or 1 week after terminating ultrasound irradiation. About 40 days after inoculation, the severity of each plant’s disease was evaluated from 0 to 4 following the indexes: 0, no symptoms; 1, lower leaves yellowing; 2, lower and upper leaves yellowing; 3, lower leaves yellowing and wilting and upper leaves yellowing; 4, all leaves wilting and yellowing or dead. Two-week ultrasound-irradiated tomato plants (cv. Moneymaker) presented significant wilt suppression when they were inoculated with Fol just after the termination of ultrasound irradiation (0 wpui) (a). Two-week ultrasound-irradiated tomato plants (cv. Moneymaker) presented wilt suppression when they were inoculated with Fol 1 week after the termination of ultrasound irradiation (1 wpui) (b). One week (1w) of irradiation also conferred a wilt control effect in cv. Moneymaker when plants were inoculated with Fol 1 week after the termination of ultrasound irradiation, but the effect of 2-week (2w) irradiation was higher (c). The severity of disease was analyzed with R, Wilcoxon rank sum test. p<0.05, bars=standard error, Asterisk means significance as compared to NI.
One-week irradiation also conferred a wilt control effect, but 2-week irradiation conferred a bigger effect (Fig. 2c).
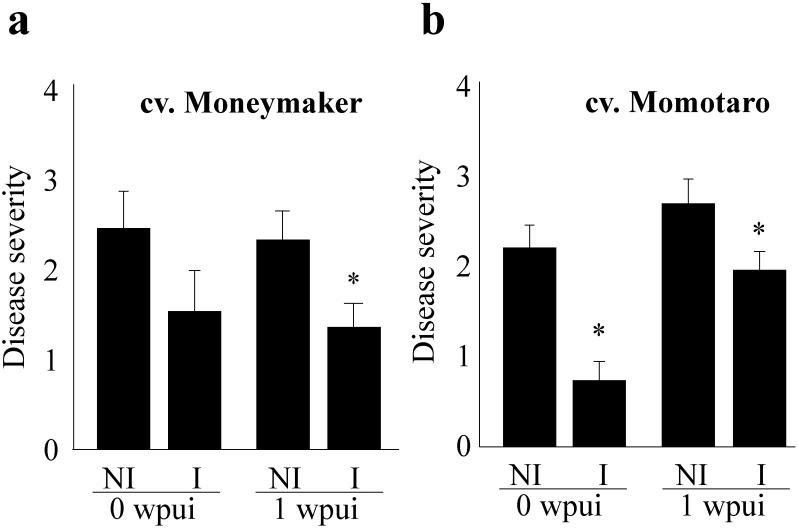
The effect of ultrasound irradiation was observed in both cultivars, Moneymaker and Momotaro, which suggested that ultrasound irradiation is applicable to preventing wilt disease in any tomato cultivar.
2. Even with irradiation for 1/6 of the time, tomato wilt was suppressed
As the number of plants irradiated with the aerial ultrasound oscillator was limited because of the limited irradiation area (50 cm in diameter at a 70 cm distance from the oscillator), we developed a traveling device that irradiates 40.5 kHz ultrasonic (intermittent pulse wave; each plant was irradiated for 15 sec with a 75 sec interval, on average) to a larger number of seedling plants (Japanese patent 2016-26268 JP), and its disease control effect was tested. With the device, the total period for the irradiation of each plant by ultrasound diminished to 1/6 of the time as compared to with the fixed device. Wilt was suppressed in both cvs. Moneymaker and Momotaro (Fig. 3) with 2 weeks of ultrasound irradiation using the traveling device, as in the 2 weeks of irradiation using the fixed device.
Fig. 3. Wilt was suppressed in tomato plants irradiated by aerial ultrasound using the traveling device where the total period of irradiation of each plant by ultrasound was as low as 1/6 of the time as compared to that with the fixed device. Tomato plants were irradiated with 40.5 kHz ultrasound for 2 weeks for 15 sec at 75-sec intervals for 2 weeks (I) or without irradiation (NI). Tomato wilt was suppressed the same as with continuous 2-week irradiation. The tomato wilt disease index was analyzed with R, Wilcoxon rank sum test. p<0.05, bars=standard error, Asterisk means significance as compared to NI.
The traveling 40.5 kHz aerial ultrasound oscillator device seems suited to producing a large number of tomato seedlings with resistance to wilt disease.
3. Ultrasound irradiation suppressed tomato powdery mildew and rice blast but did not suppress cabbage yellows
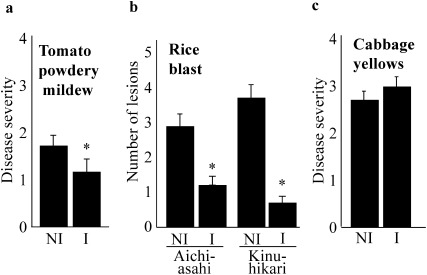
We examined whether the efficacy of ultrasound irradiation of 40.5 kHz in disease suppression depends on plant species or plant-pathogen interactions. For that purpose, we tested the disease suppression effect on tomato (cv. Momotaro) powdery mildew caused by Oidium sp., cabbage yellows caused by Foc, and rice (cvs. Aichi-asahi and Kinuhikari) blast caused by Po.
Powdery mildew and blast were significantly suppressed in ultrasound-irradiated tomato and rice plants as compared to non-irradiated plants (Fig. 4a, b). On the other hand, cabbage yellows was not suppressed by ultrasound irradiation (Fig. 4c). The irradiation of strawberry to 40.5 kHz ultrasound suppressed powdery mildew caused by Sphaerotheca aphanis (original data will be presented in another paper).20) However, lettuce powdery mildew caused by Erysiphe sp. was not suppressed by 40.5 kHz ultrasound irradiation (Fig. S2).
Fig. 4. Disease suppression by ultrasound irradiation possibly differs depending on the plant species or plant-pathogen interactions. One-week-old tomato seedlings (cv. Momotaro) were irradiated with 40.5 kHz ultrasound for two weeks and inoculated with tomato powdery mildew pathogens just after terminating ultrasound irradiation. Two weeks after inoculation, the disease incidence of each plant was graded from 0 to 4 using the following index: 0, no symptoms; 1, a foliar area of 0–5% indicating powdery mildew symptoms; 2, 6–30%; 3, 31–60%; 4, 61–100% (a). One-week-old rice seedlings (cvs. Aichi-asahi and Kinuhikari) were irradiated with ultrasound for two weeks and inoculated with rice blast pathogen (Po) just after terminating ultrasound irradiation. One week after inoculation, the lesions on the upper three leaves of each plant were counted (b). One-week-old cabbage seedlings were irradiated with ultrasound for two weeks and inoculated with cabbage yellows pathogens just after terminating ultrasound irradiation. Two weeks after inoculation, the disease incidence of each plant was graded from 0 to 4 using the following index: 0, no symptoms; 1, lower leaves yellowing; 2, lower and upper leaves yellowing; 3, lower leaves yellowing and wilting and upper leaves yellowing; 4, all leaves wilting and yellowing or dead (c). The disease indexes of tomato wilt and cabbage yellows were analyzed with R, Wilcoxon rank sum test, and the number of lesions of rice blasts was analyzed with R, t-test. p<0.05, bars=standard error, Asterisk means significance as compared to NI.
These results suggested that disease suppression by ultrasound irradiation possibly differs depending on plant species or plant-pathogen interactions.
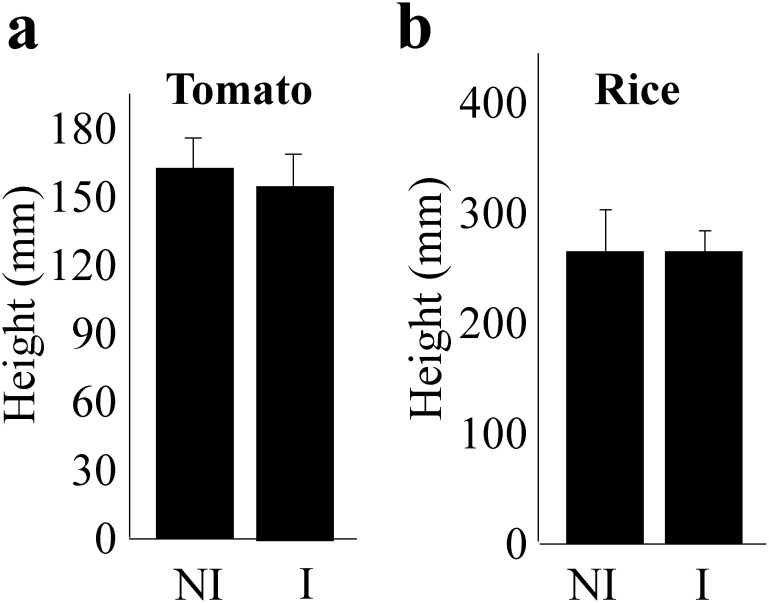
4. Ultrasound irradiation did not affect the growth of tomato and rice plants
Sound vibrations have been previously reported to promote or inhibit plant growth.21–23) For example, tomato growth was promoted by audible sound (20–2000 Hz) irradiation.22) However, no report on the effect of ultrasound on the growth of plants could be found. Furthermore, several reports have claimed that the induction of disease resistance in plants is sometimes accompanied by the inhibition of growth.24,25) Therefore, we measured the heights of tomato (cv. Momotaro) and rice (cv. Aichi-asahi) plants after 2 weeks of irradiation at 40.5 kHz ultrasound. Ultrasound conditions were identical to those described previously. No significant positive or negative effect was observed on the growth of tomato and rice plants (Fig. 5).
Fig. 5. The heights of tomato and rice plants did not change with ultrasound wave irradiation. We examined the effects of ultrasound on plant growth. The heights of tomato (cv. Momotaro) and rice (cv. Aichi-asahi) plants did not show differences as compared to NI plants. The heights of tomato and rice plants were analyzed with R, t-test. p<0.05, bars=standard error.
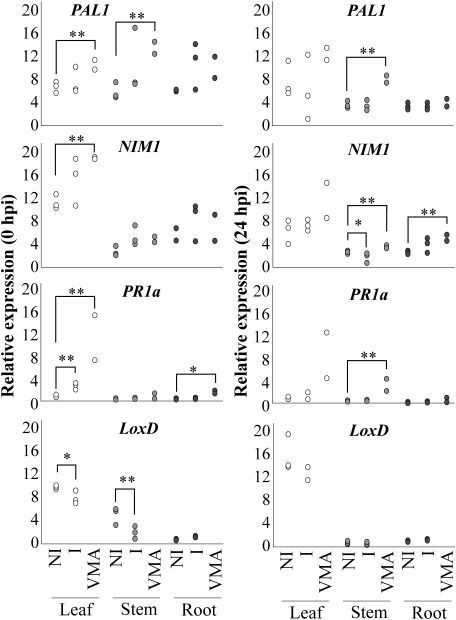
5. The SA signaling pathway was associated with the suppression of tomato wilt
As ultrasound seemed to induce disease resistance in tomatoes, we tried to profile the expression of resistance-related genes in ultrasound-irradiated tomatoes (cv. Moneymaker) by RT-qPCR. Target genes PAL1 (M83314 in GenBank), NIM1 (also known as NPR1; AY640378), and PR1a (M69247) are involved in the SA signaling pathway, and LoxD (U37840) is involved in the jasmonic acid (JA) signaling pathway (Fig. S1).26–29) Choi et al. (2017) reported that sound irradiation (1000 Hz, 3 hr/day, 10 days) primed SAR in A. thaliana, and the expression of SA signaling pathway-related genes in sound-irradiated plants was upregulated earlier than in non-irradiated plants when inoculated with B. cinerea.9)
Thus, in this study, we extracted total RNA from the leaves, stems, and roots of 40.5 kHz ultrasound-irradiated or non-irradiated tomato plants at 0 and 24 hpi with Fol. As a control, we used tomato plant foliage sprayed with 100 ppm validamycin A (VMA, Sumitomo Chemical Co.) 1 week before inoculation. VMA is known to induce SAR in tomatoes and suppress Fusarium wilt.24,25)
The expression of PR1a in leaves was significantly upregulated by ultrasound irradiation at 0 hpi, although it was lower than with VMA treatment (Fig. 6). The expression of LoxD in leaves and stems was downregulated at 0 hpi. The expression of PAL1 or NIM1 was not significantly upregulated, although the expression of both genes was upregulated by VMA in tomato leaves.
Fig. 6. The expression of PR1a possibly involved in the SA signaling pathway was upregulated in tomato plants irradiated by ultrasound. Analyses of the expression of defense-related genes were performed by RT-qPCR. Genes suggested to be involved in the SA signaling pathway—PAL1, NIM1, and PR1a—and a gene involved in the JA-signaling pathway—LoxD—were examined. PR1a was upregulated in leaves at 0 hpi, and LoxD was downregulated in leaves and stems at 0 hpi. Total RNA was isolated from leaves (white circle), stems (light gray), and roots (dark gray) of tomato plants (cv. Moneymaker) irradiated with ultrasound (40.5 kHz, ca. 100 dB, intermittent pulse wave, 2 weeks) at 0 and 24 hr post inoculation (hpi). The relative expression levels of mRNA were determined by normalizing the PCR threshold cycle number of each gene with that of the Actin2 reference gene. Three biological replicates were used in the experiments. The relative expression levels of the mRNA of I were compared to those of NI and analyzed with R, t-test. *, p<0.10; **, p<0.05, bars=standard error.
No gene in ultrasound-irradiated tomatoes showed higher expression than in non-irradiated plants at 24 hpi, while VMA treatment significantly induced the expression of all genes (PAL1, NIM1, and PR1a) involved in SAR. Especially the expression of NIM1 was downregulated by ultrasound irradiation at 24 hpi.
These results showed that ultrasound irradiation possibly induced a part of the SA signaling pathway and reduced Fusarium wilt in tomato plants, which did not contradict previous reports by Ishikawa et al. (2005),24) Di et al. (2016),30) and Choi et al. (2017).9) In our study, no gene involved in the SA signaling pathway was upregulated at 24 hpi in ultrasound-irradiated tomato plants (Fig. 6), which did not contradict a previous report where PR1 (the homolog of PR1a in tomato) in A. thaliana was downregulated when infected with F. oxysporum at 1 day post inoculation (dpi).31)
In conclusion, we found that ultrasound irradiation induced disease resistance that lasted longer than a week in tomato and rice plants. Ultrasound irradiation can provide seedlings with resistance to later attacks by pathogens.
Preliminary studies on the effect by irradiation at lower frequencies indicated that tomato wilt is suppressed in plants irradiated with not only 40.5 but also 19.8 and 28.9 kHz. Although the difference was small, 19.8 kHz showed a higher preventive effect than did 40.5 kHz, which indicated that the efficacy of the prevention effect differs depending on the frequencies of ultrasound. In the future, we are planning to optimize the wavelength of ultrasonic waves to be irradiated.
Ultrasound is easily absorbed by PVC. Therefore, in the practical use of ultrasound in a greenhouse, ultrasound can be easily shielded to prevent unexpected negative effects on humans or the environment. In the experiment using strawberries in a greenhouse, no effect was observed on the ratio of pollination by bees or the number of spider mites (personal communication from M. Arimoto and T. Nishimura).
The power consumption of the ultrasound oscillator used in our experiment is 40 W, which is the same as that of a room lamp; it can expose more than 200 plant pots (7.5 cm in diameter) under the traveling device (3 m×50 cm in the irradiation area), for example. The ultrasound device seems to be applicable in ecofriendly agricultural fields.
Further studies are required to determine more effective and efficient conditions for ultrasound irradiation, how long the induction of disease resistance lasts, and how plants recognize ultrasound stimuli. Each plant species might have a unique sensor or recognition mechanism because the efficacy of disease suppression depends on the plant species (Fig. 4). Moreover, it is necessary to examine which plants (or diseases) will respond to the application of ultrasound. In this study, we found that aerial ultrasound irradiation is effective for suppressing not only tomato powdery mildew caused by airborne Oidium sp. but also tomato wilt caused by soilborne Fol, perhaps by inducing PR1a involved in the SA signaling pathway in plants. This is consistent with previous reports that described the suppression of rice blast and tomato wilt by SAR inducers, such as PBZ and VMA.24,32) All of the pathogens that were controlled by ultrasound irradiation in this study are recognized as biotrophic. In general, plant resistance to biotrophic pathogens is mainly regulated by SA-mediated resistance, and resistance to necrotrophic pathogens is regulated by JA-mediated resistance.26) However, it is known that even parts of necrotrophic fungi have a biotrophic stage during their infections, and they are suppressed by the induction of SA-mediated resistance.33) It is expected that ultrasound irradiation suppresses diseases caused by pathogens that have a biotrophic stage during infection.
Acknowledgments
This research was supported by Grants from NARO for TY and TA. This research is partially applied to patent, 2013-035518 JP and 2016-26268 JP. Parts of contents of this research have been published as the abstracts of oral presentations.34,35)
Supplementary Data
References
- 1).A. Sham, K. Moustafa, S. Al-Ameri, A. Al-Azzawi, R. Iratni and S. AbuQamar: PLoS One 10, e0125666 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).E. W. Chehab, E. Eich and J. Braam: J. Exp. Bot. 60, 43–56 (2008). [DOI] [PubMed] [Google Scholar]
- 3).T. Arie and H. Nakashita: “Plant Activator,” Plant Protection, Tokyo, pp. 531–536 (2007) (in Japanese).
- 4).P. P. Reddy: “Plant Defense Activators,” Springer India, 2013.
- 5).K. Creath and G. E. Schwartz: J. Altern. Complement. Med. 10, 113–122 (2004). [DOI] [PubMed] [Google Scholar]
- 6).Y. C. Qin, W. C. Lee, Y. C. Choi and T.-W. Kim: Ultrasonics 41, 407–411 (2003). [DOI] [PubMed] [Google Scholar]
- 7).M. Gagliano, S. Mancuso and D. Robert: Trends Plant Sci. 17, 323–325 (2012). [DOI] [PubMed] [Google Scholar]
- 8).W. Bochu, S. Jiping, L. Biao, L. Jie and D. Chuanren: Colloids Surf. B Biointerfaces 37, 107–112 (2004). [DOI] [PubMed] [Google Scholar]
- 9).B. Choi, R. Ghosh, M. A. Gururani, G. Shanmugam, J. Jeon, J. Kim, S.-C. Park, M. J. Jeong, K. H. Han, D. W. Bae and H. Bae: Sci. Rep. 7, 2527 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).S. R. G. Beckett, S. Aspley, M. Graham and C. A. Marsden: Psychopharmacology (Berl.) 127, 384–390 (1996). [DOI] [PubMed] [Google Scholar]
- 11).A. Kershenbaum, D. T. Blumstein, M. A. Roch, Ç. Akçay, G. Backus, M. A. Bee, K. Bohn, Y. Cao, G. Carter, C. Cäsar, M. Coen, S. L. DeRuiter, L. Doyle, S. Edelman, R. Ferrer-i-Cancho, T. M. Freeberg, E. C. Garland, M. Gustison, H. E. Harley, C. Huetz, M. Hughes, J. Hyland Bruno, A. Ilany, D. Z. Jin, M. Johnson, C. Ju, J. Karnowski, B. Lohr, M. B. Manser, B. McCowan, E. Mercado 3rd, P. M. Narins, A. Piel, M. Rice, R. Salmi, K. Sasahara, L. Sayigh, Y. Shiu, C. Taylor, E. E. Vallejo, S. Waller and V. Zamora-Gutierrez: Biol. Rev. Camb. Philos. Soc. 91, 13–52 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).R. Mankin: Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 7, 001, DOI: 10.1079PAVSNNR20127001 (2012). [Google Scholar]
- 13).A. Morozova, E. Zubkov, T. Strekalova, Z. Kekelidze, Z. Storozeva, C. Schroeter, N. A. Bazhenova, K.-P. Lesch, B. Cline and V. H. Chekhonin: Prog. Neuropsychopharmacol. Biol. Psychiatry 68, 52–63 (2016). [DOI] [PubMed] [Google Scholar]
- 14).K. Inami, T. Kashiwa, M. Kawabe, A. Onokubo-Okabe, N. Ishikawa, E. R. Pérez, T. Hozumi, L. A. Caballero, F. C. de Baldarrago, M. J. Roco, K. A. Madadi, T. L. Peever, T. Teraoka, M. Kodama and T. Arie: Microbes Environ. 29, 200–210 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).T. Kashiwa, K. Inami, M. Fujinaga, H. Ogiso, T. Yoshida, T. Teraoka and T. Arie: J. Gen. Plant Pathol. 79, 412–421 (2013). [Google Scholar]
- 16).S. Urayama: J. Gen. Plant Pathol. 81, 97–102 (2015). [Google Scholar]
- 17).Y. Kanda: Bone Marrow Transplant. 48, 452–458 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).S. R. Uppalapati, P. Ayoubi, H. Weng, D. A. Palmer, R. E. Mitchell, W. Jones and C. L. Bender: Plant J. 42, 201–217 (2005). [DOI] [PubMed] [Google Scholar]
- 19).M. Du, J. Zhao, D. T. W. Tzeng, Y. Liu, L. Deng, T. Yang, Q. Zhai, F. Wu, Z. Huang, M. Zhou, Q. Wang, Q. Chen, S. Zhong, C. Li and C. Li: Plant Cell 29, 1883–1906 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).M. Arimoto, Y. Shimokawa, M. Yamamoto, Y. Enami, H. Amano, T. Iwagaya, A. Goto, T. Yoshida, D. Kawakami and T. Arie: Jpn. J. Phytopathol. 83, 196 (2017) (in Japanese). [Google Scholar]
- 21).R. H. E. Hassanien, T. Hou, Y. Li and B. Li: J. Integr. Agric. 13, 335–348 (2014). [Google Scholar]
- 22).T. Z. Hou and R. E. Mooneyham: Am. J. Chin. Med. 27, 1–10 (1999). [DOI] [PubMed] [Google Scholar]
- 23).H. Hunan Sheng nong ye ke xue yuan and J. ShiRen : Agric. Sci. Technol. 12, 847–851 (2011). [Google Scholar]
- 24).R. Ishikawa, K. Shirouzu, H. Nakashita, H.-Y. Lee, T. Motoyama, I. Yamaguchi, T. Teraoka and T. Arie: Phytopathology 95, 1209–1216 (2005). [DOI] [PubMed] [Google Scholar]
- 25).R. Ishikawa, K. Shirouzu, H. Nakashita, T. Teraoka and T. Arie: J. Pestic. Sci. 32, 83–88 (2007). [Google Scholar]
- 26).J. Glazebrook: Annu. Rev. Phytopathol. 43, 205–227 (2005). [DOI] [PubMed] [Google Scholar]
- 27).S. Hase, S. Takahashi, S. Takenaka, K. Nakaho, T. Arie, S. Seo, Y. Ohashi and H. Takahashi: Plant Pathol. 57, 870–876 (2008). [Google Scholar]
- 28).J. A. Ryals, U. H. Neuenschwander, M. G. Willits, A. Molina, H. Y. Steiner and M. D. Hunt: Plant Cell 8, 1809–1819 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Y. Bai, S. Sunarti, C. Kissoudis, R. G. F. Visser and C. G. van der Linden: Front. Plant Sci. 9, 801 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).X. Di, F. L. W. Takken and N. Tintor: Front. Plant Sci. 7, 170 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).B. N. Kidd, N. Y. Kadoo, B. Dombrecht, M. Tekeoğlu, D. M. Gardiner, L. F. Thatcher, E. A. B. Aitken, P. M. Schenk, J. M. Manners and K. Kazan: Mol. Plant Microbe Interact. 24, 733–748 (2011). [DOI] [PubMed] [Google Scholar]
- 32).K. Yoshioka, H. Nakashita, D. F. Klessig and I. Yamaguchi: Plant J. 25, 149–157 (2008). [DOI] [PubMed] [Google Scholar]
- 33).Y. Kouzai, M. Kimura, M. Watanabe, K. Kusunoki, D. Osaka, T. Suzuki, H. Matsui, M. Yamamoto, Y. Ichinose, K. Toyoda, T. Matsuura, I. C. Mori, T. Hirayama, E. Minami, Y. Nishizawa, K. Inoue, Y. Onda, K. Mochida and Y. Noutoshi: New Phytol. 217, 771–783 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Y. Kanemaru, T. Yoshida, T. Mizukami, N. Tanaka, T. Teraoka and T. Arie: Jpn. J. Phytopathol. 79, 73 (2013) (in Japanese). [Google Scholar]
- 35).D. Kawakami, T. Yoshida, T. Mizukami, H. Amano, T. Iwagaya, H. Aritake, A. Goto, Y. Enami, M. Yamamoto, M. Arimoto, T. Teraoka, K. Komatsu, T. Fukuhara and T. Arie: Jpn. J. Phytopathol. 83, 199 (2017) (in Japanese). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.