Summary
The human body houses a variety of microbial ecosystems, such as the microbiotas on the skin, in the oral cavity and in the digestive tract. The gut microbiota is one such ecosystem that contains trillions of bacteria, and it is well established that it can significantly influence host health and diseases. With the advancement in bioinformatics tools, numerous comparative studies based on 16S ribosomal RNA (rRNA) gene sequences, metabolomics, pathological and epidemical analyses have revealed the correlative relationship between the abundance of certain taxa and disease states or amount of certain causative bioactive compounds. However, the 16S rRNA‐based taxonomic analyses using next‐generation sequencing (NGS) technology essentially detect only the majority species. Although the entire gut microbiome consists of 1013 microbial cells, NGS read counts are given in multiples of 106, making it difficult to determine the diversity of the entire microbiota. Some recent studies have reported instances where certain minority species play a critical role in creating locally stable conditions for other species by stabilizing the fundamental microbiota, despite their low abundance. These minority species act as ‘keystone species’, which is a species whose effect on the community is disproportionately large compared to its relative abundance. One of the attributes of keystone species within the gut microbiota is its extensive enzymatic capacity for substrates that are rare or difficult to degrade for other species, such as dietary fibres or host‐derived complex glycans, like human milk oligosaccharides (HMOs). In this paper, we propose that more emphasis should be placed on minority taxa and their possible role as keystone species in gut microbiota studies by referring to our recent studies on HMO‐mediated microbiota formation in the infant gut.
Introduction
The gut microbial composition, which significantly influences host health and diseases (Vijay‐Kumar et al., 2010; Kau et al., 2011; Kinross et al., 2011; Iida et al., 2013; Sommer and Bäckhed, 2013), changes over time, with the most drastic changes occurring at the onset and termination of breastfeeding (Yatsunenko et al., 2012). Bifidobacteria are the first colonizers in the intestines of breastfed infants. In many cases, bifidobacteria occupy more than 70% of the total infant gut microbiota (Tannock et al., 2013; Matsuki et al., 2016; Yamada et al., 2017). The lack of a bifidobacteria‐rich gut microbiota (bifidus flora) during infancy has been shown to be linked to a variety of health conditions (Brown et al., 1989; López‐Alarcón et al., 1997; von Kries et al., 1999; Olszak et al., 2012; Cox et al., 2014), including diarrhoea, allergy, atopic dermatitis, impaired immune responses and elevated serum cholesterol levels (Kalliomäki et al., 2001; Di Gioia et al., 2014) that continue throughout adulthood. Thus, a thorough understanding of the mechanisms that shape and modulate the infant bifidus flora within the gut microbiota is an important approach to address long‐term health problems.
The key modulator within breast milk is human milk oligosaccharides (HMOs). Despite being the third most abundant solid component in breast milk after lactose (Lac) and lipids, HMOs have no nutritional value for infants because of their resistance to pancreatic digestion (Kunz et al., 2000; Urashima et al., 2012). Several groups, including our own, have found the gene sets coding for enzymes that degrade HMOs (Sela et al., 2008; Garrido et al., 2016; James et al., 2016; Katayama, 2016; Matsuki et al., 2016) and have shown that these genes are limited to the infant gut‐associated bifidobacterial species among gut microbes (Ruiz‐Moyano et al., 2013; Katayama, 2016; Thomson et al., 2017). These findings suggest that it is highly likely that HMOs serve as selective nutrients for bifidobacterial species.
The bifidus flora mainly comprises four bifidobacterial species, Bifidobacterium breve, Bifidobacterium bifidum, Bifidobacterium longum subsp. longum (B. longum) and Bifidobacterium longum subsp. infantis (B. infantis), that show varied HMO assimilation phenotypes due to their different genotypes at the species and strain levels. Although the bifidus flora in the infant gut is a relatively simple microbial community compared to that of adults at the genus level, it is highly diverse and complex at the genotype level. Our previous report shows that within the bifidus flora, the minority species/strain act as potential keystone species that promote and maintain the bifidus flora.
A keystone species is a species whose effect on the community is disproportionately large compared to its relative abundance (Paine, 1969; Power et al., 1996) and it alters their community through a variety of mechanisms. Most commonly cited examples, such as sea otters in kelp forests (Estes and Palmisano, 1974) and starfish in the rocky intertidal (Paine, 1966), are often apex predators. In the gut microbiota, however, keystone species primarily affect the community through altruistic degradation of substrates, which are otherwise recalcitrant, but are made available for other bacterial species to consume (Ze et al., 2012, 2013; Goodrich et al., 2014; Trosvik and de Muinck 2015; Centanni et al., 2018). One such recalcitrant substrate present in the infant gut ecosystem is HMOs. In human milk, HMOs with a variety of structures are included: lacto‐N‐tetraose (LNT: Galβ1‐3GlcNAcβ1‐3Galβ1‐4Glc) that contains lacto‐N‐biose I (LNB: Galβ1‐3GlcNAc) residue at the non‐reducing terminus of Lac, fucosylated LNT such as lacto‐N‐fucopentaose I (Fucα1‐2Galβ1‐3GlcNAcβ1‐3Galβ1‐4Glc) and lacto‐N‐difucohexaose I (Fucα1‐2Galβ1‐3(Fucα1‐4)GlcNAcβ1‐3Galβ1‐4Glc), and fucosylated Lac such as 2′‐fucosyllactose (Fucα1‐2Galβ1‐4Glc) and 3‐fucosyllactose (Galβ1‐4(Fucα1‐3)Glc; Fig. 1). Previously reported in vitro culture experiments showed that infant gut‐associated Bifidobacterium species have different HMO consumption behaviours (Fig. 1). For example, the fucosylated oligosaccharides were well assimilated by B. infantis and B. bifidum. However, the ability to utilize HMOs of B. longum and B. breve was restricted to LNT and LNB that is produced through LNT degradation (Asakuma et al., 2011), and only a limited number of B. longum and B. breve strains possess the enzyme set that degrades fucosylated HMOs (James et al., 2016; Matsuki et al., 2016). Despite their limited ability to degrade HMOs, B. longum and B. breve are frequently dominant species in the bifidus flora. This suggests that it is difficult to describe the mechanism of bifidus flora formation through in vitro HMO assimilation phenotype of each species and strain. Thus, we focused on the altruistic role (cross‐feeding) that minority species play within the gut microbiota.
Figure 1.
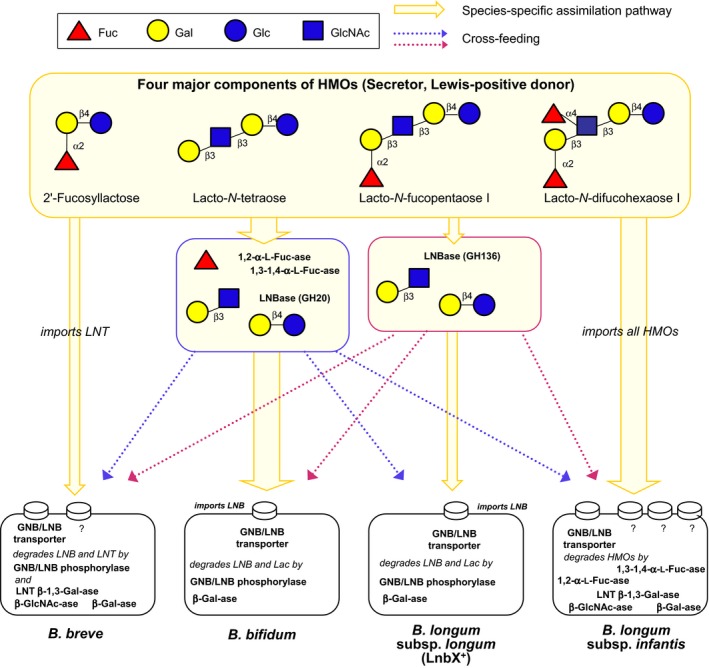
Structures of the four main human milk oligosaccharides (HMOs), and the assimilation pathways and enzymes utilized by each infant gut‐associated bifidobacterial species. Solid arrows indicate species‐specific assimilation pathways, and dotted arrows indicate potential cross‐feeding. B. breve and B. longum generally utilize LNT, while both B. infantis and B. bifidum consume a variety of HMOs. B. bifidum possesses cell surface‐attached enzymes that allow for extracellular degradation of HMOs. LNB +−B. longum degrades LNT to LNB and Lac. Degradants produced and left unconsumed by B. bifidum and B. longum may be shared among the other bifidobacteria expressing both the GNB/LNB transporters and GNB/LNB phosphorylases. This figure was modified and adapted from the review article by Katayama (2016).
Case studies
LnbX+−B. longum
A few rare strains of B. longum have the extracellular enzyme lacto‐N‐biosidase (LnbX), which degrades LNT to LNB and Lac (Fig. 1; Sakurama et al., 2013). LnbX can act not only on LNT, but also on human‐derived glycoconjugate sugars (Gotoh et al., 2015). We demonstrated that LnbX+−B. longum strains promote the growth of other bifidobacteria through cross‐feeding of LNT by‐products when co‐cultured in medium containing LNT as a carbon source. To assess the contribution of LnbX to the bifidus flora formation, we determined the prevalence of lnbX gene, B. longum (species level) and Bifidobacterium (genus level) in stools of both exclusively breastfed and mixed‐fed (formula‐ and breastfed) infants, by quantitative PCR (qPCR). As a result, we confirmed that the abundance of Bifidobacterium and B. longum in stools of breastfed infants was significantly higher than that of mixed‐fed infants. While lnbX expression was detected in five out of 10 (50%) individuals in the breastfed group, it was detected in only 17% of the individuals in the mixed‐fed group. In addition, we observed a positive correlation between the abundance of lnbX and B. longum in the stools of exclusively breastfed infants. On the other hand, no correlation was observed between the two factors for the stools of mixed‐fed infants. Interestingly, B. longum carrying the lnbX gene was, on average, only about 0.2% of the total B. longum population. These findings suggest that, although the LnbX+−B. longum strain is a minority species, it significantly contributes to the formation of bifidus flora through cross‐feeding of degraded by‐products of LNT (Yamada et al., 2017).
B. bifidum
Bifidobacterium bifidum, which is generally known to be the minority species in the bifidus flora, expresses various glycosidases as cell surface‐anchored extracellular enzymes (Katayama et al., 2004; Wada et al., 2008; Ashida et al., 2009; Miwa et al., 2010) and has high viability when cultured with HMOs (Asakuma et al., 2011). Four strains of B. bifidum isolated from infant stools produced degradants of HMOs, such as LNB, Lac, fucose and galactose in culture supernatant during the logarithmic growth phase. This suggested that B. bifidum leaves the degraded mono‐ and oligosaccharides in the medium without using them immediately. When B. longum 105‐A strain, which assimilates only LNT, was co‐cultured with B. bifidum in the presence of HMOs, its growth was remarkably promoted (Gotoh et al., 2018). Stool samples collected from infants, children and adults were cultured in the presence or absence of each of the four strains of B. bifidum in the medium containing glucose (Glc) or HMOs. The total abundance of bacteria, Bifidobacterium (genus level) and B. bifidum (species level) was measured using qPCR. As a result, when the stool samples were cultured in the media supplemented with Glc, the abundance of Bifidobacterium either significantly decreased or did not change, compared to the group without the addition of B. bifidum. On the other hand, when cultured with HMOs, the exogenous addition of B. bifidum significantly increased the total abundance and prevalence of species in the Bifidobacterium genus other than B. bifidum (Table 1). This increase was strongly promoted in the cultured sample from an infant born through Caesarean section, in which the bifidus flora was not initially confirmed (Table 1, Infant C). We found that promoting the growth of preexisting Bifidobacterium was difficult when only HMOs were added, but the addition of B. bifidum stimulated the formation of the bifidus flora (Gotoh et al., 2018).
Table 1.
Addition of B. bifidum to faecal suspensions incubated in the presence of HMOs enriches the Bifidobacterium population (species other than B. bifidum) in the culture. Prevalence was calculated by dividing total bifidobacterial 16S rRNA gene counts (except for B. bifidum) by total bacterial 16S rRNA gene counts. The data were adapted from the paper by Gotoh et al., 2018
| Bifidobacterium bifidum strain added to faecal suspension | Faecal suspension (Age/Delivery mode) | Child A (4 years/vaginal) | Child B (5 years/vaginal) | Infant C (4 months/caesarean) | Adult D (30 years/no data) | Adult E (39 years/no data) |
|---|---|---|---|---|---|---|
| None added | Total bacteria (copies/ml; ×1013) | 1.4 ± 0.5 | 1.7 ± 0.1 | 0.78 ± 0.01 | 1.7 ± 0.1 | 2.5 ± 0.0 |
| Prevalence of other bifidobacterial species in total bacteria (%) | 0.0050 | 2.6 | 0.00034 | 0.37 | 5.0 | |
| JCM1254 | Total bacteria (copies/ml×; ×013) | 2.0 ± 0.1 | 2.5 ± 0.4 | 1.7 ± 0.0 | 2.2 ± 0.1 | 2.4 ± 0.1 |
| Prevalence of other bifidobacterial species in total bacteria (%) | 0.21 | 4.3 | 0.27 | 2.4 | 3.6 | |
| JCM7004 | Total bacteria (copies/ml; × 1013) | 1.6 ± 0.1 | 2.0 ± 0.2 | 1.3 ± 0.0 | 2.2 ± 0.1 | 2.6 ± 0.1 |
| Prevalence of other bifidobacterial species in total bacteria (%) | 0.58 | 3.4 | 0.81 | 2.8 | 4.4 | |
| TMC3108 | Total bacteria (copies/ml; ×1013) | 2.2 ± 0.8 | 1.4 ± 0.1 | 1.1 ± 0.0 | 2.1 ± 0.1 | 2.7 ± 0.1 |
| Prevalence of other bifidobacterial species in total bacteria (%) | 1.0 | 4.9 | 1.6 | 0.48 | 4.4 | |
| TMC3115 | Total bacteria (copies/ml; ×1013) | 1.3 ± 0.0 | 1.6 ± 0.1 | 1.4 ± 0.0 | 2.0 ± 0.1 | 2.5 ± 0.1 |
| Prevalence of other bifidobacterial species in total bacteria (%) | 5.3 | 1.9 | 2.0 | 4.4 | 3.8 |
Caveats
Although the two above‐mentioned studies indicated that minority species had a significant effect on microbiota formation, these studies have only examined a small snapshot of 24 h. To determine whether a species acts as a keystone species with confidence, future studies will need to examine the microbiota over a longer time period and perform a community time series analysis (Trosvik and de Muinck, 2015). Furthermore, the role of a keystone species is highly context‐dependent (Power et al., 1996). Keystone species may not always be the controlling agent at all times, but rather only under certain conditions. In the examples that we raised, the presence of an exclusive carbon source like HMOs allowed B. bifidum and LnbX+−B. longum to act as a keystone species in the bifidus flora (Yamada et al., 2017; Gotoh et al., 2018).
Conclusions
In the adult intestine, members of the gut microbiome exhibit complex cross‐feeding. Previous reports show several examples of microbe–microbe relationships, such as bidirectional feeding between Bacteroides ovatus and Bacteroides vulgatus, which is mediated through inulin (Rakoff‐Nahoum et al., 2016), and between Akkermansia muciniphila and Eubacterium hallii, which is mediated through O‐glycan degradants derived from mucin and pseudovitamin B12 (Belzer et al., 2017). Unidirectional feeding was also observed between A. muciniphila and Anaerostipes caccae, and between B. adolescentis and Faecalibacterium prausnitzii (Rios‐Covian et al., 2015). These examples show that Actinobacteria, the phylum to which Bifidobacterium belongs, and Verrucomicrobia, to which Akkermansia belongs, are minority phyla in human gut microbiota that influence the abundance of species that belong to other phyla, such as Bacteroidetes and Firmicutes.
In the infant gut, the most abundant genus is generally Bifidobacterium, and the carbon source that is the most available to them comes from HMOs in breast milk. Interestingly, the four infant gut‐associated bifidobacterial species and their multiple strains have evolved different strategies to degrade HMOs and to maintain diversity. We demonstrated that B. longum strains that express lnbX and B. bifidum are potential keystone species in the establishment of the bifidus flora by providing HMO degradants for other bacterial groups to use. In other words, the cross‐feeding between minority taxa and dominant taxa is an important mechanism for the formation and maintenance of a diverse bifidus flora (Fig. 1). These findings enhance our understanding of how the bifidus flora is formed and, by conducting follow‐up microbiota studies of different individuals, can provide insight into how the physiology and ecology of the gut microbiota potentially affect human health.
Conflict of interest
None declared.
Microbial Biotechnology (2019) 12(2), 259–264
Funding Information
This study was supported in part by Grants‐in‐Aid from the Institute for Fermentation, Osaka and JSPS‐KAKENHI (15H04481 and 17K19231) to TK and by Grant‐in‐Aid for JSPS Research Fellows (17J08530) to AG.
References
- Asakuma, S. , Hatakeyama, E. , Urashima, T. , Yoshida, E. , Katayama, T. , Yamamoto, K. , et al (2011) Physiology of consumption of human milk oligosaccharides by infant gut‐associated bifidobacteria. J Biol Chem 286: 34583–34592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashida, H. , Miyake, A. , Kiyohara, M. , Wada, J. , Yoshida, E. , Kumagai, H. , et al (2009) Two distinct alpha‐L‐fucosidases from Bifidobacterium bifidum are essential for the utilization of fucosylated milk oligosaccharides and glycoconjugates. Glycobiology 19: 1010–1017. [DOI] [PubMed] [Google Scholar]
- Belzer, C. , Chia, L.W. , Aalvink, S. , Chamlagain, B. , Piironen, V. , Knol, J. , and de Vos, W.M. (2017) Microbial metabolic networks at the mucus layer lead to diet‐independent butyrate and vitamin B12 production by intestinal symbionts. MBio 8: e00770‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, K.H. , Black, R.E. , Lopez de Romaña, G. , and Creed de Kanashiro, H. (1989) Infant‐feeding practices and their relationship with diarrheal and other diseases in Huascar (Lima), Peru. Pediatrics 83: 31–40. [PubMed] [Google Scholar]
- Centanni, M. , Lawley, B. , Butts, C.A. , Roy, N.C. , Lee, J. , Kelly, W.J. , and Tannock, G.W. (2018) Bifidobacterium pseudolongum in the ceca of rats fed Hi‐Maize starch has characteristics of a keystone species in bifidobacterial blooms. Appl Environ Microbiol 84: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, L.M. , Yamanishi, S. , Sohn, J. , Alekseyenko, A.V. , Leung, J.M. , Cho, I. , et al (2014) Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158: 705–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gioia, D. , Aloisio, I. , Mazzola, G. , and Biavati, B. (2014) Bifidobacteria: their impact on gut microbiota composition and their applications as probiotics in infants. Appl Microbiol Biotechnol 98: 563–577. [DOI] [PubMed] [Google Scholar]
- Estes, J.A. , and Palmisano, J.F. (1974) Sea otters: their role in structuring nearshore communities. Science 185: 1058–1060. [DOI] [PubMed] [Google Scholar]
- Garrido, D. , Ruiz‐Moyano, S. , Kirmiz, N. , Davis, J.C. , Totten, S.M. , Lemay, D.G. , et al (2016) A novel gene cluster allows preferential utilization of fucosylated milk oligosaccharides in Bifidobacterium longum subsp. longum SC596. Sci Rep, 6, 35045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich, J.K. , Waters, J.L. , Poole, A.C. , Sutter, J.L. , Koren, O. , Blekhman, R. , et al (2014) Human genetics shape the gut microbiome. Cell 159: 789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh, A. , Katoh, T. , Sugiyama, Y. , Kurihara, S. , Honda, Y. , Sakurama, H. , et al (2015) Novel substrate specificities of two lacto‐N‐biosidases towards β‐linked galacto‐N‐biose‐containing oligosaccharides of globo H, Gb5, and GA1. Carbohyd Res 408: 18–24. [DOI] [PubMed] [Google Scholar]
- Gotoh, A. , Katoh, T. , Sakanaka, M. , Ling, Y. , Yamada, C. , Asakuma, S. , et al (2018) Sharing of human milk oligosaccharides degradants within bifidobacterial communities in faecal cultures supplemented with Bifidobacterium bifidum . Sci Rep 8: 13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida, N. , Dzutsev, A. , Stewart, C.A. , Smith, L. , Bouladoux, N. , Weingarten, R.A. , et al (2013) Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342: 967–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, K. , Motherway, M.O. , Bottacini, F. and van Sinderen, D. (2016) Bifidobacterium breve UCC2003 metabolises the human milk oligosaccharides lacto‐N‐tetraose and lacto‐N‐neo‐tetraose through overlapping, yet distinct pathways. Sci Rep, 6, 38560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliomäki, M. , Kirjavainen, P. , Eerola, E. , Kero, P. , Salminen, S. , and Isolauri, E. (2001) Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol 107: 129–134. [DOI] [PubMed] [Google Scholar]
- Katayama, T. (2016) Host‐derived glycans serve as selected nutrients for the gut microbe: human milk oligosaccharides and bifidobacteria. Biosci Biotechnol Biochem 80: 621–632. [DOI] [PubMed] [Google Scholar]
- Katayama, T. , Sakuma, A. , Kimura, T. , Makimura, Y. , Hiratake, J. , Sakata, K. , et al (2004) Molecular cloning and characterization of Bifidobacterium bifidum 1,2‐alpha‐L‐fucosidase (AfcA), a novel inverting glycosidase (glycoside hydrolase family 95). J Bacteriol 186: 4885–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau, A.L. , Ahern, P.P. , Griffin, N.W. , Goodman, A.L. , and Gordon, J.I. (2011) Human nutrition, the gut microbiome and the immune system. Nature 474: 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinross, J.M. , Darzi, A.W. , and Nicholson, J.K. (2011) Gut microbiome‐host interactions in health and disease. Genome Med 3: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kries, R. , Koletzko, B. , Sauerwald, T. , von Mutius, E. , Barnert, D. , Grunert, V. , and von Voss, H. (1999) Breast feeding and obesity: cross sectional study. BMJ (Clinical Research Ed.) 319: 147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz, C. , Rudloff, S. , Baier, W. , Klein, N. , and Strobel, S. (2000) Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr 20: 699–722. [DOI] [PubMed] [Google Scholar]
- López‐Alarcón, M. , Villalpando, S. , and Fajardo, A. (1997) Breast‐feeding lowers the frequency and duration of acute respiratory infection and diarrhea in infants under six months of age. J Nutr 127: 436–443. [DOI] [PubMed] [Google Scholar]
- Matsuki, T. , Yahagi, K. , Mori, H. , Matsumoto, H. , Hara, T. , Tajima, S. , et al (2016) A key genetic factor for fucosyllactose utilization affects infant gut microbiota development. Nat Commun 7: 11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa, M. , Horimoto, T. , Kiyohara, M. , Katayama, T. , Kitaoka, M. , Ashida, H. , and Yamamoto, K. (2010) Cooperation of β‐galactosidase and β‐N‐acetylhexosaminidase from bifidobacteria in assimilation of human milk oligosaccharides with type 2 structure. Glycobiology 20: 1402–1409. [DOI] [PubMed] [Google Scholar]
- Olszak, T. , An, D. , Zeissig, S. , Vera, M.P. , Richter, J. , Franke, A. , et al (2012) Microbial exposure during early life has persistent effects on natural killer T cell function. MBio 336: 489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine, R.T. (1966) Food web complexity and species diversity. Am Nat 100: 65–75. [Google Scholar]
- Paine, R.T. (1969) A note on trophic complexity and community stability. Am Nat 103: 91–93. [Google Scholar]
- Power, M.E. , Tilman, D. , Estes, J.A. , Menge, B.A. , Bond, W.J. , Mills, L.S. , et al (1996) Challenges in the quest for keystones. Bioscience 46: 609–620. [Google Scholar]
- Rakoff‐Nahoum, S. , Foster, K.R. , and Comstock, L.E. (2016) The evolution of cooperation within the gut microbiota. Nature 53: 255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios‐Covian, D. , Gueimonde, M. , Duncan, S.H. , Flint, H.J. and de los Reyes‐Gavilan, C.G. (2015) Enhanced butyrate formation by cross‐feeding between Faecalibacterium prausnitzii and Bifidobacterium adolescentis . FEMS Microbiol Lett, 362, fnv176. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Moyano, S. , Totten, S.M. , Garrido, D.a. , Smilowitz, J.T. , Bruce German, J. , Lebrilla, C.B. and Mills, D.A. (2013) Variation in consumption of human milk oligosaccharides by infant gut‐associated strains of Bifidobacterium breve . Appl Environ Microbiol 79, 6040–6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurama, H. , Kiyohara, M. , Wada, J. , Honda, Y. , Yamaguchi, M. , Fukiya, S. , et al (2013) Lacto‐N‐biosidase encoded by a novel gene of Bifidobacterium longum subspecies longum shows unique substrate specificity and requires a designated chaperone for its active expression. J Biol Chem 288: 25194–25206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela, D.A. , Chapman, J. , Adeuya, A. , Kim, J.H. , Chen, F. , Whitehead, T.R. , et al (2008) The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc Natl Acad Sci USA 105: 18964–18969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer, F. , and Bäckhed, F. (2013) The gut microbiota–masters of host development and physiology. Nat Rev Microbiol 11: 227–238. [DOI] [PubMed] [Google Scholar]
- Tannock, G.W. , Lawley, B. , Munro, K. , Pathmanathan, S.G. , Zhou, S.J. , Makrides, M. , et al (2013) Comparison of the compositions of the stool microbiotas of infants fed goat milk formula, cow milk‐based formula, or breast milk. Appl Environ Microbiol 79: 3040–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson, P. , Medina, D.A. , and Garrido, D. (2017) Human milk oligosaccharides and infant gut bifidobacteria: molecular strategies for their utilization. Food Microbiol 75: 37–46. [DOI] [PubMed] [Google Scholar]
- Trosvik, P. , and de Muinck, E.J. (2015) Ecology of bacteria in the human gastrointestinal tract–identification of keystone and foundation taxa. Microbiome 3: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urashima, T. , Asakuma, S. , Leo, F. , Fukuda, K. , Messer, M. , and Oftedal, O.T. (2012) The glycobiology of human milk oligosaccharides – the predominance of type I oligosaccharides is a feature specific to human breast milk. Adv Nutr 3: 473S–482S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay‐Kumar, M. , Aitken, J.D. , Carvalho, F.A. , Cullender, T.C. , Mwangi, S. , Srinivasan, S. , et al (2010) Metabolic syndrome and altered gut microbiota in mice lacking toll‐like receptor 5. Science 328: 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada, J. , Ando, T. , Kiyohara, M. , Ashida, H. , Kitaoka, M. , Yamaguchi, M. , et al (2008) Bifidobacterium bifidum lacto‐N‐biosidase, a critical enzyme for the degradation of human milk oligosaccharides with a type 1 structure. Appl Environ Microbiol 74: 3996–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, C. , Gotoh, A. , Sakanaka, M. , Hattie, M. , Stubbs, K.A. , Katayama‐Ikegami, A. , et al (2017) Molecular insight into evolution of symbiosis between breast‐fed infants and a member of the human gut microbiome Bifidobacterium longum . Cell Chem Biol 24: 515–524. [DOI] [PubMed] [Google Scholar]
- Yatsunenko, T. , Rey, F.E. , Manary, M.J. , Trehan, I. , Dominguez‐Bello, M.G. , Contreras, M. , et al (2012) Human gut microbiome viewed across age and geography. Nature 486: 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ze, X. , Duncan, S.H. , Louis, P. , and Flint, H.J. (2012) Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J 6: 1535–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ze, X. , Le Mougen, F. , Duncan, S.H. , Louis, P. , and Flint, H.J. (2013) Some are more equal than others: the role of “keystone” species in the degradation of recalcitrant substrates. Gut Microbes 4: 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]


