Abstract
STUDY QUESTION
Does administration of recombinant human granulocyte colony stimulating factor (rhG-CSF) in the first trimester improve pregnancy outcomes, among women with a history of unexplained recurrent pregnancy loss?
SUMMARY ANSWER
rhG-CSF administered in the first trimester of pregnancy did not improve outcomes among women with a history of unexplained recurrent pregnancy loss.
WHAT IS KNOWN ALREADY
The only previous randomized controlled study of granulocyte colony stimulating factor in recurrent miscarriage in 68 women with unexplained primary recurrent miscarriage found a statistically significant reduction in miscarriage and improvement in live birth rates. A further four observational studies where G-CSF was used in a recurrent miscarriage population were identified in the literature, two of which confirmed statistically significant increase in clinical pregnancy and live birth rates.
STUDY DESIGN, SIZE, DURATION
A randomized, double-blind, placebo controlled clinical trial involving 150 women with a history of unexplained recurrent pregnancy loss was conducted at 21 sites with established recurrent miscarriage clinics in the United Kingdom between 23 June 2014 and 05 June 2016. The study was coordinated by University of Birmingham, UK.
PARTICIPANTS/MATERIALS, SETTING, METHODS
One hundred and fifty women with a history of unexplained recurrent pregnancy loss: 76 were randomized to rhG-CSF and 74 to placebo. Daily subcutaneous injections of recombinant human granulocyte – colony stimulating factor 130 μg or identical appearing placebo from as early as three to five weeks of gestation for a maximum of 9 weeks. The trial used central randomization with allocation concealment. The primary outcome was clinical pregnancy at 20 weeks of gestation, as demonstrated by an ultrasound scan. Secondary outcomes included miscarriages, livebirth, adverse events, stillbirth, neonatal birth weight, changes in clinical laboratory variables following study drug exposure, major congenital anomalies, preterm births and incidence of anti-drug antibody formation. Analysis was by intention to treat.
MAIN RESULTS AND THE ROLE OF CHANCE
A total of 340 participants were screened for eligibility of which 150 women were randomized. 76 women (median age, 32[IQR, 29–34] years; mean BMI, 26.3[SD, 4.2]) and 74 women (median age, 31[IQR, 26–33] years; mean BMI, 25.8[SD, 4.2]) were randomized to placebo. All women were followed-up to primary outcome, and beyond to live birth. The clinical pregnancy rate at 20 weeks, as well as the live birth rate, was 59.2% (45/76) in the rhG-CSF group, and 64.9% (48/74) in the placebo group, giving a relative risk of 0.9 (95% CI: 0.7–1.2; P = 0.48). There was no evidence of a significant difference between the groups for any of the secondary outcomes. Adverse events (AEs) occurred in 52 (68.4%) participants in rhG-CSF group and 43 (58.1%) participants in the placebo group. Neonatal congenital anomalies were observed in 1/46 (2.1%) of babies in the rhG-CSF group versus 1/49 (2.0%) in the placebo group (RR of 0.9; 95% CI: 0.1–13.4; P = 0.93).
LIMITATIONS, REASONS FOR CAUTION
This trial was conducted in women diagnosed with unexplained recurrent pregnancy loss and therefore no screening tests (commercially available) were performed for immune dysfunction related pregnancy failure/s.
WIDER IMPLICATIONS OF THE FINDINGS
To our knowledge, this is the first multicentre study and largest randomized clinical trial to investigate the efficacy and safety of granulocyte human colony stimulating factor in women with recurrent miscarriages. Unlike the only available single center RCT, our trial showed no significant increase in clinical pregnancy or live births with the use of rhG-CSF in the first trimester of pregnancy.
STUDY FUNDING/COMPETING INTEREST(S)
This study was sponsored and supported by Nora Therapeutics, Inc., 530 Lytton Avenue, 2nd Floor, Palo Alto, CA 94301, USA. Darryl Carter was the co-founder and VP of research, Nora Therapeutics, Inc. and held shares in the company. He holds a patent for the use of recombinant human granulocyte colony stimulating factor to reduce unexplained recurrent pregnancy loss. Mark Joing, Paul Kwon and Jeff Tong were or are employees of Nora Therapeutics, Inc. No other potential conflict of interest relevant to this article was reported.
TRIAL REGISTRATION NUMBER
EUDRACT No: 2014-000084-40; ClinicalTrials.gov Identifier: NCT02156063
TRIAL REGISTRATION DATE
31 Mar 2014
DATE OF FIRST PATIENT’S ENROLMENT
23 Jun 2014
Keywords: recombinant human granulocyte colony stimulating factor, cytokine, neutropenia, recurrent pregnancy loss, semi-allogenic fetus, immune mediated miscarriages, unexplained recurrent miscarriages
Introduction
Recurrent pregnancy losses (RPL) defined as the loss of three or more pregnancies, affects ~1–3% of couples attempting to have a child (Ford and Schust, 2009). Investigations do not provide a cause for recurrent pregnancy losses in approximately half of those investigated, and such couples are said to have unexplained recurrent pregnancy losses (Jeve and Davies, 2014). Whilst a range of treatments are currently offered to couples with unexplained recurrent pregnancy losses (uRPL), no effective treatment has yet been identified (ESHRE Recurrent Pregnancy Loss Guidelines, 2017).
Immune mediated mechanisms are thought to contribute to recurrent pregnancy losses. In particular, a failure of the maternal immune system to adapt to accommodate the semi-allogenic fetus may be important. Despite a lack of good evidence of their efficacy, a range of immunomodulatory treatments including, paternal lymphocyte infusion therapy (Mowbray et al., 1985, Cavalcante et al., 2014), corticosteroids, intravenous immunoglobulin therapy (Nyborg et al., 2014), intravenous intralipid infusion (Meng et al., 2016) and anti–TNFα monoclonal antibody therapy (Clark, 2009) to modulate the maternal immune response are offered to women with recurrent pregnancy losses.
Granulocyte colony stimulating factor (G-CSF) is a cytokine conventionally viewed as important in stimulating the proliferation and differentiation of neutrophils (Thomas et al., 2002). It is widely used in treatments associated with severe chronic neutropenia and chemotherapy induced neutropenia for mobilization of neutrophils (Carton et al., 2013). In addition, G-CSF is postulated to have immunomodulatory properties by inducing peripheral regulatory T-cells and myeloid derived suppressor cells, which have an important function curbing the immune response to infection, inflammation, and autoimmunity (Williams, 2012). Furthermore, studies in both humans and animals have showed that administration of G-CSF improves endometrial thickness (Gleicher et al. 2011), ovarian follicular function and oocyte quality (Salmassi et al., 2004), which may enhance embryo implantation (Ledee et al., 2008).
A Phase 1 randomized double blind, placebo-controlled dose escalation (65 mcg, 130 mcg and 260 mcg) study of recombinant human (rh) G-CSF in 48 healthy female volunteers suggested changes in peripheral blood subsets including a temporary induction of toleragenic cell subsets and decreased percentages of pro-inflammatory and cytotoxic cell subsets, without evidence of global immune changes or suppression. These specific changes were observed only in the multidose groups and not in single dose or placebo groups (unpublished data; available on request through the corresponding author).
Furthermore, a single center, randomized controlled trial of 68 women diagnosed with recurrent pregnancy losses, suggested that rhG-CSF may be an effective treatment (Scarpellini and Sbracia, 2009). Although the clinical evidence is limited (ESHRE Recurrent Pregnancy Loss Guidelines, 2017), the use of G-CSF to treat recurrent miscarriage and implantation failure appears to be increasing. Therefore, there was an urgent need to determine the efficacy and safety of this treatment in a multicentre trial.
We conducted a multicentre, randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of recombinant human granulocyte colony stimulating factor (rhG-CSF) in women with a history of unexplained recurrent pregnancy loss to provide a definitive answer on whether rhG-CSF administration improved pregnancy outcomes.
Materials and Methods
Study design
RESPONSE study was a multicentre, double blind; placebo controlled randomized clinical trial conducted to determine the effect of recombinant human granulocyte – colony stimulating factor in women with a history of unexplained recurrent pregnancy loss. All the eligible participants gave their written informed consent. The participants in the RESPONSE trial were recruited from 21 hospitals with established recurrent pregnancy loss clinics located across the United Kingdom. Study enrollment occurred between June 2014 and June 2016. The study was approved by NRES Committee North West – Greater Manchester Central (REC Ref.No: 14/NW/0130) and the individual research and development departments at respective hospital sites.
Participants
Women were eligible for enrollment in the study: (1) if they were aged 18–37 years with a BMI of 19–35 (at the time of consent), and (2) with regular ovulatory menstrual cycles and those who were actively trying to conceive naturally after being diagnosed with a history of unexplained recurrent pregnancy loss (three or more consecutive or non-consecutive first trimester losses of which at least two were confirmed by ultrasound or by histology). Age criterion was applied because the likelihood of miscarriages due to chromosomal aberrations is higher in older women, with such miscarriages unlikely to be prevented by immune-modulation.
Participants were excluded if any of the following criteria were applicable (a) greater than 5 completed weeks of gestation (i.e. greater than 3 completed weeks since ovulation as indicated by ovulation monitoring) when presenting for randomization, (b) known karyotype abnormalities in either the participant or her current male partner, (c) congenital malformations and uncorrected major and minor uncorrected intrauterine abnormalities (as assessed by ultrasound, hysterosonography, hysterosalpingography, or hysteroscopy within 3 years prior to screening), (d) vaginal bleeding of unknown cause, (e) diagnosis of infertility, (f) current or past diagnosis of the following: systemic autoimmune disease (e.g. systemic lupus erythematosus, Hashimoto’s thyroiditis, Graves’ disease, rheumatoid arthritis), antiphospholipid syndrome, or other thrombophilic disorder, (g) presence of anti-thyroid antibodies, lupus anticoagulant, anti-cardiolipin antibodies, or anti-β2 GP1 antibodies, (h) hyperprolactinemia, (i) any uncontrolled clinically significant medical condition (e.g. asthma, type II diabetes, infection), (j) the following laboratory abnormalities at initial screening or within 3 months prior to randomization: thrombocytopenia or thrombocytosis (platelet count <75,000/μL or > 500,000/μL), neutropenia or neutrophilia (absolute neutrophil count <1500/μL or > 10,000/μL), leucopenia or leucocytosis (white blood cell count <3000/μL or >15,000/μL), and creatinine, hepatic transaminases, lactate dehydrogenase (LDH), alkaline phosphatase, or uric acid ≥1.5× upper limit of normal (ULN), (k) use of lithium within 1 month prior to screening, (l) known hypersensitivity to any rhG-CSF drug product, any of its components, or any E. coli-derived proteins, (m) history of any of the following conditions: human immunodeficiency virus (HIV) infection, (n) malignancy within the past 5 years other than treated basal cell carcinoma or squamous cell carcinoma of the skin, (o) splenomegaly or splenic rupture, (p) adult respiratory distress syndrome (ARDS), acute lung injury (ALI), or pulmonary edema, (q) sickle cell anemia, (r) acute myocardial infarction, stroke, or revascularization (coronary or cerebral), (s) previous rhG-CSF therapy for any indication, or (t) in the investigator’s opinion, any contraindication to use of the investigational drug.
Randomization, masking and procedures
Participants in RESPONSE trial were randomized to receive rhG-CSF 130 mcg or placebo in a 1:1 ratio. Stratified permuted block randomization was used with number of prior miscarriages (3, >3), and age (<35, 35–37 years) as the stratification factors. An interactive central web response system (IWRS) was used for randomization.
Eligible participants were identified from recurrent pregnancy loss clinics and underwent comprehensive screening tests for eligibility evaluation. Once eligibility was established, the participant began ovulation monitoring and attempts at spontaneous conception. The participant performed daily urine pregnancy tests from 6 days after ovulation. After reporting a positive home urine pregnancy test, the participant scheduled a visit to the study site for a repeat urine pregnancy test, randomization into the study and initiation of study drug treatment. The study site visit took place within 4 days of the positive home urine pregnancy test. Once randomized, the participant self-administered rhG-CSF or placebo as a subcutaneous injection for a maximum of 9 weeks (up to 12th week of gestation) or until pregnancy failure.
Recombinant human granulocyte colony stimulating factor (rhG-CSF) and placebo were supplied to the investigative site in single-use 1 mL prefilled syringes. Each prefilled syringe contained 0.5 mL of rhG-CSF 260 μg/mL for the 130 μg dose, or identical appearing placebo. Participants, doctors and trial nurses remained unaware of study assignments. The first dose of study drug was administered at the investigative site. All subsequent doses were administered by the participant once daily at approximately the same time each day (within 20–28 h after the previous dose).
All study data except central laboratory and immunogenicity data were recorded in an electronic case report form (eCRF). Research personnel allocated for the trial at individual sites were responsible for entering these data.
Immunogenicity and safety analysis
All participants receiving study drug had serum samples collected prior to study drug administration, and at 6, 12 and 16 weeks of gestation or 4 week post drug follow up (for participants diagnosed with pregnancy loss) for the presence of anti-drug antibodies (ADAs). Safety was monitored through the assessment of adverse events, vital signs, physical examinations and clinical laboratory variables throughout the treatment period and 4-week post drug follow-up period by a designated medical monitor. In order to minimize unnecessary exposure to study drug, any randomized participant who was no longer pregnant discontinued study drug prior to the completion of the treatment period and was followed for a minimum of 4 weeks after last dose of study drug.
An external and independent Data Monitoring Committee (DMC) facilitated close monitoring of safety data, including any deaths, serious adverse events, anti-drug antibody formation and adverse events of special interest including splenic rupture, anaphylaxis, acute respiratory distress syndrome (ARDS) or acute lung injury (ALI) and major cardiovascular event.
Study blinding
This study was randomized, double-blinded and placebo-controlled to minimize potential bias in treatment assignment, subject monitoring, and endpoint evaluations. All participants, subjects, investigative center study staff, and investigative center monitors were blinded to treatment assignment. In addition, the laboratory results for white blood cells (WBC) and WBC subset counts, alkaline phosphatase, uric acid, lactate dehydrogenase (LDH) and anti-drug antibody (ADA) was blinded, as it had the potential for unblinding the intervention.
Outcomes
The primary outcome of ongoing clinical pregnancy was assessed via ultrasound examination at 20 weeks of gestation. All participants were monitored for adverse events. All participants who received at least one dose of study drug were followed for safety for a minimum of 4 weeks following the last dose of study drug.
The secondary outcome measures were: (a) live birth, (b) ongoing clinical pregnancy at weeks 6 and 12 of gestation, (c) spontaneous pregnancy loss, (d) elective abortion, (e) stillbirth, (f) neonatal birth weight, (g) maternal adverse events and serious adverse events during the treatment period and within 4 weeks of the last dose of study drug, (h) changes in clinical laboratory variables following study drug exposure, (i) major congenital anomalies, (j) preterm births and (k) incidence of anti-drug antibody (ADA) formation.
For participants who maintained pregnancy through 20 weeks of gestation, phone visits were conducted every 8 weeks during pregnancy to assess pregnancy status/outcomes and prescription medication use. Within one month of delivery, additional information was obtained, including pregnancy outcome, gestational age at delivery, mode of delivery, birth weight and Apgar scores.
Sample size calculations
The target sample size was a total of 150 participants. Participants were randomized in a 1:1 ratio to the two treatment arms. This sample size had been selected to achieve >90% power to detect a difference in ongoing clinical pregnancy rates of 60% for the placebo group and 80% in the active treatment group. Efficacy analysis was based on an intent-to-treat principle. The difference in the primary efficacy outcome measure (ongoing clinical pregnancy rate at week 20 of gestation) between rhG-CSF and placebo was tested using a Cochran Mantel Haenszel (CMH) test.
Statistical analysis
The statistician who conducted the analysis was blinded to group allocation. Efficacy analysis was based on an intention to treat principle. The relative risks (RRs) with 95% CIs were calculated for the primary and secondary outcomes. Subgroup analysis was performed using the RR for the stratification factors. Missing data were imputed only for those with a clinical pregnancy and livebirth. This implied that summary measures were limited to participants who remained pregnant at that time point. The statistical analysis plan (SAP) is available in Supplementary material.
Results
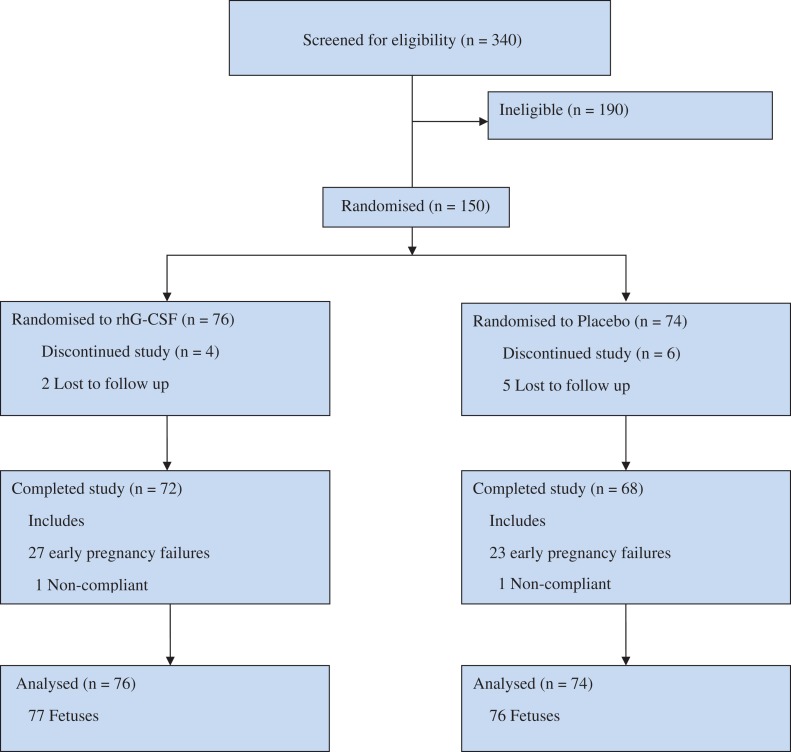
Between 23 June 2014 and 05 June 2016, a total of 340 women were screened for inclusion criteria at 21 different recruiting centers in the United Kingdom. One hundred and ninety women did not meet the inclusion criteria. One hundred and fifty women who conceived naturally and remained willing to participate in the trial were randomized to receive either rhG-CSF (76 women) or placebo (74 women) (Fig. 1). Overall, 140/150 women (93.3%) completed the study and 10/150 women (6.7%) discontinued the study prematurely for reasons including loss to follow up, withdrawal of consent, and other reasons (for e.g. use of any excluded therapy).
Figure 1.
Participant flow diagram.
All participants were followed to primary outcome, and beyond to live birth. The baseline characteristics of the study population were similar across the study groups (Table I). The median age at the time of recruitment was 30.6 years (IQR 29–34 years) and 37 participants (24.7%) had experienced more than three previous miscarriages. The mean BMI at the time of randomization was 26.06 kg/m2. Ethnicity data was available for all 150 randomized women, and of these, 134 (89%) were white, 9 (6%) were Asian, 2 (1%) were black and 5 (3%) were from other ethnic groups. Most of the women were non-smokers (123/150, 82%). Study records of concurrent medications showed that 3 (2%) participants were taking metformin at the time of participation, and 23 (15.3%) were taking low dose aspirin.
Table I.
Baseline characteristics of the participants (intention to treat analysis)a.
| rhG-CSF | Placebo | |
|---|---|---|
| Characteristics | (N = 76) | (N = 74) |
| Maternal age – yrb | ||
| Median | 32 | 31 |
| Interquartile range | 29–34 | 26–33 |
| Maternal BMI | 26.3 ± 4.2 | 25.8 ± 4.2 |
| Maternal BMI >30 – no. (%) | 17(22.4) | 13(17.6) |
| Maternal race – no. (%)c | ||
| White | 71(93.4) | 63(85.1) |
| Black | 0 | 2(2.7%) |
| Asian | 4(5.3) | 5(6.8) |
| Other, including mixed race | 1(1.3) | 1(5.4) |
| Maternal smoking – no (%) | ||
| Nonsmoker | 62(81.6) | 61(82.4) |
| <10 cigarettes/day | 10(13.2) | 7(9.5) |
| 10 to 19 cigarettes/day | 3(3.9) | 6(8.1) |
| ≥20 cigarettes/day | 1(1.3) | 0 |
| Alcohol use – no. (%)d | ||
| None | 36(47.4) | 35(47.3) |
| ≤3 units/day | 27(35.5) | 26(35.1) |
| >3 to ≥20 units/day | 13(17.1) | 13(17.6) |
| >20 units/day | 0 | 0 |
| Parity | ||
| Previous live birth – no. (%) | 38(50.0) | 37(50.0) |
| ≥4 previous miscarriages – no. (%) | 40(52.6) | 40(54.1) |
| Previous pregnancy losses – no. | ||
| Median | 4.0 | 4.0 |
| Interquartile range | 3–5 | 3–5 |
| Clinical risk factors – no. (%) | ||
| Polycystic ovaries | 2(2.6) | 6(8.1) |
| Fibroids | 5(6.6) | 3(4.1) |
| Large-loop excision of the cervical transformation zone | 2(2.6) | 8(10.8) |
| Concurrent medications – no. (%) | ||
| Metformin | 1(1.3) | 2(2.7) |
| Aspirin | 13(17.1) | 10(13.5) |
aPlus – minus values are means ± SD. The baseline data (age, body mass index [BMI; the weight in kilograms divided by the square of the height in meters], maternal race, smoking status and parity) of the participants were similar in the two study groups.
bListed is the maternal age at the time of randomization.
cRace was self-reported.
dOne unit is 10 g of pure alcohol.
Treatment compliance
Subject compliance with study drug dosing was accessed via a site review of the returned syringes and the compliance record maintained by the participant. From these results, summaries of treatment compliance and exposure were produced. In the overall population, the mean (SD) compliance rate was 98.7% (3.99).
Outcomes of the participants
The clinical pregnancy rate at 20 weeks of gestation was 59% (45/76) in the rhG-CSF group, compared with 65% (48/74) in the placebo group, giving a RR of 0.9 (95% CI: 0.7–1.2; P = 0.48) (Table II). There were no pregnancy losses in the time period from primary outcome to live birth; therefore the live birth rate was 59.2% (45/76) in the rhG-CSF group, and 64.9% (48/74) in the placebo group, giving a relative risk of 0.9 (95% CI: 0.7–1.2; P = 0.48). During the study, clinical pregnancies were confirmed by ultrasound scan at 6 weeks of gestation in 136 (90.7%) of the 150 randomized participants [67/76, 88.2% in the rhG-CSF group vs 69/74, 93.2% in the placebo group, RR of 0.9 (95% CI: 0.9–1.0; P = 0.28)]. Ongoing pregnancies were confirmed at ~12 weeks in 96 (64.0%) of the women [45/76, 59.2% in the rhG-CSF group versus 51/74, 68.9% in the placebo group, RR of 0.9 (95% CI: 0.7–1.1; P = 0.224)].
Table II.
Primary outcome and secondary outcomes of participants in this trial.
| rhG-CSF | Placebo | Relative risk | P value | |
|---|---|---|---|---|
| no./total | no. (%) (95% CI) | |||
| Primary outcome | ||||
| Live birth after 20 weeks of gestation | 45/76 (59.2) | 48/74 (64.9) | 0.9 (0.7, 1.2) | 0.48 |
| Secondary outcomes | ||||
| Pregnancy outcomes | ||||
| Clinical pregnancy at 6 weeks | 67/76 (88.2) | 69/74 (93.2) | 0.9 (0.9, 1.0) | 0.28 |
| Ongoing pregnancy at 8 weeks | 51/76 (67.1) | 59/74 (79.7) | 0.8 (0.7, 1.0) | 0.09 |
| Ongoing pregnancy at 12 weeks | 45/76 (59.2) | 51/74 (68.9) | 0.9 (0.7, 1.1) | 0.22 |
| Live birth after 24 weeks of gestation | 45/76 (59.2) | 48/74 (64.9) | 0.9 (0.7, 1.2) | 0.48 |
| Live birth after 34 weeks of gestation | 45/76 (59.2) | 42/74 (56.8) | 1.0 (0.8, 1.4) | 0.76 |
| Ectopic pregnancy | 1/76 (1.3) | 0/74 (0.0) | NA | NA |
| Miscarriagea | 28/76 (36.8) | 25/74 (33.8) | 1.1 (0.7, 1.7) | 0.70 |
| Stillbirth | 0/76 (0.0) | 0/76 (0.0) | NA | NA |
| Preterm birth (before 37 weeks 0 days of gestation) | 5/45 (11.1) | 8/48 (16.7) | 0.7 (0.3, 2.0) | 0.54 |
| Infant birth weight (g) | ||||
| Median | 3420.0 | 3300.0 | NA | NA |
| Range | 3005–3920 | 2690–3610 | NA | NA |
| Neonatal outcomesb | ||||
| Infants discharged alive from hospital | 46/46 (100.0) | 49/49 (100.0) | NA | NA |
| Any congenital anomaly | 1/46 (2.2) | 1/49 (2.0) | 0.9 (0.1, 13.4) | 0.93 |
| Adverse eventsc | n/N (%) | n/N(%) | ||
| Maternal adverse events | 52/76 (68.4) | 43/74 (58.1) | 1.2 (0.9, 1.5) | 0.20 |
| Serious adverse events | 4/76 (5.2) | 2/74 (2.7) | 1.9 (0.3, 10.3) | 0.43 |
| Incidence of anti-drug antibody formation | 0/76 (0.0) | NA | NA | NA |
aMiscarriage was defined as spontaneous loss of a pregnancy less than 24 weeks of gestation; the median gestational age at miscarriage was 6.0 weeks (interquartile range, 6–7) in the rhG-CSF and 6.5 weeks (interquartile range, 6–9) in the placebo group. There were three pregnancies of unknown location in the rhG-CSF group.
bThe end point is listed per neonate.
cPlease see Supplementary Table SI for details.
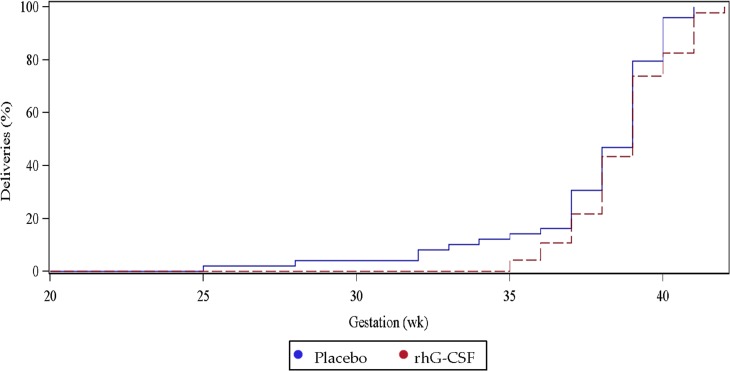
Miscarriage rates were not significantly different between the study groups (rhG-CSF 28/76, 36.8% versus placebo 25/74, 33.8%, RR of 1.1 (95% CI: 0.7–1.7, P = 0.70)). Amongst the 28 pregnancies that ended in miscarriage for participants receiving rhG-CSF, the median gestation was 6 weeks (IQR 6–7 weeks). Amongst the 25 pregnancies that ended in miscarriage for participants receiving placebo, the median gestation was 6.5 weeks (IQR 6–9 weeks). The distributions of gestational age at live birth delivery for the rhG-CSF and placebo groups are given in Fig. 2. All infants were discharged home alive from the hospital.
Figure 2.
Distribution of gestational age according to study group assignment. Only pregnancies which continued beyond 24 weeks are shown.
Adverse events (AEs) occurred in 52 (68.4%) participants in rhG-CSF group and 43 (58.1%) participants in the placebo group (Supplementary Table SI). Neonatal congenital anomalies were observed in 1/46 (2.1%) of babies in the rhG-CSF group versus 1/49 (2.0%) in the placebo group (RR of 0.9; 95% CI: 0.1–13.4; P = 0.93).
Findings of subgroup analyses are given in Supplementary Table SII; no significant subgroup effects were identified. Exploratory analyses for a number of key obstetric outcomes did not find any significant differences between the rhG-CSF and placebo arms.
Discussion
This randomized clinical trial investigating the effect of recombinant human granulocyte colony stimulating factor in the first trimester of pregnancy in women, diagnosed with unexplained recurrent pregnancy loss, found no improvement in clinical pregnancies at 20 weeks, or live births, compared to placebo.
Comparison with previous studies
The findings from this large multicentre, randomized, placebo-controlled trial do not support the findings of the only previous randomized control study evaluating recombinant G-CSF in recurrent pregnancy loss (Scarpellini and Sbracia, 2009). In this previous, smaller, single center study by Scarpellini and Sbracia, 68 women with a previous history of unexplained recurrent miscarriage were randomized to G-CSF or placebo. Women in the intervention group were started on a dose of 1 mcg/kg/day starting on sixth day after ovulation. The live birth rates in the rhG-CSF group was 82.8% versus 48.5% in the placebo group (P = 0.0061, OR = 5.1; 95% confidence interval 1.5–18.4), suggesting a statistically significant improvement in outcomes.
There are also two retrospective cohort studies in women with recurrent miscarriage which suggested improved outcomes with administration of G-CSF (Santjohanser et al., 2013, Würfel, 2013). Observational data from two separate population registry was also identified. Boxer et al., identified 224 pregnancy events in women diagnosed with chronic neutropenia and identified a decrease in abortion rates with no adverse side effects (Boxer et al., 2010). However, Zeidler et al., used data from severe chronic neutropenia international registry (SCNIR) and observed no improvement in pregnancy outcomes after administration of G-CSF (Zeidler et al., 2014). All the above studies used a varying dose and duration of G-CSF and were of poor quality (ESHRE Recurrent Pregnancy Loss Guidelines, 2017).
rhG-CSF is also widely used in assisted conception treatment. A previous review of G-CSF in reproductive medicine studies also suggested therapeutic benefit based on body weight dependent target dose or use of G-CSF as an intrauterine infusion (Cavalcante et al., 2015). This review included 1 RCT, 5 cohort studies and 1 case report in a varied range of patients. The included studies were of poor quality and the researchers called for larger well-designed studies. A more recent randomized open label clinical trial of G-CSF (using a single dose of 300 μg as an intrauterine infusion on day of oocyte recovery) in 100 infertile women undergoing in-vitro fertilization treatment did not show a benefit of G-CSF in improving pregnancy outcomes (Eftekhar et al., 2016).
Strength and limitations of this study
The strengths of our study include the multicentre study design involving 21 hospitals spread across the United Kingdom. After standard screening tests, as practiced in the United Kingdom, 150 women with unexplained recurrent pregnancy losses from different ethnic background were randomized. Thus, our study represents the largest placebo-controlled randomized control study of rhG-CSF in women with unexplained recurrent pregnancy losses. Participant compliance rate was high and all participants were followed up until completion of study endpoints, as appropriate. Unlike other studies where a varying dose of G-CSF was utilized, we initiated optimal dosage of rhG-CSF treatment as soon as the pregnancy was confirmed, which started as early as 7 days after ovulation. A Phase 1, randomized, double-blind, placebo-controlled, dose-escalation study was conducted prior to this study for dosage determination. This study consisted of six single- and multiple-dose cohorts with eight participants in each dose cohort, randomized in a 3:1 ratio to receive either rhG-CSF or placebo. Transient neutrophilia and increases in white blood cell (WBC) counts were observed following both single and multiple doses. Changes in peripheral blood cell subsets were observed consistent with supporting a state of maternal–fetal immune tolerance. These changes include the temporary induction of toleragenic cell subsets including an increase in toleragenic myeloid derived suppressor cells (MDSC) and a decrease in cytotoxic natural killer (NK) cells, without evidence of global immune changes or suppression. These changes were observed only in the multidose rhG-CSF treatment groups, and not in any placebo group. The weakness of our study was that women were not screened prior to inclusion to demonstrate immune dysfunction as the reason for their pregnancy losses. This is because there is no accepted test(s) for immune dysfunction in reproductive immunology.
There was no increased risk of congenital anomalies among offspring of women treated with rhG-CSF, although the study was not powered for such rare outcomes.
Conclusion
Among women with a history of unexplained recurrent pregnancy loss, administration of rhG-CSF in the first trimester of pregnancy, compared with placebo, did not improve the clinical pregnancy rates at 20 weeks or live birth rates.
Supplementary Material
Acknowledgements
RESPONSE was an investigator led trial sponsored by Nora Therapeutics, Inc., California, USA. We thank all the women who participated in this study; the following investigators for supervising recruitment and randomization at the study centers: Maya Chetty, Lisa Starrs, Manisha Chandra, Yakoub Khalaf, Gourab Misra, Shamma Al-Inizi, Vinita Raheja, Ayman Ewies; all the RESPONSE research nurses who assisted in the collection of data; study pharmacists; Hugh H Tilson for chairing the data and safety monitoring committee; Premier research, clinical research organization; The Miscarriage Association; and all those not otherwise mentioned above who have contributed to the RESPONSE study. The views and opinions expressed in this publication are of the authors and do not reflect those of the NHS, MHRA or Department of Health, UK. The RESPONSE trial was approved by the United Kingdom Medicines and Healthcare Products Regulatory Authority, the National Research Ethics Service, and the research and development department at each participating hospital. Recombinant human granulocyte – colony stimulating factor (rhG-CSF) and placebo were manufactured and supplied by Nora Therapeutics, 530 Lytton Avenue, second Floor, Palo Alto, CA 94301.
Appendix
RESPONSE study group consist of: A Eapen (Tommy’s National Centre for Miscarriage Research, Institute of Metabolism and Systems Research, University of Birmingham, UK & University of Iowa Hospitals and Clinics, Carver college of Medicine, Iowa, USA), M Joing, P Kwon, J Tong & D Carter (Nora Therapeutics, California, USA), E Maneta, C De Santo & F Mussai (Tommy’s National Centre for Miscarriage Research, Institute of Metabolism and Systems Research, University of Birmingham, UK), A Ahmed (Sunderland Royal Hospital, Sunderland, UK), C Bass (St. Peters Hospital, Ashford, UK), R Bender-Atik (Miscarriage Association, UK), R Bhattacharya (Derriford Hospital, Plymouth, UK), Y Cheong (University of Southampton, UK), F Dawood (Liverpool Women’s Hospital, UK), I Granne (University of Oxford, UK), P Gupta (Birmingham Heartlands Hospital, UK), A Horne (MRC Centre for Reproductive Health, Edinburgh, UK), P Manda (James Cook University Hospital, Middlesborough, UK), L Mohiyiddeen (St Mary’s Hospital, Manchester, UK), J Moore (Queens Medical Centre, Nottingham, UK), S Quenby (University Hospital, Coventry, UK), R Rai (St Mary’s Hospital, London, UK), J Shillito (St James’ Hospital, Leeds, UK), J Stewart (Royal Victoria Infirmary, Newcastle upon Tyne, UK), E Truchanowicz (Alta Innovations Ltd, UK), L Dwyer (St Mary’s Hospital, Manchester, UK), R Small (Birmingham Heartlands Hospital, UK)., L Sharpe (St. Peters Hospital, Ashford), A Smith (Liverpool Women’s Hospital, UK), D Lissauer & A Coomarasamy (Tommy’s National Centre for Miscarriage Research, Institute of Metabolism and Systems Research, University of Birmingham, UK).
Contributor Information
RESPONSE study group:
A Eapen, M Joing, P Kwon, J Tong, D Carter, E Maneta, C De Santo, F Mussai, A Ahmed, C Bass, R Bender-Atik, R Bhattacharya, Y Cheong, F Dawood, I Granne, P Gupta, A Horne, P Manda, L Mohiyiddeen, J Moore, S Quenby, R Rai, J Shillito, J Stewart, E Truchanowicz, L Dwyer, R Small, L Sharpe, A Smith, D Lissauer, and A Coomarasamy
Authors’ roles
The first, second and last author vouch for the accuracy of the data and analyses and for the fidelity of the study to the protocol. Conception of the study: Darryl Carter, Mark Joing, Paul Kwon and Jeffrey Tong. Data interpretation and writing of the report: Abey Eapen, Mark Joing, David Lissauer and Darryl Carter. Members of trial steering committee: Abey Eapen, Mark Joing and, Paul Kwon. Biomarker study: Ebtehaj Maneta, Carmela De, Francis Mussai and David Lissauer.
Funding
The RESPONSE study was sponsored and supported by Nora Therapeutics, Inc., 530 Lytton Avenue, 2nd Floor, Palo Alto, CA 94301, USA.
Conflict of interest
Darryl Carter was the co-founder and VP of research, Nora Therapeutics, Inc. and held shares in the company. He holds a patent for the use of recombinant human granulocyte colony stimulating factor to reduce unexplained recurrent pregnancy loss. Mark Joing, Paul Kwon and Jeff Tong were or are employees of Nora Therapeutics, Inc. No other potential conflict of interest relevant to this article was reported.
References
- Boxer LA, Boyard AA, Marrero TT, Alter BP, Bonilla MA, Link D, Newburger PE, Rosenberg PS, Shimamura A, Dale DC 490 outcomes of pregnancies for women with severe chronic neutropenia with and without G-CSF treatment. Blood In: 52nd ASH AnnualMeeting, Abstract 4786. 2010.
- Carton E, Bellesoeur A, Mir O. Colony-stimulating factors for febrile neutropenia. N Engl J Med 2013;369:285–286. [DOI] [PubMed] [Google Scholar]
- Cavalcante MB, Costa FD, Araujo Júnior E, Barini R. Risk factors associated with a new pregnancy loss and perinatal outcomes in cases of recurrent miscarriage treated with lymphocyte immunotherapy. J Matern Fetal Neonatal Med 2014;28:1–5. [DOI] [PubMed] [Google Scholar]
- Cavalcante MB, Costa Fda S, Barini R, Araujo Júnior E. Granulocyte colony-stimulating factor and reproductive medicine: A review. Iran J Reprod Med 2015;13:195–202. [PMC free article] [PubMed] [Google Scholar]
- Clark DA. Should anti-TNF-alpha therapy be offered to patients with infertility and recurrent spontaneous abortion? Am J Reprod Immunol 2009;61:107–112. [DOI] [PubMed] [Google Scholar]
- Eftekhar M, Hosseinisadat R, Baradaran R, Naghshineh E. Effect of granulocyte colony stimulating factor (G-CSF) on IVF outcomes in infertile women: an RCT. Int J Reprod Biomed (Yazd) 2016;14:341–346. [PMC free article] [PubMed] [Google Scholar]
- ESHRE Recurrent Pregnancy Loss Guidelines; 2017.
- Ford HB, Schust DJ. Recurrent pregnancy loss: etiology, diagnosis, and therapy. Rev Obstet Gynecol 2009;2:76–83. [PMC free article] [PubMed] [Google Scholar]
- Gleicher N, Vidali A, Barad DH. Successful treatment of unresponsive thin endometrium. Fertil Steril 2011;95:e13–e17. [DOI] [PubMed] [Google Scholar]
- Jeve YB, Davies W. Evidence-based management of recurrent miscarriages. J Hum Reprod Sci 2014;7:159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledee N, Lombroso R, Lombardelli L, Selva J, Dubanchet S, Chaouat G, Frankenne F, Foidart JM, Maggi E, Romagnani S et al. Cytokines and chemokines in follicular fluids and potential of the corresponding embryo: the role of granulocyte colony-stimulating factor. Hum Reprod 2008;23:2001–2009. [DOI] [PubMed] [Google Scholar]
- Meng L, Lin J, Chen L, Wang Z, Liu M, Liu Y, Chen X, Zhu L, Chen H, Zhang J. Effectiveness and potential mechanisms of intralipid in treating unexplained recurrent spontaneous abortion. Arch Gynecol Obstet 2016;294:29–39. [DOI] [PubMed] [Google Scholar]
- Mowbray SF, Gibbons C, Lidell H, Reginald PW, Underwood JL, Beard RW. Controlled trial of treatment of recurrent spontaneous abortion by immunization with paternal cells. Lancet 1985;1:941–943. [DOI] [PubMed] [Google Scholar]
- Nyborg KM, Kolte AM, Larsen EC, Christiansen OB. Immunomodulatory treatment with intravenous immunoglobulin and prednisone in patients with recurrent miscarriage and implantation failure after in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril 2014;102:1650–1655. [DOI] [PubMed] [Google Scholar]
- Salmassi A, Schmutzler AG, Huang L, Hedderich J, Jonat W, Mettler L. Detection of granulocyte colony-stimulating factor and its receptor in human follicular luteinized granulosa cells. Fertil Steril 2004;81:786–791. [DOI] [PubMed] [Google Scholar]
- Santjohanser C, Knieper C, Franz C, Hirv K, Meri O, Schleyer M, Würfel W, Toth B. Granulocyte-colony stimulating factor as treatment option in patients with recurrent miscarriage. Arch Immunol Ther Exp (Warsz) 2013;61:159–164. [DOI] [PubMed] [Google Scholar]
- Scarpellini F, Sbracia M. Use of granulocyte colony-stimulating factor for the treatment of unexplained recurrent miscarriage: a randomised controlled trial. Hum Reprod 2009;24:2703–2708. [DOI] [PubMed] [Google Scholar]
- Thomas J, Liu F, Link DC. Mechanisms of mobilization of hematopoietic progenitors with granulocyte colony-stimulating factor. Curr Opin Hematol 2002;9:183–189. [DOI] [PubMed] [Google Scholar]
- Williams Z. Inducing tolerance to pregnancy. N Engl J Med 2012;367:1159–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Würfel JW. G-CSF treatment of patients with recurrent implantation failures (RIF) and recurrent spontaneous abortions (RSA). J Reprod Immunol 2013;101/102:S25. [DOI] [PubMed] [Google Scholar]
- Zeidler C, Grote UA, Nickel A, Brand B, Carlsson G, Cortesão E, Dufour C, Duhem C, Notheis G, Papadaki HA et al. Outcome and management of pregnancies in severe chronic neutropenia patients by the European Branch of the Severe Chronic Neutropenia International Registry. Haematologica 2014;99:1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.