Abstract
Background: SCN5A with Brugada syndrome (BrS) is not commonly considered as an independent risk marker for subsequent cardiac events. However, the risk of SCN5A combined with other clinical characteristics has not been fully investigated.
Objectives: The aim of this study is to investigate and evaluate risk stratification and related risk factors of SCN5A in BrS.
Methods: The databases of PubMed, EMBASE, Cochrane Library, MEDLINE, Chinese National Knowledge Infrastructure (CNKI) and Wanfang Data were searched for related studies published from January 2002 to May 2018 followed by meta-analysis. The BrS patients who underwent SCN5A gene tests were included. The prognosis and risk stratification of SCN5A combined with symptoms and asymptoms diagnosis in BrS, electrophysiology study (EPS) were then investigated and evaluated. Outcomes were defined as ventricular tachycardia/fibrillation (VT/VF), sudden cardiac death (SCD).
Results: Eleven suitable studies involving 1892 BrS patients who underwent SCN5A gene tests were identified. SCN5A (+) was not considered to be a significant predictor of future cardiac events (95% CI: 0.89–2.11; P = 0.15; I2 = 0%). However, SCN5A (+) patients with symptoms at diagnosis revealed a higher prevalence of future VT/VF, SCD compared to SCN5A (–) patients with symptoms at diagnosis. (95% CI: 1.06–3.70; P = 0.03 I2 = 0%) Among asymptomatic patients, the risk did not significantly differ between SCN5A (+) patients and SCN5A (–) patients. (95% CI: 0.51–4.72; P = 0.45 I2 = 0 %). In an investigation involving patients in EPS (–) BrS electrocardiogram (ECG), the risk of SCN5A (+) is higher than that of SCN5A (–) (P < 0.001).
Conclusions: In BrS patients with symptoms at diagnosis or EPS (–), the meta-analysis suggests that SCN5A (+) are at a higher risk of arrhythmic events than SCN5A (–).
Keywords: SCN5A, brugada syndrome, electrophysiology study, arrhythmia, sudden cardiac death
Introduction
BrS is an inheritable arrhythmogenic syndrome in a structurally integrated heart. According to current guidelines, it is the features of an ST segment elevation in the precordial leads which is related to improved danger of SCD (Priori et al., 2013). SCN5A gene mutation, as a risk factor for BrS, its prognostic significance in the general population remains controversial. On one hand, the present guideline shows that SCN5A mutation status cannot be an independent predictor of future cardiac events (Priori et al., 2013). On the other hand, BrS patients with SCN5A-mediated have higher prevalence of incidences of bradyarrhythmia events and conduction abnormalities (Yamagata et al., 2017). Recently, an important study particularly reported that SCN5A was the only gene which is clinically associated with BrS among the 21 included genes (Hosseini et al., 2018). Therefore, we initially preformed a comprehensive systematic review and meta-analysis of published data to elucidate the effect on mutations in SCN5A with symptoms and EPS, among the patients with BrS.
Methods
Search Strategy and Inclusion Criteria
A comprehensive literature research on MEDLINE, Embase, CNKI, and Wanfang Data databases was performed by two investigators. We used the query terms “Brugada syndrome” and “SCN5A Mutation” to identify and retrieve all potentially relevant studies from January 2002 to May 2018. Only full-size English articles published in peer-reviewed journals were considered for this meta-analysis. Studies were considered to be suitable whether they met the following criteria:
the study was a prospective or retrospective observational study;
inclusion of subjects with BrS were as previously defined;
inclusion of patients who underwent SCN5A gene tests;
the follow- up duration was long enough that the arrhythmia events would be observed;
endpoint events [appropriate implantable cardioverter-defibrillator therapy (ICD), VF/VT, and SCD] were clearly defined;
patients with endpoint events were clearly identified if they had SCN5A mutations;
risk ratio (RR), hazard ratio (HR), odds ratio (OR), corresponding 95% confidence intervals (CIs), or necessary original data were presented.
In addition, we also contacted several corresponding authors of the studies to obtain more specific experimental data which were not included in the articles. Studies which demonstrated on only compound endpoints but particular data on all-cause mortality or different patient groups were not taken into account. In order to resolve the disagreements or uncertainties between the two investigators, a third investigator was responsible for rechecking the source data and consultation.
Data Extraction
The elements of the extracted data were included in the meta-analysis: (a) publication information: surname of first author, publication year, and location; (b) type of study: multi-center or single-center study; (c) study design; (d) follow-up duration; (f) endpoint events (arrhythmic events were defined as VT/VF, SCD, and the combination of those two during the follow-up); (g) the quality score; (h) the characteristics of the population comprising sample size, gender, age, number of subjects with and without cardiac events, number of subjects with history of sudden cardiac arrest (SCA), syncope and family history of SCD. It also included the number of subjects with ICD, number of subjects with spontaneous type 1 ECG, and non-spontaneous type 1 ECG, number of symptomatic subjects with spontaneous type 1 ECG and non-spontaneous type 1 ECG, number of subjects who underwent EPS, the number of subject with EPS positive and EPS negative, number of EPS positive subjects who underwent ex-stimulation from 1 to 3 times, number of subjects with atrial fibrillation (AF) positive, number of subjects who underwent SCN5A gene test, number of subjects with SCN5A positive and SCN5A negative, number of symptomatic subjects with SCN5A positive and SCN5A negative during follow-up, number of subjects with Fragmented QRS (f-QRS) positive and f-QRS negative, number of subjects with early repolarization (ER) positive and ER negative; (i) among SCN5A (+) subjects with future cardiac events, the number of male subjects and female subjects, the number of subjects with or without family history of SCD, spontaneous type 1 ECG, symptoms and documented AF; (j) among SCN5A (–) subjects with future cardiac events, the number of male subjects and female subjects, the number of subjects with or without family history of SCD, spontaneous type 1 ECG, and symptoms and documented AF.
Quality Assessment
The Methodological Index for Non-Randomized Studies (MINORS) was applied used for the Methodological quality to assess all studies. The use of maximum 24 points (each item scored from 0 to 2) was based on the following aspects: aim of the study, inclusion of consecutive patients, prospective data collection, appropriate endpoint to the aim of the study, unbiased evaluation of endpoints, follow-up period appropriate to the end-point, loss to follow-up no more than 5%, comparable control group, contemporary groups, base-line equivalence of groups, prospective calculation of the sample size, and use of adequate statistical analysis. After two independent investigators valued the included publications, the mean MINORS score was assessed as the final result. Studies were considered to be of low quality and high quality according to their MINORS scores of < 16 and ≥16 points, respectively (Slim et al., 2003).
Statistical Analysis
A series of meta-analysis were performed including an analysis of all the patients who underwent SCN5A gene test and 8 subgroups, using Review Manager, version 5.3.5 (Revman; The Cochrane Collaboration, Oxford, U.K.). The concludes of the cardiac events outcome are indicated as ORs with 95% CIs for each study. To assess the heterogeneity among studies, the I2 value from the chi-square test was used, which describes the percentage of the variability in effect estimates due to heterogeneity, rather than sampling error. An I2 >50% indicates at least moderate statistical heterogeneity (Higgins et al., 2003).
We extracted data from 3 studies to compare categorical variables applying either a chi-square test or Fisher test (Sacher et al., 2013; Tokioka et al., 2014; Yamagata et al., 2017). TheSPSS 17.0 statistical package (SPSS Inc., IL, USA) was used to perform the analysis. In each analysis, statistical significance for treatment effect was defined at P < 0.05.
Results
Study Selection
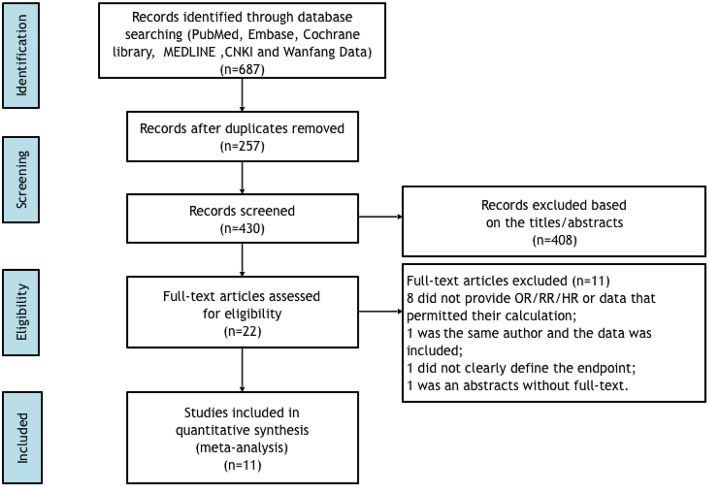
A flow chart of the data research and study selection is shown in Figure 1. We excluded 257 duplicate studies across the number of 687 records that were identified by our research criteria. After screening the titles and abstracts, 408 studies were into the discard since they were categorized as guidelines, editorials, case reports, review articles, animal studies, laboratory studies, or unrelated to the present study. Then, 22 potential relevant studies were retrieved for specific evaluation. Of these, a number of 11 studies were further excluded from further analysis because of the following reasons: 8 studies did not provide RRs, ORs, or HRs or data could be calculated, or the 95% confidence intervals; one study did not clearly define the type of abnormal QRS complex; one did not clearly define the endpoints, and one was only an abstract without full-text.
Figure 1.
Flow diagram of data search and study selection.
Eleven studies (six prospective and five retrospective) were ultimately involved in this meta-analysis constituting 1892 patients with BrS in total (Table 1) (Gasparini et al., 2002; Juang et al., 2003; Mok et al., 2004; Liang et al., 2006; Yuan et al., 2008; Priori et al., 2012; Sacher et al., 2013; Tokioka et al., 2014; Andorin et al., 2016; Calò et al., 2016; Yamagata et al., 2017). The average age of the BrS patients was from 11 to 53 years old. A spontaneous type 1 ECG pattern of BS was reported in 68.8% of patients and a SCN5A gene test was performed on 1075 patients (56.8%). A positive genetic mutation was demonstrated in 248 patients (23.1%). Among these, 41 patients (16.5%) were demonstrated to have symptoms. Whereas, a total of 127 patients (15.4%) out of 827 patients (76.9%) with a negative SCN5A gene mutation were symptomatic. The mean follow-up duration ranged from 20 to 77 months. During follow-up, 229 patients (12.1%) suffered an arrhythmia event (syncope, non-sustained VT, aborted sudden cardiac death, and appropriate ICD shocks caused by VT/VF). All involved studies were assessed as high-quality publications (average MINORS score: 15 ± 2.9). In addition, we pursued further analysis to attempt to establish the relationship among SCN5A, other clinical features and subsequent cardiac events. The clinical characteristics of the 698 BrS patients from 3 studies are summarized in Table 2 and Figure 3 (Sacher et al., 2013; Tokioka et al., 2014; Yamagata et al., 2017). It consists of 660 male patients and 37 female patients. In the SCN5A+ and SCN5A– patient groups, 23 and 83 individuals experienced subsequent cardiac events, respectively. A total of 161 patients had a family history of SCD. A spontaneous type 1 BrS ECG was demonstrated in 72% of patients and 577 patients underwent EPS in total, with 329 patients displaying positive results. With regard to documented AF, 2 of the 100 AF positive patients and 82 of the 586 AF negative patients had arrhythmia events during follow-up.
Table 1.
Study characteristics of 11 studies included in meta-analysis.
| Investigator | Location | Type of study | Study of design | Study population | Mean follow-up | Endpoint | Quality score |
|---|---|---|---|---|---|---|---|
| Gasparini et al., 2002 | Italy | SC | PS | Patients with BrS underwent a PES protocol | 20 ± 12 months | PES protocol completion/induction of sustained/reproducible nonsustained fast ventricular arrhythmia | 15 |
| Juang et al., 2003 | Taiwan | MC | RS | Patients with the diagnosis of the BrS | 29 ± 17 months | Seizure/syncope/sudden cardiac death | 14 |
| Mok et al., 2004 | Hong Kong | MC | PS | Patients with type 1 Brugada ECGs | 25.8 ± 10.9monthes | Syncope/syncopal ventricular arrhythmia/sudden death/appropriate ICD shock | 20 |
| Liang et al., 2006 | China | SC | PS | Patients with Brugada ECGs or suspected Brs | NA | Syncope/VT | 13 |
| Yuan et al., 2008 | China | SC | PS | patients with Brugada ECGs | NA | Syncope/VT | 8 |
| Priori et al., 2012 | Italy | MC | PS | Patients with type 1 ECGs, without history of cardiac arrest | 36 ± 8 months | The occurrence of VF or appropriate ICD interventions | 16 |
| Sacher et al., 2013 | France | SC | RS | Patients with type 1 Brugada ECGs withimplantable cardioverter-defibrillator | 77 ± 42 months | Aborted sudden cardiac arrest/syncope | 16 |
| Tokioka et al., 2014 | Japan | SC | RS | Patients with a Brugada-type ECG | 45.1 ± 44.3 months | VF/SCD | 16 |
| Andorin et al., 2016 | Europe | MC | RS | Patients with Brugada ECG under 19 years of age | 54 months | Sudden death/documented VT or VF/appropriate ICD shock | 15 |
| Calò et al., 2016 | Italy | MC | PS | Patients with spontaneous type 1 BrS ECG phenotype | 48 ± 38.6 months | VF/SCD | 16 |
| Yamagata et al., 2017 | Japan | MC | RS | Patients with type 1 Brugada ECG pattern | 72 months | Documented atrial fibrillation/appropriate ICD interventions | 16 |
BrS, Brugada syndrome; ECG, electrocardiogram; ICD, implantable cardioverter defibrillator; PES, Programmed Electrical Stimulation; MC, multicenter study; NA, not available; n, number; PS, prospective study; RS, retrospective study; SC, single center study; SCD, sudden cardiac death; VF, ventricular fibrillation; VT, ventricular tachycardia.
Table 2.
Patients' characteristics of 11included studies.
| Gasparini et al., 2002 | Juang et al., 2003 | Mok et al., 2004 | Liang et al., 2006 | Yuan et al., 2008 | Priori et al., 2012 | Sacher et al., 2013 | Tokioka et al., 2014 | Andorin et al., 2016 | Calò et al., 2016 | Yamagata et al., 2017 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Patients, n | 21 | 10 | 50 | 4 | 7 | 308 | 378 | 246 | 106 | 347 | 415 |
| Male/female, n | 18/3 | 10/0 | 47/3 | 4/0 | 7/0 | 247/61 | 310/68 | 236/10 | 58/48 | 272/75 | 403/12 |
| Age (years) | 34 | 46 ± 7 | 53 | 40.9 | 43.6 ± 8.7 | 47 ± 12 | 46 ± 13 | 47.6 ± 13.6 | 11.1 ± 5.7 | 45 ± 13.1 | 46 ± 14 |
| Symptomatic, n (%) | 0 (0) | 3 (30) | 30 (60) | 4 (100) | 4 (57) | 14 (4) | 46 (12) | 24 (10) | 10 (9) | 32 (9) | 62 (15) |
| History of SCA, n (%) | 1 (5) | 9 (90) | 8 (16) | NA | 3 (43) | NA | 31 (8) | 13 (5) | NA | 0 (0) | 88 (21) |
| History of sycope, n (%) | 8 (38) | 1 (10) | 12 (24) | 2 (20) | 4 (57) | 65 (21) | 181 (48) | 40 (16) | 15 (14) | 14 (4) | 99 (24) |
| Asymptomatic, n (%) | 12 (57) | 0 (0) | 30 (60) | 0 (0) | 3 (43) | 243 (80) | 166 (44) | NA | 85 (80) | 316 (91) | 228 (55) |
| Family history of SCD, n (%) | 8 (38) | 1 (10) | 7 (14) | NA | 3 (43) | NA | 111 (30) | 69 (28) | 46 (43) | 71 (20) | 64 (15) |
| Patients with ICD, n (%) | NA | 8 (80) | 8 (16) | NA | NA | 137 (44) | 308 (81) | 63 (26) | 22 (21) | 98 (28) | 241 (58) |
| Spontaneous type1 ECG, n (%) | 19 (90) | NA | 43 (86) | NA | 5 (71) | 171 (56) | 226 (60) | 156 (63) | 36 (34) | 347 (100) | 299 (72) |
| Events, n (%) | 0 (0) | NA | 17 (39) | NA | 3 (60) | 13 (8) | 35 (15) | 22 (14) | 8 (22) | 32 (10) | 48 (16) |
| Non-Spontaneous type1 ECG, n (%) | 2 (9.5) | NA | 7 (14) | NA | 0 (0) | NA | 152 (40) | 90 (37) | 70 (66) | 0 (0) | 116 (28) |
| Events,n (%) | 0 (0) | NA | 3 (43) | NA | 0 (0) | NA | 11 (7) | 2 (2) | 2 (3) | 0 (0) | 14 (12) |
| Patients ungergo EPS, n (%) | 21 (100) | 8 (80) | 30 (60) | NA | 7 (100) | NA | 310 (82) | 155 (63) | NA | 186 (54) | 339 (82) |
| EPS+, (n) | 18 (86) | 6 (75) | 19 (63) | NA | NA | NA | 228 (73) | 71 (46) | NA | 77 (41) | 191 (56) |
| up to 1 ex | 0 (0) | NA | 2 (10) | NA | NA | NA | NA | NA | NA | NA | NA |
| up to 2 ex | 12 (67) | NA | 9 (47) | NA | NA | NA | NA | NA | NA | NA | NA |
| up to 3 ex | 6 (33) | NA | 8 (42) | NA | NA | NA | NA | NA | NA | NA | NA |
| EPS-, (n) | 3 (14) | 2 (25) | 11 (37) | NA | 1 (14.3) | NA | 82 (26) | 84 (54) | NA | 109 (59) | 148 (44) |
| AF (+), n (%) | NA | NA | NA | NA | NA | NA | 32 (8) | 44 (17) | NA | NA | 64 |
| Patients undergo DNA tested, n (%) | 21 (100) | 4 (40) | 36 (72) | 4 (100) | 7 (100) | 123 (40) | 160 (42) | 123 (50) | 75 (71) | 107 (31) | 415 (100) |
| SCN5A (+), n (%) | 8 (38) | 1 (25) | 5 (14) | 1 (25) | 1 (14) | 24 (20) | 41 (26) | 17 (26) | 58 (77) | 32 (30) | 60 (14) |
| events, n (%) | 0 (0) | 1 (100) | 2 (40) | 1 (100) | 0 (0) | 3 (13) | 6 (15) | 4 (24) | 9 (16) | 2 (6) | 13 (22) |
| SCN5A (−), n (%) | 13 (62) | 3 (75) | 31 (86) | 3 (75) | 6 (86) | 99 (80) | 119 (74) | 106 (74) | 17 (23) | 75 (70) | 355 (86) |
| events, n (%) | 0 (0) | 2 (67) | 18 (58) | 3 (100) | 4 (67) | 6 (14) | 16 (13) | 19 (18) | 0 (0) | 10 (13) | 49 (14) |
| f-QRS (+), n (%) | NA | NA | NA | NA | NA | 25 (8) | NA | 78 (32) | NA | 85 (25) | NA |
| f-QRS (–), n (%) | NA | NA | NA | NA | NA | 283 (92) | NA | 158 | NA | 262 (76) | NA |
| ER (+), n (%) | NA | NA | NA | NA | NA | NA | NA | 25 (10) | NA | 30 (9) | NA |
| ER (–), n (%) | NA | NA | NA | NA | NA | NA | NA | 221 | NA | 317 (91) | NA |
Data were presented as mean ± SD, median, or percentage where possible; SCA, sudden cardiac arrest; NA, not available; SCD, sudden cardiac death; ICD, implantable cardioverter defibrillator;ECG, electrocardiogram; EPS, electrophysiological study; n, number; ex, extrastimuli; f-QRS, fragmented QRS; ER, early repolarization.
As for the symptomatic and asymptomatic BrS patients, we added an extra study based on the results from Andorin et al. and only did the meta-analysis (Andorin et al., 2016). Therefore, a total of 317 patients with symptoms at diagnosis and 456 patients without symptoms at diagnosis were identified. The symptoms were defined as patients with a history of ACA, SCD, or syncope.
SCN5A (+) and SCN5A (–) Groups
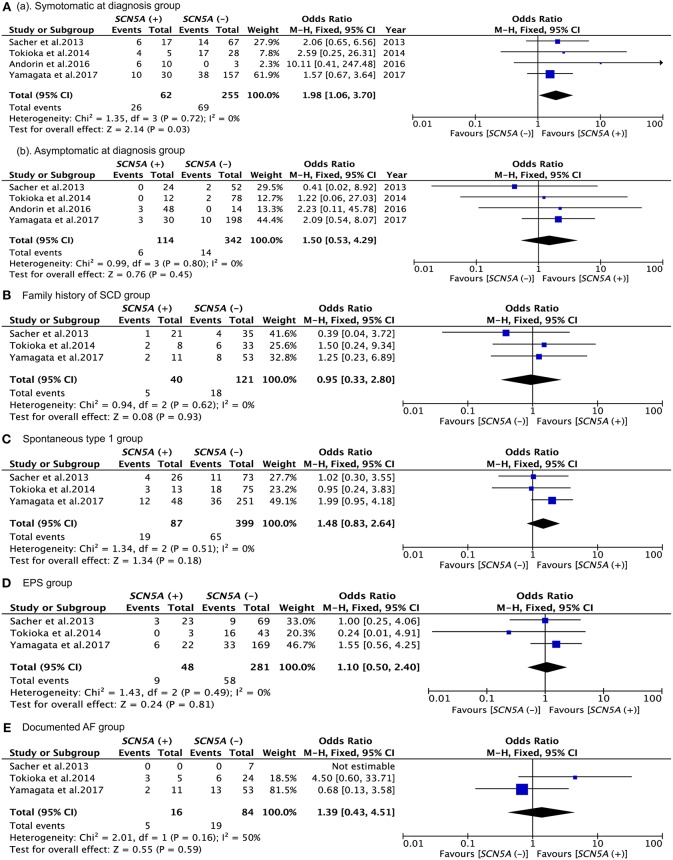
Overall, BrS patients with a positive SCN5A gene mutation were not proven to be a significant predictor of future cardiac events (OR 1.37, 95% CI: 0.89–2.11, P = 0.15; Heterogeneity: P = 0.52, I2 = 0%, Supplementary Figure 1).
Symptomatic at Diagnosis and Asymptomatic at Diagnosis Groups
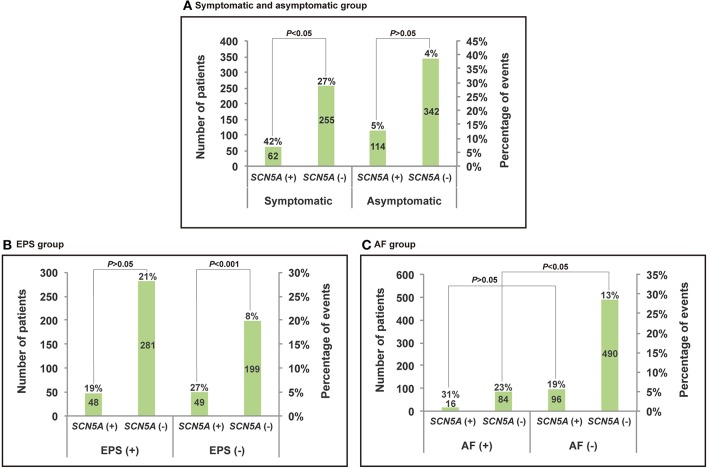
The results of the analysis are presented in Figures 2, 3 and Table 3. According to the present meta-analysis, 26 (42%) of 62 (20%) SCN5A (+) patients and 69 (27%) of 255 (80%) SCN5A (–) patients had cardiac events. A total of 6 (5%) in 114 (25%) SCN5A (+) patients and 14 (4%) in 342 (75%) SCN5A (–) patients experienced future arrhythmic events (Figure 3) (Sacher et al., 2013; Tokioka et al., 2014; Andorin et al., 2016; Yamagata et al., 2017). In comparison with the asymptomatic at diagnosis patients (OR: 1,54, 95% CI: 0,51–4,72, P = 0.45; Heterogeneity: P = 0.62, I2 = 0 %, Figure 3), SCN5A (+) patients who were symptomatic at diagnosis displayed an increased risk of arrhythmic events. (OR 1,98, 95% CI: 1,06–3,70, P = 0.03; Heterogeneity: P = 0.72, I2 = 0%, Figure 3) (Sacher et al., 2013; Tokioka et al., 2014; Andorin et al., 2016; Yamagata et al., 2017).
Figure 2.
Histogram and broken line for the comparison between events of SCN5A (+) and SCN5A (–) of subgroups. (A) Symptomatic at diagnosis group. (B) EPS group. (C) AF group.
Figure 3.
Forest plots comparing outcomes of subgroups. (Aa) Comparison between events of SCN5A (+) and SCN5A (–) in symptomatic at diagnosis group; (Ab) comparison between events of SCN5A (+) and events of SCN5A (–) in asymptomatic at diagnosis group; (B) comparison between events of SCN5A (+) and SCN5A (–) in family history of SCD group; (C) comparison between events of SCN5A (+) and SCN5A (–) in spontaneous type 1 group; (D) comparison between events of SCN5A (+) and SCN5A (–) in EPS group; (E) comparison between events of SCN5A (+) and SCN5A (–) in documented AF group.
Table 3.
Comparison between subgroup of SCN5A (+) group and SCN5A (–) group.
| SCN5A (+) | SCN5A (–) | P-value | |||
|---|---|---|---|---|---|
| EPS status | Positive | Total | 48 | 281 | 0.764 |
| Events | 9 | 58 | |||
| Negative | Total | 49 | 199 | *<0.001 | |
| Events | 13 | 15 | |||
| P-value | 0.360 | *<0.001 | |||
| Spontaneous type 1 ECG | Positive | Total | 87 | 399 | 0.215 |
| Events | 19 | 65 | |||
| Negative | Total | 31 | 181 | 0.691 | |
| Events | 4 | 19 | |||
| P-value | 0.281 | 0.090 | |||
| Documented AF status | Positive | Total | 16 | 84 | 0.495 |
| Events | 5 | 19 | |||
| Negative | Total | 96 | 490 | 0.142 | |
| Events | 18 | 64 | |||
| P-value | 0.252 | *0.021 |
Bold value means statistically significant (
P < 0.05).
Family History of SCD Group
The results of the analysis are presented in Figures 2, 3 and Table 3. During follow-up, 5 (13%) of 40 (25%) SCN5A (+) patients and 16 (13%) of 121 (75%) SCN5A (–) patients had arrhythmic events (Sacher et al., 2013; Tokioka et al., 2014; Yamagata et al., 2017). The meta-analysis result revealed that a family history of SCD had little influence on the incidence of future events among SCN5A (+) patients. (OR = 0.95, 95% CI: 0.33–2.80, P = 0.62; Heterogeneity: P = 0.93, I2 = 0%, Figure 3).
Spontaneous Type 1 BrS ECG Groups
The results of the analysis are presented in Figure 3 and Table 3. Cardiac events were documented, respectively in 22% SCN5A (+) and 16% SCN5A (–) groups, with no significant difference for patients with spontaneous type 1 BrS ECG patterns (OR = 1.48, 95% CI: 0.83–2.64, P = 0.18; Heterogeneity: P = 0.51, I2 = 0%, Figure 3) (Sacher et al., 2013; Tokioka et al., 2014; Yamagata et al., 2017). A similar result was demonstrated for the chi-square test as well. (P = 0.215 vs. P = 0.691, Table 3).
Electrophysiological Study Groups
The results of the analysis are presented in Figures 2, 3 and Table 3. SCN5A (+) and SCN5A (–) patients who were symptomatic during follow-up were presented in 19 and 21%, respectively (Figure 3) (Sacher et al., 2013; Tokioka et al., 2014; Yamagata et al., 2017). No statistically significance difference was revealed with respect to the patients with EPS positive between the SCN5A (+) group and the SCN5A (–) group. (OR = 1.12, 95 % CI: 0.51–2.44, P = 0.78; Heterogeneity: P = 0.50, I2 = 0%, Figure 3). According to the results from Table 3, SCN5A (–) BrS patients with positive EPS results had a higher prevalence for future arrhythmia events. (P = 0.764 vs. P < 0.001). Furthermore, SCN5A (+) patients with a negative EPS result had a higher prevalence of cardiac events compared to the case of for SCN5A (–) patients. (P = 0.0.360 vs. P < 0.001).
Documented AF Groups
The results of the analysis are summarized in Figures 2, 3 and Table 3 (Sacher et al., 2013; Tokioka et al., 2014; Yamagata et al., 2017). During follow-up, 6 (31%) of 16 SCN5A (+) patients and 17 (23%) of 84 SCN5A (−) patients had arrhythmic (OR = 2,10, 95% CI: 0.69–6.39, P = 0.19; Heterogeneity: P = 0.13, I2 = 50 %, Figure 3) (Sacher et al., 2013; Tokioka et al., 2014; Yamagata et al., 2017). In comparison with SCN5A (–) patients with AF, no statistically significant difference was observed for SCN5A (+) patients with AF. (P = 0.495 vs. P = 0.142). Based on the results from Table 3, SCN5A (–) patients with documented AF had a higher rate of cardiac events compared to SCN5A (–) patients without AF (P = 0.021).
Discussion
The meta-analysis yielded the following main findings: (a) the SCN5A gene mutation may not be associated with subsequent cardiac events; (b) SCN5A (+) patients with symptoms at diagnosis display a higher risk of arrhythmic events compared to SCN5A (–) patients with symptoms at diagnosis (c) Among EPS (–) BrS patients, SCN5A (+) patients have a higher prevalence of future cardiac events compared to SCN5A (–) patients. Compared with SCN5A (–) BrS patients with negative EPS results, SCN5A (–) BrS patients with positive EPS results had a higher prevalence for subsequent arrhythmia events.
The SCN5A gene locates on chromosome 3p21 and contains 28 exons spanning approximately 80 kb, and encodes α-subunit protein NaV1.5. In most situation, SCN5A mutations observed in BrS1 were loss-of-function mutations (Hedley et al., 2009), which results in the reduced availability of sodium channels, either by reducing trafficking and expression of channel on membrane surface, or through changing gate properties of channel (Remme, 2013). Variant mutations in SCN5A led to different mechanisms of action. Some mutations resulted in a reduced current density, INa, while others did not result in a decrease in INa. In a few cases, the picture was more complicated (Hedley et al., 2009). SCN5A gene mutation in particular regions also resulted in a worse outcome during follow-up. For example, pore regions documented by yamagata et al. were identified as being associated with a higher prevalence of future arrhythmia events (Yamagata et al., 2017).
SCN5A (+) and SCN5A (–) Group
The analysis included a total number of 1,075 patients from 11 studies over 10 countries. In accordance with the majority of previous study results, a negative conclusion was obtained. According to the current guideline, a SCN5A (+) is not a risk marker for the occurrence of BrS. Genetic testing is not recommended without a diagnostic ECG; unless, there was a successfully genotyped proband observed in family members (Priori et al., 2013). Based on Calò et al. study, patients who developed VF or SCD displayed a lower rate of mutations in SCN5A gene (Andorin et al., 2016). The present subgroup analysis indicated a similar result. Also, the EPS results and T waves changes on ECG did not differ significantly between the SCN5A (+) patients and the SCN5A (–) patients (Tokioka et al., 2014; Tse et al., 2018). On the other hand, the conclusion presented by Andorin et al is that an absent SCN5A mutation probably led to a lower risk of subsequent cardiac events (Andorin et al., 2016). Furthermore, yamagata et al. demonstrated that SCN5A gene mutation positive was an independent risk marker for cardiac events among all probands by applying the Cox proportional hazards model (HR = 1.1, 95% CI: 1.1 to 3.8, P = 0.02) (Yamagata et al., 2017).
Symptomatic at Diagnosis and Asymptomatic at Diagnosis Groups
Recently, Yamagata et al. demonstrated that SCN5A (+) probands, especially for mutation in the pore region presenting with prior ACA or syncope, were more likely to be associated with future cardiac events compared to SCN5A (–) BrS patients. However, no significant difference was revealed between asymptomatic SCN5A (+) probands and SCN5A (–) probands (Yamagata et al., 2017). In addition, a recent meta-analysis reported that symptomatic male BrS patients were at higher risk than asymptomatic male BrS patients (Yuan et al., 2018). Based on these results, we performed a further subgroup meta-analysis and extracted data from Yamagata et al. and three other studies involving 773 patients who underwent SCN5A gene test with either prior ACA or syncope. Interestingly, a significant difference was noted between the SCN5A (+) patients with symptoms at diagnosis and the SCN5A (–) patients with symptoms at diagnosis. With respect to BrS patients without symptoms at diagnosis, no significant difference was observed. Sacher et al. reported on a large multi-center registry on the outcome of BrS patients implanted with an ICD in France (Sacher et al., 2013). Tokioka et al. investigated the combination of ECG markers (depolarization and repolarization abnormalities) on risk assessment of VF in Japan. Both studies indicated that the role of the SCN5A mutation was of minor influence (Tokioka et al., 2014). Andorin et al. focused on BrS patients under age 19 at diagnosis in 16 European hospitals and concluded that there was a higher prevalence of SCN5A (+) pediatric patients with life-threatening arrhythmias (Andorin et al., 2016).
Some studies have reported that patients with SCN5A (+) had a higher prevalence of abnormalities in conduction (longer PQ interval, longer QRS duration, and frequent fragmentation). The decreased of sodium current reduces the action potential upstroke velocity, resulting in atrial and ventricular conduction deceleration accompanied by prolonged PR and QRS intervals (Remme, 2013). Moreover, there was sufficient background for the developed conduction abnormality leading to higher possibilities for arrhythmic events. Furthermore, it was confirmed that an abnormality in a cardiac ion channel may result in cell damage and death in patients with BrS. On this basis, it can be argued that the arrhythmic event may occur when a specific threshold of cell damage is reached, due to the severity of the ion channel protein mutation (Yamagata et al., 2017). The aforementioned facts may explain why SCN5A (+) patients with symptoms at diagnosis had a higher risk of arrhythmic events during follow-up in comparison to SCN5A (–) patients with symptoms at diagnosis. However, an opposite result was demonstrated by a recent study. It reported that 28 variants in SCN5A and other 9 genes in Human Gene Mutation Database were identified to be related to BrS, whereas, neither type 1 BrS ECG pattern nor abnormal J-point elevation in V1 and V2 was observed among genes mutations carriers. Besides, no difference was noted in susceptibility of syncope, ventricular cardiac events, or entirety mortality (Ghouse et al., 2017). On the contrary, Hosseini et al found that SCN5A was the only gene which is clinically associated with BrS among 21 included gene (Hosseini et al., 2018), thus, further studies needed to be done.
On the other hand, there was no significant difference between SCN5A (+) patients and SCN5A (–) patients who were asymptomatic at diagnosis. This may be due to the presence of a BrS-like ECG pattern in some cases including serious coronary events, imbalanced electrolyte, pharmacologic factors, pulmonary embolism, right bundle branch block, arrhythmogenic right ventricular cardiomyopathy, abnormalities in autonomic nervous system, and left ventricular hypertrophy (Shi et al., 2018). According to the specific situation, a certain number of patients might be wrongly diagnosed.
It is worth mentioning that in the meta-analysis, I2 was zero, which indicates that there was no analytical bias caused by a single dataset. Negative results were obtained according to the original data for each study, but a positive result was obtained when the sample size was expanded. This is the first time that SCN5A (+) is reported to increase the risk of future heart events among BrS patients in patients with symptoms at diagnosis.
EPS Groups
According to the 2017 guideline for ventricular cardiac events and SCD, further risk stratification in asymptomatic and spontaneous type 1 patients with EPS following programmed ventricular stimulation using single or double extrastimuli could be considered (Al-Khatib et al., 2018). On the other hand, the current guideline demonstrated that EPS inducibility appeared in a large number of BrS patients who suffered from previous sudden death or syncope (Priori et al., 2013). In addition, PES was of value particularly in patients with previous syncope: in this group, PES aided in the prevention of more than half of unnecessary ICD implants, when there was a follow-up at least within a mean of 30 months (Giustetto et al., 2009).
In the present study, we focused on the relationship between EPS and SCN5A gene mutation status. While few studies have investigated this relationship, negative conclusions were noted in Yamagata et al. (2017) and Andorin et al. (2016). Therefore, we performed a related analysis including 554 BrS patients who underwent EPS from three studies and obtained a positive result. The present analysis revealed that SCN5A (+) BrS patients with EPS negative probably have a higher prevalence of subsequent arrhythmia events, however, the risk of SCN5A (+) was not higher than that of SCN5A (–) in patients with EPS (+) BrS. This suggests that SCN5A may contribute to the occurrence of future events. According to previous studies, SCN5A (+) BrS patients had a longer HV interval during EPS compared to SCN5A (–) BrS patients or purely EPS positive BrS patients. This implies that the underlying electrophysiologic mechanisms of conduction block and ventricular arrhythmia are strongly correlated (Giustetto et al., 2009; Yamagata et al., 2017).
Study Limitation and Conclusions
In this study, the number of patients who underwent genetic testing was still limited, probably due to the high cost of the test. Secondly, the inadequacy of the original data prevented further analysis. In addition, SCN5A mutations can be variable with presumably differing effects on sodium channel function. Nevertheless, this study shows that positive mutation status is an important determinant of outcomes in particular subgroups described above. Finally, the relationship between BrS patients with symptoms and the higher frequency of future events needs to be further enhanced and additional experiments are required. SCN5A (+) as a risk marker of BrS should not be underestimated. SCN5A (+) patients with symptoms at diagnosis may have a prognosis significance for BrS. Furthermore, SCN5A (+) BrS patients with EPS (−) displayed a higher prevalence of future cardiac events.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
HB-M was employed by company Global Genetics Corp, Ventura, California, USA. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. The current work was supported by the National Natural Science Foundation Project of China (Grant Nos. 81430098 and 81670304), National high-level talent special support plan (No. W02020052), and the National clinical research base of TCM project of the State Administration of traditional Chinese medicine (JDZX2015007).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2019.00103/full#supplementary-material
References
- Al-Khatib S. M., Stevenson W. G., Ackerman M. J., Bryant W. J., Callans D. J., Curtis A. B., et al. (2018). AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart rhythm society. Circulation 138, e210on iat 10.1161/CIR.0000000000000548 [DOI] [PubMed] [Google Scholar]
- Andorin A., Behr E. R., Denjoy I., Crotti L., Dagradi F., Jesel L., et al. (2016). Impact of clinical and genetic findings on the management of young patients with Brugada syndrome. Heart Rhythm 13, 1274–1282. 10.1016/j.hrthm.2016.02.013 [DOI] [PubMed] [Google Scholar]
- Calò L., Giustetto C., Martino A., Sciarra L., Cerrato N., Marziali M., et al. (2016). A New electrocardiographic marker of sudden death in brugada syndrome: the S-Wave in Lead, I. J. Am. College Cardiol. 67, 1427–1440. 10.1016/j.jacc.2016.01.024 [DOI] [PubMed] [Google Scholar]
- Gasparini M., Priori S. G., Mantica M., Coltorti F., Napolitano C., Galimberti P., et al. (2002). Programmed electrical stimulation in Brugada syndrome: how reproducible are the results? J. Cardiovasc. Electrophysiol. 13, 880–887. 10.1046/j.1540-8167.2002.00880.x [DOI] [PubMed] [Google Scholar]
- Ghouse J., Have C. T., Skov M. W., Andreasen L., Ahlberg G., Nielsen J. B., et al. (2017). Numerous Brugada syndrome-associated genetic variants have no effect on J-point elevation, syncope susceptibility, malignant cardiac arrhythmia, and all-cause mortality. Genet. Med. 19, 521Med. B. 10.1038/gim.2016.151 [DOI] [PubMed] [Google Scholar]
- Giustetto C., Drago S., Demarchi P. G., Dalmasso P., Bianchi F., Masi A. S., et al. (2009). Risk stratification of the patients with Brugada type electrocardiogram: a community-based prospective study. Europace (2009) 11, 507–513. 10.1093/europace/eup006 [DOI] [PubMed] [Google Scholar]
- Hedley P. L., Jorgensen P., Schlamowitz S., Moolman-Smook J., Kanters J. K., Corfield V. A., et al. (2009). The genetic basis of Brugada syndrome: a mutation update. Hum. Mutat. 30, 1256–1266. 10.1002/humu.21066 [DOI] [PubMed] [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J., Altman D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327, 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini S. M., Kim R., Udupa S., Costain G., Jobling R., Liston E., et al. (2018). Reappraisal of reported genes for sudden arrhythmic death: an evidence-based evaluation of gene validity for brugada syndrome. Circulation 138, 1195–1205. 10.1161/CIRCULATIONAHA.118.03507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juang J. M., Huang S. K., Tsai C. T., Chiang F. T., Lin J. L., Lai L. P., et al. (2003). Characteristics of Chinese patients with symptomatic brugada syndrome in Taiwan. Cardiology 99, 182–189. 10.1159/000071247 [DOI] [PubMed] [Google Scholar]
- Liang P., Liu W., Hu D., Li C., Tao H., Li L. (2006). Three new mutations in the SCN5A gene of brugada syndrome. Chi. J. 34, 616–619. [PubMed] [Google Scholar]
- Mok N. S., Priori S. G., Napolitano C., Chan K. K., Bloise R., Chan H. W., et al. (2004). Clinical profile and genetic basis of Brugada syndrome in the Chinese population. Hong Kong Med. J. 10, 32–37. [PubMed] [Google Scholar]
- Priori S. G., Gasparini M., Napolitano C., Della Bella P., Ottonelli A. G., Sassone B., et al. (2012). Risk stratification in Brugada syndrome: results of the PRELUDE (PRogrammed ELectrical stimUlation preDictive valuE) registry. J. Am. College Cardiol. 59, 37–45. 10.1016/j.jacc.2011.08.064 [DOI] [PubMed] [Google Scholar]
- Priori S. G., Wilde A. A., Horie M., Cho Y., Behr E. R., Berul C., et al. (2013). HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm 10, 1932–1963. 10.1016/j.hrthm.2013.05.014 [DOI] [PubMed] [Google Scholar]
- Remme C. A. (2013). Cardiac sodium channelopathy associated with SCN5A mutations: electrophysiological, molecular and genetic aspects. J. Physiol. 591, 4099–4116. 10.1113/jphysiol.2013.256461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher F., Probst V., Maury P., Babuty D., Mansourati J., Komatsu Y., et al. (2013). Outcome after implantation of a cardioverter-defibrillator in patients with Brugada syndrome: a multicenter study-part 2. Circulation 128, 1739–1747. 10.1161/CIRCULATIONAHA.113.001941 [DOI] [PubMed] [Google Scholar]
- Shi S., Barajas-Martinez H., Liu T., Sun Y., Yang B., Huang C., et al. (2018). Prevalence of spontaneous Brugada ECG pattern recorded at standard intercostal leads: a meta-analysis. Int. J. Cardiol. 254, 151–156. 10.1016/j.ijcard.2017.11.113 [DOI] [PubMed] [Google Scholar]
- Slim K., Nini E., Forestier D., Kwiatkowski F., Panis Y., Chipponi J. (2003). Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J. Surg. 73, 712–716. 10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- Tokioka K., Kusano K. F., Morita H., Miura D., Nishii N., Nagase S., et al. (2014). Electrocardiographic parameters and fatal arrhythmic events in patients with Brugada syndrome: combination of depolarization and repolarization abnormalities. J. Am. College Cardiol. 63, 2131–2138. 10.1016/j.jacc.2014.01.072 [DOI] [PubMed] [Google Scholar]
- Tse G., Gong M., Li C. K. H., Leung K. S. K., Georgopoulos S., Bazoukis G., et al. (2018). Tpeak-Tend, Tpeak-Tend/QT ratio and Tpeak-Tend dispersion for risk stratification in Brugada Syndrome: a systematic review and meta-analysis. J. Arrhythmia 34, 587ythmia 10.1002/joa3.12118. eCollection 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K., Horie M., Aiba T., Ogawa S., Aizawa Y., Ohe T., et al. (2017). Genotype-phenotype correlation of SCN5A mutation for the clinical and electrocardiographic characteristics of probands with brugada syndrome: a japanese multicenter registry. Circulation 135, 2255–2270. 10.1161/CIRCULATIONAHA.117.027983 [DOI] [PubMed] [Google Scholar]
- Yuan B., Shan Q., Yang B., Chen M., Zou J., Chen C., et al. (2008). Detection of gene mutations of SCN5A in 7 patients with Brugada syndrome. Zhonghua Xin Xue Guan Bing Za Zhi 36, 404a X. [PubMed] [Google Scholar]
- Yuan M., Tian C., Li X., Yang X., Wang X., Yang Y., et al. (2018). Gender Differences in Prognosis and Risk Stratification of Brugada Syndrome: A Pooled Analysis of 4,140 Patients From 24 Clinical Trials. Front. Physiol. 9:1127. 10.3389/fphys.2018.01127 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.