Abstract
It is assumed that slow oscillatory up-states represent crucial time windows for memory reactivation and consolidation during sleep. We tested this assumption by utilizing closed-loop targeted memory reactivation: Participants were re-exposed to prior learned foreign vocabulary during up- and down-states of slow oscillations. While presenting memory cues during slow oscillatory up-states improved recall performance, down-state cueing did not result in a clear behavioral benefit. Still, no robust behavioral benefit of up- as compared to down-state cueing was observable. At the electrophysiological level however, successful memory reactivation during up-states was associated with a characteristic power increase in the theta and sleep spindle band. No oscillatory changes were observable for down-state cues. Our findings provide experimental support for the assumption that slow oscillatory up-states may represent privileged time windows for memory reactivation, while the interplay of slow oscillations, theta and sleep spindle activity promotes successful memory consolidation during sleep.
Introduction
The consolidation of memories critically depends on hierarchically nested oscillatory brain mechanisms. It has been proposed that the systematic interaction of neocortical slow oscillations (SOs), thalamic sleep spindles and hippocampal sharp wave ripples (SWRs) during sleep reflect the mechanistic vehicle of memory reactivation and thus sleep related consolidation1. The ~1 Hz SO represents the most prominent signature of slow wave sleep (SWS) and is generated in neocortical circuits. SOs comprise alternations between periods of neuronal membrane hyperpolarization, accompanied by widespread neuronal silence (“down-states”), followed by depolarized neuronal “up-states”, accompanied by sustained firing2. Critically, SOs are thought to coordinate spontaneous memory reactivation processes during sleep, by providing the temporal frame for active memory consolidation3. It is assumed that the SO up-states drive memory reactivation in the hippocampus together with sharp wave ripples and thalamo-cortical sleep spindles4. The formation of spindle-ripple events is suggested to enable the hippocampal-neocortical dialog and the redistribution of reactivated hippocampal memory information to neocortical long-term stores5–7.
Despite these theoretical predictions, the mechanistic role of SO up-states for memory reactivation during sleep in humans remains ambiguous. In general, the functional significance of sleep-related memory reactivation in humans has been demonstrated by a series of studies showing that inducing reactivation processes experimentally (targeted memory reactivation; TMR) improves the consolidation process and thereby affects subsequent recall performance8. TMR studies follow the rationale that memory cues associated with prior learning are presented again during subsequent non rapid eye movement (NREM) sleep to trigger reactivation processes and consequently boost later memory performance. This approach has repeatedly proven successful for context cues such as odors9,10 and for specific item cues such as sounds11–14, melodies15–17 or verbal material18–20. Importantly, all of these studies presented the memory cues at random points in time during NREM sleep, without taking the on-going oscillatory activity, specifically the phase of SOs, into account. Given the assumed role of SO up-states in driving memory reactivations during sleep, we hypothesized that experimentally aligning the memory cues to the initiation of SO up-states (negative-to-positive transition of the surface slow-wave; see21,22 should be critical for successful TMR, resulting in improved retrieval performance after sleep. In contrast, presenting memory cues at the onset of the SO down-states (positive-to-negative transition in the surface slow-wave) should block the memory benefit of TMR. We therefore used SO phase-specific targeted memory reactivation (closed-loop TMR) to test the functional role of SO up-states for memory consolidation in humans. To investigate how closed-loop TMR influences the reactivation and consolidation of memories, we used a vocabulary learning task18,20. After learning 120 Dutch-German word pairs, 16 healthy young participants slept for 3 hours in the laboratory (see ‘Materials and Methods’ section, for details). We applied an online SO detection algorithm to present subsets of the prior learned words either during the presence of SO up-states or down-states. As a control condition some of the prior learned words were not replayed at all (uncued words). After sleep, participants were tested on memory for the German translations by a cued recall procedure. We show that words presented during SO up-states were associated with a robust improvement in recall performance compared to uncued words. On a neural basis, successful up-state TMR was associated with characteristic cue-related increases in theta and sleep spindle power. Words replayed during SO down-states did not show this distinct oscillatory pattern of successful memory reactivation and also no significant performance improvement.
Results
Accuracy of the Closed-Loop Algorithm
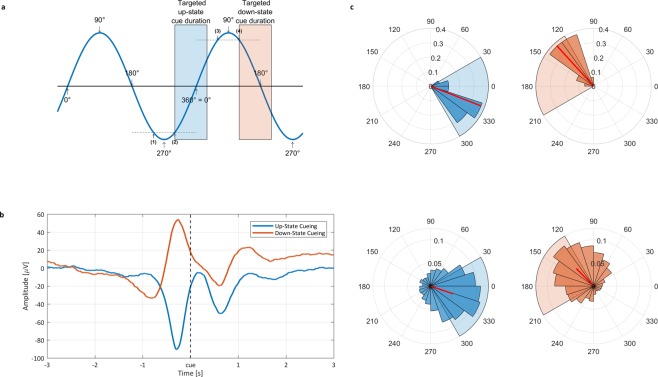
We first examined whether our algorithm correctly distinguished between words replayed during up- and down-states. We calculated ERPs separately for cues presented in the down-to-up phase transition of the cortical slow wave (targeted area for the up-state cues) and for cues presented in the up-to-down-phase transition (targeted area for the down-state cues, Fig. 1a). As expected, the ERP analysis confirmed that up- and down-state cues were played at highly distinct times of the cortical slow wave and targeted the expected areas (Fig. 1b; see Supplementary Fig. 2 for ERPs differentiated by remembered and non-remembered words). Despite the pre-stimulus peaks having clearly opposite polarities, post-stimulus ERPs of the two TMR conditions followed a similar temporal evolution. For up-state cues, the negative post-stimulus peak highly resembled an endogenous ~1 Hz slow wave. In contrast, down-state cues disrupted the natural continuation of the spontaneous slow wave. While no temporal difference was apparent for the pre-stimulus peaks (t15 = 0.07, P = 0.942), the timing of the post-stimulus ERP peaks differed significantly (t15 = 3.00, P = 0.009 for the negative peak; t15 = 2.31, P = 0.036 for the positive peak). For both the negative and positive peaks, the down-state peaks were 11 ms earlier than the up-state peaks (for additional ERP results using a bandpass filter of 0.5–1 Hz. please see Supplementary Fig. 9). It has to be noted that our ERP results might suggest that the pre-stimulus negative peak might be less pronounced as the up-state pre-stimulus negative peak. Importantly, our detection algorithm is per default agnostic to whether an up- or down-state cue will follow at the point of a given negative peak (i.e. in both cases, the EEG signal has passed the −75 µV threshold). The seemingly smaller pre-stimulus amplitude for down-state cues is solely due to the larger temporal lag between a detected peak and cue release for down-state as compared to up-state cues. Thus, the temporal position of the negative peak relative to the cue is more spread out in case of down-state cues, leading to a smaller averaged negative peak in the ERP. To further assess the accuracy of the SO detection algorithm, we determined the phase of the cortical SO at stimulus onset. On a subject level, up-state cues were associated with a mean phase angle of 338.60° ± 20.46°, while down-state cues had a mean phase angle of 132.31° ± 18.46° (see Fig. 1c, top row and Fig. 1c, bottom row, for results at the trial-by-trial level). Thus, the onset of our memory cues corresponded very accurately with the early phases of the targeted areas, assuring that mostly the whole word length (~400 ms) was played during the intended SO state (see Fig. 1a for an overview and Supplementary Fig. 4 for a phase analysis of each individual subject).
Figure 1.
Closed-loop TMR algorithm evaluation. (a) Schematic overview of the slow-wave detection algorithm and the targeted areas for up-state TMR (blue) and down-states TMR (red). (b) The ERP-analyses revealed that up-state cues were located at the down-to-up transition of the cortical slow wave (beginning of slow oscillatory up-state), and that down-state cues were played at the up-to-down transition (beginning of slow oscillatory down-state). (c) Phase angle at stimulus release. The top row illustrates the angles averaged at subject level, the bottom row shows results on trial level (red and blue shading corresponds to targeted areas in a). The left column indicates the up-state phases, while the right column shows the down-state phase angles. All data is shown for electrode Fz.
Behavioral Results
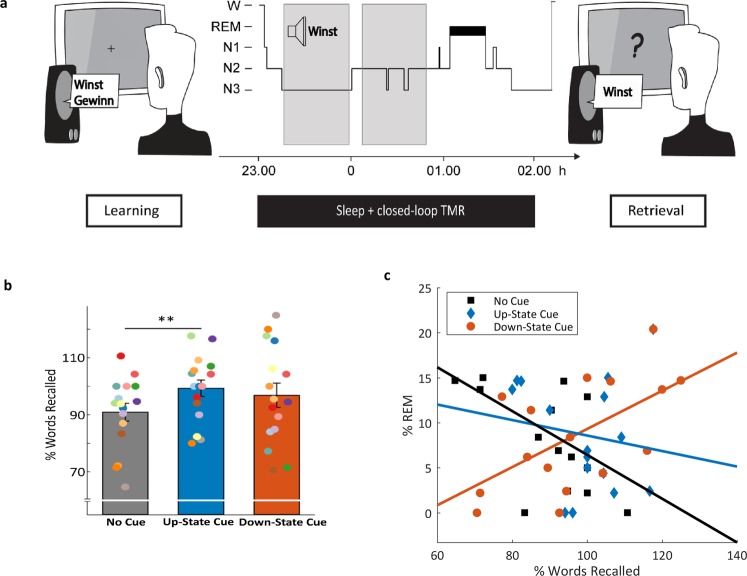
As predicted, we observed a robust improvement of memory performance when word-cues were presented during the up-state of the slow oscillation (see Fig. 2b): After the sleep interval participants remembered 99.3 ± 2.89% (range: 80–117.6) of the prior learned words which were presented during SO up-states, whereas they remembered only 90.92 ± 3.14% (range: 64.7–110.7) of the uncued words (t15 = 2.62; P = 0.019, two-tailed). In contrast, memory for words presented during SO down-states (96.83 ± 4.27%, range: 70.5–125) did not differ from uncued words (t15 = 0.93, P = 0.366). On a descriptive level, memory performance for words presented during down-states was just in-between up-state and uncued words. Furthermore, no difference in no-cue corrected memory performance was observed between up-state cued vs. down-state cued words (t15 = 0.43, P = 0.673). It must be noted that the variance for percent words remembered was also descriptively higher for words cued during the down-state than the other two categories (down: 292.29; up: 133.20; uncued: 157.58).
Figure 2.
Experimental Procedure and memory task results. (a) After studying 120 Dutch-German word pairs in the evening, participants slept for 3 hours. During NREM sleep, 40 Dutch words were presented during SO up-states and 40 Dutch words were presented during SO down-states using closed-loop TMR. 40 Dutch words were not replayed. A cued recall procedure was applied after sleep, testing the participant’s memory for the German translations. (b) Presenting prior learned words during SO up-states significantly enhanced memory performance compared to uncued words. Recall performance of words replayed during SO down-states did not differ from the two other categories. Retrieval performance is indicated as percentage of recalled German translations with performance before sleep set to 100%. Values are mean ± SEM **P ≤ 0.025. (c) Correlation between memory performance and relative time spent in REM sleep. Memory performance for words presented during down-states is positively correlated with time spent in REM sleep (r14 = 0.59, P = 0.017). There was no significant correlation for words presented during up-states (r14 = −0.16, P = 0.552), and a marginal significant negative correlation for uncued words (r14 = −0.49, P = 0.052).
In addition, we explored the associations between memory performance and time spent in the different sleep stages (for descriptive values of sleep stages, see Table 1). Interestingly, participants with high amounts of rapid eye movement (REM) sleep profited from down-state cueing, while participants with low or no REM sleep did not (r14 = 0.59, P = 0.017; see Fig. 2c; please note that no cue was presented during REM sleep). We observed no other significant correlations between any sleep stage and memory performance in any word category (all P > 0.05). We also observed no significant correlation between the number of stimuli presented during NREM sleep and memory performance, neither in the up- nor down-state category (both, P > 0.180). Descriptively, each person received 308.94 ± 18.98 cues during the night, which corresponds to about 5 repetitions of all cued words, with 50.29 ± 0.21% up-state cues and 49.71 ± 0.21% of the words presented during down states.
Table 1.
Sleep parameter.
| Total sleep duration [min] | % WASO | % N1 | % N2 | % SWS | % REM |
|---|---|---|---|---|---|
| 181.84 ± 8.55 | 5.54 ± 2.76 | 4.75 ± 0.83 | 48.41 ± 2.71 | 31.99 ± 2.54 | 8.64 ± 1.54 |
N1, N2: NREM sleep stages N1 & N2, SWS: slow-wave sleep (N3), REM: rapid eye movement sleep, WASO: wake after sleep onset. Values are means ± SEM.
EEG Results
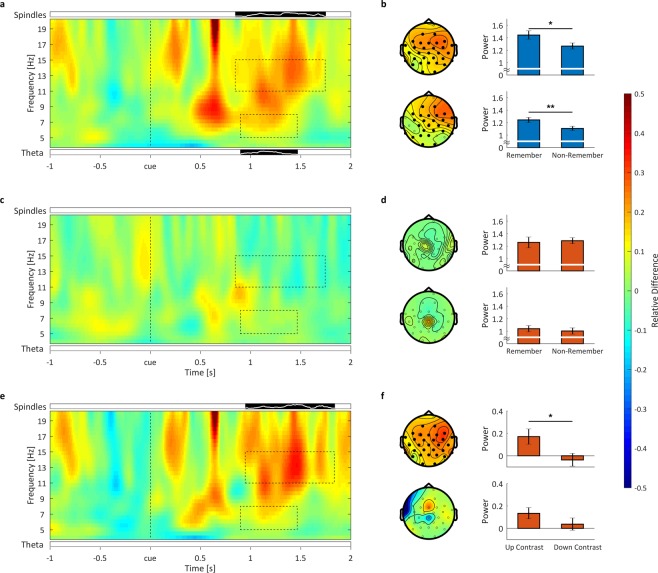
Based on our previous reports18,20, we focused the time-frequency analysis on power in the theta band (5–8 Hz) and the sleep spindle band (11–15 Hz) between the time points 0 ms and 2000 ms after stimulus onset. For memory cues played during the up-state we observed a significant increase in theta power for later remembered compared to later non-remembered words between 900 and 1470 ms involving a cluster of 26 channels (P = 0.038, see Fig. 3a,b left column, bottom row). Also in the spindle band, the overall analysis revealed a significant increase in spindle power for remembered compared to non-remembered words between 850 and 1750 ms involving all 31 electrodes (P = 0.018, see Fig. 3b left column, top row). In contrast to cues presented during SO up-states, we did not observe any significant power differences for remembered vs. non-remembered words played in the SO down-state, neither for the theta (P > 0.60) nor the spindle band (no significant cluster, P > 0.05, see Fig. 3c,d). Even a more restricted test-statistics limited to the time-range of the up-state clusters revealed no significant effect (not shown; theta: P = 0.303, spindle: no significant cluster P > 0.05). In a next step we directly compared the oscillatory fingerprint of up- and down-state reactivation, by contrasting the ‘subsequent reactivation effect’ (i.e. remembered vs. non-remembered words) between those conditions (Fig. 3e,f). Here, an increase in spindle activity for up- as compared to down-state cueing was observable, ranging from 950 to 1840 ms and involving all 31 electrodes (P = 0.021, see Fig. 3e,f top row). No significant difference emerged with regards to theta activity (P > 0.20, Fig. 3e,f bottom row). However, sleep stage-specific analyses revealed that these TMR related modulations in oscillatory power were mainly driven by cues presented during sleep stage N2 (for details see Supplementary Results and Figs 7 and 8).
Figure 3.
Oscillatory results. Time-frequency contrasts between remembered and not-remembered words in the theta and sleep spindle band for (a) up-state and (c) down-state cues, averaged over all 31 significant spindle electrodes. Black bars (significant cluster in frequency band analysis) with white lines below and above the time-frequency plot show the number of significantly differing electrodes for the theta and sleep spindle band respectively. The full height of the bar corresponds to 100% (31) electrodes. Dashed boxes indicate the areas of significant difference between remembered and not remembered words. These time-windows were used to illustrate the topographical distributions (b,d,f) left column, top row spindle band, bottom row theta band; significant electrodes shown as filled black dots). (b,d,f) right column show the mean power within the significant clusters, averaged over the significant electrodes, all frequencies and time in the sleep spindle (top) and theta (bottom) band. For up-state cueing (a) remembered words show enhanced power in the theta (5–8 Hz) as well as the sleep spindle (11–15 Hz) range compared to not-remembered words. Averaged over time, channels and frequency band, within these clusters this difference was significant in the theta band (t15 = 2.69, P = 0.008; see b right column, bottom row) and in the spindle band (t15 = 2.50, P = 0.012; see b, right column, top row). For words presented during down-states (c) no significant difference emerged between remembered and forgotten words, neither in the sleep spindle nor the theta band. Consequently, averaged activity in those clusters observed in the analysis of SO up-states did not reveal any significant differences for down-state cues, neither in the theta (t15 = 0.68, P = 0.253) nor the spindle band (t15 = −0.42, P = 0.661). The difference between the two contrasts of up and down (e) showed enhanced power in the spindle band, but not in the theta band. Averaged activity in the spindle cluster (t15 = 2.41, P = 0.015; see f right column, top row) showed a significant difference, while theta activity (averaged over the duration of the SO up-state theta cluster) showed only a statistical trend (t15 = 1.44, P = 0.085; see f right column, bottom row). Mean ± SEM are indicated. **P < 0.01; *P < 0.05.
To assess the general oscillatory difference between up- and down-state of all words (remembered and non-remembered), we ran a time frequency analysis for time-points ranging from 1 second pre-stimulus to 2 seconds after stimulus onset and including frequencies from 4 to 20 Hz, contrasting all up-state cues versus all down-state cues (see Supplementary Fig. 5). Results revealed a significant positive cluster spanning the whole segment in time, frequency and space (P = 0.001). To test the frequency bands of interest (i.e. theta and sleep spindles) in detail, we tested for frequency band specific positive clusters in the same time range and across all electrodes (see Supplementary Fig. 5a below and above time-frequency plot). We found a significant cluster from −0.64 seconds to 2 seconds in the theta band (P = 0.001). In the spindle band the cluster spanned from −0.99 to 1.01 seconds (P = 0.001). Both clusters involved all channels.
Discussion
The present study investigated for the first time the impact of SO phase-dependent memory reactivation during sleep on memory consolidation. We used a simple, yet effective algorithm to target the presentation of prior learned words specifically into the transition between neuronal quiescence and synchronized neuronal activity and vice versa (i.e. SO up- and down-states). We demonstrate that presenting memory cues during the presence of SO up-states significantly improved memory performance compared to uncued words. In contrast, presenting words during SO down-states did not exhibit such a clear beneficial effect. However, participants who spent more time in REM sleep after NREM cueing benefited more from cues presented during down-states. Our oscillatory analyses demonstrate that successful TMR during up-states was related with higher theta and spindle band activity than non-successful TMR as described previously12,18–20,23. This characteristic oscillatory pattern of successful memory reactivation during NREM sleep was not observable for the down-state condition.
In our study, we targeted the SO transition from down- to up-state (up-state condition) and up- to down-state (down-state condition) to specifically reactivate memories during sleep. We aimed for these transition periods as it has been shown that neuronal down-states occur slightly before the negative peak of the surface slow wave, while neuronal up-states start with the negative-to-positive transition of the cortical slow-wave21,22. Our algorithm accurately presented the memory cues in the intended target areas. In addition, our ERP analysis shows that, while the signals of the two cue targets differed strongly before cue onset, they followed a similar temporal evolution after cue presentation, indicating that auditory cues pushed the brains neuronal population into similar temporal successions as measured by surface EEG, regardless of the underlying endogenous brain state.
Taken together, our results suggest that SO up-states might represent privileged time windows for targeted memory reactivation and accompanying consolidation. In contrast, TMR during down-states did not ultimately block but mostly attenuated the chances of successful memory consolidation. While no consistent differences between up- and down-state TMR became apparent on a behavioral level, we found strong differences in the oscillatory fingerprint of the two TMR states.
Memory reactivation during sleep and the phase of slow oscillations
Several theoretical considerations4,5,24–27 lead to the prediction that SO up-states drive the reactivation of memories during sleep, thereby representing a critical time window for memory consolidation. During slow oscillatory up-states, more and more neurons fire synchronously21. This synchronous firing leads to a higher probability of activating a cascade of downstream–connected neurons. As memory traces are thought to be stored in the brain as interconnected neurons28–30, randomly activating a large portion of neurons during the up-state, heightens the probability that these interconnected neurons (i.e. memory traces) fire together3. We suggest that presenting auditory cues during the presence of SO up-states, activates neurons of the associated memory trace with a heightened probability. In combination with the heightened random neural activations of the up-state, a neuronal cascade involving the complete memory trace is triggered with increased probability. Interestingly, ERP associated with up-state TMR indicated a regular, uninterrupted 1 Hz oscillation after cue onset, possibly supporting the endogenous reactivation and consolidation mechanism, while down-state TMR seemed to disrupt the ongoing endogenous SO pattern. As a result of this phase reset, the evoked response of up- and down-state cues is near-perfectly aligned temporally. If, one assumes that memory traces are reactivated during this time window, the similarity in temporal evolution could in part explain the slight improvement of memory cues during down-state cueing over uncued words.
Presenting memory cues during the presence of a down-state might still activate the corresponding memory trace with an above chance probability. However, as the brain is at this point in a quiescent state, the reactivation of the specific memory trace might not be fully supported by the endogenous activity of the brain. The chance of reactivating a memory trace during the down-state, therefore remains inferior to up-state reactivation. Nevertheless, both cueing time points seem to increase the chance of reactivating a memory relative to random endogenous chance reactivations.
In contrast to our experimental findings, a recent analysis by Batterink and colleagues31 identified the SO down-state to represent the optimal phase for TMR. In a post-hoc evaluation of two previous TMR studies11,32, the authors found that the amount of forgetting was lowest for items presented just before the onset of down-states. The authors explained this result, by a potential time lag caused by auditory stimulus processing and concluded that a closed-loop TMR approach would shed further light on these findings. Our results indicate that the optimal phase for TMR and thereby memory reactivation is during down- to up-transition of the SO. However, general differences between the studies (e.g. utilized task, differing sound cues etc.) could potentially account for the timing discrepancies. Our study was specifically designed to test the difference between up-state, down-state and non-cued words on memory performance. Thus, our algorithm targeted the early stage of the up- to down- and down- to up-transition and did not aim for the pre-down-state peak interval, which was found preferable by Batterink and colleagues31.
The relationship of REM-sleep and down-state TMR
An interesting finding of our current work is that cueing success for down-state TMR was positively correlated with the relative time spent in REM sleep. Two recent studies have shown, utilizing an afternoon nap design, that REM sleep systematically influenced the effect of TMR on new word learning33 and the integration of newly learned spoken words34. This pattern of result led the authors to assume that TMR during NREM sleep might reactivate a certain memory trace, and at the same time prepare it for integration into pre-existing associative networks during the next REM sleep cycle (which might be associated with de-stabilization of a given memory trace during NREM sleep, but see)35. In the light of these results one might assume that memory cues targeted into the optimal SO up-state successfully reactivate and stabilize the memory trace immediately through the critical interplay of SO, theta and sleep spindle activity. In contrast, cues targeted into suboptimal (non-up-) states could also have the chance to reactivate the memory trace but might lack crucial stabilization processes, possibly due to the disturbance of endogenous oscillatory mechanisms. These memory traces might therefore dependent on the re-stabilization during REM sleep. Furthermore, random TMR during an afternoon might be specifically vulnerable to presenting cues during suboptimal states due to shallower afternoon sleep than nighttime sleep.
The role of theta and sleep spindle activity in memory reactivation
While the role of REM sleep in stabilizing memory representations was specifically associated with down-state TMR, successful up-state TMR was directly linked with power increases in the theta and sleep spindle range. A growing number of TMR studies18,19,23,36–38 report such elevated levels of theta and sleep spindle power to be tightly associated with cueing success, indicating a critical role of both frequency bands for the reactivation and stabilization of memories during sleep.
Here we observed significant increases in theta power for remembered as compared to non-remembered words from 0.92 to 1.48 seconds with regard to up-state cues, thus exhibiting some overlap concerning the first positive ERP-peak after stimulus onset. In addition, we found increases in sleep spindle power between 0.83 and 1.77 seconds after up-state cues, similarly coinciding with the first positive ERP-peak after stimulus onset. Critically, we observed no robust difference between remembered and non-remembered words neither in the theta nor in the sleep spindle range when it comes to down-state cues.
Nevertheless, when directly contrasting the ‘subsequent reactivation effect’ (i.e. remembered vs. not-remembered words) between the conditions of up- and down state, only a significant increase in sleep spindle power for up- as compared to down-state cues remained, while no significant difference in theta activity was observable. According to our working model, increases in theta power might indicate the reinstatement of memories by cueing, whereas related increases in spindle power might rather support the stabilization of before reactivated memories39. Thus, applying memory cues during both up- and down states might be associated with the reinstatement of specific memories. This interpretation also resonates well with a recent finding, which indicates that the function of theta activity may be to coordinate the reactivation of memories during both wakefulness and sleep, thus constituting a state-independent feature of memory reactivation40. Interestingly, theta-guided reactivation patterns autonomously re-emerged during sleep in this study at a rate of 1 Hz, suggesting a supra-ordinate coordination by SOs.
Also, the results of the current study point towards a critical role of SOs and specifically their phase, as reinstatement processes as mirrored by elevated theta activity were only behaviorally relevant when cues were applied during up-states (i.e. only here theta power distinguished reliably between later remembered and non-remembered memory items). Furthermore, up-state cues, which were played in accordance with the endogenous rhythm of the brain, seemed to recruit sleep spindles more easily than down-state cues, thereby enhancing memory consolidation in a robust fashion. Sleep spindles are assumed to promote the re-distribution of reactivated memory representations to neocortical sites41, with hippocampal reactivation signals being nested in individual spindle troughs4,5,42,43. In line with this assumption, inducing thalamic sleep spindles, when phase-locked to SO up-states enhances the oscillatory coupling between SOs, spindles and ripples and furthermore memory consolidation7,44.
Furthermore, Ngo and colleagues45,46 have previously demonstrated that entraining SOs through closed loop auditory stimulation, enhances phase-locked spindle activity and importantly memory recall after sleep. Interestingly, the associated increases in sleep spindle amplitude were positively correlated with later memory performance. These studies were able to elegantly demonstrate that elevating activity in the SO and phase-locked sleep spindle range by stimulation of SO up-states leads to a general improvement in memory performance. However, whether these effects resulted from specifically enhancing reactivation processes through up-state stimulation remained unknown. In our study, we are able to test this relation directly by targeting the memory content specifically into the proposed functional up-state of the SO and compare this to TMR in the SO down-state. As pointed out above we also found an increase in spindle power for up-state cues when contrasting later remembered and non-remembered words, providing a link between SO phases, spindles and memory performance.
Importantly, our sleep stage-specific analyses (for details see Supplementary results and Figs 7–9) suggest that these TMR related modulations in oscillatory power for both theta and sleep spindle activity were mainly driven by cues presented during sleep stage N2. Thus, only cues presented during the up-states of slow oscillations during ‘light sleep’ (i.e. K-complexes) were associated with enhanced theta and sleep spindle activity with regards to later remembered memory content as compared to none-remembered ones. In contrast to our expectations, we could not find any reliable memory-related pattern of oscillatory activity for cues presented during SWS. This finding might seem surprising, given that current models of memory consolidation assign SWS a prominent role when it comes to the re-processing of memories during sleep1.
However, our results resonate well with a recent account, suggesting that the network physiology of light sleep (i.e. N2 sleep) might be more conducive to memory consolidation via hippocampal reactivation47. Still, while our oscillatory analyses suggest that N2 sleep might be more sensitive to targeted memory reactivation, our experimental design does not allow to draw strong conclusions with regards to the behavioural outcomes. All of the TMR cues were repeatedly presented during both sleep stages N2 and SWS, making a specific differentiation impossible. Thus, future studies will need to assess in a controlled and detailed way, whether light and deep sleep differ in their capability to mediate the effects of targeted memory reactivation.
In sum, our results suggest that the slow-oscillatory up-state represents a privileged time window for enhancing memory by TMR. Still, it must be noted that the impact of up-state associated TMR did not exceed the usually described ~10 percent benefit of memory cueing in previous ‘random-phase’ TMR studies18,20. The equivalence concerning the obtained effects might result from the fact that those earlier studies featured a high stimulus repetition rate (~10 repetitions per memory cue compared to ca. 5 repetitions in the current study). In addition, presenting memory cues at suboptimal phase appears to slightly support the consolidation of memories according to our findings, especially when followed by subsequent REM sleep. As random-phase TMR allows for higher stimulus repetitions and is possibly easier to implement, it might even be the method of choice for enhancing memories during nighttime sleep in real-life.
Materials and Methods
Subjects
A total of 22 healthy, right-handed subjects (18 female, mean age = 20.85 ± 0.28) with German mother tongue and without Dutch language skills participated in the study. 6 subjects had to be excluded from the study due to technical reasons (n = 5) or because the subjects were too sensitive to the auditory cues and could not sleep (n = 1). Previous human TMR sleep studies9,11,18,20 have shown a large effect on memory, which can be adequately detected in sample sizes of 12–17 participants in a repeated measure design. We have chosen our sample size accordingly.
None of the participants was taking any medication at the time of the experiment and none had a history of any neurological or psychiatric disorders. All subjects reported a normal sleep-wake cycle and none had been on a night shift for at least 8 weeks before the experiment. On experimental days, subjects were instructed to get up at 7:00 h and were not allowed to consume caffeine or alcohol or to nap during daytime. All participants spent an adaptation night in the sleep laboratory prior to the experiment. The study was approved by the ethics committee of the Canton of Fribourg, and was performed in accordance with the approved guidelines. All subjects gave written informed consent prior to participating. After completing the whole experiment, participants received 120 Swiss Francs or course credit for participating in the study.
Design and Procedure
Participants entered the laboratory at 21:00 h. The session started with the application of the electrodes for standard polysomnography, including electroencephalographic (EEG; 32 channels, Brain Products GmbH), electromyographic (EMG), and electrocardiographic (ECG) recordings.
The encoding phase started at ~22:00 h with the learning of Dutch-German word pairs (for a detailed description see ‘Vocabulary Learning Task’ section). After completing the learning task participants went to bed at 23.00 h and were allowed to sleep for 3 h. During the 3-h retention interval, a selection of the prior learned Dutch words was presented again during sleep stages N2 and SWS. At ~2.00 h, subjects were awakened from sleep stage 1 or 2 and recall of the vocabulary was tested again (see Fig. 2a).
Vocabulary-Learning Task
The vocabulary-learning task consisted of 120 Dutch words and their German translations. There were three learning rounds. In each, Dutch words were presented aurally (duration range 300–500 ms) via loudspeakers (70 dB sound pressure level). In the first learning round, each Dutch word was followed by a fixation cross (500 ms) and subsequently by a visual presentation of its German translation (2000 ms). The inter-trial interval between consecutive word pairs was 2000–2200 ms. Subjects were instructed to memorize as many word pairs as possible. In a second round, the Dutch words were presented again followed by a question mark (ranging up to 7 seconds in duration). The participants were instructed to vocalize the correct German translation if possible or to say, “next” (German translation: “weiter”). Afterward, the correct German translation was shown again for 2000 ms, irrespective of the correctness of the given answer. In the third round, the cued recall procedure was repeated without any feedback of the correct German translation. Recall performance of the third round (without feedback) was taken as pre-retention learning performance. Here, participants recalled on average 60.5 ± 11.29 words (range 43 to 82 words) of the 120 words correctly, indicating an ideal medium task difficulty (50.42% words remembered).
Reactivation of Vocabulary
The words that would be used for TMR were selected via an algorithm that ensured that up-state presented, down-stated presented and uncued words were matched in terms of the pre-sleep memory performance. Of the 120 words learned before sleep, 2/3 of the remembered and 2/3 of the non-remembered words, totaling 80 words, were selected for cueing during sleep. The remaining 40 words were not replayed during sleep (mean remembered uncued: 20.56 ± 0.95). From all words selected for cueing, half of the remembered and half of the non-remembered words were assigned to the up-state cueing and the down-state cueing condition, respectively (mean remembered up-state: 19.88 ± 0.90; mean remembered down state: 20.00 ± 0.95 down-state). Cueing of the Dutch words started after the participants entered stable N2 sleep and was paused as soon as arousals were detected. During the 3 hour retention phase words were presented aurally via loudspeakers (55 dB sound pressure level) either during the up-state of a SO (up-state cueing) or during the down-state of a SO (down-state cueing) for a total of 90 minutes.
Online Detection Algorithm
The open-source FieldTrip toolbox48 was used to accomplish the online detection of slow oscillations and auditory replay. Slow oscillations were detected on the basis of EEG recordings from electrode site Fz, because SOs usually originate in the prefrontal cortex49. Post-hoc analysis of the phase distribution at cue onset shows an even phase distribution for all channels (See Supplementary Fig. 3). The signal was referenced to the average potential from linked mastoid electrodes and filtered between 0.2 and 4 Hz. A custom fieldtrip script running under Matlab enabled to respond to the incoming EEG data from electrode Fz in real time. To detect up- and down- states, respectively, each time the EEG signal crossed an adaptive threshold the auditory replay was triggered. The algorithm was implemented as a finite-state machine (see Supplementary Fig. 1): In the first state the algorithm tries to detect a potential slow wave by waiting for the EEG signal to go below −75 µV ((1) in Fig. 1a). If the auditory stimulus is to be played in the up-state, the presentation is triggered as soon as the EEG signal goes back above −75 µV (see position (2) in Fig. 1a). On the other hand, if the stimulus is to be released during a down-state, the following state will wait for the signal to pass into the positive domain (above 10 µV) ((3) in Fig. 1a) and then again go below the release threshold of 10 µV ((4) in Fig. 1a). At this point the auditory stimulus is played. As the duration of all words was ~400 ms, they could fit within their respective target state. After triggering the replay, an 8 second recovery period is entered before the algorithm returns to its initial state.
Recall of Vocabulary after the Retention Interval
During the recall phase, the 120 Dutch words were presented again aurally in a randomized order. The participants were asked to vocalize the correct German translation if possible. As index of memory recall of German translations across the retention interval, we calculated the relative difference between the number of correctly recalled words before and after the retention interval, with the pre-retention memory performance set to 100% (for an of memory performance see Supplementary Table 1).
Sleep EEG
Sleep was recorded by standard polysomnography including EEG, electromyographic (EMG) and electrocardiographic (ECG) recordings. EEG was recorded using a 32-channel system (EasyCap, Brain Products GmbH). Impedances were kept below 10 kOhm. Voltage was sampled at 500 Hz and initially referenced to the vertex electrode (Cz). In addition to the online identification of sleep stages, polysomnographic recordings were scored offline by 3 independent raters according to standard criteria50.
Preprocessing
EEG preprocessing was performed using Brain Vision Analyzer software (version 2.1; Brain Products, Gilching, Germany). Data were re-referenced to averaged mastoids and low passed filtered with a cutoff frequency of 30 Hz. The data was segmented into trials of 14 seconds, beginning 7000 ms before stimulus onset. Trials including artifacts (e.g. movement artifacts) were manually removed after visual inspection.
Afterwards, epochs were categorized into up- and down-state stimuli, depending on whether they were presented during up- or down-states, respectively. Furthermore, all stimuli were differentiated on a behavioral level into ‘Remember’ and ‘Non-Remember’ words. Remembered words refer to those words that were remembered at recall after sleep, while non- Remember words were not. Additionally ‘Up-All’ and’Down-All’ will be used to denote all up-state and all down-state cues irrespective of the behavioral outcome. All further analyses were done using Matlab (The Math Works Inc., Natick, MA, USA) and the FieldTrip toolbox48.
Event-Related Potentials
ERPs were analyzed using FieldTrip. Preprocessed trials were averaged and baseline corrected for each stimulus category within each subject. For baseline correction, the segment from −3 to −2 seconds was used, as the pre-stimulus time window closer to the cue-onset systematically differs between up- and down-state. Subsequently the ERPs were averaged across all subjects for each stimulus category.
Slow Wave Phase Analysis
To assess the accuracy of the closed-loop algorithm, the preprocessed data was low-pass filtered (two-pass Butterworth IIR filter with filter order 6) at 1.5 Hz.In an additional analysis, the data was band-pass filtered (two-pass Butterworth IIR filter with filter order 2) between 0.5 and 1 Hz to assess the slow wave phase. Phase information was obtained by applying a Hilbert transform to the filtered data. The angle information was then averaged within each behavioral category for each subject. Descriptive and inferential statistics were calculated using the Circular Statistics Toolbox51.
Time-Frequency Analysis
Time-frequency analysis was performed using FieldTrip. To obtain oscillatory power we used a continuous wavelet transformation (complex Morlet waveform, 5 cycles) for frequencies ranging from 4 to 20 Hz, in steps of 0.5 Hz and 10 ms. Subsequently, every frequency was normalized by dividing the data by the mean of the baseline period, ranging from −3 to −2 seconds pre-stimulus. Then the trials were averaged for each subject. For sleep stage-specific oscillatory results, see Supplementary Results and Figs 7 and 8.
Statistical Analysis
We analyzed the behavioral data using using paired t-tests corrected for two-sided testing. Pearson’s linear correlation coefficient was computed. A threshold of P = 0.05 was used to set statistical significance. For the time-frequency analysis we tested the difference between the remembered and non-remembered words with a cluster based permutation test with dependent samples and a cluster level alpha of 0.05. Monte Carlo p-values were computed on 1000 random data partitions. The critical alpha-level was set to 0.05. We first tested for significant clusters broadly from 0 to 2 seconds after stimulus onset, across all channels and across all frequencies (4 to 20 Hz), correcting for two-sided testing. In a next step, we specifically tested the frequency bands of interest (i.e. averaged theta power: 5 to 8 Hz; averaged spindle power: 11 to 15 Hz) for positive clusters, as both frequency bands have been shown to be related to cueing success in previous studies18,19,23,36–38. Testing was done independently for up- as well as down-state trials. We also tested Up-All versus Down-All to obtain the general differences in oscillatory activity between up-state and down-state TMR.
Supplementary information
Acknowledgements
B.R. is supported by the Swiss National Science Foundation (SNSF) (100014_162388) and the Clinical Research Priority Program (CRPP) “Sleep and Health” from the University of Zurich. T.S. is supported by a grant of the Swiss National Science Foundation (SNSF) (P300P1_174450).
Author Contributions
T.S., M.G. and B.R. designed the experiment, E.A.M.v.P. and T.S. carried out the experiments, M.G.,T.S. and E.A.M.v.P. analyzed the data and M.G., T.S. and B.R. wrote the paper.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Björn Rasch, Email: Bjoern.Rasch@unifr.ch.
Thomas Schreiner, Email: T.Schreiner@bham.ac.uk.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-019-39178-2.
References
- 1.Rasch B, Born J. About Sleep’s Role in Memory. Physiol. Rev. 2013;93:681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steriade M, Nuñez A, Amzica F. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J. Neurosci. 1993;13:3252–65. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diekelmann S, Born J. The memory function of sleep. Nat. Rev. Neurosci. 2010;11:114–26. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 4.Sirota A, Buzsáki G. Interaction between neocortical and hippocampal networks via slow oscillations. Thalamus Relat. Syst. 2005;3:245. doi: 10.1017/S1472928807000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staresina BP, et al. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat. Neurosci. 2015;18:1679–1686. doi: 10.1038/nn.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lörincz A, Buzsáki G. Two-phase computational model training long-term memories in the entorhinal-hippocampal region. Ann. N. Y. Acad. Sci. 2000;911:83–111. doi: 10.1111/j.1749-6632.2000.tb06721.x. [DOI] [PubMed] [Google Scholar]
- 7.Khodagholy D, Gelinas JN, Buzsáki G. Learning-enhanced coupling between ripple oscillations in association cortices and hippocampus. Science (80-.). 2017;358:369–372. doi: 10.1126/science.aan6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oudiette D, Paller KA. Upgrading the sleeping brain with targeted memory reactivation. Trends Cogn. Sci. 2013;17:142–149. doi: 10.1016/j.tics.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Rasch B, Büchel C, Gais S, Born J. Odor Cues During Slow-Wave Sleep Prompt Declarative Memory Consolidation. Science (80-.). 2007;315:1426–1429. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- 10.Rihm JS, Diekelmann S, Born J, Rasch B. Reactivating memories during sleep by odors: odor specificity and associated changes in sleep oscillations. J. Cogn. Neurosci. 2014;26:1806–18. doi: 10.1162/jocn_a_00579. [DOI] [PubMed] [Google Scholar]
- 11.Rudoy JD, Voss JL, Westerberg CE, Paller KA. Strengthening Individual Memories by Reactivating Them During Sleep. Science (80-.). 2009;326:1079–1079. doi: 10.1126/science.1179013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groch S, et al. Prior knowledge is essential for the beneficial effect of targeted memory reactivation during sleep. Sci. Rep. 2017;7:39763. doi: 10.1038/srep39763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cairney SA, Lindsay S, Sobczak JM, Paller KA, Gaskell MG. The Benefits of Targeted Memory Reactivation for Consolidation in Sleep are Contingent on Memory Accuracy and Direct Cue-Memory Associations. Sleep. 2016;39:1139–1150. doi: 10.5665/sleep.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cairney SA, Guttesen AáV, El Marj N, Staresina BP. Memory Consolidation Is Linked to Spindle-Mediated Information Processing during Sleep. Curr. Biol. 2018;28:948–954.e4. doi: 10.1016/j.cub.2018.01.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cousins JN, El-Deredy W, Parkes LM, Hennies N, Lewis PA. Cued Memory Reactivation during Slow-Wave Sleep Promotes Explicit Knowledge of a Motor Sequence. J. Neurosci. 2014;34:15870–15876. doi: 10.1523/JNEUROSCI.1011-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antony JW, Gobel EW, O’Hare JK, Reber PJ, Paller KA. Cued memory reactivation during sleep influences skill learning. Nat. Neurosci. 2012;15:1114–1116. doi: 10.1038/nn.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schönauer M, Geisler T, Gais S. Strengthening Procedural Memories by Reactivation inSleep. J. Cogn. Neurosci. 2014;26:143–153. doi: 10.1162/jocn_a_00471. [DOI] [PubMed] [Google Scholar]
- 18.Schreiner T, Lehmann M, Rasch B. Auditory feedback blocks memory benefits of cueing during sleep. Nat. Commun. 2015;6:8729. doi: 10.1038/ncomms9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehmann M, Schreiner T, Seifritz E, Rasch B. Emotional arousal modulates oscillatory correlates of targeted memory reactivation during NREM, but not REM sleep. Sci. Rep. 2016;6:39229. doi: 10.1038/srep39229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schreiner T, Rasch B. Boosting Vocabulary Learning by Verbal Cueing During Sleep. Cereb. Cortex. 2015;25:4169–4179. doi: 10.1093/cercor/bhu139. [DOI] [PubMed] [Google Scholar]
- 21.Vyazovskiy VV, et al. Cortical Firing and Sleep Homeostasis. Neuron. 2009;63:865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vyazovskiy VV, Cirelli C, Tononi G. Electrophysiological correlates of sleep homeostasis in freely behaving rats. Prog. Brain Res. 2011;193:17–38. doi: 10.1016/B978-0-444-53839-0.00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groch S, et al. Memory cueing during sleep modifies the interpretation of ambiguous scenes in adolescents and adults. Dev. Cogn. Neurosci. 2016;17:10–18. doi: 10.1016/j.dcn.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mölle M, Marshall L, Gais S, Born J. Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep. J. Neurosci. 2002;22:10941–7. doi: 10.1523/JNEUROSCI.22-24-10941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mölle M, Bergmann TO, Marshall L, Born J. Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing. Sleep. 2011;34:1411–21. doi: 10.5665/SLEEP.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–1106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 27.Mölle M, Born J. Slow oscillations orchestrating fast oscillations and memory consolidation. Prog. Brain Res. 2011;193:93–110. doi: 10.1016/B978-0-444-53839-0.00007-7. [DOI] [PubMed] [Google Scholar]
- 28.Reijmers LG, Perkins BL, Matsuo N, Mayford M. Localization of a stable neural correlate of associative memory. Science. 2007;317:1230–3. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- 29.Silva AJ, Zhou Y, Rogerson T, Shobe J, Balaji J. Molecular and cellular approaches to memory allocation in neural circuits. Science. 2009;326:391–5. doi: 10.1126/science.1174519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–385. doi: 10.1038/484410a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Batterink LJ, Creery JD, Paller KA. Phase of Spontaneous Slow Oscillations during Sleep Influences Memory-Related Processing of Auditory Cues. J. Neurosci. 2016;36:1401–9. doi: 10.1523/JNEUROSCI.3175-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Creery JD, Oudiette D, Antony JW, Paller KA. Targeted Memory Reactivation during Sleep Depends on Prior Learning. Sleep. 2015;38:755–763. doi: 10.5665/sleep.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batterink LJ, Paller KA. Sleep-based memory processing facilitates grammatical generalization: Evidence from targeted memory reactivation. Brain Lang. 2017;167:83–93. doi: 10.1016/j.bandl.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamminen J, Lambon Ralph MA, Lewis PA. Targeted memory reactivation of newly learned words during sleep triggers REM-mediated integration of new memories and existing knowledge. Neurobiol. Learn. Mem. 2017;137:77–82. doi: 10.1016/j.nlm.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Diekelmann S, Büchel C, Born J, Rasch B. Labile or stable: opposing consequences for memory when reactivated during waking and sleep. Nat. Neurosci. 2011;14:381–6. doi: 10.1038/nn.2744. [DOI] [PubMed] [Google Scholar]
- 36.Schreiner T, Rasch B. Cueing vocabulary in awake subjects during the day has no effect on memory. Somnologie - Schlafforsch. und Schlafmedizin. 2015;19:133–140. doi: 10.1007/s11818-015-0005-9. [DOI] [Google Scholar]
- 37.Oyarzún JP, Morís J, Luque D, de Diego-Balaguer R, Fuentemilla L. Targeted Memory Reactivation during Sleep Adaptively Promotes the Strengthening or Weakening of Overlapping Memories. J. Neurosci. 2017;37:7748–7758. doi: 10.1523/JNEUROSCI.3537-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farthouat J, Gilson M, Peigneux P. New evidence for the necessity of a silent plastic period during sleep for a memory benefit of targeted memory reactivation. Sleep Spindl. Cortical Up States. 2017;1:14–26. doi: 10.1556/2053.1.2016.002. [DOI] [Google Scholar]
- 39.Schreiner, T. & Rasch, B. The beneficial role of memory reactivation for language learning during sleep: A review. Brain Lang. 167 (2017). [DOI] [PubMed]
- 40.Schreiner T, Doeller CF, Jensen O, Rasch B, Staudigl T. Theta Phase-Coordinated Memory Reactivation Reoccurs in a Slow-Oscillatory Rhythm during NREM Sleep. Cell Rep. 2018;25:296–301. doi: 10.1016/j.celrep.2018.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Born J, Wilhelm I. System consolidation of memory during sleep. Psychol. Res. 2012;76:192–203. doi: 10.1007/s00426-011-0335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mölle M, Eschenko O, Gais S, Sara SJ, Born J. The influence of learning on sleep slow oscillations and associated spindles and ripples in humans and rats. Eur. J. Neurosci. 2009;29:1071–81. doi: 10.1111/j.1460-9568.2009.06654.x. [DOI] [PubMed] [Google Scholar]
- 43.Siapas AG, Wilson M. A. between Hippocampal Ripples and Cortical Spindles during Slow-Wave. Sleep. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 44.Latchoumane C-FV, Ngo H-VV, Born J, Shin H-S. Thalamic Spindles Promote Memory Formation during Sleep through Triple Phase-Locking of Cortical, Thalamic, and Hippocampal Rhythms. Neuron. 2017;95:424–435.e6. doi: 10.1016/j.neuron.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 45.Ngo H-VV, Martinetz T, Born J, Mölle M. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron. 2013;78:545–53. doi: 10.1016/j.neuron.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 46.Ngo H-VV, et al. Driving Sleep Slow Oscillations by Auditory Closed-Loop Stimulation–A Self-Limiting Process. J. Neurosci. 2015;35:6630–6638. doi: 10.1523/JNEUROSCI.3133-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Genzel L, Kroes MCW, Dresler M, Battaglia FP. Light sleep versus slow wave sleep in memory consolidation: a question of global versus local processes? Trends Neurosci. 2014;37:10–9. doi: 10.1016/j.tins.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Oostenveld R, Fries P, Maris E, Schoffelen J-MM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nir Y, et al. Regional Slow Waves and Spindles in Human Sleep. Neuron. 2011;70:153–169. doi: 10.1016/j.neuron.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iber, C., Ancoli-Israel, S., Chesson, A. & Quan, S. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specification. American Academy of Sleep Medicine (2007).
- 51.Berens P. CircStat: A MATLAB toolbox for circular statistics. J. Stat. Softw. 2009;31:1–21. doi: 10.18637/jss.v031.i10. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.