Abstract
The high prevalence of Campylobacter spp. in retail liver products was previously reported and has been linked to several outbreaks of campylobacteriosis. The main objective of this study was to investigate the influence of retail liver juices on the survivability of several strains of C. jejuni and C. coli, which were previously isolated from various retail meats at 4 °C. All tested Campylobacter strains showed higher survival in beef liver juice (BLJ) and chicken liver juice (CLJ) as compared to beef and chicken juices (BJ and CJ) or Mueller Hinton broth (MHB) at 4 °C. Overall, C. jejuni strains showed greater survival in retail liver and meat juices as compared to C. coli. CLJ enhanced biofilm formation of most C. coli strains and supported growth in favorable conditions. When diluted, retail liver and meat juices enhanced survival of Campylobacter strains at low temperatures and increased aerotolerance. In conclusion, beef and chicken liver juices enhanced the survival of C. jejuni and C. coli strains at low temperatures, which helps explain the high prevalence of Campylobacter spp. in retail liver products.
Introduction
In recent years, campylobacteriosis has been listed as a leading cause of bacterial diarrheal illnesses in the USA1. C. jejuni and C. coli are the primary causal agents for campylobacteriosis, which can lead to immunological disorders like Guillain Barre syndrome and Miller Fisher syndrome2,3. After eradication of poliomyelitis, Guillain Barre syndrome remains the leading cause for flaccid paralysis in multiple countries4–8. Campylobacter is found in various reservoirs and also occurs as a commensal organism in poultry. As a foodborne pathogen, Campylobacter is usually transmitted via the consumption of contaminated beverages and food, with the latter occurring primarily from retail meat and liver products9,10. Several outbreaks of campylobacteriosis associated with contaminated retail liver products have been reported worldwide11–17.
Retail meat and liver products remain important nutrient sources for humans and provide proteins and micronutrients that are essential for growth and immunity18. High folate content and various lipid compositions have been identified in beef and chicken liver19. The high choline content in retail liver products is beneficial to human health in terms of normal cell functioning and acetylcholine synthesis20. In spite of improvements in the handling process, retail meat and liver products may be contaminated with Campylobacter during processing in the slaughterhouse21. The high incidence of Campylobacter on the surface of retail liver suggests cross-contamination during processing; however, it is important to note that Campylobacter spp. may also occur in the internal tissues of retail meats22–24. Other foodborne pathogens such as Salmonella, Staphylococcus, hepatitis E, Escherichia spp. and Yersinia spp. are also prevalent in retail liver products worldwide25–29. The higher prevalence of Campylobacter and Staphylococcus pathogens in retail liver vs. retail meat products has been reported25,30. The high frequency of Campylobacter contamination in retail liver products30–32 indicates a risk for future outbreaks due to the consumption preference for undercooked liver products33. Up to 98% survival of Campylobacter has been reported in chicken liver dishes that are undercooked to retain a pinkish coloration33.
Although the occurrence of Campylobacter in retail meat and liver products largely depends on contamination, bacterial survival during harsh conditions ensures transmission and can result in clinical cases9,34. Campylobacter is a fastidious, microaerophilic gram-negative bacterium that grows optimally at 42 °C. It routinely encounters various stressors including temperature fluctuation and oxidative and osmotic stress34. Campylobacter copes with harsh conditions by deploying multiple survival mechanisms including the viable but nonculturable condition (VBNC), biofilm formation and aerotolerance9,34. Differential gene expression35–37 and the survival of Campylobacter in different food matrices38,39 indicates the importance of food substances on bacterial viability. Even at low temperatures (0–4 °C), Campylobacter can survive for prolonged periods in retail meat and liver38–41. Hence, the high prevalence of C. jejuni and C. coli in retail liver products is associated with enhanced survival at low temperatures. Furthermore, low numbers of Campylobacter are sufficient to cause infection42. In response to cold shock, genes related to acquisition of cryoprotectants and membrane composition remodeling were abundantly expressed in Campylobacter43. Various components in retail liver might function as cryoprotectant molecules to improve survival at low temperatures.
Biofilm production is another survival mechanism of Campylobacter during aerobic and adverse environmental conditions44. The food matrix influences the formation of Campylobacter biofilms, which occur in retail meat substances such as chicken and pork meat juices45,46. Furthermore, the retail meat environment increases adhesion and attachment of Campylobacter, thus facilitating biofilm formation45. The nutrient and iron-rich environment in retail liver products might contribute to biofilm formation by Campylobacter spp. Although oxidative stress is unfavorable for Campylobacter growth, aerotolerance in Campylobacter strains would enhance survival in aerobic conditions47. Studies on the influence of retail liver environments on aerotolerance and biofilm formation are lacking because the required assays were not readily accessible in food models (e.g. liver slices and homogenates)48,49. Liver juices in retail liver samples represent the actual environment that Campylobacter strains encounter during handling and storage; however, studies including liver juices as a food model for Campylobacter have not been previously undertaken.
In previous studies from our laboratory, a high prevalence of C. jejuni and C. coli strains were reported in retail meat and liver products30,32,50. Interestingly, C. coli was recovered from retail beef liver, although beef cuts were not contaminated30. Retail liver might contain Campylobacter in internal tissues or may get contaminated during processing. In both conditions, higher survival rate of C. coli strains in the retail liver environment compared to other food matrices might have contributed to the higher prevalence; however, a precise explanation remains unclear. Most studies on Campylobacter survival and gene expression have been conducted with C. jejuni36,37,43,51 however, it is important to note that genomic differences between Campylobacter spp52 might be associated with differential survival rates and stress tolerance mechanisms. A few studies have shown higher survival rates of C. jejuni vs. C. coli strains at a lower temperatures, although contrasting results have been reported41,48,53,54. In this study, we investigate the influence of retail meat and liver juices (chicken and beef) on different strains of C. jejuni and C. coli during biofilm formation, survival or growth at variable temperatures, and oxidative stress (aerotolerance). This study provides further insights regarding the influence of retail meat and liver environments on Campylobacter survival.
Results
Survival at 4 °C
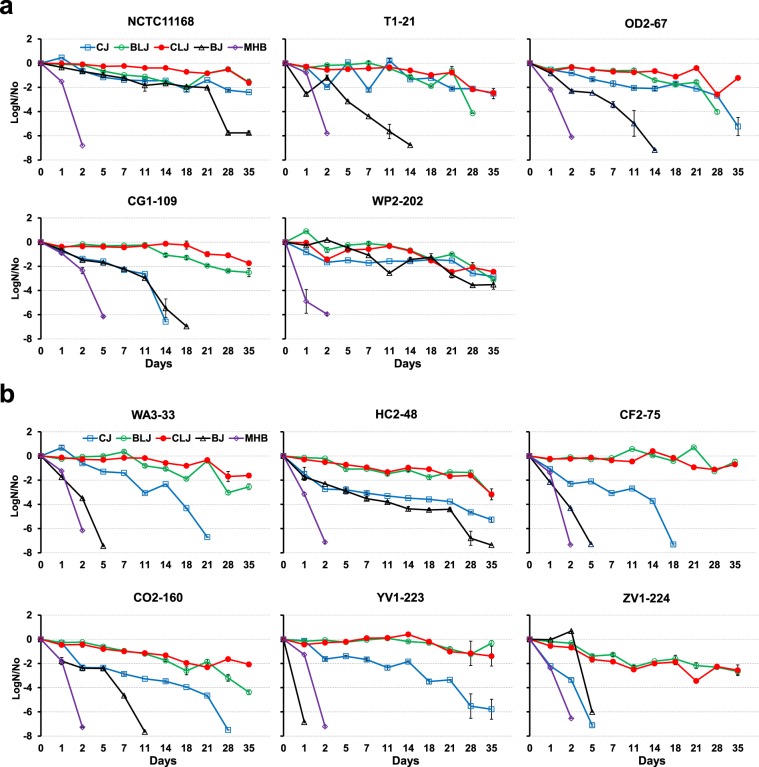
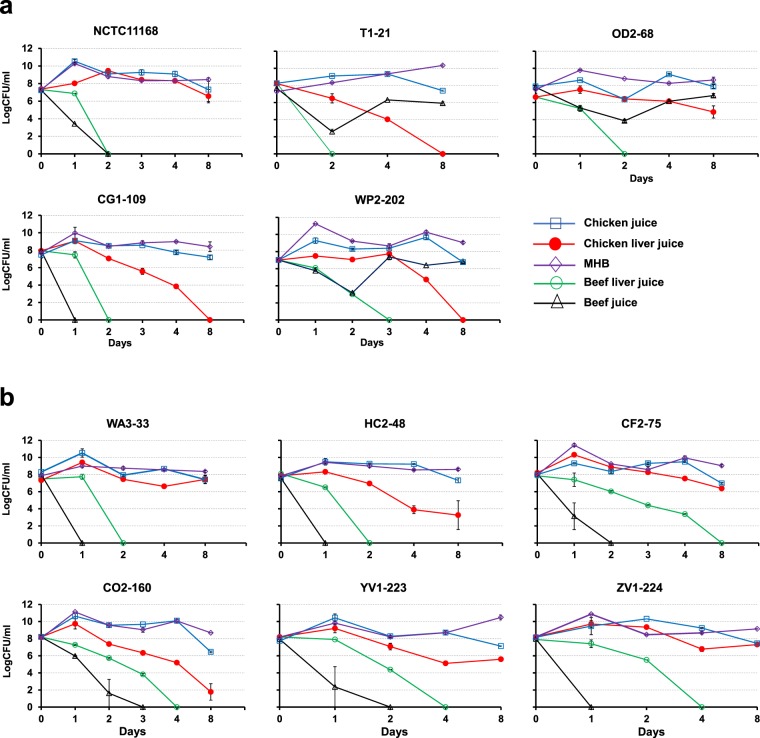
The influence of retail liver and meat juices on the survival of Campylobacter strains at 4 °C was investigated. In this experiment, we incubated eleven Campylobacter strains including reference strain C. jejuni NCTC11168 (Table 1) in retail meat and liver juices at 4 °C for five weeks. Retail meat and liver juices significantly enhanced survival (P < 0.0001) in ten of the eleven strains; an exception was C. coli YV1-223, which showed higher survival in MHB than BJ. Strains showed higher survival in BLJ and CLJ than BJ, CJ and MHB (P < 0.0001) (Fig. 1). Statistical analyses (MANOVA) showed a significant influence of retail meat and liver juices (P < 0.0001) but not the origin of juices (chicken vs. beef) on the survival of Campylobacter strains. A rapid reduction in bacterial counts (up to 5.76 log reduction) was observed for Campylobacter NCTC11168 at 28 days of incubation in BJ (Fig. 1a). A 1.57-, 1.61- and 2.41-log reduction in bacterial numbers was observed for NCTC11168 incubated in BLJ, CLJ, and CJ, respectively, at 35 days of incubation. CJ resulted in higher survival of C. jejuni strains T1-21, OD2-67, WP2-202, and NCTC11168 throughout the experiments as compared to MHB and BJ (P < 0.0001) (Fig. 1a); however, only C. coli strains HC2-48 and YV1-223 survived for 35 days in CJ (Fig. 1b). C. coli strains WA3-33, CF2-75, CO2-160, and ZV1-224 did not survive the 35-day incubation period in CJ, BJ or MHB (Fig. 1b). The overall viability of Campylobacter strains was lowest in BJ (P < 0.0001). None of the strains inoculated in MHB produced visible colonies on Mueller Hinton Agar (MHA) plates after a five-day incubation period.
Table 1.
Campylobacter strains used in this study. All Campylobacter strains (except C. jejuni NCTC11168) were isolated and whole genome sequenced in our laboratory30,32,50,78–83.
| Campylobacter strains | Source | Accession number (chromosome, plasmids) |
|---|---|---|
| C. jejuni NCTC11168 | Clinical (reference) | Al111168.1 |
| C. jejuni T1-21 | Chicken meat | CP013116.1, CP013117.1 |
| C. jejuni OD2-67 | Chicken liver | CP014744.1, CP014745.1, CP014746.1 |
| C. jejuni WP2-202 | Chicken gizzard | CP014742.1, CP014743.1 |
| C. jejuni CG1-109 | Beef liver | NA |
| C. coli WA3-33 | Chicken liver | CP017873.1, CP017874.1 |
| C. coli HC2-48 | Beef liver | CP013034.1, CP013035.1 |
| C. coli CF2-75 | Beef liver | CP013035.1, CP013036.1, CP013037.1 |
| C. coli CO2-160 | Beef liver | CP013032.1, CP013033.1 |
| C. coli YV1-223 | Pork meat | NA |
| C. coli ZV1-224 | Pork meat | CP017875.1, CP017876.1, CP017877.1 |
Figure 1.
Survival (log CFU/ml) of (a) C. jejuni and (b) C. coli strains in beef liver juice (BLJ), chicken liver juice (CLJ), beef juice (BJ), chicken juice (CJ) and MHB at 4 °C. Standard errors (vertical bars) were calculated from mean values of triplicate viable cell counts.
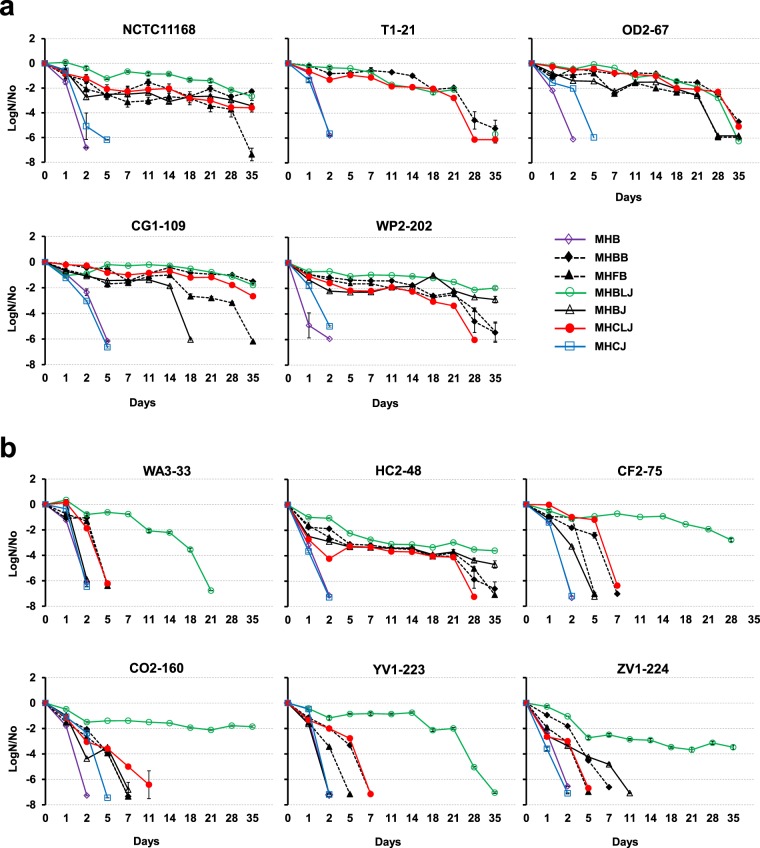
The survival of Campylobacter strains in diluted retail meat and liver juices (5% v/v in MHB) was investigated at 4 °C. With the exception of MHCJ, all diluted retail meat and liver juices significantly enhanced Campylobacter survival relative to MHB (P < 0.0001) (Fig. 2). Campylobacter strains failed to grow in MHCJ after 5 days. The addition of 5% laked horse blood and defibrinated horse blood to MHB (MHBB and MHFB, respectively) also enhanced Campylobacter survival, with most strains showing higher survival in MHBB vs. MHFB (Fig. 2). For C. jejuni strains, survival was highest in MHBLJ, MHBB and MHCLJ than unamended MHB (P < 0.0001). Survival rates in MHBB, MHBLJ, and MHCLJ were comparable to 100% BJ for several C. jejuni strains (Figs 1a, 2a). Among C. coli strains, MHBLJ promoted survival throughout the 35-day experiment. HC2-48 was the only C. coli strain producing visible colonies on MHA plates at 11 days after inoculation in MHCLJ, MHBJ, MHBB, and MHFB.
Figure 2.
Survival of (a) C. jejuni and (b) C. coli strains incubated in diluted retail meat and liver juices (5% v/v in MHB) at 4 °C. Campylobacter strains were incubated in diluted beef liver juice (MHBLJ), chicken liver juice (MHCLJ), beef juice (MHBJ), chicken juice (MHCJ), laked horse blood (MHBB), defibrinated fresh horse blood (MHFB) and MHB at 4 °C. Survival curves for C. jejuni T1-21 in MHBJ and MHFB are not available. Standard errors (vertical bars) were calculated from mean values of triplicate viable cell counts (LogCFU/ml).
Survival of C. jejuni and C. coli in various food matrices at 4 °C
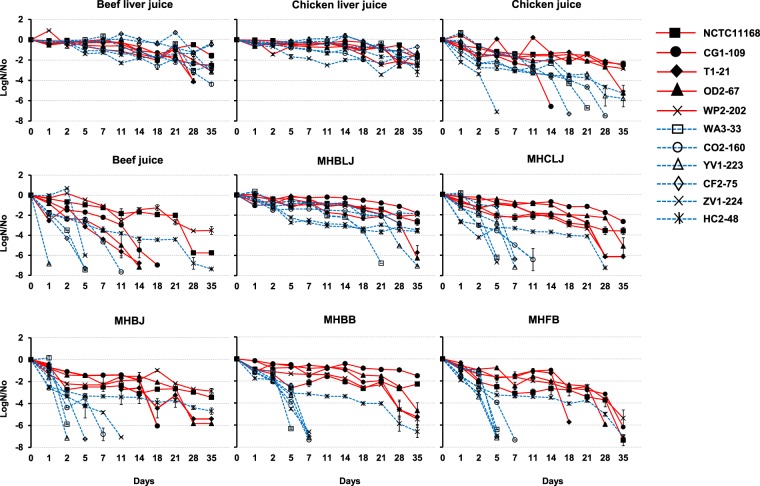
A comparative analysis of viability among Campylobacter strains was conducted using log bacterial cell count reduction rates. A higher reduction in bacterial counts (log N/No) was generally observed for C. coli strains as compared to C. jejuni in variable food matrices. Survival patterns were prolonged for all Campylobacter strains incubated in CLJ, BLJ, and MHBLJ (Fig. 3). Interestingly, the survival rate of C. coli HC2-48 was significantly higher than other C. coli strains in CJ, BJ, MHBJ, MHCLJ, MHBB, and MHFB but comparatively lower than most of the C. jejuni strains. No strains survived more than five days in MHB and MHCJ. Two tailed ANOVA for species level differences in individual juices showed significant species level differences of survival rate in retail meat juices (CJ and BJ) and all diluted juices (except MHB and MHCJ) (P < 0.0001). However, MANOVA for interaction including all experimental results for the interaction of Campylobacter species, media and time showed significant strains level differences (P < 0.0001) but not species-level differences (P = 0.38) (see Supplementary Table 1).
Figure 3.
Comparative survival analysis for C. jejuni (red lines) and C. coli (blue lines) in different retail juices and dilutions at 4 °C. Survival curves represent log reduction (CFU/ml) for each strain at 4 °C (LogN/No). Standard errors (vertical bars) were calculated from mean values of triplicate viable cell counts.
Survival at −20 °C
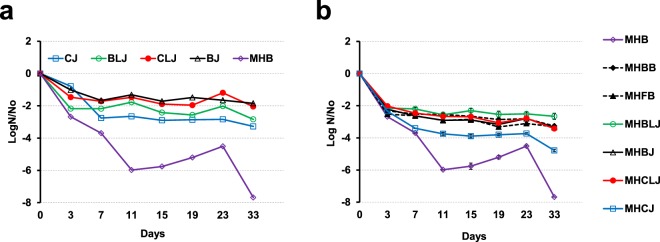
Reference strain C. jejuni NCTC11168 was used to investigate the influence of retail meat juices on the survival of Campylobacter at freezing temperature (−20 °C). We included C. jejuni NCTC11168 to compare results with a previous report of survival assay in CJ at freezing temperature (−18 °C)38. A rapid decrease in viable cell counts was observed within the first week of incubation (~3.7 logs for MHB, ~2.76 logs for CJ, ~2.17 logs for BLJ, ~1.7 logs for CLJ and ~1.67 logs for BJ); cell counts then remained relatively constant until the end of experiment in most juice matrices (Fig. 4). Diluted retail juices (MHBLJ, MHCLJ, MHBJ, and MHCJ) and horse blood (MHBB, MHFB) increased survival of C. jejuni NCTC11168 relative to the unamended MHB media at −20 °C; however, no significant differences were observed in NCTC11168 survival rates among different retail meat and liver juices.
Figure 4.
Survival curves for C. jejuni NCTC11168 (~7.6 log CFU/ml) inoculated to various full-strength (a) and diluted (b) retail meat and liver juices (5% dilution in MHB) and incubated at −20 °C. Standard errors were calculated from mean values of triplicate viable cell counts.
Growth and survival at 37 °C
The influence of retail meat and liver juices on growth and survival of Campylobacter strains (Table 1) at favorable growth temperatures was evaluated at 37 °C in microaerobic conditions. As expected, MHB supported higher growth and survival of all strains up to the end of the experiment (Fig. 5). CJ was the best matrix for growth and survival among juices. Chicken liver juice (CLJ) was more favorable for growth at 37 °C than BLJ and BJ for all C. coli strains and C. jejuni 11168 and CG1-109. None of the strains survived after four days in BLJ. C. jejuni NCTC11168 and CG1-109 and all C. coli strains failed to survive more than two days in BJ; however, C. jejuni T1-21, OD2-67, and WP2-202 survived until the end of the experiment. Interestingly, cell counts of C. jejuni T1-21 and WP2-202 decreased at 48 h and then increased, which might be caused by metabolic adaptation to the available nutrients after the initial incubation period. The lower bacterial counts in CLJ, BLJ, and BJ indicated that these matrices were less suitable food sources for growth than CJ in favorable environmental conditions (e.g. 37 °C). MANOVA showed significant species-level differences (P = 0.0035). Differences in growth on the basis of strain, juice, and origin of juice were also significant (P < 0.0001) (see Supplementary Table 2).
Figure 5.
Growth and survival of (a) C. jejuni and (b) C. coli strains in full strength (100%) retail juices and MHB at 37 °C in microaerobic condition for eight days. Statistical analyses and standard error bars are based on mean values of log CFU/ml from triplicate experiments.
Influence on biofilm formation
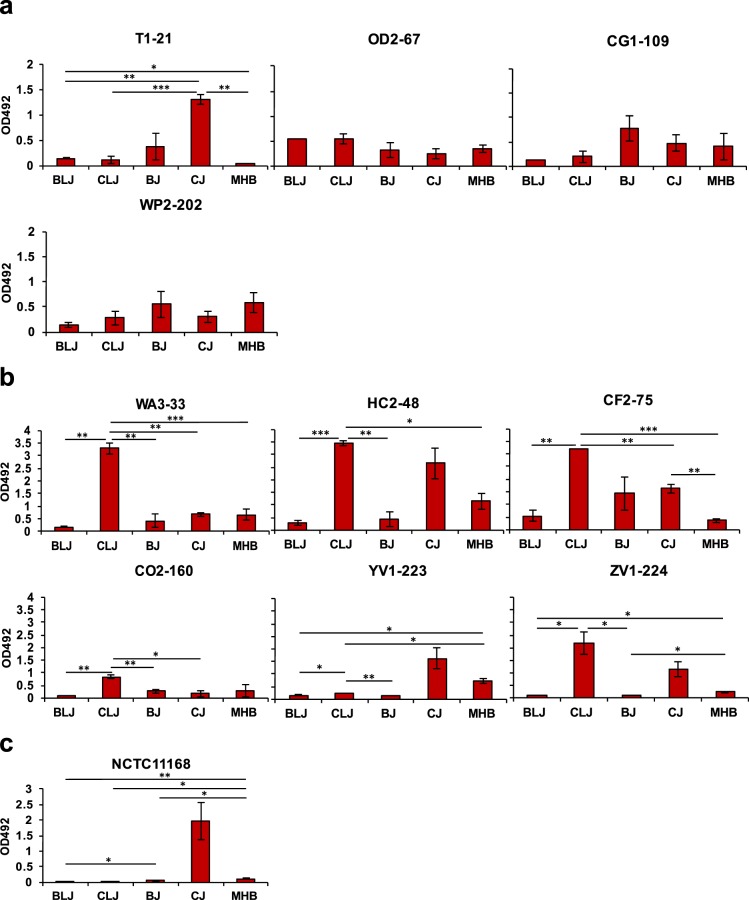
On polystyrene surfaces, incubation in CLJ significantly enhanced biofilm formation all C. coli strains except YV1-223 (Fig. 6b). Biofilm formation was higher for C. jejuni T1-21 and C. coli HC2-48, CF2-75, YV1-223 and ZV1-224 in CJ, whereas other strains did not produce significant biofilms in CJ (Fig. 6a,b). All the juices failed to promote biofilm formation in C. jejuni OD2-67, CG1-109, and WP2-202. Biofilm formation on borosilicate glass was highest when C. jejuni NCTC11168 was incubated in CJ, whereas the other juices did not promote biofilm formation (Fig. 6c). Statistical model analysis showed significant effects of strain-media (P < 0.0001) and species-media interactions on biofilm formation (P < 0.0001) (see Supplementary Table 3).
Figure 6.
Biofilm formation by (a) C. jejuni and (b) C. coli. Strains were incubated at 37 °C in MHB and retail juices (BLJ, CLJ, BJ, CJ) on polystyrene in microaerobic conditions. (c) Biofilm assay on borosilicate glass was conducted with C. jejuni NCTC11168. Error bars represent the standard error of mean values of absorbance (OD492). Significance: *P < 0.05; **P < 0.01; and ***P < 0.001.
Influence on aerotolerance
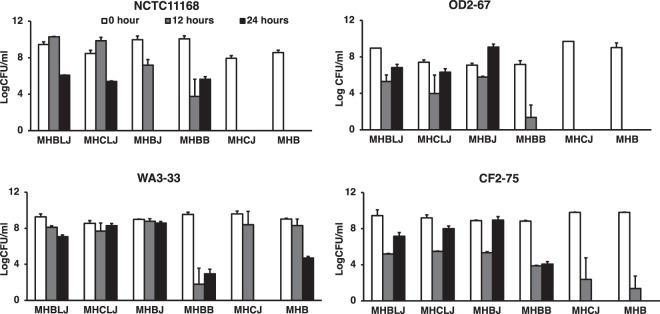
In comparison to non-amended MHB, C. jejuni NCTC11168 and OD2-67 and C. coli WA3-33 and CF2-75 showed enhanced aerotolerance in MHB amended with 10% beef and chicken liver juice (MHBLJ, MHCLJ) at 24 h of aerobic incubation (Fig. 7). The addition of laked horse blood to MHB (MHBB) enhanced survival in all strains exposed to aerobic conditions but bacterial counts were lower than MHBLJ, MHCLJ and MHBJ; this indicates the presence of an additional factor in retail liver and meat juices that enhances aerotolerance. In general, the addition of BJ to MHB also enhanced aerotolerance, but CJ and non-amended MHB did not.
Figure 7.
The influence of retail meat and liver juices on aerotolerance of C. jejuni NCTC11168 and OD2-67 and C. coli WA3-33 and CF2-75. Dilutions (10% v/v) of retail meat and liver juices in MHB were prepared and included MHBLJ (beef liver juice), MHCLJ (chicken liver juice), MHBJ (beef juice), MHCJ (chicken juice). MHBB (10% laked horse blood) and MHB (reference media) were also included in this experiment.
Discussion
Campylobacter strains routinely encounter low temperatures, and a few studies have speculated that cold tolerance and the acquisition of cryoprotectant molecules are survival mechanisms43,55. The prolonged survival of Campylobacter in cold storage conditions has been reported in media and food models38–41,48, which are influenced by the food matrix composition. Retail meat juices from chicken38,39,45, pork46 and beef56 have been used as models to represent the surface of retail meat products. Analogous to meat juice models, retail liver juice models (BLJ and CLJ) represent the retail liver environment that foodborne pathogens would encounter after contamination. Retail liver possesses choline and other nutrients which are beneficial for human health18–20. Heme and nonheme proteins and other nutrients and minerals are also highly abundant in retail meat and liver products57,58. The elevated survival of Campylobacter in retail liver juices in this study indicates that liver juices provide a nutritious and favorable environment for acquisition of cryoprotectant molecules and nutrients for metabolism59 during survival at low temperatures.
During harsh environmental conditions, Campylobacter spp. often enter the VBNC state9 and undergo a morphological change to coccoid forms59. The reported high prevalence in retail liver products30–32 and high survival rate at lower temperatures (this study) indicates that the food matrix provided by retail liver products helps retain culturable conditions of Campylobacter cells for prolonged periods. Our experimental design represents culturable cell counts of Campylobacter on MHA plates after incubation in various media at reduced temperatures. One previous report showed a rise in the C. jejuni population in inoculated chicken livers at 4 °C for a 24-h incubation, but a reduction in bacterial numbers was observed in chicken skin medallions and chicken meat60. Higher viability (culturability) of C. coli strains in retail liver juices vs. retail meat juices (Fig. 1b) correlated with the higher prevalence of reported C. coli in retail liver products as compared to retail meat products30. Similarly, the high viability of C. jejuni in retail liver and meat juices with comparable survival rates (Fig. 1a) might explain the reported high prevalence of C. jejuni in retail meats and liver products30,32,50. The decline in bacterial counts in our study when using retail liver juices is higher than previous studies, which found no significant reduction in bacterial counts in artificially-inoculated liver samples (liver slices and liver homogenate food models) at 4 °C48,49,61. Discrepancies in experimental design (food models used) and differences in the Campylobacter strains used in our study might explain the reduced survivability in our study as compared to previous reports.
During the processing and handling of retail meat and liver products, dilutions of substances naturally occur in the working environment and on the surface of retail meat and liver products. In previous studies, media supplemented with blood was compared with full-strength retail meat juices in survival assays39 however, the effect of dilutions on survival of Campylobacter at lower temperatures has not been previously studied. In this study, 5% dilutions of juices, laked blood and fresh horse blood in MHB were compared to detect the influence of the blood component and additional factor(s) in juices on the survival of Campylobacter strains at low temperatures. Enhanced survival of Campylobacter in MHB supplemented with 5% laked or fresh horse blood (MHBB or MHFB) (Fig. 2) demonstrates the influence of blood components on survival. The higher survival of selected strains in MHBB vs. MHFB indicates that the lysed blood environment improves survival. The prolonged storage of retail meat and liver products at lower temperatures promotes lysis due to the freeze-thaw process, which creates a more favorable environment for contaminant Campylobacter cells. In our study, the higher survival in MHBLJ than MHBB or MHFB for most Campylobacter strains (Fig. 2) indicates that BLJ contains other essential nutrients in addition to blood components. For C. jejuni strains, all diluents except MHCJ enhanced survival (Fig. 2a); however, only MHBLJ improved the survival of all C. coli strains (Fig. 2b). MHBJ enhanced the higher survival of some C. jejuni strains more than 100% BJ, which might be due to a more balanced nutritional composition in MHBJ vs. full-strength BJ. None of the Campylobacter strains survived well in MHCJ at low temperatures (Fig. 2), which shows the potential loss of the protective or favorable environment observed with full-strength CJ (Fig. 1). These results indicate different nutritional or environmental requirements among Campylobacter spp. for survival at low temperatures.
Significantly higher survival of C. jejuni than C. coli in CJ, BJ, and diluted juices (p < 0.0001) (Fig. 3) supports our contention that the two Campylobacter spp. have different nutritional requirements. Our results suggest that C. coli might require more nutritional support than C. jejuni strains. Hence, highly nutrient rich conditions (like BLJ and CLJ) might have supported similar survival rate for both species which differed for other juices with different nutritional contents. A similar inference was made in a previous report showing the higher survival of C. jejuni than C. coli in water samples maintained at 4 °C and 20 °C53. MANOVA for interaction of species, strains, media and time by including all results for survival at 4 °C, also showed significant strain level differences (P < 0.0001). C. jejuni strains were previously shown to be more acid tolerant than C. coli62. Among C. jejuni strains, used clinical strains survived better than poultry strains at low temperatures (4 °C and 10 °C) in a previous report63. Likewise, a waterborne C. jejuni strain showed better survival in defined fresh water media at 4 °C than a foodborne strain51. In contrast, a few reports have shown similar survival rates for both species40,41 and higher survival rate of C. coli at low temperatures in different food models64.
At freezing temperatures (−20 °C), all retail liver and meat juices and diluents enhanced survival of C. jejuni NCTC11168 relative to MHB (Fig. 4). Hence, retail liver juices likely function as a protective food matrix composition for Campylobacter spp. at subzero temperatures. Similar inference for the presence of protective materials in CJ for Campylobacter at freezing temperature had been proposed in a previous report38. The rapid decrease of bacterial numbers after inoculation into juices maintained at −20 °C (Fig. 4) is similar to previous studies where a rapid decline in bacterial numbers was observed as early as 0.5 h after inoculation49,54,65.
At a favorable growth temperature (37 °C), CJ promoted growth at 37 °C for Campylobacter strains (Fig. 5a,b), which agrees with previous reports documenting the favorable nutrient composition of CJ for enhanced growth39. Chicken liver juice also supported growth and higher survival of all Campylobacter strains in comparison to BLJ and BJ (Fig. 5a,b). Although BLJ enhanced survival of Campylobacter strains at low temperatures (Figs 1, 3, 4), it did not support growth at 37 °C. Hence, BLJ might provide cryoprotectant molecules and required nutrients for survival at low temperatures, but these factors are not conducive for growth or potentially toxic at favorable temperatures.
To our knowledge, our study is the first to use retail liver juices to investigate the influence of retail liver environments on biofilm formation and aerotolerance, which was not feasible with previously used food models (liver slices and liver homogenates)48,49,61. Chicken juice induced high levels of biofilm formation for C. jejuni strains NCTC11168 (borosilicate glass surface) and T1-21 (polystyrene) and C. coli strains HC2-48, CF2-75, YV1-223 and ZV1-224 (polystyrene) (Fig. 6a–c). In a previous report, full strength as well as 5% dilution of CJ was shown to enhance the attachment of Campylobacter strains to abiotic surfaces and biofilm formation45. It has been found that CJ environment enhances biofilm formation of both motile and non-motile variants of Campylobacter. Among tested juices, remarkably high biofilm formation was seen for most C. coli strains in CLJ (Fig. 6b), but C. jejuni biofilms were not significantly different in CLJ vs. MHB (Fig. 6a,c). In general, we observed higher biofilm formation among C. coli than C. jejuni strains in retail juices, which agrees with a previous study45. Significant strain-dependent differences in biofilm formation were observed in our study for the various retail liver and meat juices (P < 0.0001). Extracellular DNA (eDNA) has been shown to be a major component in biofilm formation of Campylobacter, where DNase and eDNase (from Campylobacter strains) treatment could rapidly remove or inhibit Campylobacter biofilms66,67. DNA components available in retail meat and liver juices after lysis of blood, meat and liver cells might enhance biofilm formation of foodborne pathogens like Campylobacter. Feng et al. previously reported higher biofilm formation of Campylobacter spp. in polymicrobial environments than in monomicrobial conditions68. Hence, other bacterial contaminants found in retail liver products25,69 could contribute to biofilm formation by Campylobacter in the retail liver environment.
Although Campylobacter spp. are microaerophilic, aerotolerant strains show enhanced survival in aerobic conditions47. The oxidative stress response is also associated with the mechanistic basis of iron acquisition70. Heme-containing proteins in retail meat and liver products57,58 function as cofactors for important enzymes in the oxidative stress response, including catalase and superoxide dismutase70. Iron content in media influences the aerotolerance mechanism of Campylobacter regulated by regulatory proteins (PerR and Fur) and genes like ferroxoxin (fdxA) and alkyl hydroperoxide reductase (ahpC)71–75. Hence, the aerotolerance observed for the four Campylobacter strains in our study (Fig. 7) might be related to the iron and nutrient content found in diluted (10%) retail meat and liver juices. Although diluted CJ did not enhance aerotolerance; other diluted retail meat and liver juices enhanced survival in aerobic conditions, possibly because of their higher nutrient composition or iron content. It is also important to note that overly high iron levels can promote the formation of toxic superoxide radicals that may be detrimental to Campylobacter metabolism70. We mention this because it could explain the survival data shown in Fig. 5, where full-strength juices resulted in reduced bacterial counts relative to MHB at 37 °C. The iron content of retail meat and liver juices might also play a significant role in survival at lower temperatures, since the oxidative stress response is activated during cold shock43.
Functional metabolic activities and genomic expression data have been reported in Campylobacter at lower temperatures (4 or 5 °C) in various growth conditions35–37. Similarly, differential expression of genes related to quorum sensing and glycosylation of flagellin have been reported in CJ when compared to artificial Brain heart infusion media36. A variety of genes were essential for survival at low temperatures in nutrient-rich or nutrient-poor media55. All transcriptomic and genome fit analyses of Campylobacter at low temperatures have been conducted with C. jejuni9,36,43,51,55. Genomic differences between C. jejuni and C. coli strains52 might contribute to differences in survival. Further investigations are needed to validate the effect of genomic differences on survival at low temperatures.
In conclusion, our results show that retail liver juices enhanced the survival of all Campylobacter strains at low temperatures, whereas other retail meat juices and dilutions had differential effects on survival. This is a highly relevant finding with respect to food safety since retail liver juices represent an environment encountered by foodborne Campylobacter after contamination. Overall, C. jejuni strains showed greater survival at 4 °C in chicken juice, beef juice, and diluted retail meat and liver juices as compared to C. coli. Chicken liver juice enhanced biofilm formation of most C. coli strains and supported growth in favorable growth conditions. Further investigations are needed to explore the mechanisms by which the retail liver environment is enhancing the survival of Campylobacter at 4 °C.
Methodology
Bacterial strain and growth conditions
Campylobacter isolates (four C. jejuni and six C. coli strains) were used in this study (Table 1) and were previously isolated from retail meat and liver products30,32,50. C. jejuni NCTC11168 (clinical isolate) was used as a reference strain. The eleven strains were subcultured from −70 °C stock cultures and grown on Mueller Hinton Agar (MHA) supplemented with 5% laked horse blood at 42 °C for 48 h in microaerobic conditions (6% O2, 13% CO2, 81% N2, Thermo Forma incubator, model 3130). Prior to harvesting bacterial cells, strains were further subcultured for 18 h on a fresh plate of MHA with 5% laked horse blood for survival, biofilm and aerotolerance assays. Bacterial cells were harvested in phosphate buffered saline (PBS) (pH 7.4), and cell suspensions were adjusted to OD600 = 0.1. In general, bacterial inoculum was prepared similarly for each assay, including survival and growth at variable temperatures, biofilm formation, and aerotolerance.
Preparation of retail meat and liver juices
For food models, retail meat and liver juices were prepared. Chicken juice (CJ) was prepared as described previously38,39. Briefly, frozen retail whole chickens without giblets were purchased from various retail meat shops and thawed overnight at room temperature. A similar procedure was used to obtain beef liver juice (BLJ) and chicken liver juice (CLJ) from frozen beef liver slices and chicken livers, respectively. Beef juice (BJ) was collected from retail meat shops after opening packets containing big chunks of beef cuts. Juices were collected aseptically in sterile containers and stored at −20 °C prior to further processing. After thawing overnight at 4 °C, CJ was centrifuged at 10,000 rpm for 15 min, whereas other meat and liver juices (CLJ, BLJ, and BJ) were centrifuged at 15,000 rpm for 30 min to exclude larger particles. Juices were filter-sterilized with a 0.45 µm membrane filter (Nalgene Rapid-Flow) and stored at −20 °C. The absence of any microbial contaminants in filtered retail meat and liver juices was confirmed by culturing (in aerobic, microaerobic and anaerobic conditions at 25 °C, 37 °C and 42 °C) in MHA supplemented with 5% laked horse blood. Microaerobic and anaerobic incubation at variable temperatures were done in gas jars containing microaerobic gas generating kits and anaerobic gas generating kits (Mitsubishi Gas Chemical, New York, NY, USA) respectively. Dilutions of meat and liver juices (5% or 10% v/v) were also prepared with MHB for survival studies at low temperature and aerotolerance assays. Similarly, dilutions of laked horse blood and fresh horse blood (5%) in MHB were also included to study whether the blood in retail meat and liver products influenced the survival of Campylobacter at low temperatures.
Survival at 4 °C
Appropriate volumes of cell suspensions were added to pre-incubated juices and dilutions to create bacterial concentrations of approximately 7 logs CFU/ml. Strains and log CFU/ml were as follows: C. jejuni NCTC11168, 6.63; T1-21, 6.6; OD2-67, 6.98; CG1-109, 6.73; WP2-202, 6.17; and C. coli WA3-33, 7.02; HC2-48, 7.28; CF2-75, 7.17; CO2-160, 7.47; YV1-223, 7.14; and ZV1-224, 6.85. MHB was used as a reference medium. An equal volume of inoculated media and juices were filled to the rim of 5 ml disposable polystyrene test tubes with caps and incubated at 4 °C to ensure microaerobic conditions. At specified time intervals, 10 µl samples were taken and serially diluted with 0.1% peptone in saline solution. Two spots of the 10 µl sample from each dilution were spotted onto MHA and incubated at 42 °C for at least 48 h. Viable cell counts were taken, and data analysis was performed with mean values of triplicate experiments.
Survival at −20 °C
C. jejuni NCTC11168 (7.6 log CFU/ml) was used as inoculum in this study for investigating the influence of retail meat and liver juices at freezing temperature. 500 µl of inoculated meat and liver juices (100%), a 5% dilution of meat and liver juices, and MHB were dispensed into 2 ml Eppendorf tubes and maintained at −20 °C. At allocated times, triplicate samples were thawed at room temperature for 10 min and serial dilutions in 0.1% peptone saline were plated. Viable cell counts were taken as described previously.
Survival at 37 °C
Inoculated juices and MHB (1.5 ml) were dispensed into a 96-well storage plate, (Square Well, 2.2 ml) and incubated at 37 °C in microaerobic conditions. 40 µl samples were removed at specified time intervals and serial dilutions were prepared as described previously. MHA plates spotted with serial dilutions were incubated at 37 °C in microaerobic conditions for at least 48 h before viable cell counts were determined.
Biofilm formation
Suspensions of all Campylobacter strains were prepared and adjusted to OD600 = 0.1 in PBS from an 18-h culture. Biofilm assays were prepared as described previously76. Briefly, the cell suspension was diluted to 1::10 in meat and liver juice, and MHB was used as a reference media. Biofilm formation on glass surfaces was investigated by incubating 1 ml of C. jejuni NTC11168-inoculated media and juices at 37 °C in 10 ml borosilicate glass tubes. After a 72 h-incubation, bacterial cell suspensions were removed, and wells were washed twice with 1.2 ml of sterile PBS (pH 7.4); plates were agitated gently to dislodge unbound cells. MHB (1.2 ml) supplemented with Triphenyl Tetrazolium Chloride (TTC) (0.05% w/v) was added to each well and incubated for 72 h at 37 °C. The other Campylobacter strains (excluding C. jejuni NCTC11168) were used to evaluate biofilm formation on polystyrene surfaces. Samples (150 µl) were incubated in sterile polystyrene 96-well microtiter plates at 37 °C in microaerobic conditions. After a 72-h incubation, cell suspensions were removed, and wells were washed twice with 180 µl sterile PBS (pH 7.4) and agitated gently to remove unbound cells. MHB (180 µl) supplemented with TTC was added to each well and incubated for 72 h at 37 °C. The remaining MHB/TTC solution was then removed, and wells were air-dried. Bound TTC dye was dissolved using a solution containing acetone (20%) and ethanol (80%); absorbance was measured at 492 nm with an Appliskan Multimode Microplate Reader (Thermo Scientific). All experiments were conducted in triplicate and repeated two or more times.
Influence of liver and meat juices on aerotolerance
Aerotolerance assays were conducted using four Campylobacter strains (C. jejuni NCTC11168, C. jejuni OD2-67, C. coli WA3-33, and C. coli CF2-75) as described previously47 with minor modifications. Similar approach has been used for aerotolerance assay in previous reports with incubation temperature at 37 °C or 42 °C71,72,74,77. Sterilized retail meat and liver juices were mixed with MHB to prepare 10% meat and liver juices. We used 10% dilutions because full-strength (100%) retail meat and liver juices coagulated more quickly, thus hindering assays of viable cell counts. Bacteria were removed from 18-h subcultures on MHA supplemented with 5% laked horse blood. Bacterial suspensions were then diluted to OD600 = 0.2 in PBS (pH 7.4). Bacterial suspensions (1 ml) were added to 9 ml of preincubated, diluted juices and then incubated aerobically at 42 °C, with agitation at 200 rpm (New Brunswick I2400 Incubator Shaker). 50 µl samples were removed at 0, 12 and 24 h, and viable cell counts were evaluated on MHA as described previously. All experiments were performed in triplicate.
Statistical analysis
For survival assays, statistical analysis was conducted with log CFU/ml values of triplicate experiments. Statistical test used with survival data was a repeated measures MANOVA with Strain effect (both C. jejuni and C. coli strains), Gowth-Media effect, Time effect (cultures repeatedly sampled over time), Strain × Growth-Media interaction, Strain × Time interaction, Growth-Media × Time interaction, and Strain × Growth-Media × Time interaction. The standard methods for approximating the F statistic were used (Wilks’ Lambda test, Pillai’s Trace test, Hotelling–Lawley test and Roy’s Max Root) when needed, which all gave the same statistical conclusion throughout our statistical analysis. For analysis of species level effect on survival, similar statistical test with the survival data was used by a repeated measures MANOVA with species replacing the strain effect (i.e. Species effect, Growth-Media effect, Time effect, Species × Growth-Media interaction, Species × Time interaction, Growth-Media × Time interaction, and Species × Growth-Media × Time interaction).
The initial data analysis was performed with respect to the origin of the Campylobacter strains (beef, chicken and pork) and the type of retail liver juice added to the growth media (BLJ, CLJ or none). Repeated measures MANOVA was also done with Origin effect, Juice effect, Time effect, Origin × Juice interaction, Origin × Time interaction, Juice × Time interaction, and Origin × Juice × Time interaction. We then examined whether liver juice specifically was significant [retail liver juice (regardless of origin or none)] by replacing juice origin (beef and chicken) in the analysis. For survival and growth at 37 °C, statistical analysis mirrored that of the survival experiment at 4 °C.
For biofilm assays, the study was conducted with mean values of absorbance (OD492). An ANOVA with Strain effect, Growth-Media effect, and Strain × Growth-Media interaction was performed. Student’s t-test and two-tailed ANOVA were conducted for each assay of survival and biofilm as needed.
Electronic supplementary material
Acknowledgements
The authors would like to acknowledge financial support from the Research Office of The University of Tulsa (Tulsa, OK, USA) for granting Anand B. Karki a student research grant.
Author Contributions
Research design and manuscript preparation: A.B.K. and M.K.F. Experimental procedures: A.B.K. Statistical analysis: H.W. and A.B.K.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-35820-7.
References
- 1.Dewey-Mattia, D., Manikonda, K. & Vieira, A. Surveillance for Foodborne Disease Outbreaks United States, 2014: Annual Report. 1–24, 10.1371/journal.pone.0183641 (2016). [DOI] [PMC free article] [PubMed]
- 2.Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet. 2016;388:717–727. doi: 10.1016/S0140-6736(16)00339-1. [DOI] [PubMed] [Google Scholar]
- 3.Ang CW, et al. Guillain-Barré syndrome- and Miller Fisher syndrome-associated Campylobacter jejuni lipopolysaccharides induce Anti-GM1 and Anti-GQ1b antibodies in rabbits. Infect. Immun. 2001;69:2462–2469. doi: 10.1128/IAI.69.4.2462-2469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Islam Z, et al. Campylobacter jejuni infection and Guillain-Barré syndrome: An emerging cause of acute flaccid paralysis after the eradication of poliomyelitis in Bangladesh. Int. J. Infect. Dis. 2016;45:176. doi: 10.1016/j.ijid.2016.02.414. [DOI] [Google Scholar]
- 5.Saraswathy TS, et al. Acute flaccid paralysis surveillance: Looking beyond the global poliomyelitis eradication initiative. Southeast Asian J. Trop. Med. Public Health. 2008;39:1033–1039. [PubMed] [Google Scholar]
- 6.Lam RMK, et al. Surveillance of acute flaccid paralysis in Hong Kong: 1997 to 2002. Hong Kong Med. J. 2005;11:164–173. [PubMed] [Google Scholar]
- 7.Naeini AE, Ghazavi M, Moghim S, Sabaghi A, Fadaei R. Acute flaccid paralysis surveillance: A 6 years study, Isfahan, Iran. Adv. Biomed. Res. 2015;4:99. doi: 10.4103/2277-9175.156670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jasem J, et al. Guillain-Barré syndrome as a cause of acute flaccid paralysis in Iraqi children: a result of 15 years of nation-wide study. BMC Neurol. 2013;13:195. doi: 10.1186/1471-2377-13-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bronowski C, James CE, Winstanley C. Role of environmental survival in transmission of Campylobacter jejuni. FEMS Microbiol. Lett. 2014;356:8–19. doi: 10.1111/1574-6968.12488. [DOI] [PubMed] [Google Scholar]
- 10.Taylor EV, et al. Common source outbreaks of Campylobacter infection in the USA, 1997-2008. Epidemiol. Infect. 2013;141:987–996. doi: 10.1017/S0950268812001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Leary MC, Harding O, Fisher L, Cowden J. A continuous common-source outbreak of campylobacteriosis associated with changes to the preparation of chicken liver pâté. Epidemiol. Infect. 2009;137:383–388. doi: 10.1017/S0950268808001003. [DOI] [PubMed] [Google Scholar]
- 12.Tompkins B, et al. Multistate outbreak of Campylobacter jejuni infections associated with undercooked chicken livers–northeastern United States, 2012. MMWR. Morb. Mortal. Wkly. Rep. 2013;62:874–6. [PMC free article] [PubMed] [Google Scholar]
- 13.Lahti E, Löfdahl M, Ågren J, Hansson I, Olsson Engvall E. Confirmation of a Campylobacteriosis Outbreak Associated with Chicken Liver Pâté Using PFGE and WGS. Zoonoses Public Health. 2017;64:14–20. doi: 10.1111/zph.12272. [DOI] [PubMed] [Google Scholar]
- 14.Edwards DS, et al. Campylobacteriosis outbreak associated with consumption of undercooked chicken liver pté in the East of England, September 2011: Identification of a dose-response risk. Epidemiol. Infect. 2014;142:352–357. doi: 10.1017/S0950268813001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Little CL, Gormley FJ, Rawal N, Richardson JF. A recipe for disaster: Outbreaks of campylobacteriosis associated with poultry liver pate in England and Wales. Epidemiol. Infect. 2010;138:1691–1694. doi: 10.1017/S0950268810001974. [DOI] [PubMed] [Google Scholar]
- 16.Inns T, Foster K, Gorton R. Cohort study of a campylobacteriosis outbreak associated with chicken liver parfait, United Kingdom, june 2010. Eurosurveillance. 2010;15:1–4. doi: 10.2807/ese.15.44.19704-en. [DOI] [PubMed] [Google Scholar]
- 17.Scott MK, et al. Notes from the field: campylobacteriosis outbreak associated with consuming undercooked chicken liver pâté - Ohio and Oregon, December 2013-January 2014. MMWR. Morb. Mortal. Wkly. Rep. 2015;64:399. [PMC free article] [PubMed] [Google Scholar]
- 18.Biesalski HK. Meat as a component of a healthy diet - Are there any risks or benefits if meat is avoided in the diet? Meat Sci. 2005;70:509–524. doi: 10.1016/j.meatsci.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 19.Vahteristo L, Ollilainen V, Varo P. HPLC determination of folate in liver and liver products. J. Food Sci. 1996;61:523–526. doi: 10.1111/j.1365-2621.1996.tb13148.x. [DOI] [Google Scholar]
- 20.Zeisel SH, Mar M-H, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003;133:1302–1307. doi: 10.1093/jn/133.5.1302. [DOI] [PubMed] [Google Scholar]
- 21.Zbrun MV, et al. Antimicrobial resistance in thermotolerant Campylobacter isolated from different stages of the poultry meat supply chain in Argentina. Food Control. 2015;57:136–141. doi: 10.1016/j.foodcont.2015.03.045. [DOI] [Google Scholar]
- 22.Luber P, Bartelt E. Enumeration of Campylobacter spp. on the surface and within chicken breast fillets. J. Appl. Microbiol. 2007;102:313–318. doi: 10.1111/j.1365-2672.2006.03105.x. [DOI] [PubMed] [Google Scholar]
- 23.Whyte R, Hudson JA, Graham C. Campylobacter in chicken livers and their destruction by pan frying. Lett. Appl. Microbiol. 2006;43:591–595. doi: 10.1111/j.1472-765X.2006.02020.x. [DOI] [PubMed] [Google Scholar]
- 24.Cox NA, et al. Natural presence of Campylobacter spp. in various internal organs of commercial broiler breeder hens. Avian Dis. 2006;50:450–453. doi: 10.1637/7481-120205R.1. [DOI] [PubMed] [Google Scholar]
- 25.Abdalrahman L, Wells H, Fakhr M. Staphylococcus aureus is More Prevalent in Retail Beef Livers than in Pork and other Beef Cuts. Pathogens. 2015;4:182–198. doi: 10.3390/pathogens4020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilhelm B, et al. Survey of Canadian retail pork chops and pork livers for detection of hepatitis E virus, norovirus, and rotavirus using real time RT-PCR. Int. J. Food Microbiol. 2014;185:33–40. doi: 10.1016/j.ijfoodmicro.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 27.von Altrock A, Roesler U, Merle R, Waldmann K-H. Prevalence of pathogenic Yersinia enterocolitica strains on liver surfaces of pigs and their antimicrobial susceptibility. J. Food Prot. 2010;73:1680–3. doi: 10.4315/0362-028X-73.9.1680. [DOI] [PubMed] [Google Scholar]
- 28.Ruzauskas M. et al. Prevalence and antimicrobial resistance of E. coli isolated from chicken liver sold in retail markets. Vet. IR Zootech. Mes Zoot52, (2010).
- 29.Kapondorah TL, Sebunya TK. Occurrence of Salmonella species in raw chicken livers purchased from retail shops in Gaborone, Botswana. J. Anim. Vet. Adv. 2007;6:87–89. [Google Scholar]
- 30.Noormohamed A, Fakhr MK. A higher prevalence rate of Campylobacter in retail beef livers compared to other beef and pork meat cuts. Int. J. Environ. Res. Public Health. 2013;10:2058–2068. doi: 10.3390/ijerph10052058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strachan NJC, et al. Source attribution, prevalence and enumeration of Campylobacter spp. from retail liver. Int. J. Food Microbiol. 2012;153:234–236. doi: 10.1016/j.ijfoodmicro.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 32.Noormohamed A, Fakhr MK. Incidence and Antimicrobial Resistance Profiling of Campylobacter in Retail Chicken Livers and Gizzards. Foodborne Pathog. Dis. 2012;9:617–624. doi: 10.1089/fpd.2011.1074. [DOI] [PubMed] [Google Scholar]
- 33.Jones AK, et al. Restaurant cooking trends and increased risk for Campylobacter infection. Emerg. Infect. Dis. 2016;22:1208–1215. doi: 10.3201/eid2207.151775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bolton DJ. Campylobacter virulence and survival factors. Food Microbiology. 2015;48:99–108. doi: 10.1016/j.fm.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 35.Wieler LH, Laturnus C. Classification and Typing of Campylobacter: Application of Genotypical Methods in Veterinary Medicine. Genome Lett. 2003;2:53–61. [Google Scholar]
- 36.Ligowska, M., Cohn, M. T., Stabler, R. A., Wren, B. W. & Brøndsted, L. Effect of chicken meat environment on gene expression of Campylobacter jejuni and its relevance to survival in food. Int. J. Food Microbiol. 145 (2011). [DOI] [PubMed]
- 37.Bronowski C, et al. Campylobacter jejuni transcriptome changes during loss of culturability in water. PLoS One. 2017;12:1–22. doi: 10.1371/journal.pone.0188936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birk T, et al. A comparative study of two food model systems to test the survival of Campylobacter jejuni at −18 degrees C. J. Food Prot. 2006;69:2635–2639. doi: 10.4315/0362-028X-69.11.2635. [DOI] [PubMed] [Google Scholar]
- 39.Birk T, Ingmer H, Andersen MT, Jørgensen K, Brøndsted L. Chicken juice, a food-based model system suitable to study survival of Campylobacter jejuni. Lett. Appl. Microbiol. 2004;38:66–71. doi: 10.1046/j.1472-765X.2003.01446.x. [DOI] [PubMed] [Google Scholar]
- 40.El-Shibiny A, Connerton P, Connerton I. Survival at refrigeration and freezing temperatures of Campylobacter coli and Campylobacter jejuni on chicken skin applied as axenic and mixed inoculums. Int. J. Food Microbiol. 2009;131:197–202. doi: 10.1016/j.ijfoodmicro.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 41.Solow BT, Cloak OM, Fratamico PM. Effect of temperature on viability of Campylobacter jejuni and Campylobacter coli on raw chicken or pork skin. J. Food Prot. 2003;66:2023–2031. doi: 10.4315/0362-028X-66.11.2023. [DOI] [PubMed] [Google Scholar]
- 42.Black RE, Levine MM, Clements ML, Hughes TP, Blaser MJ. Experimental Campylobacter jejuni Infection in Humans. J. Infect. Dis. 1988;157:472–479. doi: 10.1093/infdis/157.3.472. [DOI] [PubMed] [Google Scholar]
- 43.Stintzi, A. & Whitworth, L. Investigation of the Campylobacter jejuni cold shock response by global transcript profiling. Genome Lett. 2, 18–27 (2003).
- 44.Reuter M, Mallett A, Pearson BM, Van Vliet AHM. Biofilm formation by Campylobacter jejuni is increased under aerobic conditions. Appl. Environ. Microbiol. 2010;76:2122–2128. doi: 10.1128/AEM.01878-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown HL, et al. Chicken juice enhances surface attachment and biofilm formation of Campylobacter jejuni. Appl. Environ. Microbiol. 2014;80:7053–7060. doi: 10.1128/AEM.02614-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, et al. Effects of meat juice on biofilm formation of Campylobacter and Salmonella. Int. J. Food Microbiol. 2017;253:20–28. doi: 10.1016/j.ijfoodmicro.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 47.Oh E, McMullen L, Jeon B. High prevalence of hyper-aerotolerant Campylobacter jejuni in retail poultry with potential implication in human infection. Front. Microbiol. 2015;6:1–8. doi: 10.3389/fmicb.2015.01263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanninen ML. Survival of Campylobacter jejuni/coli in ground refrigerated and in ground frozen beef liver and in frozen broiler carcasses. Acta Vet. Scand. 1981;22:566–577. doi: 10.1186/BF03548680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore JE, Madden RH. Survival of Campylobacter coli in porcine liver. Food Microbiol. 2001;18:1–10. doi: 10.1006/fmic.2000.0367. [DOI] [Google Scholar]
- 50.Noormohamed A, Fakhr MK. Prevalence and Antimicrobial Susceptibility of Campylobacter spp. in Oklahoma Conventional and Organic Retail Poultry. Open Microbiol. J. 2014;8:130–7. doi: 10.2174/1874285801408010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trigui H, et al. Phenotypic and Transcriptomic Responses of Campylobacter jejuni Suspended in an Artificial Freshwater Medium. Front. Microbiol. 2017;8:1781. doi: 10.3389/fmicb.2017.01781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fouts, D. E. et al. Major structural differences and novel potential virulence mechanisms from the genomes of multiple Campylobacter species. PLoS Biol. 3 (2005). [DOI] [PMC free article] [PubMed]
- 53.Korhonen LK, Martikainen PJ. Comparison of the survival of Campylobacter jejuni and Campylobacter coli in culturable form in surface water. Can. J. Microbiol. 1991;37:530–533. doi: 10.1139/m91-089. [DOI] [PubMed] [Google Scholar]
- 54.Ivić-Kolevska S, Miljković-Selimović B, Kocić B, Kolevski G. Survival of Campylobacter jejuni and Campylobacter coli in chicken liver at frozen storage temperatures. J. Hyg. Eng. Des. 2015;10:10–15. [Google Scholar]
- 55.De Vries SPW, et al. Genome-wide fitness analyses of the foodborne pathogen Campylobacter jejuni in in vitro and in vivo models. Sci. Rep. 2017;7:1–17. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Midelet G, Carpentier B. Transfer of microorganisms, including Listeria monocytogenes, from various materials to beef. Appl. Environ. Microbiol. 2002;68:4015–4024. doi: 10.1128/AEM.68.8.4015-4024.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taniguchi CN, Dobbs J, Dunn MA. Heme iron, non-heme iron, and mineral content of blood clams (Anadara spp.) compared to Manila clams (V. philippinarum), Pacific oysters (C. gigas), and beef liver (B. taurus) J. Food Compos. Anal. 2017;57:49–55. doi: 10.1016/j.jfca.2016.12.018. [DOI] [Google Scholar]
- 58.Pretorius B, Schönfeldt HC, Hall N. Total and haem iron content lean meat cuts and the contribution to the diet. Food Chem. 2016;193:97–101. doi: 10.1016/j.foodchem.2015.02.109. [DOI] [PubMed] [Google Scholar]
- 59.Hazeleger W, et al. Temperature-dependent membrane fatty acid and cell physiology changes in coccoid forms of Campylobacter jejuni. Temperature-Dependent Membrane Fatty Acid and Cell Physiology Changes in Coccoid Forms of Campylobacter jejuni. Appl. Environ. Microbiol. 1995;61:2713–2719. doi: 10.1128/aem.61.7.2713-2719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kocic B, Ivic-Kolevska S, Miljkovic-Selimovic B, Milosevic Z. Survival of Campylobacter jejuni in chicken meat, chicken skin and chicken liver at low temperatures. Bratisl. Lek. Listy. 2012;113:354–6. doi: 10.4149/bll_2012_080. [DOI] [PubMed] [Google Scholar]
- 61.Firlieyanti AS, Connerton PL, Connerton IF. Campylobacters and their bacteriophages from chicken liver: The prospect for phage biocontrol. Int. J. Food Microbiol. 2016;237:121–127. doi: 10.1016/j.ijfoodmicro.2016.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shaheen BW, Miller ME, Oyarzabal OA. In vitro survival at low pH and acid adaptation response of Campylobacter jejuni and Campylobacter coli. J. Food Saf. 2007;27:326–343. doi: 10.1111/j.1745-4565.2007.00083.x. [DOI] [Google Scholar]
- 63.Chan KF, Le Tran H, Kanenaka RY, Kathariou S. Survival of clinical and poultry-derived isolates of Campylobacter jejuni at a low temperature (4 degrees C) Appl. Environ. Microbiol. 2001;67:4186–91. doi: 10.1128/AEM.67.9.4186-4191.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oyarzabal OA, Oscar TP, Speegle L, Nyati H. Survival of Campylobacter jejuni and Campylobacter coli on retail broiler meat stored at −20, 4, or 12 degrees C and development of Weibull models for survival. J. Food Prot. 2010;73:1438–1446. doi: 10.4315/0362-028X-73.8.1438. [DOI] [PubMed] [Google Scholar]
- 65.Harrison D, Corry JEL, Tchórzewska MA, Morris VK, Hutchison ML. Freezing as an intervention to reduce the numbers of campylobacters isolated from chicken livers. Lett. Appl. Microbiol. 2013;57:206–213. doi: 10.1111/lam.12098. [DOI] [PubMed] [Google Scholar]
- 66.Brown, H. L., Hanman, K., Reuter, M., Betts, R. P. & van Vliet, A. H. M. Campylobacter jejuni biofilms contain extracellular DNA and are sensitive to DNase I treatment. Front. Microbiol. 6 (2015). [DOI] [PMC free article] [PubMed]
- 67.Brown, H. L., Reuter, M., Hanman, K., Betts, R. P. & Van Vliet, A. H. M. Prevention of biofilm formation and removal of existing biofilms by extracellular dnases of Campylobacter jejuni. PLoS One, 10 (2015). [DOI] [PMC free article] [PubMed]
- 68.Feng J, et al. Chemical, physical and morphological properties of bacterial biofilms affect survival of encased Campylobacter jejuni F38011 under aerobic stress. Int. J. Food Microbiol. 2016;238:172–182. doi: 10.1016/j.ijfoodmicro.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 69.Abdalrahman L, Fakhr M. Incidence, Antimicrobial Susceptibility, and Toxin Genes Possession Screening of Staphylococcus aureus in Retail Chicken Livers and Gizzards. Foods. 2015;4:115–129. doi: 10.3390/foods4020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van Vliet AHM, Ketley JM, Park SF, Penn CW. The role of iron in Campylobacter gene regulation, metabolism and oxidative stress defense. FEMS Microbiol. Rev. 2002;26:173–186. doi: 10.1111/j.1574-6976.2002.tb00609.x. [DOI] [PubMed] [Google Scholar]
- 71.Baillon MLA, Van Vliet AHM, Ketley JM, Constantinidou C, Penn CW. An iron-regulated alkyl hydroperoxide reductase (AhpC) confers aerotolerance and oxidative stress resistance to the microaerophilic pathogen Campylobacter jejuni. J. Bacteriol. 1999;181:4798–4804. doi: 10.1128/jb.181.16.4798-4804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Vliet AHM, Baillon MLA, Penn CW, Ketley JM. The iron-induced ferredoxin FdxA of Campylobacter jejuni is involved in aerotolerance. FEMS Microbiol. Lett. 2001;196:189–193. doi: 10.1111/j.1574-6968.2001.tb10563.x. [DOI] [PubMed] [Google Scholar]
- 73.Butcher J, Handley RA, van Vliet AHM, Stintzi A. Refined analysis of the Campylobacter jejuni iron-dependent/independent Fur- and PerR-transcriptomes. BMC Genomics. 2015;16:498. doi: 10.1186/s12864-015-1661-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Handley RA, et al. PerR controls oxidative stress defence and aerotolerance but not motility-associated phenotypes of Campylobacter jejuni. Microbiol. (United Kingdom) 2015;161:1524–1536. doi: 10.1099/mic.0.000109. [DOI] [PubMed] [Google Scholar]
- 75.Van Vliet AHM, Baillon MLA, Penn CW, Ketley JM. Campylobacter jejuni contains two fur homologs: Characterization of iron-responsive regulation of peroxide stress defense genes by the PerR repressor. J. Bacteriol. 1999;181:6371–6376. doi: 10.1128/jb.181.20.6371-6376.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brown HL, van Vliet AHM, Betts RP, Reuter M. Tetrazolium reduction allows assessment of biofilm formation by Campylobacter jejuni in a food matrix model. J. Appl. Microbiol. 2013;115:1212–1221. doi: 10.1111/jam.12316. [DOI] [PubMed] [Google Scholar]
- 77.Oh E, McMullen L, Jeon B. Impact of oxidative stress defense on bacterial survival and morphological change in Campylobacter jejuni under aerobic conditions. Front. Microbiol. 2015;6:1–8. doi: 10.3389/fmicb.2015.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marasini, D. & Fakhr, M. K. Complete genome sequences of plasmid-bearing Campylobacter coli and Campylobacter jejuni strains isolated from retail chicken liver. Genome Announc. 5 (2017). [DOI] [PMC free article] [PubMed]
- 79.Marasini D, Fakhr MK. Complete Genome Sequences of Campylobacter jejuni Strains OD267 and WP2202 Isolated from Retail Chicken Livers and Gizzards Reveal the Presence of Novel 116-Kilobase and 119-Kilobase Megaplasmids with Type VI Secretion Systems. Genome Announc. 2016;4:e01060–16. doi: 10.1128/genomeA.01060-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marasini D, Fakhr MK. Complete Genome Sequences of Campylobacter jejuni Strains Isolated from Retail Chicken and Chicken Gizzards. Genome Announc. 2017;5:e01351–17. doi: 10.1128/genomeA.01351-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marasini D, Fakhr MK. Whole-Genome Sequencing of a Campylobacter jejuni Strain Isolated from Retail Chicken Meat Reveals the Presence of a Megaplasmid with Mu-Like Prophage and Multidrug Resistance Genes. Genome Announc. 2016;4:e00460–16. doi: 10.1128/genomeA.00460-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marasini, D. & Fakhr, M. K. Complete genome sequences of plasmid-bearing multidrug-resistant Campylobacter jejuni and Campylobacter coli strains with type VI secretion systems, isolated from retail turkey and pork. Genome Announc. 5 (2017). [DOI] [PMC free article] [PubMed]
- 83.Marasini D, Fakhr MK. Complete Genome Sequences of the Plasmid-Bearing Campylobacter coli Strains HC2-48, CF2-75, and CO2-160 Isolated from Retail Beef Liver. Genome Announc. 2016;4:e01004–16. doi: 10.1128/genomeA.01004-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.