Abstract
Endometriosis is an enigmatic condition with an unknown etiology and a poorly understood pathogenesis. It is considered to appear from the interplay of many genetic and environmental factors, affecting up to 10% of women and represents a major cause of pain and infertility. The familial association of endometriosis, as demonstrated through monozygotic twin and family studies suggests a genetic contribution to the disease, with further case-control and genome-wide association studies (GWAS) detecting various endometriosis risk factors. In a recent study, we described a unique, three-generation family of Cretan origin (Greece) with 7 females with surgically confirmed endometriosis (grandmother, 3 daughters and 3 granddaughters). All the affected members of this family displayed a variety of clinical manifestations and complications. In the present study, to further analyze the genetic variants conferring the risk of developing endometriosis, whole exome sequencing (WES) was performed, using the AmpliSeq technology on the Ion Proton platform. An initial analysis of 64 variants that were detected across the 14 genes previously confirmed to be associated with endometriosis, did not identify any deleterious exonic variants in these genes. However, further analysis revealed 2 hemizygous deletions in the grandmother that segregate in several of her affected offspring. The first deletion was found in the UGT2B28 locus, spanning 7 informative sequence variants across at least 14 kb. The second deletion, located in USP17L2, spans 3 informative variants across at least 2 kb. On the whole, the findings of the presents study implicate 2 additional genes in the pathogenesis of endometriosis, apart from those already identified by GWAS.
Keywords: endometriosis, gene variants, whole exome sequencing, family history
Introduction
Endometriosis is a benign, common gynecologic disorder, affecting 6–10% of women during their reproductive years (1). Chronic pelvic pain, dyspareunia, dysmenorrhea and infertility represent the main manifestations (2). The absence of a timely and non-invasive diagnostic tool is presently the greatest barrier to the identification and treatment of endometriosis.
Endometriosis is characterized by a multifactorial pattern of inheritance, influenced by multiple genetic and environmental factors, as documented initially by the elevated incidence of severe endometriosis in the first-degree relatives of affected women compared to women lacking a positive family history (3,4). Furthermore, two monozygotic twin-based and family studies have demonstrated an approximately 50% heritability for endometriosis (5,6). Case-control studies, genome-wide association studies (GWAS) and meta-analyses have led to the identification of disease-risk loci that alter a woman's risk of developing the disorder and provide new insight into potential pathways leading to endometriosis. Particularly, GWAS in several series of samples have identified single nucleotide polymorphisms (SNPs) that are either directly or indirectly implicated in matrix remodeling, cell cycle regulation and signaling, cell adhesion, hormone receptors and metabolism, transcription regulation and inflammation, as well as immune and oxidative stress processes (7). However, all these data only account for approximately 5% of the disease variance (8). It is possible that rare and recent familial mutations, not detectable by GWAS, are responsible for part of the missing heritability. Thus, recent findings have suggested an increased burden of rare coding variants in genes involved in the development of complex diseases, apart from the established common variants identified by GWAS (9). Despite the continued progress being made in resolving the genetic underpinnings of endometriosis, a comprehensive list of genetic loci is far from being completed, and the functional role of causal variants remain poorly understood.
Recent progress in next generation sequencing provides us with the opportunity to search for less common variants with significant effects. In line with the aforementioned assumptions, whole exome sequencing (WES) provides a hypothesis-neutral approach. Accordingly, WES is a procedure that allows for the purification by sequence capture of all exonic regions of a genome and their further processing by next-generation sequencing (NGS) (10). Thus, prompted by the data collected by an initial genetic analysis (11) of a three-generation family of 7 affected women with surgically confirmed endometriosis and aiming to explore the contribution of functional coding variants to this disease, in the present study, we used WES to identify inherited rare, endometriosis-associated exonic variants in the patients of this family.
Patients and methods
Patients and study design
A three-generation family with 7 affected members is shown in Fig. 1. Case no. 1 was the first individual in the family to be diagnosed with endometriosis and underwent surgical hysterectomy at the age of 32 due to stage IV bilateral ovarian endometriosis. Her mother (not shown in the pedigree) had 4 children with no gynecological problems. However, her 3 daughters (case nos. 2–4) and 3 granddaughters (case nos. 5–7) have all been surgically diagnosed with endometriosis (11,12). Further clinical details regarding the disease severity of the affected members of the family have been previously reported in the study by Matalliotakis et al (11). In addition to endometriosis, case no. 1 has been diagnosed with 14 other morbidities, including Crohn's disease, interstitial cystitis, bronchial asthma, cardiovascular diseases, lupus erythematosus and multiple sclerosis (12).
Figure 1.
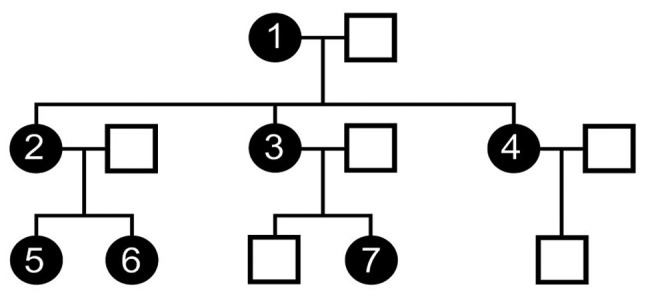
The three-generation family with 7 women affected with endometriosis, which was analyzed by whole exome sequencing. Filled circles represent women with endometriosis and open squares represent males. The available family members studied are indicated as case nos. 1–7. The figure has been adapted from a previous study (11).
The Ethics Committee of Venizeleio General Hospital of Heraklion (Heraklion, Greece) (ECHR no. 46/6686) approved the overall study and written informed consent was obtained from all the patients. The medical records were collected by the clinicians and pathologists, including surgical procedures and findings.
WES
Venous blood samples (5 ml per patient) were collected from the family members and stored at −20°C. Genomic DNA was isolated from peripheral blood leukocytes by using a commercial kit (PureLink® Genomic DNA mini kit; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's instructions. Exome sequencing was conducted using the AmpliSeq technology on Ion Proton platform (Thermo Fisher Scientific, Inc.). A series of filters was applied in order to reduce sequencing artifacts. Following sequence assembly using Torrent software, variant annotation was performed using ANNOVAR (hg19 reference; http://annovar.openbioinformatics.org/en/latest/). Variants were determined using Ion Proton protocol and confirmed using the Genome Analysis Toolkit pipeline. Identity-by-descent (IBD) was calculated using PLINK (1.07) (13) and compared to the expected values for all relative-pairs in the pedigree.
Results
Common endometriosis-associated genetic variants
In the framework of a preliminary analysis conducted (data not shown), we first detected 64 variants across the 14 genes confirmed thus far to be associated with endometriosis (8). However, the vast majority of these variants were common in the population [minor allele frequency (MAF) >1%)] and, as a consequence, are not considered to be disease-causing. Moreover, we did not find any evidence of any deletions in these well-analyzed 14 genes.
Two rare variants (rs763439987 and rs139078629) were observed in the FN1 gene (MAF <1%), with each of the variants present in a single member of the family. Segregation analysis revealed that both variants appeared to be paternally inherited and in linkage disequilibrium (LD) (detailed data not shown).
Novel genetic variants
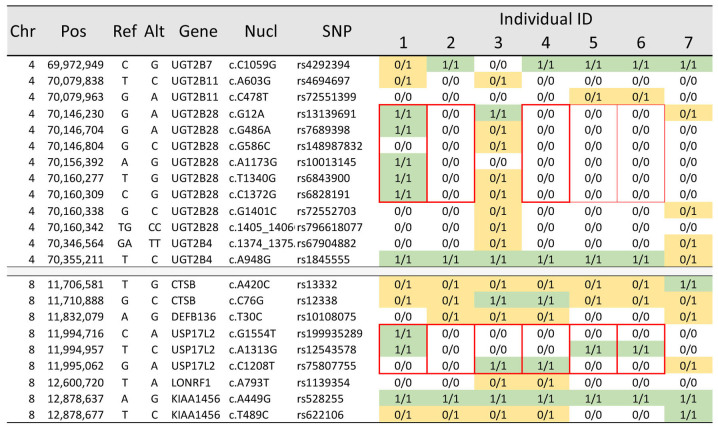
In a further in-depth analysis of normally segregating rare variants, we identified approximately 20,000 exonic variants in each of the 7 individuals, and almost 34,000 variants combined across the pedigree, which was in line with our expectations. IBD and segregation analysis confirmed all individual associations and the overall pedigree structure. We identified 2 hemizygous deletions segregating in this three-generation family (Fig. 2). A deletion was found in UDP glucuronosyltransferase family 2 member B28 (UGT2B28) genomic region, spanning 7 informative sequence variants across at least 14 kb, as well as a deletion in ubiquitin specific peptidase 17-like family member 2 (USP17L2) genomic region spanning 3 informative variants across at least 2 kb. Both deletions were present in the affected grandmother and segregated in as many as 4 and 5 of her descendants, respectively.
Figure 2.
The chromosomal position and characteristics of the genetic variants surrounding the hemizygous deletions of the UGT2B28 and USP17L2 genes are shown to the left, and the genotypes for each of the 7 affected women are shown to the right. Bold red boarders indicate the extent of the deletion and the individuals that carry the deletions. Thin red boarders indicate possible carriers of the deletion. The fields in the table shown with 0/0 are interpreted as wild-type homozygous, fields with 0/1 as heterozygotes (yellow), and those with 1/1 as homozygous for the alternate allele (green). SNP, single nucleotide polymorphisms.
Discussion
Recent advances in genome sequencing technologies provide many opportunities for the characterization of individual genomic landscapes and for the identification of mutations relevant for diagnosis and therapy. In particular, WES has become a popular approach in the human genetics community considering its moderate costs, the amount of data that can be managed reasonably and the straightforward interpretation of the accumulated results upon analysis. The progress of high-throughput sequencing has led geneticists to directly perform WES for identifying the candidate variants in monogenic, as well as multifactorial diseases (14), for elucidating the genetic basis of some genetically heterogeneous disorders (15) and for an improved diagnosis of patients (16). In this framework, we hypothesized that WES of the affected members of a rare, three-generation family with 7 members with endometriosis, would be more likely to facilitate the identification of common and rare deleterious genetic variants that contribute to disease risk.
The current analysis demonstrated the absence of any deleterious exonic variants in genes confirmed thus far to be associated with endometriosis (8). However, the results obtained implicate the UGT2B28 and USP17L2 genes in the pathogenesis of endometriosis. Neither of the genes has previously been reported to be associated with endometriosis, at least to the best of our knowledge. UGT2B28 is a member of the UGT2B gene family, which is known to be involved in the metabolism of steroid hormones and several other xenobiotics (17) and is widely expressed in the liver, as well as extrahepatic steroid target tissues (18). Germ line copy-number variation in the UGT2B28 gene has been associated with Addison's disease (19), while common polymorphisms and copy number variation of the gene affect steroid hormone abundance and has been implicated in the increase of prostate cancer risk (18,20). Deletions of this gene have been reported to be predictors of the prostate-specific antigen (PSA) level in clinically localized prostate cancer following surgical treatment (21). Based on the aforementioned findings, the important role of UGT2B28 in the regulation of the hormonal exposure of steroid target cells has been suggested, and it has also been suggested that the alteration of UGT2B28 function may affect the metabolism of sex hormones (22). However, the mechanisms through which UGT2B28 may affect the pathogenesis of endometriosis are not yet clear.
USP17L2 (also known as DUB3, DUB-3 or USP17) is a member of the USP17 subfamily of USPs, consisted by cysteine proteases (23) regulating key cellular processes, such as cell growth and survival (24). It is transiently induced in response to cytokines and, when it is constitutively expressed, can block growth factor-dependent proliferation (24). USP17L2 has deubiquitinating activity and its overexpression leads to apoptosis and cell death (25). Ubiquitin has been found to be expressed in endometriotic cells and, as suggested, may contribute to a reduced sensitivity of ectopic endometrial tissue to apoptosis (26). Thus far, USP17L2 has been suggested to possibly be a valuable biomarker for the prediction of ovarian cancer prognosis and its inhibition may be a potential strategy for ovarian cancer treatment (27). This gene plays a central role in the regulation of the transcription factors, Slug and Twist. Particularly, it has been found that USP17L2 interacts with and stabilizes Slug and Twist through de-ubiquitination (28). As is known, Slug, Snail and Twist are three key epithelial-to-mesenchymal transition transcription factors (EMT-TFs) that are tightly regulated via ubiquitination and degradation. Furthermore, the aberrant expression/activation of EMT-TFs can give rise to tumor angiogenesis, invasion and metastasis (29,30).
EMT can be considered as a cellular de-differentiation process providing cells with the increased plasticity required during embryonic development and tissue remodeling, due to the similarities in the migratory and invasive characteristics that the cells obtain during these processes (31,32). We have previously proposed that the pathogenesis of endometriosis involves the loss of mesothelial barrier integrity due to the activation of the EMT repair mechanism (33). As a consequence, in the absence of the mesothelial barrier, endometrial cells can more readily adhere to the underlying peritoneal stroma and establish endometrial lesions. Thus, we speculate that the dosage-dependent loss of USP17L2 affects mesothelial integrity and increases the risk of developing endometriosis.
Furthermore, in the framework of the present WES, two rare variants (rs763439987 and rs139078629) were observed in the FN1 gene to be paternally inherited. Although both of the hemizygous deletions clearly are inherited from the grandmother of the family under investigation, any paternally inherited risk-variants, such as the rare FN1 haplotype, must also be considered.
In conclusion, the present study identified, for the first time, to the best of our knowledge, a role of the UGT2B28 and USP17L2 genes in endometriosis and underlines the power of WES to decipher complex phenotypes. However, the precise underlying mechanisms remain elusive. The present findings may enhance our biological insight of endometriosis and may contribute towards novel strategies regarding improved patient care. Further in-depth analysis of normally segregating rare variants is ongoing.
Acknowledgements
Not applicable.
Funding
Juneau Biosciences provided funding for the sequencing.
Availability of data and materials
All data generated or analyzed during the present study are included in this published article or are available from the corresponding author on reasonable request.
Authors' contributions
HMA, RC, KW, GNG and CM conceived and designed the study and drafted the manuscript. HMA, RC, KW and MIZ performed the experiments. MM, RC, CM, GNG and MIZ searched the literature. IM, CM and MM obtained the clinical data. CM, RC, MIZ, DAS, GNG and IM analyzed and interpreted the data. DAS, HMA, MM, MIZ and IM critically revised the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The Ethics Committees of the Human Research at Venizeleio General Hospital of Heraklion (ECHR no. 46/6686) approved the overall study and written informed consent was obtained from all the patients.
Patient consent for publication
Not applicable.
Competing interests
HMA, RC and KW are employed by Juneau Biosciences who provided salary support for the study. DAS is the Editor-in-Chief for the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article.
References
- 1.Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511–519. doi: 10.1016/j.fertnstert.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halis G, Arici A. Endometriosis and inflammation in infertility. Ann N Y Acad Sci 1034. 2004:300–315. doi: 10.1196/annals.1335.032. [DOI] [PubMed] [Google Scholar]
- 3.Simpson JL, Elias S, Malinak LR, Buttram VC., Jr Heritable aspects of endometriosis. I. Genetic studies. Am J Obstet Gynecol. 1980;137:327–331. doi: 10.1016/0002-9378(80)90917-5. [DOI] [PubMed] [Google Scholar]
- 4.Hadfield RM, Mardon HJ, Barlow DH, Kennedy SH. Endometriosis in monozygotic twins. Fertil Steril. 1997;68:941–942. doi: 10.1016/S0015-0282(97)00359-2. [DOI] [PubMed] [Google Scholar]
- 5.Treloar SA, O'Connor DT, O'Connor VM, Martin NG. Genetic influences on endometriosis in an Australian twin sample. sueT@qimr.edu.au. Fertil Steril. 1999;71:701–710. doi: 10.1016/S0015-0282(98)00540-8. [DOI] [PubMed] [Google Scholar]
- 6.Saha R, Pettersson HJ, Svedberg P, Olovsson M, Bergqvist A, Marions L, Tornvall P, Kuja-Halkola R. Heritability of endometriosis. Fertil Steril. 2015;104:947–952. doi: 10.1016/j.fertnstert.2015.06.035. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi H, Imanaka S, Nakamura H, Tsuji A. Understanding the role of epigenomic, genomic and genetic alterations in the development of endometriosis (review) Mol Med Rep. 2014;9:1483–1505. doi: 10.3892/mmr.2014.2057. [DOI] [PubMed] [Google Scholar]
- 8.Sapkota Y, Steinthorsdottir V, Morris AP, Fassbender A, Rahmioglu N, De Vivo I, Buring JE, Zhang F, Edwards TL, Jones S, et al. iPSYCH-SSI-Broad Group Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat Commun. 2017;8:15539. doi: 10.1038/ncomms15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao L, Emond MJ, Louie T, Cheadle C, Berger AE, Rafaels N, Vergara C, Kim Y, Taub MA, Ruczinski I, et al. National Heart, Lung, and Blood Institute GO Exome Sequencing Project Identification of rare variants in ATP8B4 as a risk factor for systemic sclerosis by whole-exome sequencing. Arthritis Rheumatol. 2016;68:191–200. doi: 10.1002/art.39449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng SB, Turner EH, Robertson PD, Flygare SD, Bigham AW, Lee C, Shaffer T, Wong M, Bhattacharjee A, Eichler EE, et al. Targeted capture and massively parallel sequencing of 12 human exomes. Nature. 2009;461:272–276. doi: 10.1038/nature08250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matalliotakis M, Zervou MI, Matalliotaki C, Arici A, Spandidos DA, Matalliotakis I, Goulielmos GN. Genetic association study in a three-generation family with seven members with endometriosis. Mol Med Rep. 2017;16:6077–6080. doi: 10.3892/mmr.2017.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matalliotaki C, Matalliotakis M, Zervou MI, Trivli A, Matalliotakis I, Mavromatidis G, Spandidos DA, Albertsen HM, Chettier R, Ward K, Goulielmos GN. Co-existence of endometriosis with 13 non-gynecological co-morbidities: Mutation analysis by whole exome sequencing. Mol Med Rep. 2018;18:5053–5057. doi: 10.3892/mmr.2018.9521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng SB, Buckingham KJ, Lee C, Bigham AW, Tabor HK, Dent KM, Huff CD, Shannon PT, Jabs EW, Nickerson DA, et al. Exome sequencing identifies the cause of a mendelian disorder. Nat Genet. 2010;42:30–35. doi: 10.1038/ng.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girard SL, Gauthier J, Noreau A, Xiong L, Zhou S, Jouan L, Dionne-Laporte A, Spiegelman D, Henrion E, Diallo O, et al. Increased exonic de novo mutation rate in individuals with schizophrenia. Nat Genet. 2011;43:860–863. doi: 10.1038/ng.886. [DOI] [PubMed] [Google Scholar]
- 16.Choi M, Scholl UI, Ji W, Liu T, Tikhonova IR, Zumbo P, Nayir A, Bakkaloğlu A, Ozen S, Sanjad S, et al. Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proc Natl Acad Sci USA. 2009;106:19096–19101. doi: 10.1073/pnas.0910672106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tephly TR, Burchell B. UDP-glucuronosyltransferases: A family of detoxifying enzymes. Trends Pharmacol Sci. 1990;11:276–279. doi: 10.1016/0165-6147(90)90008-V. [DOI] [PubMed] [Google Scholar]
- 18.Guillemette C, Lévesque E, Harvey M, Bellemare J, Menard V. UGT genomic diversity: Beyond gene duplication. Drug Metab Rev. 2010;42:24–44. doi: 10.3109/03602530903210682. [DOI] [PubMed] [Google Scholar]
- 19.Brønstad I, Wolff AS, Løvås K, Knappskog PM, Husebye ES. Genome-wide copy number variation (CNV) in patients with autoimmune Addison's disease. BMC Med Genet. 2011;12:111. doi: 10.1186/1471-2350-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karypidis AH, Olsson M, Andersson S-O, Rane A, Ekström L. Deletion polymorphism of the UGT2B17 gene is associated with increased risk for prostate cancer and correlated to gene expression in the prostate. Pharmacogenomics J. 2008;8:147–151. doi: 10.1038/sj.tpj.6500449. [DOI] [PubMed] [Google Scholar]
- 21.Nadeau G, Bellemare J, Audet-Walsh É, Flageole C, Huang SP, Bao BY, Douville P, Caron P, Fradet Y, Lacombe L, et al. Deletions of the androgen-metabolizing UGT2B genes have an effect on circulating steroid levels and biochemical recurrence after radical prostatectomy in localized prostate cancer. J Clin Endocrinol Metab. 2011;96:E1550–E1557. doi: 10.1210/jc.2011-1049. [DOI] [PubMed] [Google Scholar]
- 22.Belledant A, Hovington H, Garcia L, Caron P, Brisson H, Villeneuve L, Simonyan D, Têtu B, Fradet Y, Lacombe L, et al. The UGT2B28 sex-steroid inactivation pathway is a regulator of steroidogenesis and modifies the risk of prostate cancer progression. Eur Urol. 2016;69:601–609. doi: 10.1016/j.eururo.2015.06.054. [DOI] [PubMed] [Google Scholar]
- 23.Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, Bernards R. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123:773–786. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Burrows JF, McGrattan MJ, Rascle A, Humbert M, Baek KH, Johnston JA. DUB-3, a cytokine-inducible deubiquitinating enzyme that blocks proliferation. J Biol Chem. 2004;279:13993–14000. doi: 10.1074/jbc.M311291200. [DOI] [PubMed] [Google Scholar]
- 25.Shin JM, Yoo KJ, Kim MS, Kim D, Baek KH. Hyaluronan- and RNA-binding deubiquitinating enzymes of USP17 family members associated with cell viability. BMC Genomics. 2006;7:292. doi: 10.1186/1471-2164-7-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ilad RS, Fleming SD, Bebington CR, Murphy CR. Ubiquitin is associated with the survival of ectopic stromal cells in endometriosis. Reprod Biol Endocrinol. 2004;2:69. doi: 10.1186/1477-7827-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou B, Shu B, Xi T, Su N, Liu J. Dub3 expression correlates with tumor progression and poor prognosis in human epithelial ovarian cancer. Biomed Pharmacother. 2015;70:84–89. doi: 10.1016/j.biopha.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Lin Y, Wang Y, Shi Q, Yu Q, Liu C, Feng J, Deng J, Evers BM, Zhou BP, Wu Y. Stabilization of the transcription factors slug and twist by the deubiquitinase dub3 is a key requirement for tumor metastasis. Oncotarget. 2017;8:75127–75140. doi: 10.18632/oncotarget.20561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blanco MJ, Moreno-Bueno G, Sarrio D, Locascio A, Cano A, Palacios J, Nieto MA. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene. 2002;21:3241–3246. doi: 10.1038/sj.onc.1205416. [DOI] [PubMed] [Google Scholar]
- 30.Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 31.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 32.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Albertsen HM, Ward K. Genes linked to endometriosis by GWAS are integral to cytoskeleton regulation and suggests that mesothelial barrier homeostasis is a factor in the pathogenesis of endometriosis. Reprod Sci. 2017;24:803–811. doi: 10.1177/1933719116660847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the present study are included in this published article or are available from the corresponding author on reasonable request.