Abstract
The process of liver fibrosis is reversible and involves a recovery phase. In the present study, the potential side effects of combination leflunomide and methotrexate (LEF+MTX), a conventional rheumatoid arthritis therapy used in the resolution of liver fibrosis, was investigated. In a carbon tetrachloride-induced liver fibrosis model, the results of hepatic pathology demonstrated that the LEF+MTX combination delayed the recovery of fibrosis, although the activation of hepatic stellate cells in vitro was inhibited. A total of four liver fibrosis-associated indicators, hyaluronic acid, laminin, procollagen type III and collagen IV, maintained high levels in the serum of LEF+MTX-treated mice, while detection of bone marrow-driven monocytes in the blood by flow cytometry indicated that they were significantly decreased. Notably, the results of immunofluorescence staining of hepatic myeloid cells and detection of vascular growth factor A (VEGF-A) in blood and liver suggested that the reduced degeneration of collagen in liver sinusoids was associated with decreased myeloid cell adhesion and the downregulation of VEGF-A in the liver. The present results suggested that in certain cases, treatment with LEF+MTX may impede the recovery of hepatic fibrosis-associated diseases in mice.
Keywords: leflunomide, methotrexate, liver fibrosis, myeloid cell, vascular growth factor
Introduction
Chronic liver disease, the leading cause of mortality and morbidity globally, is characterized as fibrogenesis, which increases the risk of liver cirrhosis and liver failure (1). Persistent liver injury leads to unresolved inflammation and the activation of myofibroblasts, which express extracellular matrix (ECM) components to trigger the generation of liver fibrosis (2). However, observations in clinical studies and experimental animal models suggest that the process of liver fibrosis is dynamic and reversible, indicating that it contains at least two phases, the developmental and recovery phases (3). Therefore, the guidance of clinical medication may need to be upgraded according to the different phases of this disease, which might have an influence on the side-effects of treatments.
Leflunomide (LEF) is an anti-rheumatic drug with immunosuppressive and anti-inflammatory functions (4). Teriflunomide, the metabolite of LEF, induces cell cycle arrest in vigorously dividing lymphocytes, and not in non-lymphatic cells (5). Methotrexate (MTX) is a chemotherapy agent used for autoimmune diseases due to its inhibitory effect on lymphocyte activation (6). Of note, following failure of monotherapy, LEF is used in combination with MTX, which has been demonstrated to be effective and safe (6). However, clinical studies suggest that this combination is associated with a significantly increased risk of leucopenia and silent liver fibrosis in patients with rheumatoid arthritis (RA) (7,8). Chronic liver diseases frequently overlap with patients suffering from rheumatoid disease, which requires the disease-modifying anti-rheumatic drugs to withstand an excessive autoimmune response (9). Thus, studying the multiple effects of the anti-rheumatic drugs in the process of liver fibrosis is of importance. Additionally, previous studies highlighted that the resolution of liver fibrosis is mediated by myeloid cell-induced sinusoidal remodeling and the involvement of macrophages (10,11). Therefore, the potential side effects of the LEF+MTX combination during the spontaneous recovery phase of liver fibrosis in mice was investigated.
In the present study, it was demonstrated that the LEF+MTX treatment impeded the step of hepatic fibrosis recovery. The underlying mechanism was associated with hindering ECM degradation and sinusoidal remodeling induced by the reduced adhesion of myeloid cells. The present study elucidated the side effects of the LEF+MTX combination in hepatic fibrosis recovery and provided evidence for its use in clinical therapy.
Materials and methods
Animal experiment
A total of 50, 6-week old C57BL/6 mice weighing ~20 g were obtained from the Shanghai Model Organisms Center, Inc. (Shanghai, China). Mice were divided into 4 groups, 6 mice per group, for 2 individual experiments. All animal experiments were approved by the Committee on Laboratory Animal Care of Fujian Medical University (Fuzhou, China) and performed according to the ‘ARRIV’ guidelines (12). Female mice aged 8 weeks were intraperitoneally injected with 200 µl 25% carbon tetrachloride (CCl4)/olive oil (2 ml/kg) three times per week for 12 weeks to induce hepatic fibrosis. A group of mice served as the control, termed the NC group. The degree of liver fibrosis in the mice was evaluated by histology. Subsequently, the mice were randomly divided into two groups: A group received LEF (15 mg/kg, oral administration) and MTX (intraperitoneal administration of 0.15 mg/Kg,) every day for four weeks and a group that received the relevant vehicle of LEF and MTX via the same routes. Leflunomide (solvent, 0.5% sodium carboxymethylcellulose) and methotrexate (solvent, PBS) were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Hematoxylin-eosin staining
Whole liver tissues were fixed in 4% paraformaldehyde at room temperature overnight and embedded in paraffin. Sections (5 µm) of paraffin-embedded tissues were subjected to hematoxylin staining for 8 min at room temperature. Following washing in water for 1 h and dehydration in ethyl alcohol for 10 min, the sections were subjected to eosin staining for 3 min at room temperature. Following dehydration in ethyl alcohol, the sections were mounted to detect pathological alterations. The images (magnification, ×200) were obtained by an Axio Vert.A1 inverted microscope (Zeiss AG, Oberkochen, Germany).
Masson staining
Masson staining was performed with a Masson-Goldner staining kit (Merck KGaA), following the manufacturer's protocol, to detect collagen deposition in liver tissues. Briefly, rehydrated sections (5 µm) were staining with Weigert's iron hematoxylin staining solution for 5 min, followed by staining with Azophloxine solution for 10 min, Tungstophosphoric acid orange G solution for 1 min and Light green SF solution for 2 min. Washes with 1% acetic acid were performed between the staining steps. Following dehydration in ethyl alcohol, the sections were mounted. All the procedures were performed on room temperature. The images (magnification, ×200) were obtained by an Axio Vert.A1 inverted microscope (Zeiss AG, Oberkochen, Germany).
Immunofluorescence
Liver tissues were fixed in 4% paraformaldehyde and cut using a cryostat. Sections (10 µm) were permeabilized with PBS containing 0.5% Triton X-100. Following blocking with PBS/5% bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 2 h at room temperature, the sections were incubated with Alexa-Fluor488-conjugated rat anti-mouse cluster of differentiation (CD)11b antibody (1:200; cat. no. 101219; clone M1/70; BioLegend, Inc., San Diego, CA, USA) at 37°C for 1 h in the dark. The sections were counterstained with DAPI (BioLegend, Inc.) for 15 min at room temperature. The images (magnification, ×200) were visualized by a Zeiss LSM700 confocal microscope (Zeiss AG).
ELISA
Serum (~5 µl) contents were detected by procollagen type III (cat. no. CSB-E17125m), collagen type IV (cat. no. CSB-E08884m), hyaluronic acid (cat. no. CSB-E08121m) and laminin (cat. no. CSB-EL012725MO) ELISA kits (all Cusabio, Wuhan, China), according to the manufacturer's protocols. To detect the expression levels of liver vascular endothelial growth factor (VEGF) protein, liver tissues were homogenized in radioimmunoprecipitation (RIPA; Cusabio Technology LLC, Wuhan, China) buffer on ice, and the supernatants of the tissue homogenate were measured using a VEGF ELISA kit (cat. no. CSB-E04756m; Cusabio, Wuhan, China).
Flow cytometry
Whole blood samples (100 µl) were incubated with red blood cell lysis buffer (BD Pharmingen; BD Biosciences, San Jose, CA, USA). Following centrifugation at 500 × g for 15 min at 4°C, the cells were washed, stained with purified anti-CD16/32 (1/200; clone 2.4G2; cat. no. 553141; BD Pharmingen; BD Biosciences), and subsequently stained with fluorescein isothiocyanate-conjugated CD11b (1:400; clone M1/70; cat. no. 557396; BD Pharmingen; BD Biosciences) and allophycocyanin-conjugated Ly6C (1:20; clone HK1.4; cat. no. 560595; BD Pharmingen; BD Biosciences) antibodies for 30 min at 4°C. The cells were washed, resuspended in 200 µl FACS buffer (BD Pharmingen; BD Biosciences) and analyzed with a FACSAria (BD Biosciences). FlowJo software (version 10; FlowJo, LLC, Ashland, OR, USA) was used for analysis. The number (N) cells per ml in blood was calculated by the formula: N=collected cells × 200/loading volume × 10.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) in liver tissues
Sections of liver tissues (4 mm) were rapidly frozen in liquid nitrogen and homogenized in 1 ml TRIzol® reagent (Thermo Fisher Scientific, Inc.). The RNA was exacted following the manufacturer's protocol, and cDNA was synthesized using SuperScriptIIreverse transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.) and oligodT primers (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The primers were as follows: VEGF-A forward, 5′-ATCCGCATGATCTGCATGG-3′ and reverse, 5′-ATCCGCATGATCTGCATGG-3′; beta-actin forward, 5′-CCACCATGTACCCAGGCATT-3′ and reverse, 5′-CGGACTCATCGTACTCCTGC-3′. RT-qPCR was performed with cDNA in 15 µl reactions using SYBR-Green Master Mix (Promega Corporation, Madison, WI, USA). The thermocycling conditions were: 94 °C for 10 min, 94°C for 30 sec, 60°C for 30 sec and 72°C for 30 sec, repeated for 35 cycles. The mRNA levels were first calculated using the 2−ΔΔCq method and then normalized to β-actin level (13).
Statistical analysis
All graphs were manufactured using GraphPad Prism version 7.0 software (GraphPad Software, Inc., La Jolla, CA, USA). Statistical analysis was analyzed by one-way analysis of variance followed by Tukey's multiple comparisons test. Data are expressed as the mean ± standard deviation and collected from at least 6 mice. P<0.05 was considered to indicate a statistically significant difference.
Results
Combination LEF+MTX impedes the recovery of liver pathology in the liver fibrosis in mice
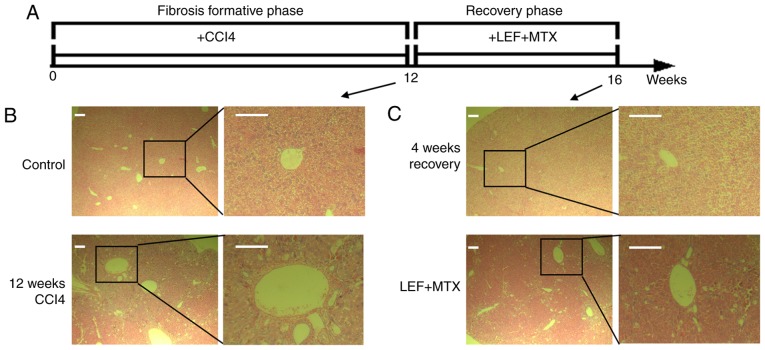
The animal experiments were performed as illustrated in Fig. 1A. After 12 weeks of CCl4 administration, five mice were randomly selected for the determination of liver pathology, and illustrating a marked alteration in the liver histology (Fig. 1B). Subsequently, the mice with liver fibrosis were divided into two groups with or without treatment with the LEF+MTX combination, and underwent recovery for 4 weeks. A completely spontaneous recovery was found in the mice without further intervention (Fig. 1C, upper panel). By contrast, the mice that received the LEF+MTX combination exhibited severe liver pathology compared with the control group (Fig. 1C, lower panel). These results demonstrated that the LEF+MTX combination had an inhibitory effect on the recovery of liver fibrosis.
Figure 1.
Mice with liver fibrosis receiving the LEF+MTX combination exhibit severe liver injury after 4 weeks of recovery. (A) Schematic representation of the animal experiments. (B) Representative images from the control and model mice (n=5). (C) Representative images from mice treated with LEF+MTX and mice that experienced spontaneous recovery (n=6). Scale bar, 200 µm. CCl4, carbon tetrachloride; LEF, leflunomide; MTX, methotrexate.
A high level of collagen degradation is observed in mice receiving LEF+MTX treatment
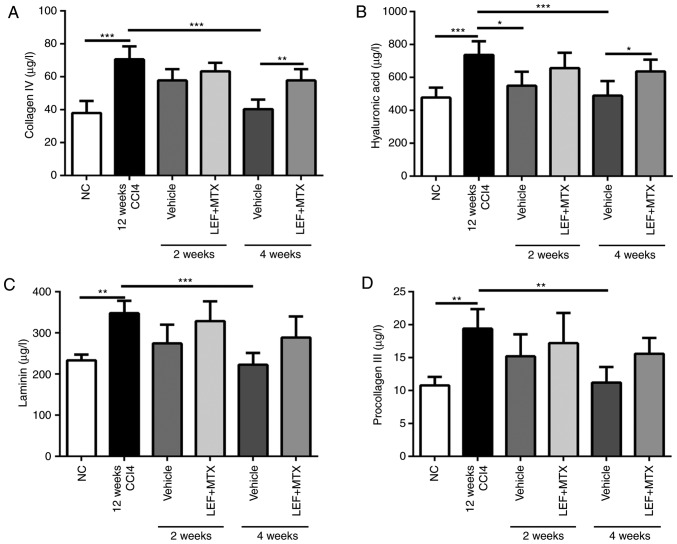
Liver fibrosis levels are accompanied by increasing levels of serum indicators, including hyaluronic acid, laminin, procollagen type III and collagen IV (14). The mice which underwent 12 weeks of CCl4 administration demonstrated a marked increase in serum parameters. Following grouping, the mice without LEF+MTX treatment exhibited a continuous decline of these indicators, which almost returned to their normal levels after 4 weeks (Fig. 2). By contrast, no significant decrease of these indicators was observed in the mice that received the LEF+MTX combination. In addition, compared with mice without LEF+MTX treatment, mice that were administrated with LEF+MTX combination showed the higher levels of collagen IV and hyaluronic acid.
Figure 2.
Serum collagen is sustained at high levels in mice treated with LEF+MTX. The serum was collected at weeks 2 and 4 during the recovery phase. The levels of (A) collagen IV, (B) hyaluronic acid, (C) laminin and (D) procollagen III in serum were measured by ELISA. Data are presented as the mean ± standard deviation (n=6). *P<0.05, **P<0.01 and ***P<0.001. CCl4, carbon tetrachloride; LEF, leflunomide; MTX, methotrexate; NC, normal control.
Reduced monocytes in blood are associated with the impaired resolution of fibrosis
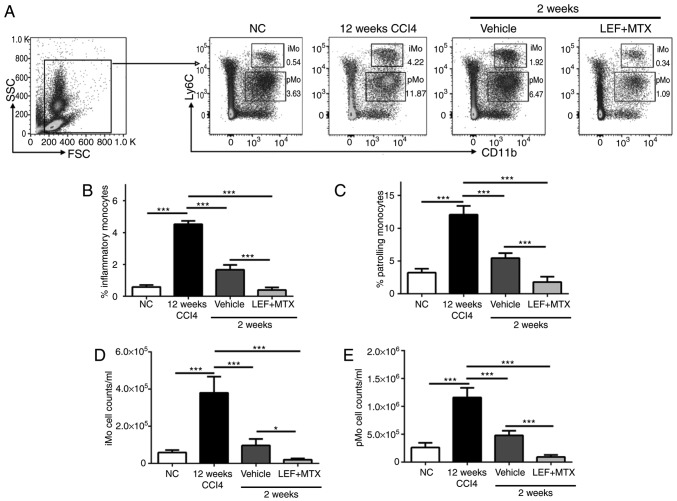
It has been demonstrated that combination LEF+MTX has a potent effect on the inhibition of leucopoiesis (7). The absolute numbers and percentages of monocytes in blood from the mice that experienced recovery at 2 weeks were measured. The gating strategy used to identify the inflammatory monocytes (iMo, CD11b+Ly6Chigh) and the patrolling monocytes (pMo, CD11b+Ly6Cint) is illustrated in Fig. 3A. Treating mice with CCl4 for 12 weeks significantly increased the number of monocytes in the blood. After 2 weeks of recovery, iMo and pMo were decreased in the mice, whether or not they were treated with LEF+MTX (Fig. 3B-E). Compared with the vehicle group, either the absolute numbers or cell percentages of iMo and pMo were decreased in the LEF+MTX-treated mice. In summary, the LEF+MTX combination significantly reduced the levels of two monocyte subsets in the mice that underwent fibrosis recovery, and this may cause the impaired recruitment of monocytes to the site of liver fibrosis, although this requires further investigation.
Figure 3.
Monocyte subsets are significantly decreased following treatment with LEF+MTX. Whole blood (200 µl) was collected, and the red blood cells were removed. (A) The gating strategy and representative images of flow cytometry for CD11b+Ly6C+ cells. The percentages of (B) iMo (CD11b+Ly6Chigh) and of (C) pMo (CD11b+Ly6Cint) were measured by flow cytometry. The absolute numbers of (D) iMo and (E) pMo are also presented. Data are presented as the mean ± standard deviation (n=6). *P<0.05 and ***P<0.001. CCl4, carbon tetrachloride; CD, cluster of differentiation; iMo, inflammatory monocytes; LEF, leflunomide; MTX, methotrexate; NC, normal control; pMo, patrolling monocytes; FSC, forward scatter; SSC, side scatter.
Combination LEF+MTX suppresses fibrosis resolution mediated by myeloid cells during the recovery phase
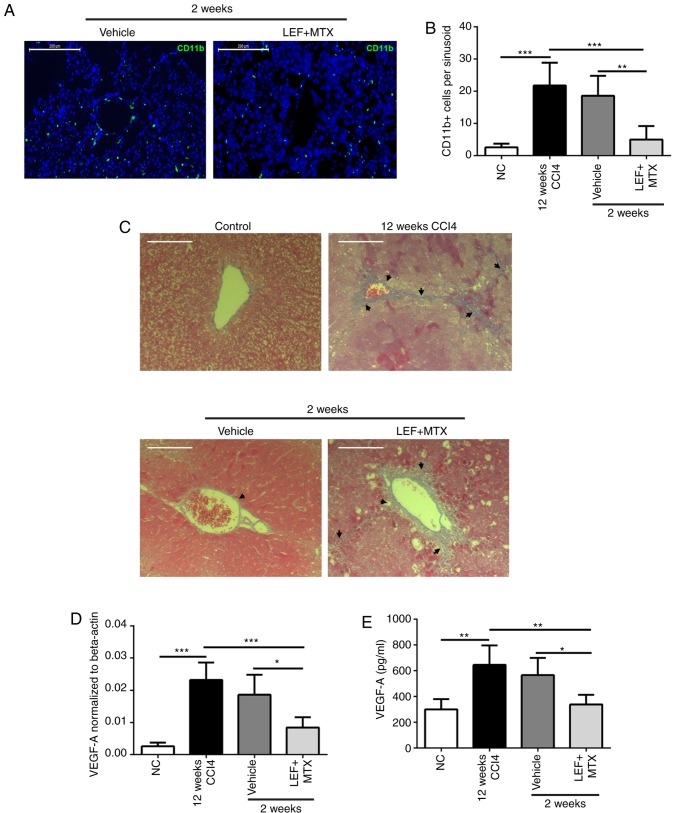
To reveal the underlying association between myeloid cells in the blood and fibrosis resolution, CD11b immunohistochemistry and Masson's trichrome staining were performed on the liver tissues. The results demonstrated that during the recovery phase, abundant CD11b+ cells were adhered to the sinusoidal endothelium, while the LEF+MTX treatment significantly reduced the number of CD11b+ cells per sinusoid (Fig. 4A and B). Notably, the results of the Masson's trichrome staining suggested a marked degradation of the ECM in liver sinusoids from mice that experienced the spontaneous recovery, although not in the mice treated with the LEF+MTX combination (Fig. 4C). Subsequently, the expression levels of VEGF-A, a cytokine involved in angiogenesis which is also present in this phase, were investigated (10). The mRNA and protein expression levels of VEGF-A demonstrated that compared with the mice that had undergone recovery at 2 weeks, the VEGF-A expression levels in the LEF+MTX-treated mice were decreased (Fig. 4D and E). These results indicated that the LEF+MTX combination may suppress the presence of myeloid cells in the liver sinusoidal endothelium, impeding the fibrosis recovery.
Figure 4.
Combination LEF+MTX suppresses myeloid cell adhesion to the sinusoidal endothelium and hinders fibrosis resolution. (A) Representative images of the liver sinusoidal endothelium illustrating the CD11b+ cells (green). (B) CD11b+ cells per liver sinusoid were calculated. (C) Representative images of Masson staining. (D) mRNA and (E) protein expression levels of VEGF-A were detected by reverse transcription-quantitative polymerase chain reaction and ELISA, respectively. Data are presented as the mean ± standard deviation (n=6). *P<0.05, **P<0.01 and ***P<0.001. Scale bar, 200 µm. CCl4, carbon tetrachloride; CD, cluster of differentiation; LEF, leflunomide; MTX, methotrexate; NC, normal control; VEGF, vascular endothelial growth factor.
Discussion
Hepatic fibrosis, a pathological consequence of chronic liver diseases with differing etiologies, is characterized by the deposition of ECM produced by activated hepatic stellate cells in response to persistent inflammation and unremitting injury (2). A previous clinical study demonstrated that treatment with combined LEF and MTX in patients with RA led to an increased risk of silent liver fibrosis (8). In the present study, part of the underlying mechanism of this was assessed in experimental animals.
Previous studies have proposed that LEF exerts an inhibitory effect on the formation of hepatic fibrosis, accelerating the fibrosis recovery that involves the enhanced apoptosis of hepatic stellate cells (15,16). Additionally, MTX is associated with the increased morbidity of hepatic fibrosis and increased myelosuppression, which is characterized by fewer white blood cells in the blood, particularly myeloid cell subsets (17,18). In the recovery phase of liver fibrosis, it was observed that the LEF+MTX combination significantly impeded the resolution of the fibrotic scar, and the underlying mechanism may be associated with impaired myeloid cell-induced remodeling of the liver sinusoids. Inflammatory monocytes selectively respond to infection and tissue damage, and are also able to digest damaged tissue (19,20). pMo, with attenuated inflammatory properties, exhibit a fundamental characteristic of patrolling the endothelium and, notably, they are the principal producers of VEGF-A and drive healing via the accumulation of myofibroblasts, angiogenesis and the deposition of ECM (20). These characteristics of monocyte subsets support the observation that the LEF+MTX combination inhibited the number of monocytes in circulation, leading to fewer monocytes reaching the site of liver fibrosis recovery. A previous study on the recovery of liver fibrosis suggested that myeloid cell-driven VEGF is indispensable for the degradation of the ECM in liver sinusoids, and that this process is dependent on the regulation of sinusoidal cells (10). This notion is consistent with the present data, in that decreased expression of liver VEGF-A was associated with impaired ECM degradation. In addition, a previous in vivo study suggested that LEF administration increases hepatic exposure to MTX, which may lead to additional liver injury (21). This observation may be another reason why the LEF+MTX administration impeded fibrosis recovery.
In the present study, it was demonstrated that during the recovery phase of CCl4-induced liver fibrosis, the LEF+MTX treatment impeded fibrosis resolution by reducing the number of myeloid cells, which may promote the revascularization of sinusoidal endothelial cells, leading to reduced ECM degradation. The present study highlighted a potential side effect of the LEF+MTX combination in the context of recovery from hepatic fibrosis-associated diseases.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
ML, RG and ZS performed the animal experiments and analyzed data. ML, SK and DZ contributed to the conception and design of this study. ML drafted this manuscript. All authors have reviewed this manuscript and approved for publication.
Ethics approval and consent to participate
All animal experiments were approved by the Committee on Laboratory Animal Care of Fujian Medical University (Fuzhou, China) and performed according to the ‘ARRIV’ guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests
References
- 1.Patten DA, Shetty S. Chronic liver disease: Scavenger hunt for novel therapies. Lancet. 2018;391:104–105. doi: 10.1016/S0140-6736(17)32671-5. [DOI] [PubMed] [Google Scholar]
- 2.Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 3.Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: Immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14:181–194. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- 4.Janssen NM, Genta MS. The effects of immunosuppressive and anti-inflammatory medications on fertility, pregnancy, and lactation. Arch Intern Med. 2000;160:610–619. doi: 10.1001/archinte.160.5.610. [DOI] [PubMed] [Google Scholar]
- 5.Dougados M, Emery P, Lemmel EM, Zerbini CA, Brin S, van Riel P. When a DMARD fails, should patients switch to sulfasalazine or add sulfasalazine to continuing leflunomide? Ann Rheum Dis. 2005;64:44–51. doi: 10.1136/ard.2003.016709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wessels JA, Huizinga TW, Guchelaar HJ. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2008;47:249–255. doi: 10.1093/rheumatology/kem279. [DOI] [PubMed] [Google Scholar]
- 7.Rozman B. Clinical pharmacokinetics of leflunomide. Clin Pharmacokinet. 2002;41:421–430. doi: 10.2165/00003088-200241060-00003. [DOI] [PubMed] [Google Scholar]
- 8.Lee SW, Park HJ, Kim BK, Han KH, Lee SK, Kim SU, Park YB. Leflunomide increases the risk of silent liver fibrosis in patients with rheumatoid arthritis receiving methotrexate. Arthritis Res Ther. 2012;14:R232. doi: 10.1186/ar4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferri C, Sebastiani M, Antonelli A, Colaci M, Manfredi A, Giuggioli D. Current treatment of hepatitis C-associated rheumatic diseases. Arthritis Res Ther. 2012;14:215. doi: 10.1186/ar3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kantari-Mimoun C, Castells M, Klose R, Meinecke AK, Lemberger UJ, Rautou PE, Pinot-Roussel H, Badoual C, Schrödter K, Österreicher CH, et al. Resolution of liver fibrosis requires myeloid cell-driven sinusoidal angiogenesis. Hepatology. 2015;61:2042–2055. doi: 10.1002/hep.27635. [DOI] [PubMed] [Google Scholar]
- 11.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI200522675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, National Centre for the Replacement, Refinement and Reduction of Amimals in Research Animal research: Reporting in vivo experiments-the ARRIVE guidelines. J Cereb Blood Flow Metab. 2011;31:991–993. doi: 10.1038/jcbfm.2010.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Fontana RJ, Dienstag JL, Bonkovsky HL, Sterling RK, Naishadham D, Goodman ZD, Lok AS, Wright EC, Su GL, HALT-C Trial Group Serum fibrosis markers are associated with liver disease progression in non-responder patients with chronic hepatitis C. Gut. 2010;59:1401–1409. doi: 10.1136/gut.2010.207423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao HW, Li J, Chen JQ, Xu SY. Inhibitory effect of leflunomide on hepatic fibrosis induced by CCl4 in rats. Acta Pharmacol Sin. 2004;25:915–920. [PubMed] [Google Scholar]
- 16.Tang X, Yang J, Li J. Accelerative effect of leflunomide on recovery from hepatic fibrosis involves TRAIL-mediated hepatic stellate cell apoptosis. Life Sci. 2009;84:552–557. doi: 10.1016/j.lfs.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Bjorkman DJ, Boschert M, Tolman KG, Clegg DO, Ward JR. The effect of long-term methotrexate therapy on hepatic fibrosis in rheumatoid arthritis. Arthritis Rheum. 1993;36:1697–1701. doi: 10.1002/art.1780361208. [DOI] [PubMed] [Google Scholar]
- 18.Bilasy SE, Essawy SS, Mandour MF, Ali EA, Zaitone SA. Myelosuppressive and hepatotoxic potential of leflunomide and methotrexate combination in a rat model of rheumatoid arthritis. Pharmacol Rep. 2015;67:102–114. doi: 10.1016/j.pharep.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Landsman L, Varol C, Jung S. Distinct differentiation potential of blood monocyte subsets in the lung. J Immunol. 2007;178:2000–2007. doi: 10.4049/jimmunol.178.4.2000. [DOI] [PubMed] [Google Scholar]
- 20.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Ma L, Lin Y, Liu X, Xiao L, Zhang Y, Xu Y, Zhou H, Pan G. Leflunomide increases hepatic exposure to methotrexate and its metabolite by differentially regulating multidrug resistance-associated protein Mrp2/3/4 transporters via peroxisome proliferator-activated receptor α activation. Mol Pharmacol. 2018;93:563–574. doi: 10.1124/mol.117.110593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.