Abstract
Endothelial dysfunction is a key pathophysiological step in early stage diabetes mellitus (DM) macrovascular complications and is also crucial in the inflammatory mechanisms of macrovascular complications. However, there is currently no effective intervention to improve endothelial dysfunction associated with DM macrovascular complications. Astragaloside IV (AS-IV), which can be extracted from the traditional Chinese medicine Astragalus membranaceus, has potential therapeutic effects on DM and its complications. The present study evaluated the effect of AS-IV on high glucose-induced human umbilical vein endothelial cell (HUVEC) injury and its possible mechanism. The result indicated that AS-IV has a significant protective effect on high glucose-induced HUVEC injury. AS-IV could significantly promote cell proliferation, reduce apoptosis and decrease the protein and mRNA expression levels of tumor necrosis factor-α and interleukin-1β in HUVECs. Furthermore, AS-IV could decrease the expression of phosphorylated c-Jun NH2-terminal kinase (JNK) phosphorylated apoptosis signal-regulating kinase 1, cytochrome c, cleaved-caspase-9, cleaved-caspase-3 and the relative ratio of B-cell lymphoma-2 associated X protein/B-cell lymphoma-2 in HUVECs. In conclusion, the present study demonstrated that AS-IV could suppress apoptosis and inflammatory reactions promoted by high glucose conditions in HUVECs by inhibiting the JNK signaling pathway. These findings suggest that AS-IV could inhibit the process of endothelial dysfunction in diabetic macrovascular complications.
Keywords: astragaloside IV, high glucose, c-Jun NH2-terminal kinase pathway, cell apoptosis, inflammation
Introduction
Macrovascular complications, mainly characterize the pathological change of atherosclerosis, are primarily associated with cardiovascular and cerebrovascular diseases. In addition, as the most common complications of DM, they are also the main causes of fatality and disability in DM patients (1). Endothelial dysfunction is a crucial pathophysiological step in the early stages of macrovascular complications and is an initial event of atherogenesis (2). Under normal conditions, apoptosis and the proliferation rate of vascular endothelial cells tends to be low in order to maintain the balance and the stability of blood vessels (3). Increased endothelial cell apoptosis is one of the predominant characteristics of endothelial dysfunction, which may promote smooth muscle cell proliferation and migration, increase blood coagulation, enhance leukocyte infiltration into the endothelium, and give rise to endothelial dysfunction and atherothrombosis (4). Research has indicated the role of inflammation in the pathogenesis of macrovascular complications of DM (5–7). Furthermore, glucotoxicity and inflammation can destroy endothelial function, leading to endothelial dysfunction and atherogenesis (8–10). Previous studies have demonstrated that high concentrations of glucose promote inflammatory damage in vascular endothelial cells in vitro (11–13). Therefore, inhibiting inflammation and decreasing cell apoptosis, which improve vascular endothelial dysfunction, are important to the treatment of macrovascular complications of DM.
C-Jun NH2-terminal kinase (JNK) is a type of serine/threonine protein kinase and a member of the family of mitogen-activated protein kinase (MAPK) (14). JNK is closely associated with cell differentiation, apoptosis, stress response and the occurrence and development of various human diseases (15). Inflammatory cytokines are the predominant activators of the JNK signaling pathway, which is a key regulator of pro-inflammatory gene expression (16,17). It has previously been suggested that the activated JNK pathway contributes to vascular cell apoptosis and endothelial dysfunction under the stimulation of inflammatory cytokines (18–22). Furthermore, the JNK signaling pathway mediates apoptosis by modulating the activities of mitochondrial pro-apoptotic and anti-apoptotic proteins in the cytoplasm (23). Therefore, inhibition of JNK signaling pathway activation is an important target for improving vascular endothelial cell disorder in macrovascular complications of DM.
Radix Astragali, a famous Traditional Chinese Medicine, is the dry root of the perennial herbaceous plant, Astragalus menabranaceus (Fisch.) Bunge. Astragaloside IV (AS-IV) is one of the primary effective components of Astragali Radix that has potent protective effects on cardiovascular disease, DM and its complications (24). Furthermore, Astragali Radix has been reported to have a series of pharmacological actions, including anti-inflammatory and antioxidant effects, and can improve endothelial dysfunction and neovascularization associated with DM and its complications (25). Previous studies have revealed that AS-IV improves vascular endothelial dysfunction in diabetic rats through inhibiting the inflammatory pathways (26) and inhibiting inflammatory gene expression in lipopolysaccharide (LPS)-treated mice (27). However, few studies have investigated the protective effects and mechanisms of AST-IV with regard to glucotoxicity and inflammation in endothelial dysfunction in vitro. The present study aimed to determine whether AS-IV could prevent high glucose-induced cell apoptosis and inflammatory reactions through inhibition of the JNK signaling pathway in human umbilical vein endothelial cells (HUVECs). In addition, an accessible therapeutic strategy was suggested for the clinical treatment of diabetic macrovascular complications.
Materials and methods
Chemicals and drugs
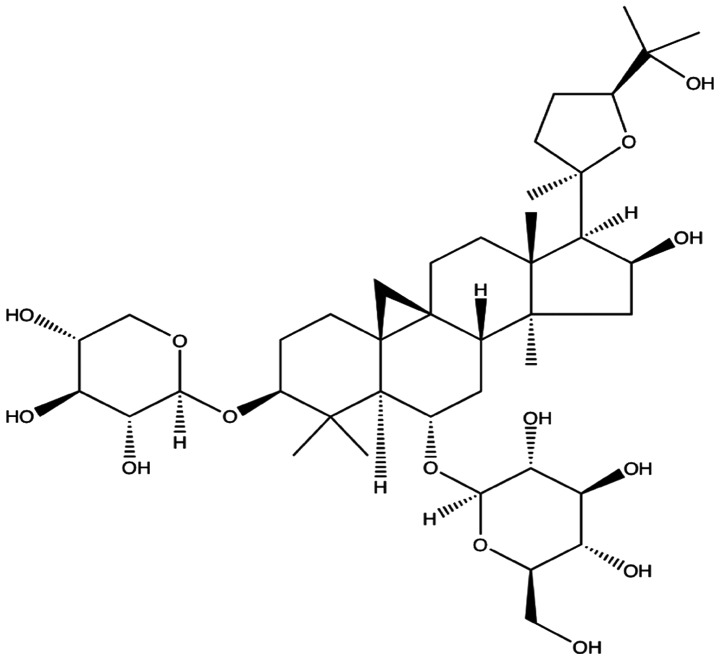
AS-IV (Fig. 1) is a white crystalline powder and insoluble compound with >98% purity. AS-IV has the molecular formula C41H68O14 and a molecular weight of 784.97 Da. AS-IV was purchased from the National Institute for Food and Drug Control (Beijing, China). The compound was dissolved in dimethyl sulfoxide (DMSO) to a concentration of 250 µM in a stock solution. The stock solution was diluted with Dulbecco's modified Eagle's medium (DMEM). The final DMSO concentration did not exceed 0.5% (v/v).
Figure 1.
Chemical structure of astragaloside IV.
Anti-phosphorylated (phospho/p)-JNK, anti-JNK, anti-cleaved-caspase-3, anti-cleaved-caspase-9 and anti-β-actin were obtained from Abcam (Cambridge, UK). Phospho-apoptosis signal-regulating kinase-1 (ASK1), ASK1, cytochrome c (Cyt-c) antibody, B-cell lymphoma-2-associated X protein (Bax) and B-cell lymphoma-2 (Bcl-2) antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). SP600125, penicillin/streptomycin solution, 0.05% trypsin-EDTA, DMSO and MTT were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). DMEM and fetal bovine serum were purchased from HyClone (Logan, UT, USA). Radioimmunoprecipitation assay lysis and extraction buffer, horseradish peroxidase-conjugated AffiniPure goat anti-mouse immunoglobulin (Ig)G and anti-rabbit IgG antibodies and D-glucose were purchased from OriGene Technologies, Inc., (Beijing, China). Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) were obtained from Absin Bioscience, Inc. (Shanghai, China; http://www.absin.cn/). A Hoechst 33258 kit was purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). ELISA kits for IL-1β (catalog no. SBJH0292) and TNF-α (catalog no. SBJH0038) were purchased from the Nanjing SenBeiJia Biological Technology Co., Ltd. (Nanjing, China). Polymerase chain reaction (PCR) primers were obtained from Sangon Biotech Co., Ltd. (Shanghai, China).
Cell culture and treatment
HUVECs were obtained from the Fudan Institutes of Biomedical Sciences Cell Resource Center (The Institutes of Biomedical Sciences, Fudan University, Shanghai, China) and cultured in DMEM supplemented with 10% fetal bovine serum, 100 µg/ml streptomycin and 100 U/ml penicillin in a humidified atmosphere containing 5% CO2 at 37°C. Experiments were performed with cells at 80% confluence.
The first series of experiments were designed to confirm the optimal glucose concentration to induce the cellular damage model and the optimal AS-IV concentration to protect against damaged cell. Doses of 5.55, 11.1, 22.2, 33.3 and 44.4 mM glucose were added to HUVECs (2×105 cells/ml), which were cultured for 24, 48 and 72 h. Doses of 0, 6.25, 12.5, 25, 50, 100, 200, 400, 800 and 1,600 µM AS-IV were added to HUVECs, which were cultured for 48 h in the solution of 5.55 mM glucose. In addition, doses of 25, 50, 100 µM AS-IV were added to HUVECs, which were cultured for 48 h in a solution of 33.3 mM glucose.
The second series of experiments were designed to examine the protective effect and possible mechanism of AS-IV. Cells were divided into five groups at a density of 2×105 cells/ml: i) The control group, where cells were cultured with 5.55 mM glucose and treated with 30 mM mannitol to balance the osmotic pressure in culture medium for 48 h; ii) model group, where cell damage was induced by incubation with 33.3 mM glucose for 48 h; iii) the SP600125 inhibitor group, where cells were cultured with 33.3 mM glucose for 48 h prior to the addition of 20 µM SP600125 (JNK inhibitor); iv) AS-IV group, where cells were cultured in 33.3 mM glucose for 48 h prior to the addition of 50 µM AS-IV; and v) the AS-IV+SP600125 group, where cells were cultured in 33.3 mM glucose for 48 h prior to the addition of 50 µM AS-IV and 20 µM SP600125. SP600125 was dissolved in DMSO (the final concentration did not exceed 0.5%).
Cell viability assay
HUVECs in the logarithmic growth phase were seeded in 96-well culture plates at 2×105 cells/well. Following respective group treatments, 20 µl MTT (5 mg/ml) was added to 100 µl of culture medium in each well. A total of 4 h following this, the medium was removed and 150 µl DMSO was added into each well. The absorbance was measured at 490 nm using an automated microplate reader (BioTek Instruments, Inc., Winooski, VT, USA). A total of five independent experiments were repeated in each group. Cell viability was calculated according to the following formula: Cell viability (%)=average absorbance of the treated group/average absorbance of the control group ×100%.
Cell apoptosis assay
Apoptosis was assessed with the Hoechst 33258 staining method, followed by photofluorography. Following the respective group treatments, the nutrient solution was removed and cells were washed twice with PBS. Subsequently, the cells were fixed with 40 g/l paraformaldehyde fluid at 4°C for 10 min. Slides were then washed twice with PBS. Once PBS was removed, the nuclear DNA was stained with 5 mg/ml Hoechst 33258 at room temperature for 10 min and then visualized under a fluorescence microscope (BX50-FLA; Olympus Corporation, Tokyo, Japan). The viable HUVECs exhibited a uniform blue fluorescence throughout the nucleus, whereas the apoptotic cells had fragmented and condensed nuclei. The experiment was carried out three times.
Apoptosis rate was analyzed by labeling cells with Annexin V-FITC apoptosis assay kit (cat.no. abs50001; Absin Bioscience, Inc., Shanghai, China; www.absin.cn/). Following the treatment, cells were collected using EDTA-free trypsin to digest the cells, followed by centrifugation at 211 × g for 10 min at 4°C to collect the cells. Cells were suspended with 500 µl binding buffer at a concentration of 1×106 cells/ml and subsequently stained with 5 µl annexin V-FITC at 4°C in the dark for 15 min followed by 10 µl PI at 4°C in the dark for 5 min. Following this, cells were immediately examined using a flow cytometer (Beckman Coulter, Inc., Brea, CA, USA) with FlowJo software (version 7.6.1; Tree Star, Inc., Ashland, OR, USA).
ELISA analysis
The levels of IL-1β and TNF-α in culture supernatant were determined using commercial ELISA kits according to the manufacturers' protocols and determined using a microplate reader at 450-nm wavelength (Multiskan FC; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from HUVECs using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Template cDNA was prepared using a RevertAid First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.). Total RNA (5 µg) was reverse transcribed to cDNA with 5X Reaction Buffer (4 µl), Oligo(dT)18 (1 µl), RevertAid Reverse Transcriptase (1 µl), RiboLock RNase Inhibitor (1 µl), dNTP Mix (2 µl) and ddH2O to supplement to a total volume of 20 µl. The synthesis temperature protocol was 60 min at 42°C, 5 min at 70°C and 1 min at 4°C. The qPCR using the Quanti Nova SYBR Green PCR kit (Qiagen GmbH, Hilden, Germany). qPCR was performed using an ABI Prism 7500 Sequence Detection System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The sequences of the primers were as follows: 5′-TCAGCCAATCTTCATTGCTC-3′ (forward) and 5′-GCCATCAGCTTCAAAGAACA-3′ (reverse) for interleukin (IL)-1β; 5′-TTTGATCCCTGACATCTGGA-3′ (forward) and 5′-GGCCTAAGGTCCACTTGTGT-3′ (reverse) for tumor necrosis factor (TNF)-α; and 5′-GGGAAATCGTGCGTGACATTAAGG-3′ (forward) and 5′-CAGGAAGGAAGGCTGGAAGAGTG-3′ (reverse) for β-actin. The reaction conditions included 95°C for 10 min, followed by 40 cycles of 95°C for 30 sec, 58°C for 1 min and 72°C for 30 sec. Reaction specificity was confirmed by melting curve analysis. Gene expression profiles were normalized to β-actin and calculated using the 2−ΔΔCq method (28) for each sample.
Western blot analysis
Total proteins were extracted with a radioimmunoprecipitation assay buffer (OriGene Technologies, Beijing, China). Proteins from each experimental group were quantified using the bicinchoninic acid assay (BestBio, Shanghai, China). An equal amount of protein (30 µg) was loaded and subjected to 10% SDS-PAGE. Gels were run at 120 V for 1.5 h and transferred to polyvinylidene fluoride membranes at 120 V for 2 h. Non-specific protein binding was blocked with 5% non-fat dried milk in Tris-buffered saline with Tween 20 (TBST; 10 mM Tris-HCl, pH 7.4, 50 mM NaCl, 0.05% Tween-20) for 2 h at room temperature. Membranes were incubated with anti-phospho-JNK (1:1,000; rabbit monoclonal antibody, cat. no. ab124956), anti-JNK (1:1,000; cat. no. ab179461), anti-cleaved-caspase-3 (1:1,000; cat. no. ab49822), anti-cleaved-caspase-9 (1:1,000; cat. no. ab2324), anti-phospho-ASK1 (1:1,000; cat. no. 3764), anti-ASK1 (1:1,000; cat. no. 3762), anti-Cyt-c (1:1,000; cat. no. 11940), anti-Bax (1:1,000; cat. no. 2772), anti-Bcl-2 (1:1,000; cat. no. 3498) and anti-β-actin (1:1,000; cat. no. ab179467) overnight at 4°C. After washing with TBST, the membranes were incubated with horseradish peroxidase (HRP)conjugated goat anti-rabbit IgG secondary antibody (1:20,000; cat. no. TA130015, OriGene Technologies) at room temperature for 2 h. The signal was detected using an enhanced chemiluminescence substrate kit (GE Healthcare, Chicago, IL, USA). According to the manufacturer's protocol, immunoreactive bands of proteins were scanned with a digital gel imaging and analysis system (ProteinSimple, San Jose, CA, USA).
Statistical analysis
Data were expressed as the mean ± standard deviation of three independent experiments and analyzed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). The Student's ttest was used for comparison between two groups. Multiple comparisons were achieved using one-way analysis of variance with the StudentNewmanKeuls test. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of glucose on HUVEC viability
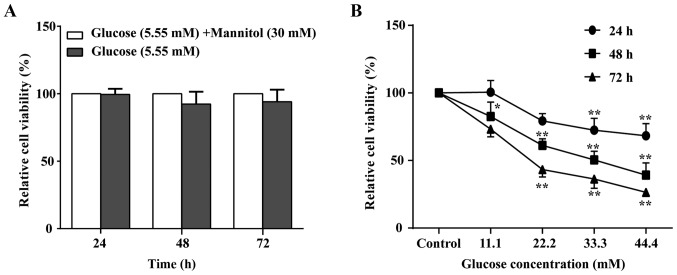
To confirm the optimal glucose concentration to induce the cellular damage model, the MTT assay was performed to observe HUVEC viability alterations in cells treated with glucose for 24, 48 and 72 h at different concentrations (5.55–44.4 mM). As indicated in Fig. 2, glucose exhibited a time- and concentration-dependent inhibitory effect on cell viability. The 50% inhibitory concentration (IC50) value for the HUVECs was 66.90 mM at 24 h of glucose treatment; however, the maximal glucose treatment still failed to reach half of cell inhibition. The IC50 value was 21.89 mM at 72 h of glucose treatment; however, the cells were unstable. Notably, the IC50 value was 32.46 mM glucose treatment for 48 h and the cells were stable. Combining the results of previous studies (29,30) with the present study results, the cell state was the most stable with 33.3 mM glucose treatment for 48 h. Therefore, 33.3 mM glucose for 48 h was selected to induce the cellular damage model. In addition, there was no significant difference in HUVEC viability between cells treated with glucose mixed with mannitol and cells that received glucose alone (Fig. 2).
Figure 2.
Effect of glucose on HUVEC viability. (A) Effects of mannitol on the viability of HUVECs at 24, 48 and 72 h. (B) Effects of glucose on the viability of HUVECs at 24, 48 and 72 h using different concentrations of glucose. Data of three experiments were presented as the mean ± standard deviation. *P<0.05 and **P<0.01 vs. the Control group (5.55 mM glucose concentration). HUVECs, human umbilical vein endothelial cells; AS-IV, astragaloside IV.
Effect of AS-IV on HUVEC viability
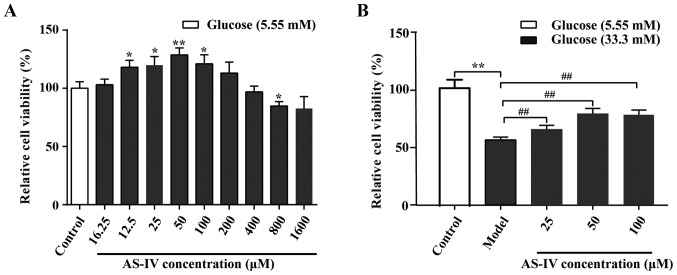
The AS-IV cell toxicity assay was performed (Fig. 3A). Data indicated that AS-IV could enhance cell viability over a specific concentration range (16–200 µM). At higher concentrations (800 µM), AS-IV significantly inhibited the cell viability compared with the control group (P<0.05). The effects of AS-IV on HUVEC viability under high glucose conditions were indicated (Fig. 3B). Data revealed that the cell viability was optimal when the concentration of AS-IV was 50 µM (P<0.01). Therefore, the 50 µM AS-IV was used in the later experiments.
Figure 3.
Effect of AS-IV on HUVEC viability. (A) Effects of AS-IV on the viability of HUVECs in 5.55 mM glucose at 48 h. (B) Effects of AS-IV on the viability of HUVECs in 33.3 mM glucose at 48 h. Data of three experiments were presented as the mean ± standard deviation. *P<0.05 and **P<0.01 vs. the Control group (5.55 mM glucose concentration); ##P<0.01 vs. the Model group (33.3 mM glucose concentration). HUVECs, human umbilical vein endothelial cells; AS-IV, astragaloside IV.
Effect of AS-IV on cell apoptosis in HUVECs stimulated with high glucose conditions
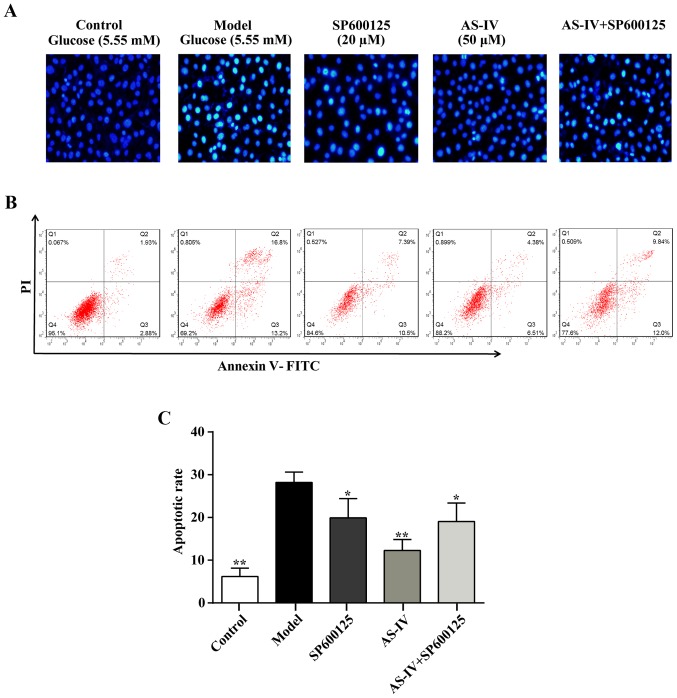
Hoechst 33258 staining was performed to identify the nuclear morphology associated with apoptotic cell death. Exposure to high glucose conditions for 48 h increased the number of apoptotic cells (Fig. 4) and nuclei pyknosis was observed. Furthermore, a large number of chromosomal contractions could be observed in the model group. Furthermore, specific apoptotic cell nuclei were light blue and an apoptotic body was occasionally visible when compared with the control group. The increased number of apoptotic cells was reversed with AS-IV treatment (Fig. 4). Similarly, the apoptosis rate of HUVECs was significantly decreased with AS-IV pre-treatment according to annexin V-FITC and PI staining analysis (P<0.05; Fig. 4B and C).
Figure 4.
Effect of AS-IV on apoptosis in human umbilical vein endothelial cells stimulated with high glucose conditions. (A) Apoptotic status was determined using a fluorescent microscope (magnification, ×200). (B) Apoptotic rates were assessed using annexin V-FITC and PI staining analysis. (C) Bar graph of apoptotic rates. Data of three experiments were presented as the mean ± standard deviation. *P<0.05 and **P<0.01 vs. the Model group. AS-IV, astragaloside IV; FITC, fluorescein isothiocyanate; PI, propidium iodide.
Effect of AS-IV on high glucose-stimulated inflammatory mediators
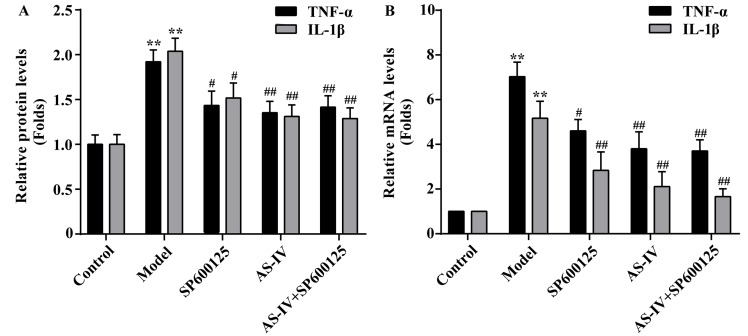
The cytokine levels of IL-1β and TNF-α in HUVECs were measured using an ELISA assay. As indicated in Fig. 5A, treatment of HUVECs with high glucose resulted in the significantly increased IL-1β and TNF-α levels compared with the control (P<0.01). However, IL-1β and TNF-α levels were significantly reduced following pre-treatment with AS-IV (P<0.01).
Figure 5.
Effect of AS-IV on the inflammatory response of human umbilical vein endothelial cells stimulated with high glucose conditions. (A) Expression levels of IL-1β and TNF-α protein. (B) Expression levels of IL-1β and TNF-α mRNA. β-actin was used as an internal reference. Data of three experiments were presented as the mean ± standard deviation. **P<0.01 vs. the Control group; #P<0.05 and ##P<0.01 vs. the Model group. AS-IV, astragaloside IV; TNF, tumor necrosis factor; IL, interleukin.
Effect of AS-IV on the mRNA expression levels of IL-1β and TNF-α
To further investigate the expression of inflammatory factors, mRNA expression levels of IL-1β and TNF-α were measured by RT-qPCR. As indicated in Fig. 5B, stimulation with high glucose increased IL-1β and TNF-α mRNA expression levels in HUVECs. However, pretreatment with AS-IV with could significantly decrease the mRNA expression levels of IL-1β and TNF-α in HUVECs (P<0.01).
Effect of AS-IV on the expression of the JNK signaling pathway
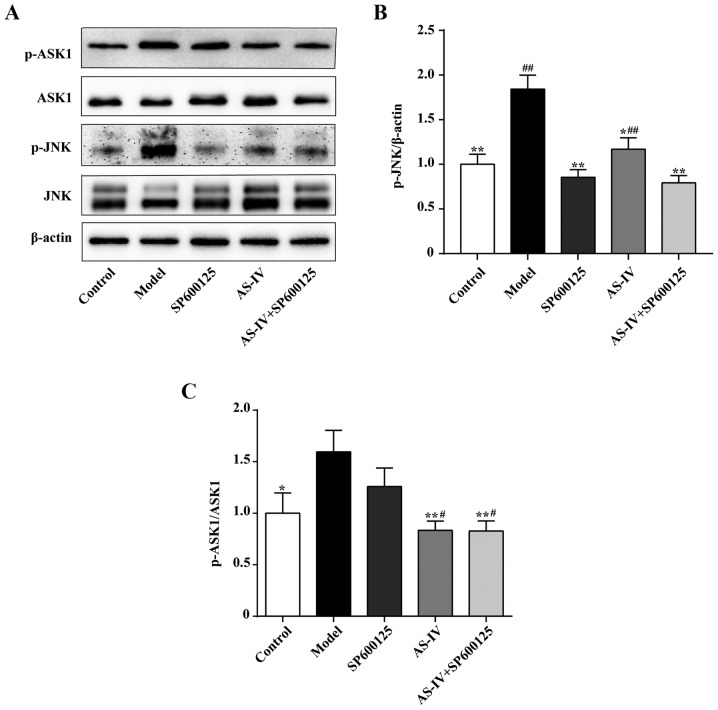
The JNK signaling pathway helps regulate the inflammatory response. Western blot analysis was used to detect the protein expression levels of JNK, p-JNK, ASK1 and p-ASK1 in HUVECs in order to investigate the mechanism of action of AS-IV. As indicated in Fig. 6, stimulation with high glucose increased JNK and ASK1 phosphorylation in HUVECs. AS-IV and the combination of AS-IV and JNK inhibitor could decrease JNK and ASK1 phosphorylation (P<0.01 or P<0.05). Notably, the JNK inhibitor could significantly decrease JNK phosphorylation (P<0.01); however, the JNK inhibitor was unable to significantly decrease ASK1 phosphorylation.
Figure 6.
Effect of AS-IV on expression of JNK pathway components. (A) Western blot analysis of p-ASK1, ASK1, p-JNK and JNK protein. (B) JNK phosphorylation. (C) ASK1 phosphorylation. Data of three experiments were presented as the mean ± standard deviation. *P<0.05 and **P<0.01 vs. the Model group; #P<0.05 and ##P<0.01 vs. the SP600125 group. AS-IV, astragaloside IV; JNK, c-Jun NH2-terminal kinase; ASK1, apoptosis signal-regulating kinase-1; p-, phosphorylated-.
Effect of AS-IV on the expression levels of Bax and Bcl-2
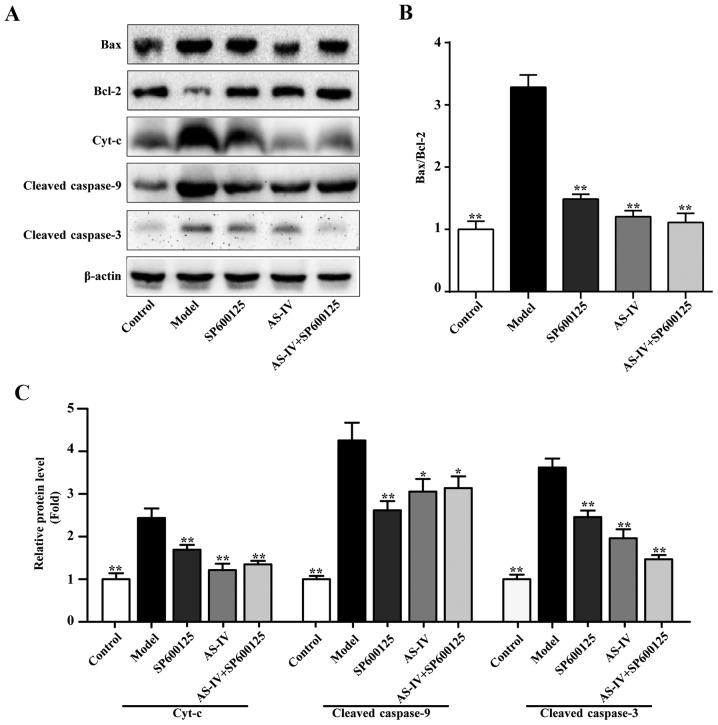
In order to determine the effect of AS-IV on the protein expression levels of components associated with apoptosis and the JNK signaling pathway in HUVECs, the Bax and Bcl-2 protein expression levels were assessed. As indicated in Fig. 7, stimulation with high glucose increased the protein expression levels of Bax and decreased the expression levels Bcl-2 protein in HUVECs. AS-IV, the JNK inhibitor and the combination of AS-IV and JNK inhibitor could decrease the protein expression levels of Bax, increased the protein expression levels of Bcl-2 and significantly decreased the ratio of Bax/Bcl-2 (P<0.01; Fig. 7).
Figure 7.
Mechanism of AS-IV on cell apoptosis of human umbilical vein endothelial cells stimulated with high glucose conditions. (A) Western blot analysis of Bax, Bcl-2 Cyt-c, cleaved-caspase-9 and cleaved-caspase-3. (B) Ratio of Bax/Bcl-2. (C) Expression levels of Cyt-c, cleaved-caspase-9 and cleaved-caspase-3. Data of three experiments were presented as the mean ± standard deviation. *P<0.05 and **P<0.01 vs. the Model group. AS-IV, astragaloside IV; Bax, B-cell lymphoma-2-associated X protein; Bcl-2, B-cell lymphoma-2.
Effects of AS-IV on the expression levels of Cyt-c, cleaved-caspase-9 and cleaved-caspase-3
Apoptosis regulatory proteins Bax and Bcl-2 are associated with the cell mitochondrial apoptotic pathways. Therefore, western blot analysis was performed to detect the expression of a series of key proteins involved in the mitochondrial apoptosis pathway, including Cyt-c, cleaved-caspase-9 and cleaved-caspase-3. As indicated in Fig. 7, stimulation with high glucose increased the protein expression levels of Cyt-c, cleaved-caspase-9 and cleaved-caspase-3; however, AS-IV, JNK inhibitor and the combination of AS-IV with JNK inhibitor could significantly decrease the expression levels of these proteins (P<0.05).
Discussion
The glucotoxicity of diabetes induces sustained hyperglycemia, but also participates in the development of chronic diabetic complications (31,32). A large amount of medical evidence indicates that glucotoxicity is associated with endothelial dysfunction, which is an important factor involved in the development of macrovascular and microvascular diabetic complications (32,33). Notably, a number of diabetic incidents are associated with inflammatory reactions (34–36). The inflammatory response is the important mechanism of endothelial dysfunction that can be promoted by glucotoxicity. Upon exposure to high glucose conditions, HUVECs produce high levels of proinflammatory cytokines, including TNF-α and IL-1β (37,38). In the present study, the results indicated that high glucose conditions decreased the HUVEC survival rate, increased cell apoptosis and promoted the protein and mRNA expression levels of TNF-α and 1L-1β. Previous studies have demonstrated that AS-IV inhibits inflammation and protects endothelial function (26,39–41). AS-IV has also been revealed to decrease the serum levels of TNF-a in diabetic rats (39) and can inhibit the expression of inflammatory factors in TNF-α-induced HUVECs (40). Furthermore, AS-IV can improve the structure of the aortic endothelium wall, inhibit over-activation of monocyte chemoattractant protein-1 and TNF-α in diabetic rats (26) and protect endothelial cells from high glucose-induced barrier impairment (41). In the present study, no direct cytotoxic effect of ASIV was identified in HUVECs. Additionally, the present findings suggested that ASIV enhanced the HUVEC viability under high glucose conditions but also exerted an inhibitory effect on high-glucose-induced HUVEC apoptosis and inflammation. Notably, SP600125, an inhibitor of JNK, inhibited high-glucose-induced TNF-α and IL-1β expression levels and cell apoptosis. Similar effects were also observed with AS-IV and SP600125 treatment. These results suggest that the effects of AS-IV are associated with the JNK signaling pathway.
The JNK signaling pathway is sensitive to the inflammatory response in diabetes complications. It has previously been suggested that, under the stimulation of inflammatory cytokines, which are involved in the endothelial dysfunction process, the JNK signaling pathway is activated and raises the number of endothelial cells damaged and cell apoptosis (18–20). Inflammatory cytokines are the primary activators of the JNK signaling pathway, which is a key regulator of pro-inflammatory gene expression (16). It has been reported that TNF-α induces increased phosphorylation of JNK in HUVECs (42). ASK1 is a member of the MAPK kinase kinase family, which activates JNK in response to a variety of stress stimuli (43). A previous study revealed that high glucose conditions induced the increase of ASK1 expression and its activity in HUVECs (44). In the present study, high glucose conditions in HUVECs resulted in increased ASK1 phosphorylation and JNK expression. However, a significant decrease in JNK and ASKI phosphorylation was indicated following treatment with AS-IV. In addition, an inhibitor of JNK, SP600125 (45), was used as a reference to compare the effectiveness of ASIV in the JNK signaling pathway. The results indicated that AS-IV could significantly inhibit the phosphorylation of high glucoseinduced JNK and ASK1, as well as the expression of TNF-α and IL-1β. Furthermore, SP600125 significantly suppressed the phosphorylation of JNK but not ASK1. Compared with SP600125, the inhibitory effect of AS-IV on JNK phosphorylation was decreased compared with SP600125; however, there was no notable difference between SP600125 or AS-IV in combination with SP600125. Furthermore, SP600125 and the combination of AS-IV with SP600125 had the ability to inhibit ASK1 phosphorylation, whereas SP600125 did not.
The increase of endothelial cell apoptosis is one of the primary characteristics of endothelial dysfunction (4) and one of the most important effects on HUVECs in high glucose conditions (46). The present data indicated that high glucose conditions induced cell apoptosis. The JNK signaling pathway activates apoptotic signaling, either by promoting the expression of apoptotic genes in the nucleus or through modulating the activities of associated mitochondrial apoptotic proteins by phosphorylation events in the cytoplasm (23). In higher organisms, mitochondria-mediated apoptosis is the predominant pathway of cellular apoptosis (32). The pathways associated with mitochondrial JNK-mediated apoptosis include intrinsic apoptosis and extrinsic apoptosis pathways (23). In the intrinsic apoptosis pathway, JNK activates the internal cell mitochondrial apoptotic pathway and changes the mitochondrial structure and function, causing the release of Cyt-c into the cytoplasm (47). Following this, Cyt-c and an adaptor protein are combined with active caspase-9, forming the apoptotic bodies Cyt-c, caspase-9 and apoptotic protease activating factor-1, which further activates caspase-3 and causes cell apoptosis (48). Furthermore, JNK can adjust the activity of Bcl-2 members by promoting the activation of apoptosis proteins Bax, which are dimers in the cytoplasm. This subsequently inhibits the protein expression of Bcl-2 and regulates cell apoptosis (48,49). In the present study, the expression levels of Bax, Cyt-c, cleaved-caspase-9 and cleaved-caspase-3 were increased and Bcl-2 expression was decreased in high glucose-stimulated HUVECs. However, the expression levels of Bax, Cyt-c, cleaved-caspase-9 and cleaved-caspase-3 were reduced and Bcl-2 expression was increased by treatment with ASIV. Compared with ASIV, the effects of SP600125 and the combination of AS-IV with SP600125 were not significantly different. This suggests that the ability of AS-IV and JNK inhibitors in regulating apoptotic proteins is similar. These data strongly support the mitochondria-mediated apoptosis pathway being involved in the pathogenesis of injury induced by high glucose conditions and ASIV could reduce cell apoptosis associated with the mitochondria-mediated apoptosis pathway. However, the effect of ASIV on mitochondrial morphology and mitochondrial membrane potential requires further observation in future studies to identify the mechanism of apoptosis inhibition.
In conclusion, the present study indicates that high glucosestimulation in HUVECs causes decreased cell activity, increased the expression of apoptosis and inflammatory factors and activated the JNK signaling pathway, and mitochondrial apoptosis pathway. The findings suggested that ASIV may be valuable for the prevention and treatment of diabetic vascular complications by reducing cell apoptosis and inflammatory reactions through the inhibition of the JNK signaling pathway and mitochondria-mediated apoptosis pathway. However, other relevant mechanisms underlying the effects of ASIV require further investigation.
Acknowledgements
The authors would like to thank the Key Laboratory of Xinan Medicine of Chinese Ministry of Education (Hefei, China) for their technical assistance.
Funding
The present study was supported by grants from the National Natural Science Foundation of China (grant nos. 81774286, 81573944 and 81273646), the Scientific Research Base of Traditional Chinese Medicine Clinical of State Administration of Traditional Chinese Medicine (grant no. JDZX2015001) and the National Basic Public Health Services Project of State Administration of Traditional Chinese Medicine (grant no. 20131012).
Availability of data and materials
The datasets used during the present study are available from the corresponding author on reasonable request.
Authors' contributions
ZF, GS and LY conceived the present study and edited the manuscript. LY, QW, YH, SY, LW, MH and YL performed the experiments and acquisition of data. ML and AJ were involved in analysis and interpretation of data. All authors discussed the results and implications and commented on the manuscript at all stages, as well as in the final approval of the version to be published.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Huang D, Refaat M, Mohammedi K, Jayyousi A, Al Suwaidi J, Abi Khalil C. Macrovascular complications in patients with diabetes and prediabetes. Biomed Res Int 2017. 2017:7839101. doi: 10.1155/2017/7839101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaur R, Kaur M, Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: Molecular insights and therapeutic strategies. Cardiovasc Diabetol. 2018;17:121. doi: 10.1186/s12933-018-0763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galley HF, Webster NR. Physiology of the endothelium. Br J Anaesth. 2004;93:105–113. doi: 10.1093/bja/aeh163. [DOI] [PubMed] [Google Scholar]
- 4.Wang Q, Zhang M, Ding Y, Wang Q, Zhang W, Song P, Zou MH. Activation of NAD(P)H oxidase by tryptophan-derived 3-hydroxykynurenine accelerates endothelial apoptosis and dysfunction in vivo. Circ Res. 2014;114:480–492. doi: 10.1161/CIRCRESAHA.114.302113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 6.Donath MY. Targeting inflammation in the treatment of type 2 diabetes: Time to start. Nat Rev Drug Discov. 2014;13:465–476. doi: 10.1038/nrd4275. [DOI] [PubMed] [Google Scholar]
- 7.Yan SF, Ramasamy R, Naka Y, Schmidt AM. Glycation, inflammation, and RAGE: A scaffold for the macrovascular complications of diabetes and beyond. Circ Res. 2003;93:1159–1169. doi: 10.1161/01.RES.0000103862.26506.3D. [DOI] [PubMed] [Google Scholar]
- 8.Haidari M, Zhang W, Willerson JT, Dixon RA. Disruption of endothelial adherens junctions by high glucose is mediated by protein kinase C-beta-dependent vascular endothelial cadherin tyrosine phosphorylation. Cardiovasc Diabetol. 2014;13:105. doi: 10.1186/1475-2840-13-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sajja RK, Prasad S, Cucullo L. Impact of altered glycaemia on blood-brain barrier endothelium: An in vitro study using the hCMEC/D3 cell line. Fluids Barriers CNS. 2014;11:8. doi: 10.1186/2045-8118-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao XY, Wang XF, Li L, Zhang L, Shen DL, Li DH, Jin QS, Zhang JY. Effects of high glucose on human umbilical vein endothelial cell permeability and myosin light chain phosphorylation. Diabetol Metab Syndr. 2015;7:98. doi: 10.1186/s13098-015-0098-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen G, Chen Y, Chen H, Li L, Yao J, Jiang Q, Lin X, Wen J, Lin L. The effect of NF-kappaB pathway on proliferation and apoptosis of human umbilical vein endothelial cells induced by intermittent high glucose. Mol Cell Biochem. 2011;347:127–133. doi: 10.1007/s11010-010-0620-5. [DOI] [PubMed] [Google Scholar]
- 12.Kageyama S, Yokoo H, Tomita K, Kageyama-Yahara N, Uchimido R, Matsuda N, Yamamoto S, Hattori Y. High glucose-induced apoptosis in human coronary artery endothelial cells involves up-regulation of death receptors. Cardiovas Diabetol. 2011;10:73. doi: 10.1186/1475-2840-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ning RB, Zhu J, Chai DJ, Xu CS, Xie H, Lin XY, Zeng JZ, Lin JX. RXR agonists inhibit high glucose-induced upregulation of inflammation by suppressing activation of the NADPH oxidase-nuclear factor-kappaB pathway in human endothelial cells. Genet Mol Res. 2013;12:6692–6707. doi: 10.4238/2013.December.13.3. [DOI] [PubMed] [Google Scholar]
- 14.Owen GR, Achilonu I, Dirr HW. High yield purification of JNK1beta1 and activation by in vitro reconstitution of the MEKK1-->MKK4-->JNK MAPK phosphorylation cascade. Protein Expr Purif. 2013;87:87–99. doi: 10.1016/j.pep.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK)-from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/S0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- 16.Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2012;4(pii):a006049. doi: 10.1101/cshperspect.a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foletta VC, Segal DH, Cohen DR. Transcriptional regulation in the immune system: All roads lead to AP-1. J Leukoc Biol. 1998;63:139–152. doi: 10.1002/jlb.63.2.139. [DOI] [PubMed] [Google Scholar]
- 18.Kralisch S, Sommer G, Stangl V, Köhler U, Kratzsch J, Stepan H, Faber R, Schubert A, Lössner U, Vietzke A, et al. Secretory products from human adipocytes impair endothelial function via nuclear factor kappaB. Atherosclerosis. 2008;196:523–531. doi: 10.1016/j.atherosclerosis.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Shen C, Li Q, Zhang YC, Ma G, Feng Y, Zhu Q, Dai Q, Chen Z, Yao Y, Chen L, et al. Advanced glycation endproducts increase EPC apoptosis and decrease nitric oxide release via MAPK pathways. Biomed Pharmacother. 2010;64:35–43. doi: 10.1016/j.biopha.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Yamawaki H, Saito K, Okada M, Hara Y. Methylglyoxal mediates vascular inflammation via JNK and p38 in human endothelial cells. Am J Physiol Cell Physiol. 2008;295:C1510–C1517. doi: 10.1152/ajpcell.00252.2008. [DOI] [PubMed] [Google Scholar]
- 21.Breton-Romero R, Feng B, Holbrook M, Farb MG, Fetterman JL, Linder EA, Berk BD, Masaki N, Weisbrod RM, Inagaki E, et al. Endothelial dysfunction in human diabetes is mediated by Wnt5a-JNK signaling. Arterioscler Thromb Vasc Biol. 2016;36:561–569. doi: 10.1161/ATVBAHA.115.306578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabio G, Davis RJ. TNF and MAP kinase signalling pathways. Semin Immunol. 2014;26:237–245. doi: 10.1016/j.smim.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhanasekaran DN, Reddy EP. JNK-signaling: A multiplexing hub in programmed cell death. Genes Cancer. 2017;8:682–694. doi: 10.18632/genesandcancer.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Hou X, Xu R, Liu C, Tu M. Research review on the pharmacological effects of astragaloside IV. Fundam Clin Pharmacol. 2017;31:17–36. doi: 10.1111/fcp.12232. [DOI] [PubMed] [Google Scholar]
- 25.You LZ, Lin YX, Fang ZH, Shen GM, Zhao JD, Wang TT. Research advances on astragaloside-IV in treatment of diabetes mellitus and its complications pharmacological effects. Zhongguo Zhong Yao Za Zhi. 2017;42:4700–4706. doi: 10.19540/j.cnki.cjcmm.20171010.007. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 26.Yin Y, Qi F, Song Z, Zhang B, Teng J. Ferulic acid combined with astragaloside IV protects against vascular endothelial dysfunction in diabetic rats. Biosci Trends. 2014;8:217–226. doi: 10.5582/bst.2014.01081. [DOI] [PubMed] [Google Scholar]
- 27.Zhang WJ, Frei B. Astragaloside IV inhibits NF-κB activation and inflammatory gene expression in LPS-treated mice. Mediators Inflamm 2015. 2015:274314. doi: 10.1155/2015/274314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Zhong W, Zou G, Gu J, Zhang J. L-arginine attenuates high glucose-accelerated senescence in human umbilical vein endothelial cells. Diabetes Res Clin Pract. 2010;89:38–45. doi: 10.1016/j.diabres.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Sheu ML, Chiang CK, Tsai KS, Ho FM, Weng TI, Wu HY, Liu SH. Inhibition of NADPH oxidase-related oxidative stress-triggered signaling by honokiol suppresses high glucose-induced human endothelial cell apoptosis. Free Radic Biol Med. 2008;44:2043–2050. doi: 10.1016/j.freeradbiomed.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Campos C. Chronic hyperglycemia and glucose toxicity: Pathology and clinical sequelae. Postgrad Med. 2012;124:90–97. doi: 10.3810/pgm.2012.11.2615. [DOI] [PubMed] [Google Scholar]
- 32.Ceriello A. Point: Postprandial glucose levels are a clinically important treatment target. Diabetes Care. 2010;33:1905–1907. doi: 10.2337/dc10-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grassi D, Desideri G, Necozione S, Ruggieri F, Blumberg JB, Stornello M, Ferri C. Protective effects of flavanol-rich dark chocolate on endothelial function and wave reflection during acute hyperglycemia. Hypertension. 2012;60:827–832. doi: 10.1161/HYPERTENSIONAHA.112.193995. [DOI] [PubMed] [Google Scholar]
- 34.Jourdan T, Godlewski G, Cinar R, Bertola A, Szanda G, Liu J, Tam J, Han T, Mukhopadhyay B, Skarulis MC, et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat Med. 2013;19:1132–1140. doi: 10.1038/nm.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei X, Song H, Yin L, Rizzo MG, Sidhu R, Covey DF, Ory DS, Semenkovich CF. Fatty acid synthesis configures the plasma membrane for inflammation in diabetes. Nature. 2016;539:294–298. doi: 10.1038/nature20117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang W, Wang WH, Azadzoi KM, Dai P, Wang Q, Sun JB, Zhang WT, Shu Y, Yang JH, Yan Z. Alu RNA accumulation in hyperglycemia augments oxidative stress and impairs eNOS and SOD2 expression in endothelial cells. Mol Cell Endocrinol. 2016;426:91–100. doi: 10.1016/j.mce.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Wu Z, He Y, Zhang H, Tian L, Zheng C, Shang T, Zhu Q, Li D, He Y. Humanin prevents high glucose-induced monocyte adhesion to endothelial cells by targeting KLF2. Mol Immunol. 2018;101:245–250. doi: 10.1016/j.molimm.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Zhang WJ, Hufnagl P, Binder BR, Wojta J. Antiinflammatory activity of astragaloside IV is mediated by inhibition of NF-kappaB activation and adhesion molecule expression. Thromb Haemost. 2003;90:904–914. doi: 10.1160/TH03-03-0136. [DOI] [PubMed] [Google Scholar]
- 40.Gui D, Huang J, Guo Y, Chen J, Chen Y, Xiao W, Liu X, Wang N. Astragaloside IV ameliorates renal injury in streptozotocin-induced diabetic rats through inhibiting NF-κB-mediated inflammatory genes expression. Cytokine. 2013;61:970–977. doi: 10.1016/j.cyto.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 41.Li HB, Ge YK, Zhang L, Zheng XX. Astragaloside IV improved barrier dysfunction induced by acute high glucose in human umbilical vein endothelial cells. Life Sci. 2006;79:1186–1193. doi: 10.1016/j.lfs.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 42.Lo HM, Lai TH, Li CH, Wu WB. TNF-α induces CXCL1 chemokine expression and release in human vascular endothelial cells in vitro via two distinct signaling pathways. Acta Pharmacol Sin. 2014;35:339–350. doi: 10.1038/aps.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hattori K, Naguro I, Runchel C, Ichijo H. The roles of ASK family proteins in stress responses and diseases. Cell Commun Signal. 2009;7:9. doi: 10.1186/1478-811X-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yokoi T, Fukuo K, Yasuda O, Hotta M, Miyazaki J, Takemura Y, Kawamoto H, Ichijo H, Ogihara T. Apoptosis signal-regulating kinase 1 mediates cellular senescence induced by high glucose in endothelial cells. Diabetes. 2006;55:1660–1665. doi: 10.2337/db05-1607. [DOI] [PubMed] [Google Scholar]
- 45.Shin M, Yan C, Boyd D. An inhibitor of c-jun aminoterminal kinase (SP600125) represses c-Jun activation, DNA-binding and PMA-inducible 92-kDa type IV collagenase expression. Biochim Biophys Acta 1589. 2002:311–316. doi: 10.1016/s0167-4889(02)00195-7. [DOI] [PubMed] [Google Scholar]
- 46.Zhu W, Yuan Y, Liao G, Li L, Liu J, Chen Y, Zhang J, Cheng J, Lu Y. Mesenchymal stem cells ameliorate hyperglycemia-induced endothelial injury through modulation of mitophagy. Cell Death Dis. 2018;9:837. doi: 10.1038/s41419-018-0861-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tournier C. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 48.Chauhan D, Li G, Hideshima T, Podar K, Mitsiades C, Mitsiades N, Munshi N, Kharbanda S, Anderson KC. JNK-dependent release of mitochondrial protein, Smac, during apoptosis in multiple myeloma (MM) cells. J Biol Chem. 2003;278:17593–17596. doi: 10.1074/jbc.C300076200. [DOI] [PubMed] [Google Scholar]
- 49.Kim BJ, Ryu SW, Song BJ. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J Biol Chem. 2006;281:21256–21265. doi: 10.1074/jbc.M510644200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the present study are available from the corresponding author on reasonable request.