Abstract
Polycystic ovary syndrome (PCOS) is a condition in which a woman's levels of the sex hormones (estrogen and progesterone) are out of balance, leading to the growth of ovarian cysts. PCOS can affect the menstrual cycle, fertility, cardiac function and even appearance of women. Therefore, we aimed to explore the genetic polymorphism of the melatonin receptors 1A and 1B in obese patients with PCOS to identify a new theoretical basis for its treatment. Patients presenting with PCOS (n=359) were enrolled and classified into an obese OB-PCOS group [body mass index (BMI) of PCOS patients ≥25 kg/m2] or a nonobese NOB-PCOS group, and 215 oviduct infertile patients who experienced normal ovulation were used as the control group. All baseline characteristics, endocrine hormone levels, lipid and glucose metabolism, and insulin indices were measured. The genotypes of rs2119882 within the MTNR1A gene and of rs10830963 within the MTNR1B gene were determined by PCR-RFLP; the genotype frequency and the difference in the distribution of allele frequency were compared. For rs2119882, C allele carriers who were not diagnosed with PCOS had an increased risk of developing PCOS, and C allele carriers with PCOS had an increased risk of developing OB-PCOS. For rs10830963, G allele carriers who were not diagnosed with PCOS had an increased risk of developing PCOS. The TT genotype in rs2119882 and the CC genotype in rs10830963 were protective factors for OB-PCOS, and increased levels of LH, testosterone, and estradiol and abnormal menstruation were key risk factors for PCOS. Furthermore, the TT genotype at the rs2119882 site was the key protective factor for OB-PCOS patients. Our study found that MTNR1A rs2119882 and MTNR1B rs10830963 could increase the risk for PCOS and cause glycolipid metabolism disorder in PCOS patients.
Keywords: polycystic ovary syndrome, MTNR1A, MTNR1B, glycolipid metabolism disorder, risk factors
Introduction
Polycystic ovary syndrome (PCOS) is a main hyperandrogenic disorder in which women present with unbalanced hormone levels (1). It is generally acknowledged as the most prevalent disorder in the female endocrine systems at the reproductive age, with still undefined diagnostic criteria and limited sample methodology (2). Up to 10% of women at the reproductive age develop PCOS, which is a main cause of infertility, obstetrical complications, cardiovascular disease, type 2 diabetes mellitus and eating disorders (3). Apart from that, women with PCOS, to a large extent, exhibit symptoms of anxiety and depression, which lead to an impaired health-related quality of life (4). Clomiphene citrate has been used as the first-line oral medication therapy to benefit fertility, followed by gonadotrophins and laparoscopic ovarian surgery or possibly metformin as the second-line treatment for clomiphene citrate-resistant PCOS women (5). More recently, vitamin D was found to be effective for women diagnosed with PCOS by improving their menstrual frequency and glucose metabolism (6). Insulin resistance, obesity and increased cardiometabolic risk factors are some of the risks associated with PCOS (7), thus making the enrollment of obese (OB)-PCOS patients in our study important. Additionally, the association between the melatonin receptor 1A (MTNR1A) gene polymorphism and PCOS has already been indicated (8). Therefore, the present study was designed to combine the elements of the MTNR polymorphism with obesity to ascertain whether they are related in PCOS.
Melatonin (N-acetyl-5-methoxytryptamine), the neurohormone of the pineal gland, which is also produced by many other tissues and cells, acts through G protein-coupled receptors and is present in peripheral tissues and in different areas of the central nervous system (9). The MTNR1A receptor is one of the many melatonin receptors that may influence the risk of calcium nephrolithiasis (10). The effects of MTNR1A polymorphisms on the fertility rate after artificial insemination (AI) treatment in Sarda sheep was also previously evaluated (11), which paved the way for exploring the relationship between MTNR1A and PCOS. The symptom of infertility was associated with an elevated risk of type 2 diabetes (T2DM), which could result from glycolipid metabolism disorder (12,13). In addition, previous evidence also revealed that a common variant in MTNR1B is related to an increased risk of T2DM (14). Furthermore, the actions of melatonin were reported to be mainly regulated by interacting with specific receptors bound to the membrane, MT1 and MT2, of which the MT1 receptor has been found to be expressed in ovary tissues (15). Intriguingly, Slominski et al (16) found that intracellular reactive oxygen species exposed to ultraviolet B (UVB) were greatly decreased in cells treated with melatonin or its metabolites, which is a process that is independent of the specific receptors bound to the membrane. As one of the risk factors (already mentioned above) for PCOS occurrence, infertility, which is possibly caused by disordered glycolipid metabolism, could be involved in PCOS development. The present study was performed to ascertain whether MTNR1A (rs2119882) and MTNR1B (rs10830963) polymorphisms are protective or harmful for obese PCOS patients.
Materials and methods
Ethics statement
This study was approved by the Ethics Committee of Dongying People's Hospital, and all experimental procedures were conducted under the strict supervision of the committee. Written informed consent was obtained from all patients or their parents or guardians.
Study subjects
From March 2013 to May 2015, a total of 359 patients diagnosed with PCOS and receiving treatment at Dongying People's Hospital were enrolled in our study. The diagnostic criteria for PCOS were in line with the revised 2003 Rotterdam ESHRE/ASRM-Sponsored PCOS consensus (17): i) female patients demonstrating sporadic ovulation and (or) anovulation; ii) female patients demonstrating clinical and (or) biochemical indices of heightened androgen levels with no other pathogenic factors such as Cushing's syndrome or ovarian tumors; and iii) female patients demonstrating polycystic ovarian change along with 12 2- to 9-mm ovarian follicles in at least one ovary. Three months before the experiment, all included subjects did not take any medicine that could affect their reproductive hormones or glycolipid metabolism. A body mass index (BMI) ≥25 kg/m2 was used as the standard for classifying obesity (18), according to which the selected patients were classified into the obese PCOS patients (OB-PCOS) group or the nonobese PCOS patients (NOB-PCOS) group. There were 168 patients in the OB-PCOS group and 191 in the NOB-PCOS group, and 215 oviduct infertile patients with normal ovulation comprised the control group. The average age of the three groups were, respectively, 25.7±4.1, 25.3±3.7 and 25.1±3.2 years.
Analysis of the clinical features
Patients in the OB-PCOS and NOB-PCOS groups were examined to ascertain whether they had abnormal menstruation or infertility. At the same time, the Ferriman-Galley score and the Rosenfield acne score were calculated (19,20). Based on the Ferriman-Galley test, patients with an F-G score <7 were considered normal and those with an F-G score >9 were considered hirsute. After fasting for 12 h, venous blood was drawn from patients in each group on the 3rd-5th day of menstruation or at a time when patients who suffered from abnormal menstruation or amenorrhea showed no dominant follicles. Chemiluminescent enzyme immunoassay was used to detect follicle stimulating hormone (FSH), luteinizing hormone (LH), testosterone and estradiol levels, and a Roche automatic biochemical analyzer was used for detecting the total cholesterol (TC), triglycerides, high density lipoprotein (HDL) and low density lipoprotein (LDL) of patients in both groups. On the same day of blood drawing, the weight, height, waistline and hipline of each patient were measured, recorded and used to calculate the BMI and the waist-to-hip ratio (WHR) according to the following formulas: BMI=weight (kg)/height (m); WHR=waistline (cm)/hipline (cm). From the drawn venous blood, 2 ml was removed combined with ethylenediaminetetraacetic acid (EDTA) for anticoagulation, and preserved at a temperature of −80°C.
Gene extraction, amplification and sequencing
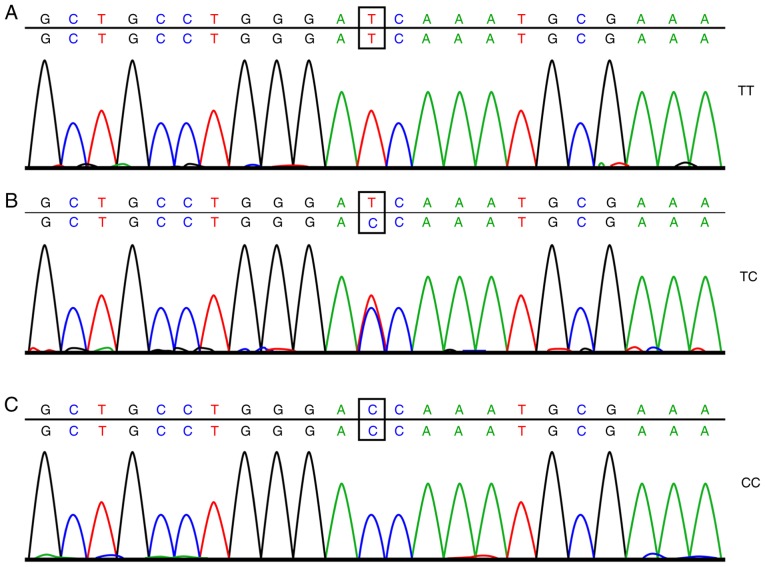
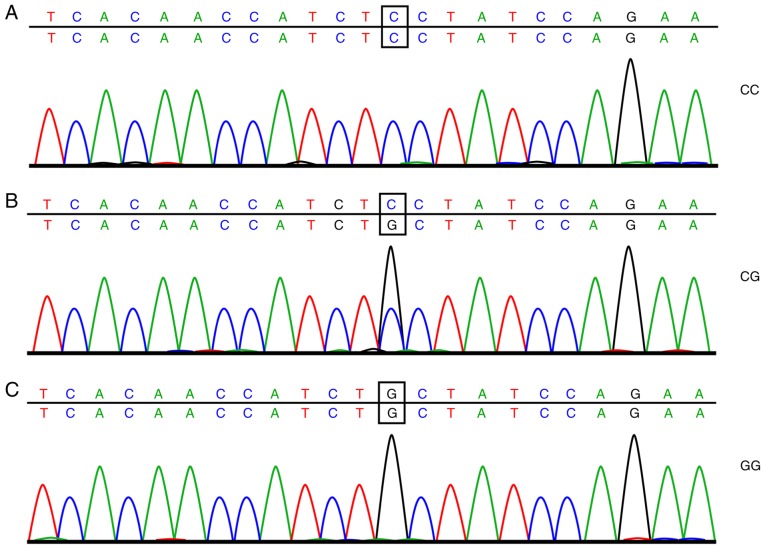
A total of 2 ml of frozen EDTA anticoagulant was thawed at room temperature. The total DNA of the drawn blood was extracted using a DNA extraction kit (Omega Bio-Tek, Inc., Norcross, GA, USA) following the manufacturer's instructions. Subsequently, SYBR Green polymerase chain reaction (PCR; ABI Company, Oyster Bay, NY, USA) amplification and analysis of the dissolving curve were carried out, with the amplification conditions as follows: 10 µl reaction system, predenaturation at 95°C for 5 min, denaturation at 95°C for 15 sec, and annealing at 60°C for 30 sec; the cycle was repeated 30 times. The analysis conditions were as follows: 95°C for 15 sec, 60°C for 60 sec, 95°C for 15 sec, and 60°C for 15 sec. Next, 6% of the DNA samples was selected for direct sequencing following the subsequent conditions for the PCR: Predenaturation at 95°C for 5 min, 35 cycles of denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec, and extension at 72°C for 7 min. After the PCR, analysis of the dissolving curve was carried out, and when the dissolving curve displayed a single peak, the PCR was determined as successful and the purification and sequencing could be carried out. The sample was purified by ethanol and then dissolved in 10 µl deionized formamide to undergo sequencing. The results of the sample sequencing are shown in Figs. 1 and 2. The amplification primer of SYBR Green PCR and the sequencing primers were both synthesized by Shanghai Bioengineering Technology Co., Ltd., (Shanghai, China) with the detailed sequence displayed in Table I.
Figure 1.
Sequencing results of the rs2119882 site of the MTNR1A gene: (A) wild-type homozygous genotype TT at the rs2119882 site of the MTNR1A gene, (B) heterozygous genotype TC at the rs2119882 site of the MTNR1A gene and (C) mutant homozygous genotype CC at the rs2119882 site of the MTNR1A gene.
Figure 2.
Sequencing results of the rs10830963 site of the MTNR1B gene: (A) wild-type homozygous genotype CC at the rs10830963 site of the MTNR1B gene, (B) heterozygous genotype CG at the rs10830963 site of the MTNR1B gene and (C) mutant homozygous genotype GC at the rs10830963 site of the MTNR1B gene.
Table I.
Primer sequences for the SNP sites.
| Site | Primer sequence (5′-3′) | |
|---|---|---|
| rs2119882 | SYBR green PCR | Forward CTCTCTGGGATGGGACTTTTCACC |
| Reverse CGCGGGCAGGGCGGCCTAATCTCATTTCGCATTGGG | ||
| Reverse TGCGGGCCTAATCTCATTTCGCATTGGA | ||
| Sequencing primers | Forward GCCTGCGTCCTCATCTTCAC | |
| Reverse GTTCCGATACACCGACAGGAT | ||
| rs10830963 | SYBR green PCR | Forward GCCCCCAGTGATGCTAAGAATTCA |
| Reverse GGCGGGCAGGCAGTTACTGGTTCTGGATCGC | ||
| Reverse CGCGGGCAGGGCGGCAGGCAGTTACTGGTTCTGGATCGG | ||
| Sequencing primers | Forward GATCCAGGTGGGTAGAAGGTC | |
| Reverse CCCCTGCAAACTTCGTCCT |
SNP, single nucleotide polymorphism; SYBR Green PCR, SYBR Green polymerase chain reaction.
Detection of endocrine hormone
Chemiluminescent enzyme immunoassay was used to detect the levels of hormones, including FSH, LH, testosterone, and estradiol, in patients with different genotypes in the PCOS groups. In brief, the patient's sample and alkaline phosphate-coupled hormone were incubated in a bead (Biolabs Technology Co., Ltd., Beijing, China)-containing reaction tube for 60 min at 37°C. Then the unbounded enzyme-labeled conjugates and unbounded samples were removed by centrifugation at 37°C at 225 × g for 10 min. Substrates were added and the reaction tube was incubated at 37°C for 5 min. The photon number was measured by photomultiplier tube (PTM). The binding complex measured by a luminometer and the photon number are inversely proportional to the concentration of hormones.
Detection of lipid metabolism
Fasting venous blood was drawn from patients in the two groups in the early morning and was centrifuged at 1,610 × g for 15 min to collect the serum. Levels of TC, TG, HDL, and LDL in the serum of patients with different genotypes were measured in the PCOS groups. Fasting blood lipid levels of patients were detected using an automatic biochemical analyzer (AU640; Olympus, Hamburg, Germany) and Olympus special test paper.
Detection of glycometabolism index and homeostasis model assessment-insulin resistance index (HOMA-IRI)
PCOS patients were administered an oral glucose tolerance test (OGTT) to measure their glycometabolism. Three to seven days before the experiment, PCOS patients stopped taking all medication that could affect sugar tolerance (increase or decrease in blood sugar), such as acyeterion, diuretics, β-adrenergic blocking agents, phenytoin, and nicotinic acid. Three days before the experiment, patients consumed more than 300 g of carbohydrate in their diets. On the first day of the experiment, venous blood was extracted from patients after fasting for 10–14 h. The glucose oxidase method (using special reagents from Olympus) was used to detect the plasma glucose content, and the chemiluminescence method (Beckman Coulter, Inc., Brea, CA, USA) was used for plasma insulin detection. Within 5 min of the blood draw, patients were asked to consume 300 ml of glucose water containing 75 g glucose powder. At 0.5, 1, 2 and 3 h after glucose water consumption, venous blood was extracted to measure its content of glucose and insulin. During the entire experiment, the enrolled patients did not drink tea or coffee, smoke, or do any strenuous exercise. The OGTT area under the glucose curve and the area under the insulin curve were calculated according to the trapezoid method (21): Area under the glucose curve = (G0 + G180)/2 + G30 + G60 + G120 and the area under the insulin curve = (S0 + S180)/2 + S30 + S60 + S120 (G0, G30, G60, and G120 were the blood sugar values at different time points; S0, S30, S60, S120, and S180 were the insulin values detected at different time points). According to the levels of fasting blood sugar and fasting insulin, the values of HOMA-IRI, pancreatic islet β-cell function (HOMA-β%), and insulin sensitivity index (HOMA-ISI) were calculated and compared (22). HOMA-IRI = FBG × FINS/22.5; HOMA-ISI = 1/FBG × FINS; and HOMA-β% = 20 × FINS/(FBG-3.5).
Statistical analysis
Statistical analysis was performed using SPSS 21.0 software (IBM Corporation Armonk, NY, USA). Data are presented as the mean ± standard deviation. Comparisons between two groups were conducted by t-tests; comparisons among multiple groups were assessed by one-way analysis of variance (ANOVA) for variance analysis and significance test. Data are presented as ratios or percentages and were compared and analyzed by the Chi-square test. Logistic regression analysis was performed to analyze the risk factors associated with the occurrence of PCOS and OB-PCOS, and P<0.05 was considered to indicate a statistically significant result.
Results
Comparison of general data among the OB-PCOS, NOB-PCOS and control groups
A total of 215 patients were included in the control group, with an average age of 25.1±3.2 years; there were 191 patients in the NOB-PCOS group, with a mean age of 25.3±3.7 years, and there were 168 patients in the OB-PCOS group, with an average age of 25.7±4.1 years. The above information shows that there was no difference in age among the patients in the three groups (P>0.05). Patients in the OB-PCOS and NOB-PCOS groups had more cases of abnormal menstruation, hirsutism, acne, and infertility; higher levels of LH, testosterone, and estradiol; and lower levels of FSH than patients in the control group (P<0.05). Patients in the OB-PCOS group exhibited higher BMIs, WHRs, TC, TG and LDL than patients in the NOB-PCOS and control groups did, the lowest HDL levels exhibited in the NOB-PCOS and control groups (P<0.05; Table II).
Table II.
Higher levels of BMI, WHR, TC, TG and LDL but a lower HDL level in the OB-PCOS group when compared with these levels in the NOB-PCOS and control groups.
| Items | Control group (n=215) | NOB-PCOS group (n=191) | OB-PCOS group (n=168) | P-valuea | P-valueb | P-valuec |
|---|---|---|---|---|---|---|
| Age (years) | 25.1±3.2 | 25.3±3.7 | 25.7±4.1 | 0.559 | 0.109 | 0.332 |
| BMI (kg/m2) | 21.02±2.14 | 21.16±2.08 | 25.05±2.49 | 0.505 | <0.001 | <0.001 |
| WHR | 0.73±0.05 | 0.74±0.06 | 0.77±0.12 | 0.068 | <0.001 | 0.003 |
| Abnormal menstruation (yes/no) | 11/204 | 30/161 | 28/140 | <0.001 | <0.001 | 0.805 |
| Hairiness (yes/no) | 17/198 | 92/99 | 80/88 | <0.001 | <0.001 | 0.917 |
| Acne (yes/no) | 25/190 | 104/87 | 91/77 | <0.001 | <0.001 | 0.957 |
| Infertile (yes/no) | 12/203 | 31/160 | 29/139 | 0.001 | <0.001 | 0.794 |
| FSH (IU/l) | 7.50±2.12 | 5.44±1.67 | 5.36±1.62 | <0.001 | <0.001 | 0.646 |
| LH (IU/l) | 8.03±2.35 | 13.05±4.22 | 13.21±4.27 | <0.001 | <0.001 | 0.722 |
| Testosterone (nmol/l) | 1.13±0.21 | 1.71±0.47 | 1.73±0.48 | <0.001 | <0.001 | 0.691 |
| Estradiol (ng/ml) | 41.18±3.99 | 54.63±4.73 | 55.15±4.69 | <0.001 | <0.001 | 0.297 |
| TC (mmol/l) | 2.28±0.39 | 2.35±0.37 | 2.54±0.45 | 0.065 | <0.001 | <0.001 |
| TG (mmol/l) | 0.83±0.05 | 0.84±0.06 | 0.88±0.12 | 0.068 | <0.001 | <0.001 |
| HDL (mmol/l) | 1.57±0.23 | 1.54±0.24 | 1.32±0.22 | 0.199 | <0.001 | <0.001 |
| LDL (mmol/l) | 1.25±0.11 | 1.27±0.19 | 1.67±0.42 | 0.189 | <0.001 | <0.001 |
PCOS, polycystic ovary syndrome; OB, obesity; NOB, non-obesity; BMI, body mass index; WHR, waist-to-hip ratio; FSH, follicle-stimulating hormone; LH, luteotropic hormone; TC, total cholesterol; TG, triglyceride; HDL, high density lipoprotein; LDL, low density lipoprotein.
vs. the NOB-PCOS and control groups
vs. the OB-PCOS and control groups
vs. the OB-PCOS and NOB-PCOS groups; independent sample t-test was adopted for comparison of measurement data between two groups; Chi-square test was performed for enumeration data.
rs2119882 C allele carrier or rs10830963 G allele carrier has increased risk for the occurrence of PCOS
A goodness-of-fit test was conducted for the Hardy-Weinberg equilibrium test, and the result showed that the genotype distribution of rs2119882 in the MTNR1A gene and rs10830963 in the MTNR1B gene was in line with Hardy-Weinberg equilibrium (P>0.05). The research samples used were random samples with representative characteristics of the group.
The genotype of rs2119882 and the frequency distribution of allele genes were notably different among the control, NOB-PCOS and OB-PCOS groups. The CC genotype of OB-PCOS patients displayed a higher percentage than that of the NOB-PCOS patients (42.26 vs. 28.80%), and the TC + CC genotype of NOB-PCOS patients was higher than that of the patients in the control group (79.06 vs. 67.91%), which revealed that carrying a C allele in the human gene can possibly increase the risk of PCOS [odds ratio (OR)=1.411; 95% confidence interval (CI)=1.070–1.860, P=0.015] and increase the risk of PCOS patients developing obesity (OR=1.442, 95% CI=1.069–1.945, P=0.016). The genotype of rs10830963 and the frequency distribution of allele genes in the control and NOB-PCOS groups were significantly different, with patients in the NOB-PCOS group exhibiting a markedly higher ratio of the GG genotype and G allele than patients in the control group (16.23 vs. 4.65% and 39.01 vs. 25.81%, respectively), thus showing that patients carrying the G allele are at greater risk of developing PCOS (OR=1.838, 95% CI=1.364–2.476, P<0.001). In addition, it was shown that the genotype of rs10830963 and the frequency distribution of allele genes in the NOB-PCOS and OB-PCOS group were not significantly different (P>0.05; Tables III and IV).
Table III.
Comparisons of genotype and allele frequency distribution in rs2119882 of MTNR1A and rs10830963 of MTNR1B between the NOB-PCOS group and the control group.
| SNP | Control group (n=215) | NOB-PCOS group (n=191) | P-value | OR (95% CI) |
|---|---|---|---|---|
| rs2119882 | ||||
| TT | 69 (32.09) | 40 (20.94) | Ref | |
| TC | 97 (45.12) | 96 (50.26) | 0.029a | 1.707 (1.055–2.762) |
| CC | 49 (22.79) | 55 (28.80) | 0.018a | 1.936 (1.120–3.349) |
| TC+CC | 146 (67.91) | 151 (79.06) | 0.011a | 1.784 (1.136–2.801) |
| T | 235 (54.65) | 176 (46.07) | Ref | |
| C | 195 (43.35) | 206 (53.93) | 0.015a | 1.411 (1.070–1.860) |
| rs10830963 | ||||
| CC | 114 (53.02) | 73 (38.22) | Ref | |
| CG | 91 (42.33) | 87 (45.55) | 0.058 | 1.493 (0.985–2.262) |
| GG | 10 (4.65) | 31 (16.23) | <0.001a | 4.841 (2.239–10.470) |
| CG+GG | 101 (46.98) | 118 (61.78) | 0.003a | 1.824 (1.228–2.712) |
| C | 319 (74.19) | 233 (60.99) | Ref | |
| G | 111 (25.81) | 149 (39.01) | <0.001a | 1.838 (1.364–2.476) |
SNP, single nucleotide polymorphism; PCOS, polycystic ovary syndrome; NOB, non-obesity; OR, odds ratio; 95% CI, 95% confidence intervals; Ref, reference.
P<0.05; Chi-square test was performed for enumeration data.
Table IV.
Comparisons of genotype and allele frequency distribution in rs2119882 of MTNR1A and rs10830963 of MTNR1B between the NOB-PCOS group and the OB-PCOS group.
| SNP | NOB-PCOS group (n=191) | OB-PCOS group (n=168) | P-value | OR (95% CI) |
|---|---|---|---|---|
| rs2119882 | ||||
| TT | 40 (20.94) | 28 (16.67) | Ref | |
| TC | 96 (50.26) | 69 (41.07) | 0.928 | 1.027 (0.579–1.822) |
| CC | 55 (28.80) | 71 (42.26) | 0.044a,b | 1.844 (1.014–3.353) |
| TC + CC | 151 (79.06) | 140 (83.33) | 0.302 | 1.325 (0.776–2.262) |
| T | 176 (46.07) | 125 (37.20) | Ref | |
| C | 206 (53.93) | 211 (62.80) | 0.016a,b | 1.442 (1.069–1.945) |
| rs10830963 | ||||
| CC | 73 (38.22) | 52 (30.95) | Ref | |
| CG | 87 (45.55) | 82 (48.81) | 0.239 | 1.323 (0.829–2.110) |
| GG | 31 (16.23) | 34 (20.24) | 0.159 | 1.540 (0.843–2.814) |
| CG + GG | 118 (61.78) | 116 (69.05) | 0.149 | 1.380 (0.890–2.140) |
| C | 233 (60.99) | 186 (55.36) | Ref | |
| G | 149 (39.01) | 150 (44.64) | 0.126 | 1.261 (0.937–1.698) |
PCOS, polycystic ovary syndrome; NOB, non-obesity; OB, obesity; SNP, single nucleotide polymorphisms; OR, odds ratio; 95% CI, 95% confidence intervals; Ref, reference.
P<0.05 vs. PCOS and control groups
P<0.05 vs. OB-PCOS and control groups; Chi-square test was performed for enumeration data.
Differences in endocrine index and index of lipid metabolism between NOB-PCOS and OB-PCOS patients carrying the TC + CC genotype
NOB-PCOS patients carrying the TC + CC genotype presented significantly decreased FSH levels; notably increased levels of LH, testosterone and estradiol (P<0.05); and nonsignificant differences in glycometabolism, lipid metabolism and insulin homeostasis indices when compared with the NOB-PCOS patients carrying the TT genotype in the rs2119882 gene (P>0.05). Compared with patients carrying the CC genotype in rs10830963, patients with the CG + GG genotype demonstrated no significant differences in any of the above indices (all P>0.05; Table V).
Table V.
NOB-PCOS patients carrying the TC + CC genotype have decreased FSH level and significantly increased levels of LH, testosterone and estradiol.
| rs2119882 | rs10830963 | |||||
|---|---|---|---|---|---|---|
| TT | TC + CC | P-value | CC | CG + GG | P-value | |
| FSH (IU/l) | 5.92±1.96 | 5.31±1.57 | 0.039a | 5.34±1.72 | 5.50±1.64 | 0.521 |
| LH (IU/l) | 10.53±4.89 | 13.72±3.77 | <0.001a | 12.98±3.88 | 13.09±4.43 | 0.862 |
| Testosterone (nmol/l) | 1.56±0.48 | 1.75±0.46 | 0.022a | 1.66±0.50 | 1.74±0.45 | 0.254 |
| Estradiol (ng/ml) | 50.21±3.86 | 55.80±4.23 | <0.001a | 55.45±4.49 | 54.12±4.82 | 0.059 |
| TC (mmol/l) | 2.41±0.38 | 2.33±0.37 | 0.228 | 2.37±0.36 | 2.34±0.38 | 0.589 |
| TG (mmol/l) | 0.85±0.07 | 0.84±0.06 | 0.367 | 0.85±0.06 | 0.84±0.06 | 0.264 |
| HDL (mmol/l) | 1.51±0.24 | 1.55±0.24 | 0.349 | 1.52±0.25 | 1.55±0.23 | 0.398 |
| LDL (mmol/l) | 1.29±0.15 | 1.27±0.20 | 0.556 | 1.28±0.18 | 1.27±0.19 | 0.719 |
| Glucose (mmol/l) | ||||||
| OGTT 0 min | 4.76±0.37 | 4.74±0.42 | 0.784 | 4.73±0.38 | 4.75±0.43 | 0.745 |
| OGTT 30 min | 7.81±1.52 | 7.85±1.56 | 0.885 | 7.61±1.59 | 7.98±1.51 | 0.109 |
| OGTT 60 min | 9.25±1.33 | 9.33±1.51 | 0.761 | 9.27±1.42 | 9.33±1.50 | 0.784 |
| OGTT120 min | 6.70±1.46 | 6.42±1.56 | 0.308 | 6.35±1.53 | 6.56±1.55 | 0.362 |
| OGTT 180 min | 4.90±1.37 | 5.09±1.51 | 0.472 | 5.09±1.49 | 5.03±1.48 | 0.786 |
| Insulin (mU/l) | ||||||
| OGTT 0 min | 8.37±1.56 | 8.70±1.42 | 0.202 | 8.82±1.22 | 8.51±1.57 | 0.152 |
| OGTT 30 min | 59.37±25.42 | 60.84±17.85 | 0.675 | 60.10±16.92 | 60.79±21.17 | 0.814 |
| OGTT 60 min | 66.43±21.13 | 74.01±22.47 | 0.056 | 76.37±23.44 | 69.97±21.39 | 0.054 |
| OGTT 120 min | 56.04±1.46 | 56.69±13.83 | 0.767 | 57.50±13.39 | 55.96±13.69 | 0.447 |
| OGTT 180 min | 24.75±1.37 | 25.75±9.91 | 0.526 | 24.67±10.03 | 26.08±9.39 | 0.327 |
| HOMA-IRI | 1.57±0.45 | 1.59±0.37 | 0.772 | 1.61±0.39 | 1.58±0.39 | 0.606 |
| HOMA-β% | 3.07±0.57 | 3.03±0.62 | 0.713 | 3.05±0.63 | 3.03±0.60 | 0.826 |
| HOMA-ISI | 162.20±37.85 | 171.09±36.75 | 0.178 | 171.92±8.29 | 167.57±36.34 | 0.432 |
PCOS, polycystic ovary syndrome; NOB, non-obesity; OB, obesity; BMI, body mass index; WHR, waist-to-hip ratio; FSH, follicle-stimulating hormone; TC, total cholesterol; TG, triglyceride; HDL, high density lipoprotein; LDL, low density lipoprotein; HOMA-IRI, homeostasis model assessment-insulin resistance index; HOMA-β%, pancreatic islet β-cell function; HOMA-ISI, insulin sensitivity index.
P<0.05; independent sample t-test was adopted for comparison of measurement data between two groups.
Compared with the OB-PCOS patients carrying the TT genotype in rs2119882, OB-PCOS patients carrying the TC + CC genotype had no significantly different endocrine indices (FSH, LH, testosterone, and estradiol) or indices of lipid metabolism (TC, TG, LDL and HDL) (P>0.05); increased fasting glucose levels and insulin levels from the OGTT at 30, 60 and 120 min and OGTT area under the glucose curve (P<0.05); no significantly different glucose levels from the OGTT at 30, 60, 120 and 180 min (P>0.05); markedly increased HOMA-IRI; and significantly decreased levels of HOMA-β% and HOMA-ISI (P<0.05). Compared with patients carrying the CC genotype in rs10830963, patients with the CG + GG genotype had no significantly different endocrine indices (FSH, LH, testosterone, and estradiol) or indices of lipid metabolism (TC, TG, LDL and HDL) (P>0.05), with an increased HOMA-IRI level and significantly decreased levels of HOMA-β% and HOMA-ISI (all P<0.05; Table VI).
Table VI.
OB-PCOS patients carrying the TC + CC genotype have increased fasting glucose level, insulin level, OGTT area under the glucose curve, HOMA-IRI, and decreased levels of HOMA-β% and HOMA-ISI.
| rs2119882 | rs10830963 | |||||
|---|---|---|---|---|---|---|
| TT | TC + CC | P-value | CC | CG + GG | P-value | |
| FSH (IU/l) | 5.47±1.52 | 5.34±1.64 | 0.699 | 5.30±1.48 | 5.39±1.68 | 0.739 |
| LH (IU/l) | 12.23±4.17 | 13.41±4.28 | 0.183 | 12.32±4.87 | 13.61±3.93 | 0.07 |
| Testosterone (nmol/l) | 1.67±0.44 | 1.74±0.49 | 0.484 | 1.80±0.56 | 1.70±0.44 | 0.214 |
| Estradiol (ng/ml) | 54.58±5.70 | 55.26±4.48 | 0.486 | 55.45±4.49 | 55.14±4.65 | 0.969 |
| TC (mmol/l) | 2.63±0.36 | 2.52±0.47 | 0.244 | 2.53±0.41 | 2.55±0.47 | 0.791 |
| TG (mmol/l) | 0.90±0.14 | 0.87±0.12 | 0.242 | 0.90±0.16 | 0.87±0.11 | 0.16 |
| HDL (mmol/l) | 1.36±0.20 | 1.31±0.22 | 0.267 | 1.33±0.22 | 1.31±0.22 | 0.587 |
| LDL (mmol/l) | 1.57±0.47 | 1.69±0.41 | 0.169 | 1.68±0.47 | 1.67±0.40 | 0.888 |
| Glucose (mmol/l) | ||||||
| OGTT 0 min | 4.66±0.80 | 5.01±0.56 | 0.006a | 5.04±0.56 | 4.91±0.64 | 0.208 |
| OGTT 30 min | 9.27±1.33 | 9.58±1.69 | 0.362 | 9.59±1.68 | 9.50±1.63 | 0.744 |
| OGTT 60 min | 9.44±1.56 | 9.92±1.47 | 0.12 | 9.67±1.54 | 9.91±1.47 | 0.337 |
| OGTT 120 min | 7.74±1.40 | 7.31±1.54 | 0.173 | 7.28±1.71 | 7.42±1.43 | 0.582 |
| OGTT 180 min | 5.50±1.30 | 5.22±1.31 | 0.303 | 5.23±1.17 | 5.29±1.37 | 0.784 |
| Insulin (mU/l) | ||||||
| OGTT 0 min | 8.35±2.08 | 9.78±1.67 | <0.001a | 9.71±1.82 | 9.47±1.82 | 0.431 |
| OGTT 30 min | 60.68±23.77 | 74.47±23.42 | 0.005a | 71.44±23.41 | 72.50±24.31 | 0.792 |
| OGTT 60 min | 67.06±29.84 | 87.94±22.92 | <0.001a | 85.67±23.57 | 83.92±26.18 | 0.68 |
| OGTT 120 min | 58.88±14.53 | 74.21±14.52 | <0.001a | 74.53±14.99 | 70.37±15.71 | 0.109 |
| OGTT 180 min | 28.96±10.31 | 29.98±8.65 | 0.582 | 28.20±8.93 | 30.53±8.86 | 0.118 |
| HOMA-IRI | 2.34±0.57 | 2.73±0.56 | 0.001a | 2.39±0.58 | 2.79±0.54 | <0.001a |
| HOMA-β% | 2.57±0.43 | 2.20±0.45 | <0.001a | 2.46±0.52 | 2.17±0.41 | <0.001a |
| HOMA-ISI | 97.24±15.95 | 85.22±13.39 | <0.001a | 93.64±14.84 | 84.34±13.46 | <0.001a |
PCOS, polycystic ovary syndrome; NOB, non-obesity; OB, obesity; BMI, body mass index; WHR, waist-to-hip ratio; FSH, follicle-stimulating hormone; TC, total cholesterol; TG, triglyceride; HDL, high density lipoprotein; LDL, low density lipoprotein; HOMA-IRI, homeostasis model assessment-insulin resistance index; HOMA-β%, pancreatic islet β-cell function; HOMA-ISI, insulin sensitivity index.
P<0.05; independent sample t-test was adopted for comparison of measurement data between two groups.
Haplotype TC decreases the occurrence of PCOS in normal individuals
SHEs is software (http://analysis.bio-x.cn/myAnalysis.php) was used to analyze the rs2119882 site in the MTNR1A gene and the rs10830963 site in the MTNR1B gene concerning their linkage disequilibrium and haplotype. As shown in the results, there was rather intensive linkage disequilibrium between the two sites, and the haplotype analysis was carried out. The TC haplotype was found to be able to decrease the risk of PCOS in normal individuals (OR=0.709, 95% CI=0.538–0.935, P=0.015), while the CG haplotype increased the risk of PCOS in normal individuals (OR=1.838, 95% CI=1.364–2.476, P<0.001; Table VII). Among all the three haplotypes from rs2119882 and rs10830963, the TC haplotype decreased the risk of PCOS in normal individuals (OR=0.693, 95% CI=0.514–0.935, P=0.016), with the other three haplotypes demonstrating no relationship with the occurrence of OB-PCOS (Table VIII).
Table VII.
Haplotype analysis of rs2119882 site of the MTNR1A gene and rs10830963 site of the MTNR1B gene in the control and NOB-PCOS groups.
| Haplotype | Control group (n=215) | NOB-PCOS group (n=191) | χ2 | P-value | OR (95% CI) |
|---|---|---|---|---|---|
| CC | 84 (0.195) | 57 (0.149) | 3.001 | 0.083 | 0.722 (0.500–1.045) |
| CG | 111 (0.258) | 149 (0.390) | 16.172 | <0.001 | 1.838 (1.364–2.476) |
| TC | 235 (0.547) | 176 (0.461) | 5.955 | 0.015 | 0.709 (0.538–0.935) |
PCOS, polycystic ovary syndrome; NOB, non-obesity; OR, odds ratio; 95% CI, 95% confidence intervals. Chi-square test (χ2) was performed for enumeration data.
Table VIII.
Haplotype analysis of rs2119882 site of the MTNR1A gene and rs10830963 site of the MTNR1B gene in OB-PCOS and NOB-PCOS patients.
| Haplotype | NOB-PCOS group (n=136) | OB-PCOS group (n=108) | χ2 | P-value | OR (95% CI) |
|---|---|---|---|---|---|
| CC | 57 (0.149) | 61 (0.182) | 1.361 | 0.243 | 1.265 (0.852–1.878) |
| CG | 149 (0.390) | 150 (0.446) | 2.338 | 0.126 | 1.261 (0.937–1.698) |
| TC | 176 (0.461) | 125 (0.372) | 5.778 | 0.016 | 0.693 (0.514–0.935) |
PCOS, polycystic ovary syndrome; NOB, non-obesity; OB-obesity; OR, odds ratio; 95% CI, 95% confidence intervals. Chi-square test (χ2) was performed for accounting data.
The rs2119882 TT genotype and the rs10830963 CC genotype are crucial protective factors for PCOS
Dichotomous logistic regression analysis was conducted to analyze the risk factors for NOB-PCOS and OB-PCOS, including BMI, WHR, FSH, LH, testosterone, estradiol, TC, TG, HDL, LDL, abnormal menstruation, hirsutism, acne, infertility and the polymorphism of the rs2119882 site and the rs10830963 site. The results showed that FSH, LH, testosterone, estradiol, abnormal menstruation, and polymorphisms at the rs2119882 and rs10830963 sites were significantly correlated with PCOS occurrence, among which the TT genotype in rs2119882 and the CC genotype in rs10830963 were important protective factors for PCOS, and increased levels of LH, testosterone, estradiol and abnormal menstruation were key risk factors for PCOS (all P<0.05; Table IX).
Table IX.
TT genotype in the rs2119882 site and the CC genotype in the rs10830963 site are protective for NOB-PCOS but increased levels of LH, testosterone and estradiol 2 are risk factors for NOB-PCOS.
| 95% CI for Exp (B) | |||||||
|---|---|---|---|---|---|---|---|
| Factor | B | SE | Wald | Sig. | Exp (B) | Lower | Upper |
| FSH | −0.528 | 0.198 | 7.128 | 0.008 | 0.59 | 0.4 | 0.869 |
| LH | 0.269 | 0.115 | 5.439 | 0.02 | 1.308 | 1.044 | 1.639 |
| Testosterone | 3.33 | 1.262 | 6.966 | 0.008 | 27.928 | 2.356 | 331.042 |
| Estradiol | 1.074 | 0.224 | 22.966 | <0.001 | 2.928 | 1.887 | 4.542 |
| Irregular period | 2.819 | 1.358 | 4.308 | 0.038 | 16.752 | 1.17 | 239.84 |
| rs2119882 | 2.291 | 0.9 | 6.473 | 0.011 | 9.885 | 1.692 | 57.733 |
| rs10830963 | −1.975 | 0.875 | 5.097 | 0.024 | 0.139 | 0.025 | 0.771 |
PCOS, polycystic ovary syndrome; NOB, non-obesity; FSH, follicle-stimulating hormone; LH, luteotropic hormone; B, partial regression coefficient; SE, standard error; Sig, significance; OR, odds ratio; 95% CI, 95% confidence interval.
Values of BMI, WHR, TC, TG, HDL, and LDL along with the polymorphism of the rs2119882 site were notably associated with OB-PCOS. Specifically, high values for BMI, WHR, TC, TG and LDL were significant risk factors for OB-PCOS, and the TT genotype at the rs2119882 site was the key protective factor for OB-PCOS (all P<0.05; Table X).
Table X.
Increased levels of BMI, WHR, TC, TG and LDL are risk factors for OB-PCOS occurrence while the TT genotype in the rs2119882 site is a protective factor for OB-PCOS.
| 95% CI for exp (B) | |||||||
|---|---|---|---|---|---|---|---|
| Factor | B | SE | Wald | Sig. | Exp (B) | Lower | Upper |
| BMI | 0.198 | 0.076 | 6.822 | 0.009 | 1.219 | 1.051 | 1.415 |
| WHR | 4.937 | 2.178 | 5.139 | 0.023 | 139.374 | 1.951 | 9,954.773 |
| TC | 2.11 | 0.452 | 21.749 | <0.001 | 8.244 | 3.397 | 20.008 |
| TG | 5.747 | 2.103 | 7.467 | 0.006 | 313.363 | 5.078 | 19,336.553 |
| HDL | −5.313 | 0.81 | 42.975 | <0.001 | 0.005 | 0.001 | 0.024 |
| LDL | 5.303 | 0.687 | 59.579 | <0.001 | 201.032 | 52.289 | 772.893 |
| rs2119882 | −0.807 | 0.389 | 4.3 | 0.038 | 0.446 | 0.208 | 0.957 |
PCOS, polycystic ovary syndrome; OB, obesity; BMI, body mass index; WHR, waist-to-hip ratio; FSH, follicle-stimulating hormone; TC, total cholesterol; TG, triglyceride; HDL, high density lipoprotein; LDL, low density lipoprotein; B, partial regression coefficient; SE, standard error; Sig, significance; OR, odds ratio; 95% CI, 95% confidence interval.
Discussion
Polycystic ovary syndrome (PCOS) is one of the most prevalent endocrinopathies in women of child-bearing age and is characterized by hyperinsulinemia, hyperandrogenemia and disordered adipokine secretion of adipose tissue (23). Generally, efficient therapies for PCOS include the use of hormonal treatments, the intake of oral contraceptives and other medicines, and the performance of hormone replacement, yet all of these treatments have been found to result in certain side effects such as increasing the severity of diabetes and changing the patient's lifestyle (24). For this reason, developing a better understanding of the risk factors for PCOS as well as trying to determine more efficient and safer methods to treat and diagnose PCOS is urgent. Therefore, the present study was conducted to ascertain whether polymorphisms of the MTNR genes would be protective or harmful for obese (OB)-PCOS patients.
From the comparison of the general measurement data between patients in the OB-PCOS and NOB-PCOS group, it was noted that abnormal menstruation, hirsutism, acne, infertility and elevated levels of LH, testosterone and estradiol were biomarkers for PCOS. As one of the causes for irregular menstruation, PCOS is also characterized by its high occurrence (25). Previous research has demonstrated that women with PCOS are more likely to suffer from hirsutism and acne and are even at greater risk of developing diabetes and cancer (26). Previous studies have also confirmed the relationship between BMI, LH, FSH and the clinical symptoms of PCOS patients (27,28). It is possible that women diagnosed with PCOS may present increased levels of estradiol and testosterone when compared with eumenorrheic ovulatory women (29). Our study also revealed that patients in the OB-PCOS group had significantly higher values for BMI, WHR, TC, TG and LDL but lower HDL than women in the NOB-PCOS group. Njelekela et al discovered in their study that obese women would likely have notably higher mean serum TG, TC, and LDL-C and an increased prevalence of dyslipidemia (30). In addition, BMI, WHR and waist circumference were known as three important identifiers of PCOS patients (31). Additionally, another study demonstrated that PCOS patients demonstrate the most prevalent MS components such as central obesity, decreased HDL-C, and increased TG, BP and fasting glucose levels, which was consistent with our results (32). Melatonin is the essential hormone secreted by the pineal gland in both humans and mammals and plays a critical part in regulating circadian rhythm, sleep and reproduction (33). It is known that epidermal melatonin production varies with sex, age, and race and that the melatonin produced locally could regulate the peripheral clock, thus influencing melanocytic activities (34). In mammals, melatonin metabolism is cell type-dependent and could occur directly at the production site; the production of 6-hydroxymelatonin is relatively high in normal epidermal melanocytes (35).
In our study, the OB-PCOS group was found to have a higher percentage of CC genotypes than the NOB-PCOS group, while the carrying of the C allele was observed to increase the occurrence of PCOS; thus, OB-PCOS patients have a higher PCOS incidence due to the CC genotype. In a recent study, MTNR1A polymorphisms (rs2119882, rs13140012, rs6553010) were correlated with a higher risk of oral cancer (36). CC genotype carriers were discovered to have higher metabolic and clinical risk factors than did carriers of the TC and TT genotypes, thus making the rs2119882 an important risk factor for OB-PCOS (37). In addition, compared with patients carrying the TT genotype at the rs2119882 site, OB-PCOS patients carrying the CT + CC genotype had increased fasting glucose levels, insulin levels, and HOMA-IRI and decreased HOMA-β% and HOMA-ISI. As mentioned above, the CC genotype carrier was responsible for PCOS occurrence. A recent study has demonstrated that rs2119882 of MTNR1A was correlated with schizophrenia in a recessive model (CC vs. TT/TC, P=0.013, OR=1.69, 95% CI=1.12–2.55) (38). The increased HOMA-IR index was associated with IL28b, with 68% genotype CC and 45% genotype CT/TT, making the relationship responsible for sustained viral response (39). Decreased HOMA-β% and HOMA-ISI and increased HOMA-IR were detected in cases of pregnancy-induced hypertension as a result of insulin resistance (40). Symptoms of insulin resistance, β-cell dysfunction and hyperinsulinemia are commonly noted in PCOS patients (41), which could better explain the altered levels of HOMA-IR, HOMA-β%, and HOMA-ISI. Patients with the TT genotype in rs2119882 and the CC genotype in rs10830963 displayed significant effects reducing PCOS. Subjects in the Mulao population carrying the TT genotype in rs2144300 exhibited lower TG levels (42), which could be beneficial for PCOS treatment. PCOS patients carrying the CG and GG genotypes in rs10830963 had increased HOMA-IR, plasma glucose levels and AUC from the OGTT compared to patients with the CC genotype, thus providing evidence for the protective effect of CC in rs10830963 for PCOS (43).
Taken together, our findings in the present study provide evidence for the relationship between a polymorphism of melatonin receptor genes (rs2119882 and rs10830963) and lipid metabolism disorder in PCOS patients. Specifically, the CC genotype in rs2119882 and the TC + CC genotype in OB-PCOS patients could increase the occurrence of PCOS; the higher values for BMI, WHR, TC, TG, and LDL were risk factors for OB-PCOS, and TT in rs2119882 and CC in rs10839063 were protective factors for OB-PCOS. In regards to the limitations of PCOS diagnosis and therapy, more studies need to be carried out to better our understanding of PCOS and determine more efficient PCOS therapies. In addition, due to limited time and energy, we failed to explore the interaction between genetic polymorphisms of melatonin and serum melatonin levels, which is expected to be investigated in the near future. Additionally, the therapeutic or protective effects of melatonin or its metabolites remain an important clinical challenge. Thus, it is necessary to determine the role of the membrane bound receptors for melatonin metabolites, which is a difficult task due to the similar protective activities of melatonin metabolites on melatonin.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Authors' contributions
XHX and LCK conceived and designed the study. XHX and HMW performed the experiments and collected the data. LCK and HMW wrote the paper and interpreted the data. CMB and XCS obtained and validated the results them, reviewed them and contributed to discussions. All authors contributed to revision of the manuscript, and read and approved the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Dongying People's Hospital, and all experimental procedures were conducted under the strict supervision of committee. Written informed consent was obtained from all patients or their parents or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing interests.
References
- 1.Swaroop A, Jaipuriar AS, Gupta SK, Bagchi M, Kumar P, Preuss HG, Bagchi D. Efficacy of a novel fenugreek seed extract (Trigonella foenum-graecum, Furocyst) in polycystic ovary syndrome (PCOS) Int J Med Sci. 2015;12:825–831. doi: 10.7150/ijms.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544–551. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 3.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: Etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 4.Stener-Victorin E, Holm G, Janson PO, Gustafson D, Waern M. Acupuncture and physical exercise for affective symptoms and health-related quality of life in polycystic ovary syndrome: Secondary analysis from a randomized controlled trial. BMC Complement Altern Med. 2013;13:131. doi: 10.1186/1472-6882-13-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costello MF, Misso ML, Wong J, Hart R, Rombauts L, Melder A, Norman RJ, Teede HJ. The treatment of infertility in polycystic ovary syndrome: A brief update. Aust N Z J Obstet Gynaecol. 2012;52:400–403. doi: 10.1111/j.1479-828X.2012.01448.x. [DOI] [PubMed] [Google Scholar]
- 6.Wehr E, Pieber TR, Obermayer-Pietsch B. Effect of vitamin D3 treatment on glucose metabolism and menstrual frequency in polycystic ovary syndrome women: A pilot study. J Endocrinol Invest. 2011;34:757–763. doi: 10.3275/7748. [DOI] [PubMed] [Google Scholar]
- 7.Gourgari E, Spanakis E, Dobs AS. Pathophysiology, risk factors, and screening methods for prediabetes in women with polycystic ovary syndrome. Int J Womens Health. 2016;8:381–387. doi: 10.2147/IJWH.S104825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C, Shi Y, You L, Wang L, Chen ZJ. Melatonin receptor 1A gene polymorphism associated with polycystic ovary syndrome. Gynecol Obstet Invest. 2011;72:130–134. doi: 10.1159/000323542. [DOI] [PubMed] [Google Scholar]
- 9.Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR. Melatonin-a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93:350–384. doi: 10.1016/j.pneurobio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 10.McClay DR. Evolutionary crossroads in developmental biology: Sea urchins. Development. 2011;138:2639–2648. doi: 10.1242/dev.048967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carcangiu V, Luridiana S, Vacca GM, Daga C, Mura MC. A polymorphism at the melatonin receptor 1A (MTNR1A) gene in Sarda ewes affects fertility after AI in the spring. Reprod Fertil Dev. 2011;23:376–380. doi: 10.1071/RD10014. [DOI] [PubMed] [Google Scholar]
- 12.Tobias DK, Gaskins AJ, Missmer SA, Hu FB, Manson JE, Buck Louis GM, Zhang C, Chavarro JE. History of infertility and risk of type 2 diabetes mellitus: A prospective cohort study. Diabetologia. 2015;58:707–715. doi: 10.1007/s00125-015-3493-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren T, Zhu Y, Kan J. Zanthoxylum alkylamides activate phosphorylated AMPK and ameliorate glycolipid metabolism in the streptozotocin-induced diabetic rats. Clin Exp Hypertens. 2017;39:330–338. doi: 10.1080/10641963.2016.1259332. [DOI] [PubMed] [Google Scholar]
- 14.Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spégel P, Bugliani M, Saxena R, Fex M, Pulizzi N, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41:82–88. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol Cell Endocrinol. 2012;351:152–166. doi: 10.1016/j.mce.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slominski AT, Zmijewski MA, Semak I, Kim TK, Janjetovic Z, Slominski RM, Zmijewski JW. Melatonin, mitochondria, and the skin. Cell Mol Life Sci. 2017;74:3913–3925. doi: 10.1007/s00018-017-2617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ying X, Qian Y, Jiang Y, Jiang Z, Song Z, Zhao C. Association of the apolipoprotein B/apolipoprotein A-I ratio and low-density lipoprotein cholesterol with insulin resistance in a Chinese population with abdominal obesity. Acta Diabetol. 2012;49:465–472. doi: 10.1007/s00592-012-0419-9. [DOI] [PubMed] [Google Scholar]
- 18.Chin R, Miyazaki S. Criteria of obesity and obesity disease in Japan. Nihon Rinsho. 2009;67:297–300. (In Japanese) [PubMed] [Google Scholar]
- 19.Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–1447. doi: 10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- 20.Lucky AW, McGuire J, Rosenfield RL, Lucky PA, Rich BH. Plasma androgens in women with acne vulgaris. J Invest Dermatol. 1983;81:70–74. doi: 10.1111/1523-1747.ep12539043. [DOI] [PubMed] [Google Scholar]
- 21.Stumvoll M, Mitrakou A, Pimenta W, Jenssen T, Yki-Järvinen H, Van Haeften T, Renn W, Gerich J. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care. 2000;23:295–301. doi: 10.2337/diacare.23.3.295. [DOI] [PubMed] [Google Scholar]
- 22.Abbasi F, Blasey C, Feldman D, Caulfield MP, Hantash FM, Reaven GM. Low circulating 25-hydroxyvitamin D concentrations are associated with defects in insulin action and insulin secretion in persons with prediabetes. J Nutr. 2015;145:714–719. doi: 10.3945/jn.114.209171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macut D, Bjekić-Macut J, Rahelić D, Doknić M. Insulin and the polycystic ovary syndrome. Diabetes Res Clin Pract. 2017;130:163–170. doi: 10.1016/j.diabres.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Genazzani AR, Genazzani AD. Front Gynecol Endocrinol. Springer International Publishing; 2015. Polycystic ovary syndrome: From contraception to hormone replacement therapy. [DOI] [Google Scholar]
- 25.Wu D, Kimura F, Takashima A, Shimizu Y, Takebayashi A, Kita N, Zhang G, Murakami T. Intake of vinegar beverage is associated with restoration of ovulatory function in women with polycystic ovary syndrome. Tohoku J Exp Med. 2013;230:17–23. doi: 10.1620/tjem.230.17. [DOI] [PubMed] [Google Scholar]
- 26.Costello M, Shrestha B, Eden J, Sjoblom P, Johnson N. Insulin-sensitising drugs versus the combined oral contraceptive pill for hirsutism, acne and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. Cochrane Database Syst Rev. 2007:CD005552. doi: 10.1002/14651858.CD005552.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Esmaeilzadeh S, Andarieh MG, Ghadimi R, Delavar MA. Body mass index and gonadotropin hormones (LH & FSH) associate with clinical symptoms among women with polycystic ovary syndrome. Glob J Health Sci. 2014;7:101–106. doi: 10.5539/gjhs.v7n2p101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramezanali F, Khalili G, Arabipoor A, Bagheri Lankarani N, Moini A. Relationships between serum luteinizing hormone level, endometrial thickness and body mass index in polycystic ovary syndrome patients with and without endometrial hyperplasia. Int J Fertil Steril. 2016;10:36–41. doi: 10.22074/ijfs.2016.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawwass JF, Sanders KM, Loucks TL, Rohan LC, Berga SL. Increased cerebrospinal fluid levels of GABA, testosterone and estradiol in women with polycystic ovary syndrome. Hum Reprod. 2017;32:1450–1456. doi: 10.1093/humrep/dex086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Njelekela MA, Negishi H, Nara Y, Sato T, Tomohiro M, Kuga S, Noguchi T, Kanda T, Yamori M, Mashalla Y, et al. Obesity and lipid profiles in middle aged men and women in Tanzania. East Afr Med J. 2002;79:58–64. doi: 10.4314/eamj.v79i2.8901. [DOI] [PubMed] [Google Scholar]
- 31.Kalani Z, Salimi T, Rafiei M. Comparison of obesity indexes BMI WHR and WC in association with Hypertension: Results from a blood pressure status survey in Iran. J Cardiovasc Dis Res. 2015;6:72–77. doi: 10.5530/jcdr.2015.2.5. [DOI] [Google Scholar]
- 32.Zaki M, Kholoussi S, Ismail S, Raouf HA, Helwa I, Hassan N, Youness E, Mohamed NA, Kamal S, Yousef W, et al. Metabolic abnormalities in young Egyptian women with polycystic ovary syndrome and their relation to ADIPOQ, gene variants and body fat phenotype. Egypt J Med Hum Genet. 2015;16:367–374. doi: 10.1016/j.ejmhg.2015.05.007. [DOI] [Google Scholar]
- 33.Jaworek J, Nawrot-Porabka K, Leja-Szpak A, Bonior J, Szklarczyk J, Kot M, Konturek SJ, Pawlik WW. Melatonin as modulator of pancreatic enzyme secretion and pancreatoprotector. J Physiol Pharmacol. 2007;58(Suppl 6):S65–S80. [PubMed] [Google Scholar]
- 34.Slominski AT, Hardeland R, Zmijewski MA, Slominski RM, Reiter RJ, Paus R. Melatonin: A cutaneous perspective on its production, metabolism, and functions. J Invest Dermatol. 2018;138:490–499. doi: 10.1016/j.jid.2017.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slominski AT, Semak I, Fischer TW, Kim TK, Kleszczynski K, Hardeland R, Reiter RJ. Metabolism of melatonin in the skin: Why is it important? Exp Dermatol. 2017;26:563–568. doi: 10.1111/exd.13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin FY, Lin CW, Yang SF, Lee WJ, Lin YW, Lee LM, Chang JL, Weng WC, Lin CH, Chien MH. Interactions between environmental factors and melatonin receptor type 1A polymorphism in relation to oral cancer susceptibility and clinicopathologic development. PLoS One. 2015;10:e0121677. doi: 10.1371/journal.pone.0121677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song X, Sun X, Ma G, Sun Y, Shi Y, Du Y, Chen ZJ. Family association study between melatonin receptor gene polymorphisms and polycystic ovary syndrome in Han Chinese. Eur J Obstet Gynecol Reprod Biol. 2015;195:108–112. doi: 10.1016/j.ejogrb.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 38.Park HJ, Park JK, Kim SK, Cho AR, Kim JW, Yim SV, Chung JH. Association of polymorphism in the promoter of the melatonin receptor 1A gene with schizophrenia and with insomnia symptoms in schizophrenia patients. J Mol Neurosci. 2011;45:304–308. doi: 10.1007/s12031-011-9522-6. [DOI] [PubMed] [Google Scholar]
- 39.Del Campo JA, Ampuero J, Rojas L, Conde M, Rojas A, Maraver M, Millán R, García-Valdecasas M, García-Lozano JR, González-Escribano MF, Romero-Gómez M. Insulin resistance predicts sustained virological response to treatment of chronic hepatitis C independently of the IL28b rs12979860 polymorphism. Aliment Pharmacol Ther. 2013;37:74–80. doi: 10.1111/apt.12113. [DOI] [PubMed] [Google Scholar]
- 40.Chen Z, Liu W, Sun X, Zhu L. Clinical study on the association between pregnancy-induced hypertension and insulin resistance. Exp Ther Med. 2017;13:2065–2070. doi: 10.3892/etm.2017.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diamanti-Kandarakis E. Insulin resistance in PCOS. Endocrine. 2006;30:13–17. doi: 10.1385/ENDO:30:1:13. [DOI] [PubMed] [Google Scholar]
- 42.Li Q, Yin RX, Yan TT, Miao L, Cao XL, Hu XJ, Aung LH, Wu DF, Wu JZ, Lin WX. Association of the GALNT2 gene polymorphisms and several environmental factors with serum lipid levels in the Mulao and Han populations. Lipids Health Dis. 2011;10:160. doi: 10.1186/1476-511X-10-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li C, Shi Y, You L, Wang L, Chen ZJ. Association of rs10830963 and rs10830962 SNPs in the melatonin receptor (MTNR1B) gene among Han Chinese women with polycystic ovary syndrome. Mol Hum Reprod. 2011;17:193–198. doi: 10.1093/molehr/gaq087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the present study are included in this published article.