Abstract
Dendropanax morbifera (D. morbifera), known as Dendro, means ‘omnipotent drug’ (Panax), and has been called the panacea tree. Various studies on D. morbifera are currently ongoing, aiming to determine its medicinal uses. The present study investigated the anti-inflammatory effects and underlying mechanism of a natural extract of D. morbifera leaves (DPL) in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages. In the present study, the following assays and models were used: MTT assay, nitric oxide (NO) assay, western blotting, ELISA and mouse models of atopic dermatitis. DPL extract markedly reduced the production of NO, inducible NO synthase and interleukin-6, as well as the nuclear translocation of nuclear factor-κB (NF-κB). Additionally, the LPS-induced activation of extracellular signal-regulated kinase 1/2 (ERK1/2), P38 and c-Jun N-terminal kinase (JNK) was suppressed by DPL extract. Taken together, these results indicate that NF-κB, ERK1/2, P38 and JNK may be potential molecular targets of DPL extract in the LPS-induced inflammatory response. Subsequently, the present study investigated the effects of DPL extract in a 2,4-dinitrochlorobenzene-induced atopic dermatitis mouse model. Ear thickness, serum immunoglobulin E levels and histological analysis revealed that the DPL extract was effective in attenuating the inflammatory response. These results indicate that DPL extract has anti-inflammatory potential and may be developed as a botanical drug to treat atopic dermatitis.
Keywords: Dendropanax morbifera, anti-inflammation, nuclear factor-κB, mitogen-activated protein kinase
Introduction
Recently, more studies investigating natural materials have been conducted in response to the increased public interest in healthy skin. There is particular interest in the prevention and treatment of various skin diseases, including atopic dermatitis and inflammatory acne (1). Atopic dermatitis (AD), also known as inflammatory dermatitis, is characterized by severe itching, chronic edema and skin rashes (2). Although the etiology is not clearly understood, it is known that AD is associated with immunological abnormalities, abnormal skin barriers, and genetic and environmental factors (3). Exposure to allergens induces atopy, leading to an imbalance between T helper cell (Th) 1 and Th2 cytokines, and the overproduction of Th2 cytokines stimulates B-cells to increase the generation of immunoglobulin E (4). Increased immunoglobulin E (IgE) induces the exocytosis of compounds such as histamine in skin mast cells, causing edema and itching, thereby aggravating AD (4). Treatment methods for dermatitis include moisturizing dry skin, the use of steroids as anti-inflammatory agents, the application or administration of antihistamines and immunosuppressive agents. Although these drugs may relieve certain symptoms, long-term use of topical steroids may additionally lead to thinning of the skin with subsequent bleeding (5,6). Therefore, a number of ongoing studies are attempting identify functional substances from safe and effective natural products to prevent AD without side effects (7,8).
The inflammatory response is a physiological protective activity in the human body that recognizes external physical and chemical stimuli, and employs a defensive mechanism to restore damaged tissues (9). Inflammatory reactions are categorized as acute or chronic depending on their activation and duration (10). When an inflammatory reaction occurs, inflammatory mediators such as nuclear factor-κB (NF-κB), nitric oxide (NO) and inflammatory cytokines are secreted. NF-κB is widely distributed and is known as a regulator of immune and inflammatory responses (11,12). NO, an indicator of the inflammatory response, is synthesized by NO synthase (NOS) from L-arginine. There are three types of NOS: Endothelial, neuronal and inducible NOS (iNOS). Of these, NO produced by iNOS serves an important pathological role (13). Excessive NO production by iNOS induced by lipopolysaccharide (LPS) or inflammatory cytokines exacerbates the inflammatory response, resulting in tissue damage, gene mutations and nerve damage (14). Cyclooxygenase (COX) is an enzyme that catalyzes the conversion of arachidonic acid to prostaglandin. COX-2 is induced by various stimuli including inflammation, growth factors and cytokines released by cancer cells (15). The proinflammatory cytokines that are enriched during inflammation include tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-1β, and are secreted by macrophages to mediate various inflammatory responses that induce NO generation. Excessive production of NO and cytokines can lead to genetic alterations, and tissue and nerve damage, with fatal consequences for the host (16). Therefore, regulation of the inflammatory response is important for maintaining homeostasis, and an anti-inflammatory effect may be achieved by inhibiting the production of mediators such as NO and cytokines, which are produced in inflammatory reactions (17).
Other signaling molecules involved in inflammatory reactions include mitogen-activated protein kinases (MAPKs), which can be characterized as three different types of serine/threonine protein kinases: Extracellular signal-regulated protein kinase (ERK), stress-activated c-Jun N-terminal kinase (JNK) and P38 kinases. Each type of kinase has a different activity, and all 3 types are known to carry out signal transduction in various cellular activities, including proliferation, differentiation, cell death and inflammation (18). Efficient inhibition of these inflammatory mediators may be an important strategy in the development of anti-inflammatory drugs.
Dendropanax morbifera (D. morbifera) is one of the world's rarest warm climate trees. There is only one species in one genus in Korea, and it is found across a broad area of western and southern coastal Korea encompassing Jeju Island, Wando, Geomundo and Haenam; however, the native distribution has been impacted by overharvesting and urbanization, and D. morbifera now only grows in a limited area (19). D. morbifera, known as Dendro, means ‘omnipotent drug’ (Panax), and has been called the panacea tree. Various ongoing studies on D. morbifera are aiming to determine its medicinal uses (20–24). D. morbifera leaves are composed of 70.2% water, 1.2% protein, 2.7% fat, 1.7% ash, 56.9 mg% vitamin C and 746 mg% water soluble tannins; 32 types of substances have been identified with gas chromatography-mass spectrometry. Among these substances are β-selinene and capnellene-8-one, which are sesquiterpenes with two ring structures, as well as many unidentified volatile components (20). When D. morbifera bark is scarred, it produces a golden resinous solution that contains benzoic acid, a unique aromatic component (21,22).
Recent studies have revealed that polyacetylene compounds isolated from D. morbifera leaves have anti-complement activity, whereas ethanol extracts promote the actions of B- and T-cells (20,23). D. morbifera sap inhibited melanin biosynthesis by reducing the expression of tyrosinase in melanin synthesis, and the ethyl acetate fraction extracted from D. morbifera leaves also exhibited cell protective and skin whitening effects, such as antioxidant properties and tyrosinase inhibition (24). However, few studies or animal experiments have been conducted on the mechanism underlying its anti-inflammatory properties.
In the present study, the effects of D. morbifera leaf (DPL) extract on the production of NO, cytokine secretion and inflammation-associated protein expression was investigated in activated RAW264.7 macrophages by inducing inflammation with LPS. The anti-inflammatory effect of DPL was also determined in a 2,4-dinitrochlorobenzene (DNCB)-induced AD animal model.
Materials and methods
Chemicals, drugs and antibodies
Dulbecco's modified Eagle's medium (DMEM), penicillin-streptomycin, and 10% fetal bovine serum (FBS) were purchased from Welgene (Gyeongsan, Korea). LPS, MTT and dimethyl sulfoxide (DMSO) were purchased from Merck KGaA (Darmstadt, Germany). The nitrate/nitrite colorimetric assay kit was obtained from Cayman Chemical Company (Ann Arbor, MI, USA). The mouse TNF ELISA (cat. no. 560478) and mouse IL-6 ELISA kits (cat. no. 555240) were purchased from BD Biosciences (San Jose, CA, USA). β-actin (cat. no. 4967), iNOS (cat. no. 2982), COX-2 (cat. no. 4842), phosphorylated (p)-NF-κB-p65 (cat. no. 3033), NF-κB-p65 (cat. no. 8242), p-NF-κB inhibitor-α (IκB-α; cat. no. 4812), IκB-α (cat. no. 5209) p-ERK1/2 (cat. no. 4376), ERK1/2 (cat. no. 9194), p-P38 MAPK (cat. no. 9211), P38 MAPK (cat. no. 8690), p-JNK (cat. no. 4668), JNK (cat. no. 9252) and anti-rabbit horseradish peroxidase (HRP; cat. no. 7074) antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Plant materials and extraction
The D. morbifera leaves used in the experiments were collected from the native areas of Jeju Island. The collected D. morbifera leaves (100 g) were pulverized and then fermented with 70% ethanol and tertiary distilled water at a ratio of 4:6 for 10 days at room temperature, then subjected to hydrothermal extraction. The extracted solution was filtered using Whatman No. 1 disc paper. Then the extract was concentrated under reduced pressure using a rotary vacuum evaporator (R-220; BUCHI Corporation, New Castle, DE, USA), and the remaining solution was boiled again to obtain an extract (25). The extracts were mixed with distilled water (DW) and refrigerated.
Cell culture and stimulation
The RAW264.7 macrophage line was obtained from the Korean Cell Line Bank (Korean Cell Line Research Foundation, Seoul, Korea), and maintained in DMEM supplemented with 5% FBS/1% penicillin-streptomycin at 37°C in a 5% CO2 humidified air environment. The cells were incubated for 24 h in medium supplemented with 10% FBS. Subsequently, the RAW 264.7 cells were pre-treated with doses of DPL (0, 100, 200, 300, 400 and 500 µg/ml) for 2 h at 37°C and treated with LPS (1 µg/ml) for 24 h at 37°C, in serum-free media.
Cell viability assay
RAW264.7 cells were seeded in a 96-well plate at a density of 1×105 cells/ml and a volume of 200 µl/well. Following incubation for 24 h at 37°C, the cells were treated with DPL extract (mixed with DW) and negative control (DW) at various concentrations (DPL 0, 100, 200, 300, 400 and 500 µg/ml) for 24 h at 37°C, followed by the addition of 5 mg/ml MTT solution to each well, and the plates were further incubated for 2 h at 37°C. The supernatant was removed and 200 µl DMSO was added to each well to solubilize the water-insoluble purple formazan crystals. The absorbance at a wavelength of 595 nm was measured using a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The percentage of viable cells compared with that of untreated control cells was then estimated.
Measurement of NO
RAW264.7 cells were seeded (1×105/ml) and cultured in 96-well plates. Following incubation for 24 h at 37°C, the cells were treated with DPL extract at the indicated concentrations (DPL 0, 100, 200, 300 and 400 µg/ml) for 2 h in serum-free medium prior to the addition of LPS (1 µg/ml). Following a 24-h incubation at 37°C, the supernatants were measured for NO production using the nitrate/nitrite assay kit (Cayman Chemical Company). NO was measured as the accumulation of nitrite and nitrate reductase, which were determined spectrophotometrically using Griess reagent, included in the nitrate/nitrite assay kit, at an optical density of 540 nm.
Determination of TNF-α and IL-6 production
RAW 264.7 cells were pre-treated with doses of DPL (0, 100, 200, 300 and 400 µg/ml) for 2 h and treated with LPS (1 µg/ml) for 24 h at 37°C. Subsequently, production of the proinflammatory cytokines TNF-α and IL-6 in the culture medium was determined using commercially available ELISA kits (BD Biosciences), according to the manufacturer's protocol.
Western blot analysis
Cells were preincubated with various concentrations of DPL extract (0, 200 and 400 µg/ml) for 2 h prior to a 24-h incubation at 37°C with LPS (1 µg/ml), and subsequently harvested. Western blot assays were performed as previously described (26). The membranes were incubated with the primary antibodies specific for β-actin (1:10,000), p-NF-κB-p65 (1:1,000), NF κB-p65 (1:1,000), p-IκB-α (1:1,000), IκB-α (1:1,000) p-ERK1/2 (1:1,000), ERK1/2 (1:1,000), p-P38 MAPK (1:1,000), P38 MAPK (1:1,000), p-JNK (1:1,000) and JNK (1:1,000) overnight at 4°C with gentle shaking. Following incubation with the primary antibodies, the membranes were incubated with HRP-conjugated anti-rabbit IgG secondary antibodies (1:1,000) for 2 h at room temperature with gentle shaking. The membranes were washed three times for 10 min in TBS containing 0.1% Tween-20. The bands were detected using enhanced chemiluminescence western blotting detection reagents (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to the manufacturer's protocol. β-actin was used as a loading control. Band density was measured using ImageJ software (version 1.48; National Institutes of Health, Bethesda, MD, USA).
Animals
A total of 30 BALB/c female mice (age, 4 weeks; body weight, 16–18 g) were purchased from the Nara Biotech, Co., Ltd. (Seoul, South Korea) and maintained at 23±5°C at 40±10% relative humidity with a 12-h light/dark cycle (artificial lighting from 8:00 a.m. to 8:00 p.m.) in facilities approved by the Companion and Laboratory Animal Science Department of Kongju National University (Chungnam, Korea). The animals were housed in cages and allowed access to sterilized water and commercial rodent chow (Biopia, Seoul, Korea) ad libitum. All animal experiments were performed with the approval of the Institutional Animal Care and Use Committee of Kong-Ju National University (approval no. KNU_2017-10; Yesan, Korea) and the institutional guidelines were adhered to.
Induction of AD
AD was induced in BALB/c mice as previously described, with minor modifications (27). Briefly, BALB/c mice were divided into seven groups (n=5/group): Group I (control treatment; 3:1 acetone/olive oil solution; 20 µl/ear), group II (1% DNCB; 20 µl/ear), groups III (dexamethasone; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany; 50 µg/20 µl/ear; positive control), group IV (DPL high; 20 µl/ear, undiluted solution), group V (DPL medium; 20 µl/ear, 0.5× undiluted solution/DW) and group VI (DPL low; 20 µl/ear, 0.25× undiluted solution/DW) received DPL extract or combinations as indicated. To induce AD, the surfaces of both ears of the mice (not anesthetized) were stripped with surgical tape. Following this, 1% DNCB dissolved in a 3:1 acetone/olive oil solution was painted on each ear. The DPL extract was applied at three concentrations: High (undiluted solution mixed with an equal volume of 3:1 solution of acetone and olive oil, v/v), medium (0.5× undiluted solution/DW mixed with an equal volume of 3:1 solution of acetone and olive oil, v/v) or low (0.25× undiluted solution/DW mixed with an equal volume of 3:1 solution of acetone and olive oil, v/v). DNCB was applied every 2 days starting from 3 days prior to measurements. Dexamethasone and DPL extract were applied every 2 days from day 0. Following 24 h of DNCB application, the thickness of the ears was measured with a vernier caliper (Mitutoyo, Kawasaki, Japan). The mice were sacrificed on Day 10. Blood samples were then collected from the abdominal aorta, and the plasma was stored at −70°C until further analysis. Following sacrifice, the ears were excised and subjected to histopathological analysis.
Measurement of Ig levels
Blood samples were obtained from each treatment group 10 days following AD induction. Total serum IgE levels were measured using an ELISA kit (cat. no. K3231082; Komabiotech, Seoul, Korea), according to the manufacturer's protocol.
Histological observations
The ears were immediately fixed in 10% formaldehyde (1 week at room temperature) following excision, and embedded in paraffin. Blocks were then cut into 5-µm-thick slices. To measure epidermal thickening, hematoxylin and eosin (H&E) staining was performed (6 h at room temperature). To evaluate mast cells, the skin sections were stained with toluidine blue (TB; 6 h at room temperature). The sections were examined under a light microscope (magnification, ×200; Olympus CH30; Olympus Corporation, Tokyo, Japan).
Statistical analysis
The results are expressed as the mean ± standard deviation. The experiments were repeated three times. Statistical analyses were performed using Prism (version 5; GraphPad Software, Inc., La Jolla, CA, USA) Differences between the mean values for the individual groups were assessed by one-way analysis of variance with Dunnett's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of DPL extract on the viability of RAW264.7 macrophages
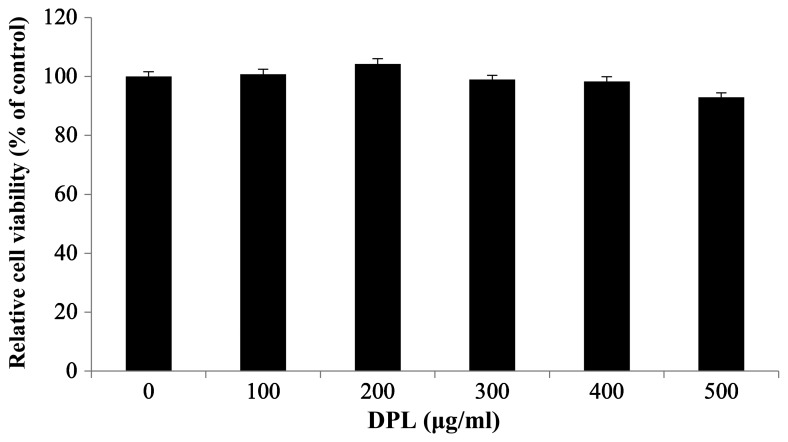
To investigate the effect of DPL extract on the survival of RAW264.7 macrophages, an MTT assay was performed. Cell viability was measured once RAW264.7 cells were treated with 0, 100, 200, 300, 400 and 500 µg/ml DPL extract and cultured for 24 h. There were no differences in cell viability at any concentration, confirming that the DPL extract did not affect the survival of RAW264.7 cells (Fig. 1). However, cell viability following treatment with DPL at a concentration of 500 µg/ml was decreased compared with other concentrations. Therefore, concentrations of 100, 200, 300 and 400 µg/ml were used.
Figure 1.
Effects of DPL extract on the viability of RAW264.7 cells. RAW264.7 cells (1×105 cells/ml) were treated with 100, 200, 300, 400 and 500 µg/ml DPL extract for 24 h, and cell viability was determined by an MTT assay. Each bar represents the mean ± standard deviation calculated from three independent experiments. DPL, Dendropanax morbifera leaf.
Effects of DPL extract on LPS-induced NO production
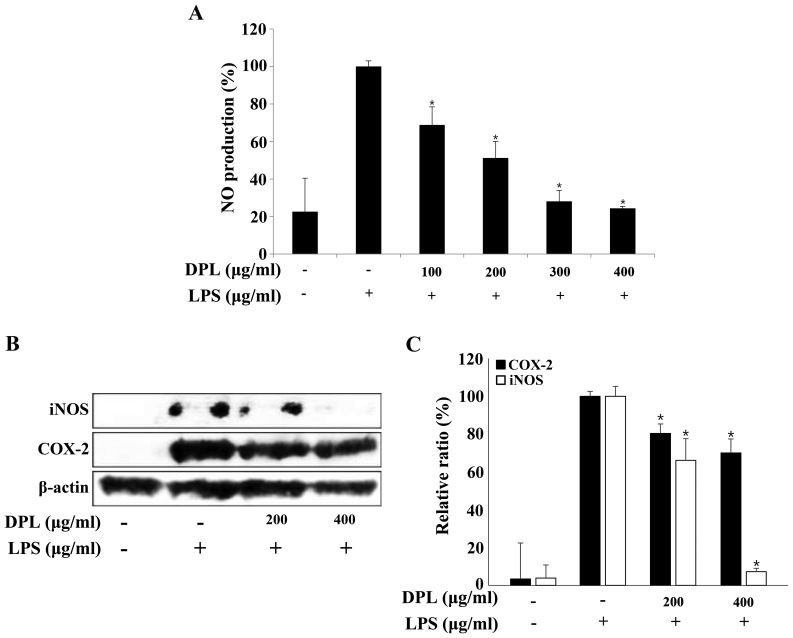
NO is an inorganic compound produced by NO synthase. It is involved in many biological processes including the immune response, cytotoxicity and vascular relaxation, and it also maintains cell function and cytotoxicity depending on the concentration (28). The present study selected the concentration of DPL extract that had no effect on the survival of RAW264.7 macrophages and performed a NO assay. RAW264.7 cells were treated with DPL extract (100, 200, 300 and 400 µg/ml) for 2 h before treatment with LPS (1 µg/ml), and the concentration of NO was measured (Fig. 2A). The NO concentration in RAW264.7 cells stimulated with LPS was ~5 times higher compared with that observed in the untreated control group. In the groups treated with 100, 200, 300 and 400 µg/ml DPL extract with LPS, the NO concentrations were 68.9, 51.2, 28.1 and 24.4%, respectively, indicating that the NO concentration decreased in a DPL concentration-dependent manner when compared with the group treated with LPS alone (Fig. 2A).
Figure 2.
Effects of DPL extract on LPS-induced iNOS and COX-2 protein levels and NO production in RAW264.7 cells. (A) RAW264.7 cells were pre-incubated with 100, 200, 300, and 400 µg/ml DPL extract for 2 h, and then treated with 1 µg/ml LPS for an additional 24 h. NO was measured using the Griess reaction. (B) The cells were sampled and lysed following 24 h treatment, and iNOS and COX-2 protein levels were determined by western blotting. β-actin was used as a loading control. (C) Data analysis was performed using ImageJ software by measuring the integrated band densities following background subtraction. Each bar represents the mean ± standard deviation calculated from three independent experiments. *P<0.05 vs. LPS only treatment. DPL, Dendropanax morbifera leaf; LPS, lipopolysaccharide; iNOS, inducible nitric oxide synthase; NO, nitric oxide; COX2, cyclooxygenase 2.
Effects of DPL extract on LPS-induced iNOS and COX-2 protein expression
The protein expression levels of iNOS and COX-2, which are known to activate inflammation, were assessed by western blotting. The protein expression levels of iNOS and COX-2 were increased in the LPS-treated group when compared with the control group, and these levels were significantly decreased in a concentration-dependent manner in the groups treated with different concentrations of DPL (Fig. 2B and C).
Effects of DPL extract on LPS-induced proinflammatory cytokine production
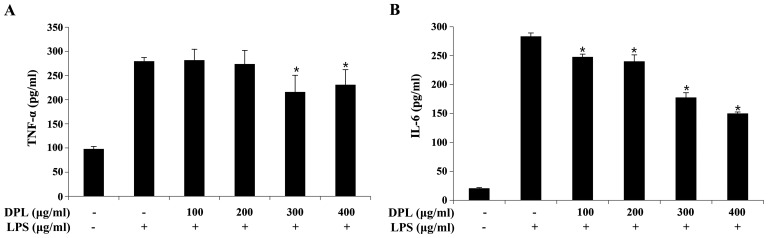
The expression of TNF-α and IL-6 was quantified by ELISA to assess the anti-inflammatory effects of DPL extract. When compared with the expression levels in the group treated with LPS alone, the expression of TNF-α was significantly decreased following treatment with 300 and 400 µg/ml DPL, and the expression of IL-6 was significantly decreased in a concentration-dependent manner (Fig. 3).
Figure 3.
Effects of DPL extract on LPS-induced pro-inflammatory cytokine production in RAW264.7 cells. RAW264.7 cells were treated with LPS (1 µg/ml) in the presence or absence of DPL extract at the indicated concentrations for 24 h. (A) TNF-α and (B) IL-6 in the culture supernatant were measured by ELISA. Each bar represents the mean ± standard deviation calculated from three independent experiments. *P<0.05 vs. LPS only treatment. DPL, Dendropanax morbifera leaf; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor-α; IL, interleukin.
Effects of DPL extract on the activation of NF-κB in LPS-stimulated RAW264.7 macrophages
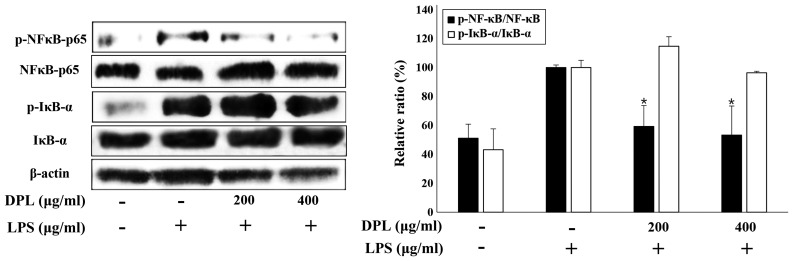
NF-κB is activated by IκB-α and acts as an inflammatory mediator (11). To investigate the activation of the well-known inflammatory mediators, NF-κB and IκB-α, the present study performed western blot analysis. The expression of NF-κB and IκB-α was increased in the LPS-treated group when compared with the untreated control group. Comparisons of the LPS and 200 or 400 µg/ml DPL extract groups and the LPS-only group revealed that the activation of NF-κB was significantly decreased with DPL extract treatment, whereas the activation of IκB-α was not significantly changed (Fig. 4).
Figure 4.
Effects of DPL extract on the degradation or phosphorylation of NF-κB/p65 and IκB-α protein in LPS-induced RAW264.7 cells. RAW264.7 cells were treated with LPS (1 µg/ml) in the presence or absence of DPL extract at the indicated concentrations for 24 h. NF-κB phosphorylation and IκB-α degradation were assessed by western blotting. Data analysis was performed using ImageJ software by measuring the integrated band densities following background subtraction. Each bar represents the mean ± standard deviation calculated from three independent experiments. *P<0.05 vs. LPS only treatment. DPL, Dendropanax morbifera leaf; LPS, lipopolysaccharide; NF-κB, nuclear factor-κB; IκB-α, NF-κB inhibitor-α; p-, phosphorylated.
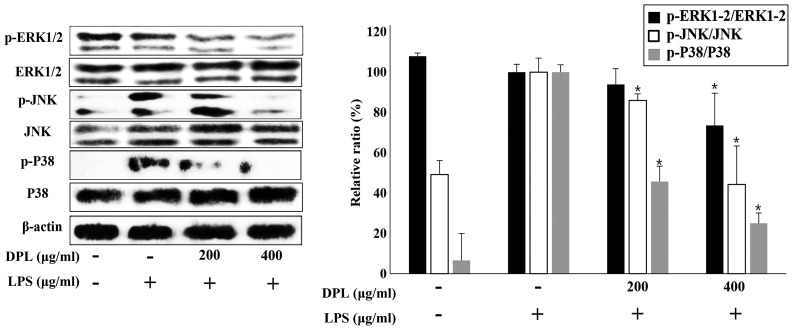
Effects of DPL extract on LPS-induced NF-κB and MAPK phosphorylation
MAPK is a typical signaling molecule that affects the activation of NF-κB. When MAPK is phosphorylated and activated in the cell, it influences the production of various inflammatory mediators. When inflammation occurs, MAPKs such as P38 and JNK are activated in macrophages, further activating the inflammatory response (18). To confirm if the expression of MAPKs is involved in the inflammatory response, the present study performed western blot analysis. Comparing the LPS-only group with the untreated control group, the expression of p-ERK was not significantly changed, but the expression levels of p-JNK and p-P38 were increased (Fig. 5). When assessing DPL extract treatment, compared with the group treated with LPS-only, the expression levels of p-ERK, p-JNK and p-P38 were significantly downregulated in the 400 µg/ml DPL-treated group; the levels of p-JNK and p-P38 were also significantly downregulated in the 200 µg/ml DPL-treated group. Therefore, p-JNK and p-P38, in particular, exhibited more significant decreases in expression than p-ERK.
Figure 5.
Effects of DPL extract on the MAPK signaling pathway in LPS-induced RAW264.7 cells. RAW264.7 cells were treated with LPS (1 µg/ml) in the presence or absence of DPL extract at the indicated concentrations for 24 h. MAPKs were assessed by western blot analysis of whole cell lysates. Each bar represents the mean ± standard deviation calculated from three independent experiments. *P<0.05 vs. LPS only treatment. DPL, Dendropanax morbifera leaf; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; ERK1/2, extracellular signal-regulated kinase 1/2; JNK, c-Jun N-terminal kinase; p-, phosphorylated.
Effect of DPL extract on DNCB-induced AD-like skin lesions in BALB/c mice
DNCB is a typical compound with a benzene ring that triggers a local immune response to induce inflammatory dermatitis (25,29,30). Therefore, to determine the effect of DPL extract on induced AD, the present study measured ear thickness in mice following treatment with DNCB and DPL extract. The DPL extract was applied to the ears at three concentrations: High (undiluted solution), medium (1:2 dilution), or low (1:4 dilution). A schematic of the experimental procedure is presented in Fig. 6A. The ear thickness of the DPL-treated group was decreased when compared with that of the DNCB-induced AD group (Fig. 6B). In addition, the AD symptoms were more effectively reduced in the DPL low group than in the DPL high and medium groups. Next, the present study measured the morphological changes in the lymph nodes of induced AD mice. The results revealed that the lymph nodes were increased in the DNCB-treated group when compared with the untreated control group, whereas, the DPL-treated groups exhibited a decreased size compared with DNCB, particularly in the DPL low group (Fig. 6C). In the induced AD mice, IgE levels were significantly decreased in the DPL-treated groups when compared with the DNCB-treated group (Fig. 6D). These results confirmed that DPL extracts attenuated the symptoms of AD in mice.
Figure 6.
Effects of DPL extract on DNCB-induced AD in BALB/c mice. (A) Schematic diagram presenting the timeline employed for the induction of AD and treatment with DPL extract. (B) Ear thickness during the course of AD. (C) The lymph nodes were photographed to record morphological changes. (D) Levels of serum IgE were measured by ELISA. Blood samples were collected on day 10 post-induction. Data are expressed as the mean ± standard error of the mean. *P<0.05 vs. DNCB. DPL, Dendropanax morbifera leaf; LPS, lipopolysaccharide; DNCB, 2,4-dinitrochlorobenzene; AD, atopic dermatitis; IgE, immunoglobulin E.
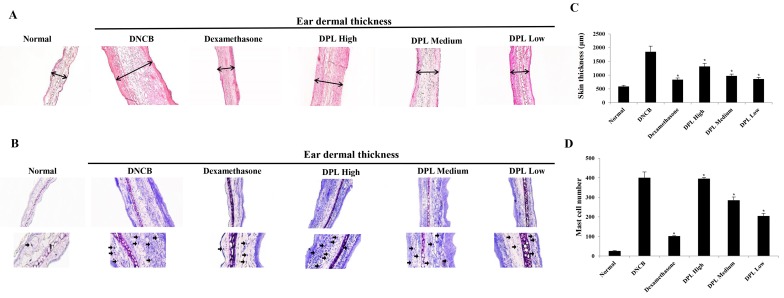
Effects of DPL extract on DNCB-induced immune cell infiltration in BALB/c mice
When AD is induced, the secretion of vasodilators such as proteases and histamine by mast cells increase (31). The ears of the induced AD mice were excised to examine the thickness of the ear tissue and mast cell secretion. Firstly, the morphological changes in the ear tissues were evaluated by H&E staining. The ear dermal thickness of induced AD mice were increased, whereas the ear dermal thickness was significantly decreased in the DPL-treated group (Fig. 7A and C). Additionally, the present study measured mast cell secretion by TB staining, which revealed that mast cell secretions were increased in AD mice, whereas mast cell secretions were significantly decreased in the DPL-treated group (Fig. 7B and D). These results indicate that DPL reduces inflammation and mast cell secretions, and suppresses AD symptoms.
Figure 7.
Effects of DPL extract on tissue inflammation and the infiltration of immune cells in atopic dermatitis mice. (A) Hematoxylin and eosin (red) and (B) toluidine blue (blue) staining was performed, and the cells were examined under a light microscope (magnification, ×200). The arrows indicate mast cells. (C) Skin thickness and (D) the numbers of mast cells were measured. Data are expressed as the mean ± standard error of the mean. *P<0.05 vs. DNCB. DPL, Dendropanax morbifera leaf; LPS, lipopolysaccharide; DNCB, 2,4-dinitrochlorobenzene.
Discussion
AD is an inflammatory skin disease that causes symptoms such as severe itching, chronic edema and skin rash. The pathogenesis of this disease has not been elucidated to date, but immunological abnormalities, and environmental and genetic factors are known to be involved (3). Contact with atopic allergens alters the balance of inflammatory cytokines and stimulates B-cells to produce IgE, which mediates the mast cell secretion of histamine in the skin, causing edema, itchy skin, inflammatory reactions and the release of inflammatory mediators such as NF-κB, NO and inflammatory cytokines (4). Immunosuppressive agents such as steroids or antihistamines are used to treat inflammatory skin diseases, but owing to the induced side effects, these agents are inadequate for long-term treatment. In this regard, studies are being conducted to identify potentially safe and effective natural products with no side effects (5,6).
Among such natural sources, D. morbifera, which means ‘omnipotent drug (Panax)’, has been called a panacea, and has been the subject of various studies investigating medicinal plants (20–24). As a result, 32 different substances have been identified in D. morbifera. Extracts from D. morbifera leaves are known to activate B- and T-cells (20,23), inhibit the synthesis of melanin, have antioxidant properties, and protect and whiten skin (24). Although D. morbifera has exhibited potential anti-inflammatory effects, few studies have been conducted to identify a clear mechanism relevant to AD. The present study investigated the effects of DPL extract on the production of NO, cytokine secretion and inflammation-associated protein expression in activated RAW264.7 macrophages by inducing inflammation with LPS, and investigated its anti-inflammatory effects in DNCB-induced AD.
Firstly, the toxicity of DPL was evaluated in RAW264.7 macrophages by MTT assay. No cytotoxicity was observed at a concentration of 500 µg/ml. This has already been reported (32), and further experiments were conducted at concentrations of 400 µg/ml or less.
NO, a known indicator of the inflammatory response, is synthesized by NOS from L-arginine. There are three types of NOS: Endothelial, neuronal, and iNOS, and among these, NO produced by iNOS serves an important pathological role (13). In the present study, the NO production in LPS-induced RAW264.7 cells was significantly reduced by treatment with different concentrations of DPL. A previous study also demonstrated that NO expression was inhibited in extracts of D. morbifera stems (25). These results suggest that DPL extract inhibited the LPS-induced expression of NO in RAW264.7 cells.
iNOS and COX-2 serve important roles in the production of NO, and the regulation of iNOS and COX-2 expression is a known strategy for alleviating inflammatory diseases (14,15). In the present study, western blotting was performed to examine the expression of these proteins. The expression levels of iNOS and COX-2 were significantly increased in LPS-stimulated RAW264.7 cells when compared with untreated cells, whereas in the group treated with 200 and 400 µg/ml DPL the expression levels of iNOS and COX-2 were decreased. In particular, the expression of iNOS was decreased in a concentration-dependent manner. A previous study also reported significant inhibition of COX-2 and iNOS expression, consistent with the results obtained in the present study (33). These results suggest that DPL extracts may regulate the expression of the inflammatory mediators iNOS and COX-2, and regulate the expression of iNOS, thereby suppressing NO production and alleviating the inflammatory response.
The inflammatory cytokines TNF-α and IL-6 serve important roles in the inflammatory process, causing inflammation through trauma and stress, and regulating the expression of inflammatory mediators (34). In the present study, the expression of TNF-α and IL-6 was measured, revealing that TNF-α expression was significantly decreased with high concentrations of DPL, and the expression of IL-6 was decreased in a concentration-dependent manner when compared with RAW264.7 cells stimulated with LPS and DPL extract. Previous studies have also reported significantly reduced IL-6 expression following treatment with different concentrations of D. morbifera extract (20,25). These results indicate that DPL extract may inhibit the inflammatory response by regulating the expression of IL-6 in RAW264.7 cells stimulated with LPS.
TNF-α and IL-6 activate inflammation by activating NF-κB attached to IκB-α (11). Western blotting was performed to examine the activation of NF-κB and IκB-α. The induction of p-NF-κB appeared to be increased following treatment with LPS; by contrast, the induction of p-NF-κB was lower in the groups treated with 200 and 400 µg/ml DPL than in the group stimulated with LPS. Yu et al (34) demonstrated that olefolioside A, a component of the D. morbifera extract, inhibited the activation of NF-κB and IκB-α. These results suggest that DPL inhibited the activation of NF-κB, which induced an inflammatory response in RAW264.7 macrophages stimulated with LPS.
MAPK, a typical signaling molecule that affects the activation of NF-κB, is phosphorylated and activated in cells, and influences various inflammatory mediators. When inflammatory responses are induced, MAPK activators such as P38 and JNK are activated in macrophages (18). In the present study, RAW264.7 cells stimulated with LPS were treated with different concentrations of DPL extract and the activation of ERK, P38, and JNK was assessed. The expression of p-ERK, p-P38 and p-JNK was significantly decreased by 400 µg/ml DPL treatment. A previous study also revealed that D. morbifera extract modulates the expression of MAPK (34). These results suggest that DPL extract may modulate the signaling pathway of MAPK and inhibit the inflammatory response in LPS-stimulated macrophages.
BALB/c mice were treated with DNCB, which is known to induce inflammatory dermatitis, to induce AD. DPL extract was then applied to the ear and the thickness of the ear was assessed. Ear thickness was significantly lower in the DPL-treated group than in the group treated with DNCB. The ears in the DPL low-dose group exhibited a larger decrease in thickness than the medium- and high-dose groups. This suggested that the higher the concentration of DPL extract (the closer to undiluted solution), the higher the viscosity, which decreased its penetration; thus, groups treated with lower concentrations of DPL exhibited higher effects owing to the low viscosity. DPL was less effective than dexamethasone, which was used as a positive control. However, the dexamethasone groups observed cutaneous atrophy and erythema. This indicated that DPL may have potential for therapeutic use with fewer side effects (cutaneous atrophy and erythema) than dexamethasone with repeated use. There were significantly fewer morphological changes in the lymph nodes of the DPL-treated group than in the DNCB-treated group, and IgE was significantly decreased in the DPL-treated group. Additionally, morphological changes in the epidermis and corium of ear tissues, and the secretion of immune-activated mast cells were measured by H&E and TB staining. The ears of DNCB-treated mice were thicker than those of DPL-treated mice, and the mast cell distribution was significantly decreased in the DPL-treated group. Lee et al (35) reported that Dendropanax exhibited anti-inflammatory effects in pneumonia, and Jung et al (36) confirmed that Dendropanax relieves nephritis. These results suggest that DPL extract alleviated DNCB-induced atopic skin disease.
In conclusion, the present study investigated the anti-inflammatory effects of DPL extract. It was observed that D. morbifera regulated the expression of NO and IL-6, and the signaling pathway of NF-κB and MAPK in LPS-stimulated RAW264.7 macrophages in vitro. The present study also confirmed that DNCB-induced inflammatory dermatitis was mitigated by DPL in vivo, without side effects. These results suggest that DPL extract merits future research and development as a natural anti-inflammatory or functional drug. In addition, the investigation of the components of D. morbifera will be required in the future as well as studying the mechanisms underlying its anti-inflammatory effects.
Acknowledgements
Not applicable.
Funding
The present study was supported by a research grant from MBG group (Daejeon, Republic of Korea).
Availability of data and materials
Not applicable.
Authors' contributions
GSC, DPL, SMK, CHK and JYJ conceived and designed the experiments. GSC, ESY, SHK and JSW performed the experiments. GSC, DPL, ESY and JYJ analyzed the data. DPL, CHK, HJK and SMK contributed in analyzing the data and provided reagents and materials. GS wrote the manuscript.
Ethics approval and consent to participate
All animal experiments were performed with the approval of the Institutional Animal Care and Use Committee of Kong-Ju National University (approval no. KNU_2017-10; Yesan, Korea) and the institutional guidelines were adhered to.
Patient consent for publication
Not applicable.
Competing interests
The authors Dong-pyo Lim, Sae-man Kim and Chang-hyun Kim are all affiliated with MBG group (Daejeon, Republic of Korea); this company also provided financial support for the present study. The authors declare that they have no competing interests.
References
- 1.Urabe K, Aroca P, Tsukamoto K, Mascagna D, Paulumbo A, Prota G, Hearing VJ. The inherent cytotoxicity of melanin precursors: A revision. Biochim Biophys Acta 1221. 1994:272–278. doi: 10.1016/0167-4889(94)90250-x. [DOI] [PubMed] [Google Scholar]
- 2.Voss GT, Oliveira RL, de Souza JF, Duarte LFB, Fajardo AR, Alves D, Luchese C, Wilhelm EA. Therapeutic and technological potential of 7-chloro-4-phenylselanyl quinoline for the treatment of atopic dermatitis-like skin lesions in mice. Mater Sci Eng C Mater Biol Appl. 2018;84:90–98. doi: 10.1016/j.msec.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 3.Hwang SW, Kang JH, Seol JE, Seo JK, Lee DBR, Sung HS. The correlation between SCORAD index and instrumental assessment in evaluation of atopic dermatitis severity. Korean J Dermatol. 2010;48:266–271. (In Korean) [Google Scholar]
- 4.Leung DY. Pathogenesis of atopic dermatitis. J Allergy Clin Immun. 1999;104:S99–S108. doi: 10.1016/S0091-6749(99)70051-5. [DOI] [PubMed] [Google Scholar]
- 5.Leung V, Hartwell R, Yang H, Ghahary A, Ko F. Bioactive nanofibres for wound healing applications. Journal of Fiber Bioengineering and Informatics. 2011;4:1–14. doi: 10.3993/jfbi04201101. [DOI] [Google Scholar]
- 6.Guglielmetti S, Dart JK, Calder V. Atopic keratoconjunctivitis and atopic dermatitis. Curr Opin Allergy Clin Immunol. 2010;10:478–485. doi: 10.1097/ACI.0b013e32833e16e4. [DOI] [PubMed] [Google Scholar]
- 7.Kim BJ, Son WR, Choi MO, Jo SK, Jung HK, Lee JT, Kim HY, Kwoen DJ. Anti-atopic effects of Castanea crenata inner shell extracts fermented by Lactobacillus bifermentans. J Korean Soc Food Sci Nutr. 2013;42:1378–1386. doi: 10.3746/jkfn.2013.42.9.1378. [DOI] [Google Scholar]
- 8.Kang BK, Kim KBWR, Kim MJ, Bark SW, Pak WM, Kim BR, Ahn NK, Choi YU, Bae NY, Park JH, et al. Anti-atopic activity of tuna heart ethanol extract. J Korean Soc Food Sci Nutr. 2015;44:1–6. doi: 10.3746/jkfn.2015.44.1.001. [DOI] [Google Scholar]
- 9.Ferero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1beta generation. Clin Exp Immunol. 2007;147:227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zamora R, Vodovotz Y, Billiar TR. Inducible nitric oxide synthase and inflammatory diseases. Mol Med. 2000;6:347–373. doi: 10.1007/BF03401781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, Humpert PM, Chen J, Hong M, Luther T, Henle T, Klöting I, et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes. 2001;50:2792–2808. doi: 10.2337/diabetes.50.12.2792. [DOI] [PubMed] [Google Scholar]
- 12.Hattori Y, Hattori S, Sato N, Kasai K. High-glucose-induced nuclear factor kappaB activation in vascular smooth muscle cells. Cardiovasc Res. 2000;46:188–197. doi: 10.1016/S0008-6363(99)00425-3. [DOI] [PubMed] [Google Scholar]
- 13.Yun HY, Dawson VL, Dawson TM. Neurobiology of nitric oxide. Crit Rev Neurobiol. 1996;10:291–316. doi: 10.1615/CritRevNeurobiol.v10.i3-4.20. [DOI] [PubMed] [Google Scholar]
- 14.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. doi: 10.1096/fasebj.6.12.1381691. [DOI] [PubMed] [Google Scholar]
- 15.Seibert K, Zhang Y, Leahy K, Hauser S, Masferrer J, Perkins W, Lee L, Isakson P. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci USA. 1994;91:12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hippeli S, Elastner EF. Inhibition of biochemical model reactions for inflammatory processes by plant extracts: A review on recent developments. Free Radic Res. 1999;31(Suppl):S81–S87. doi: 10.1080/10715769900301361. [DOI] [PubMed] [Google Scholar]
- 17.Yoon YI, Chung MY, Hwang JS, Goo TW, Ahn MY, Lee YB, Han MS, Yun EY. Anti-inflammatory effect of Oxya chinensis sinuosa ethanol extract in LPS-induced RAW 264.7 cells. J Life Sci. 2014;24:370–376. doi: 10.5352/JLS.2014.24.4.370. [DOI] [Google Scholar]
- 18.Cobb MH. MAP kinase pathways. Prog Biophys Mol Biol. 1999;71:479–500. doi: 10.1016/S0079-6107(98)00056-X. [DOI] [PubMed] [Google Scholar]
- 19.Lee MK, Lee IS, Lee JS. For the utilization of native plant resources as high-value materials: Evaluation of demelanizing activity of Dendropanax morbifera in Bogildo. J Korean Island. 2013;25:227–240. (In Korean) [Google Scholar]
- 20.Lee SH, Lee HS, Park YS, Hwang B, Kim JH, Lee HY. Screening of immune activation activities in the leaves of Dendropanax morbifera Lev. Korean J Medicinal Crop Sci. 2002;10:109–115. (In Korean) [Google Scholar]
- 21.Castro Aceituno V, Ahn S, Simu SY, Wang C, Mathiyalagan R, Yang DC. Silver nanoparticles from Dendropanax morbifera Léveille inhibit cell migration, induce apoptosis, and increase generation of reactive oxygen species in A549 lung cancer cells. In Vitro Cell Dev Biol Anim. 2016;52:1012–1019. doi: 10.1007/s11626-016-0057-6. [DOI] [PubMed] [Google Scholar]
- 22.Choi SK. Growth characteristics of Dendropanax morbifera LEV. in Wando area of Korea. Korean J Crop Sci. 2003;48:434–437. (In Korean) [Google Scholar]
- 23.Park BY, Min BS, Oh SR, Kim JH, Kim TJ, Kim DH, Bae KH, Lee HK. Isolation and anticomplement activity of compounds from Dendropanax morbifera. J Ethnopharmacol. 2004;90:403–408. doi: 10.1016/j.jep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Park SA, Park J, Park CI, Jie YJ, Hwang YC, Kim YH, Jeon SH, Lee HM, Ha JH, Kim KJ, Park SN. Cellular antioxidant activity and whitening effects of Dendropanax morbifera leaf extracts. Korean J Microbiol Biotechnol. 2013;41:407–415. doi: 10.4014/kjmb.1311.11001. [DOI] [Google Scholar]
- 25.Im KJ, Jang SB, Yoo DY. Anti-cancer effects of Dendropanax Morbifera extract in MCF-7 and MDA-MB-231 cells. J Korean Obstet Gynecol. 2015;28:26–39. doi: 10.15204/jkobgy.2015.28.2.026. [DOI] [Google Scholar]
- 26.Lee HN, Shin SA, Choo GS, Kim HJ, Park YS, Kim BS, Kim SK, Cho SD, Nam JS, Choi CS, et al. Anti-inflammatory effect of quercetin and galangin in LPS-stimulated RAW264.7 macrophages and DNCB-induced atopic dermatitis animal models. Int J Mol Med. 2018;41:888–898. doi: 10.3892/ijmm.2017.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu YN, Zha WJ, Ma Y, Chen FF, Zhu W, Ge A, Zeng XN, Huang M. Galangin attenuates airway remodelling by inhibiting TGF-β1-mediated ROS generation and MAPK/Akt phosphorylation in asthma. Sci Rep. 2015;5:11758. doi: 10.1038/srep11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeong DH, Kim KB, Kim MJ, Kang BK, Ahn DH. Anti-inflammatory activity of methanol extract and n-hexane fraction mojabanchromanol b from Myagropsis myagroides. Life Sci. 2014;114:12–19. doi: 10.1016/j.lfs.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 29.Kim BA, Yang JC, Park CI. Effect of Hwangryunhaedok-tang extracts on DNCB-induced allergic contact dermatitis. Kor J Herbology. 2009;24:1–5. (In Korean) [Google Scholar]
- 30.Park SO, Park BS, Ryu CM, Ahn YS. Effect of herb extracts mixed with Houttuynia cordata on antiatopic dermatitis in DNCB-induced BALB/c mouse. J Kor Oil Chemists Soc. 2012;2:175–183. (In Korean) [Google Scholar]
- 31.Lim SJ, Kim M, Randy A, Nam EJ, Nho CW. Effects of Hovenia dulcis Thunb. extract and methyl vanillate on atopic dermatitis-like skin lesions and TNF-α/IFN-γ-induced chemokines production in HaCaT cells. J Pharm Pharmacol. 2016;68:1465–1479. doi: 10.1111/jphp.12640. [DOI] [PubMed] [Google Scholar]
- 32.Park SY, Karthivashan G, Ko HM, Cho DY, Kim J, Cho DJ, Ganesan P, Su-Kim I, Choi DK. Aqueous extract of Dendropanax morbiferus leaves effectively alleviated neuroinflammation and behavioral impediments in MPTP-induced parkinson's mouse model. Oxid Med Cell Longev 2018. 2018:3175214. doi: 10.1155/2018/3175214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyun TK, Ko YJ, Kim EH, Chung IM, Kim JS. Anti-inflammatory activity and phenolic composition of Dendropanax morbifera leaf extracts. Ind Crop Pro. 2015;74:263–270. doi: 10.1016/j.indcrop.2015.05.002. [DOI] [Google Scholar]
- 34.Yu HY, Kim KS, Lee YC, Moon HI, Lee JH. Oleifolioside A, a new active compound, attenuates LPS-Stimulated iNOS and COX-2 expression through the downregulation of NF-κB and MAPK activities in RAW 264.7 macrophages. Evid Based Complement Alternat Med 2012. 2012:637512. doi: 10.1155/2012/637512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JW, Ryu HW, Lee SU, Son TH, Park HA, Kim MO, Yuk HJ, Ahn KS, Oh SR. Protective effect of polyacetylene from Dendropanax morbifera Leveille leaves on pulmonary inflammation induced by cigarette smoke and lipopolysaccharide. J Fun Foods. 2017;32:358–366. doi: 10.1016/j.jff.2017.03.007. [DOI] [Google Scholar]
- 36.Jung HY, Kwon HJ, Hahn KR, Yoo DY, Kim W, Kim JW, Kim YJ, Yoon YS, Kim DW, Hwang IK. Dendropanax morbifera Léveille extract ameliorates cesium-induced inflammation in the kidney and decreases antioxidant enzyme levels in the hippocampus. Mol Cel Toxicol. 2018;14:193–199. doi: 10.1007/s13273-018-0021-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.