Abstract
Although ticagrelor has been demonstrated to possess an anti-atherosclerosis (AS) effect, its underlying mechanism remains unclear. In the present study, it was investigated whether ticagrelor reduces oxidized low-density lipoprotein (ox-LDL)-induced endothelial cell apoptosis, an initial step for the development of AS, and alleviates AS in apolipoprotein-E-deficient (ApoE−/−) mice by inhibiting the expression of proprotein convertase subtilisin/kexin type 9 (PCSK9). The human endothelial cell line EAhy926 was treated with ox-LDL, ox-LDL + ticagrelor (40 µmol/l) and ox-LDL + ticagrelor (60 µmol/l) for 24 h. Cell apoptosis was detected using Annexin V-fluorescein isothiocyanate/propidium iodide staining. The expression levels of PCSK9, apoptosis-associated proteins and signaling pathways were determined by reverse transcription-quantitative polymerase chain reaction and western blotting. ApoE−/− mice fed a high-fat diet were used to induce an AS model. After 20 weeks, ApoE−/− mice were randomly assigned to receive saline or ticagrelor intragastrically for 10 days. The formation of atherosclerotic plaques was detected by hematoxylin and eosin staining. The expression of PCSK9 in the arterial tissues was measured by immunohistochemistry. The results demonstrated that treatment with ticagrelor was able to decrease ox-LDL-induced apoptosis in a concentration-dependent manner (40 µmol/l vs. ox-LDL, 17.58±2.66 vs. 27.25±5.54%; 60 µmol/l vs. ox-LDL, 12.26±1.54 vs. 27.25±5.54%). The mRNA and protein expression level of PCSK9 significantly decreased following treatment with ticagrelor, accompanied with upregulation of B-cell lymphoma (Bcl) 2 and downregulation of Bcl-2 associated X, apoptosis regulator, caspase-3, p38, phosphorylated-(p) p38, p-c-Jun N-terminal kinases (JNK), p-extracellular signal-regulated kinases and the ratio of p-JNK to JNK. Histological analysis of arterial tissues revealed ticagrelor markedly decreased the atherosclerotic plaque area and inhibited the expression of PCSK9. The present results suggested that ticagrelor may alleviate AS via downregulation of PCSK9-mediated endothelial cell apoptosis, which may be JNK-dependent.
Keywords: ticagrelor, atherosclerosis, proprotein convertase subtilisin/kexin type 9, endothelial cells, apoptosis
Introduction
Atherosclerosis (AS) is one of the most prevalent diseases in elderly people. AS is characterized by the accumulation of lipids in the intima of large- and medium-sized arteries (1) (including coronary or carotid arteries); this leads to a compromised flow to target organs, resulting in hypoxic and ischemic cardiovascular events, including myocardial infarction and stroke, which are two leading causes of mortality worldwide (2,3). Therefore, the development of treatments to prevent the formation and control the progression of atherosclerotic lesions has attracted considerable attention.
Accumulating evidence has demonstrated that oxidized low-density lipoprotein-(ox-LDL)-induced endothelial dysfunction is the initial step in the development of AS (4). By binding to the lectin-like endothelial ox-LDL receptor, ox-LDL promotes the production of reactive oxygen species and activates the endoplasmic reticulum stress response, which sequentially phosphorylates the mitogen-activated protein kinase (MAPK) cascade and mediates nuclear factor (NF)-κB activation to upregulate the expression of pro-apoptotic proteins, including caspase-3, caspase-9, caspase-12 and Bcl-2 associated X, apoptosis regulator (Bax), ultimately leading to apoptosis of endothelial cells (4–6). Endothelial cell apoptosis may destroy the integrity of endothelium and increase vascular permeability, which facilitates the infiltration and deposition of local lipids within the arterial wall and finally results in atherogenesis (7). Furthermore, disruption of the endothelial lining may additionally cause the plaque instability and rupture that triggers the coagulation cascade and platelet aggregation, eventually increasing the formation of atherothrombosis and resulting in sudden mortality (8–10). Therefore, inhibition of endothelial dysfunction may represent a promising approach for the treatment of AS (11–13).
In addition to platelets, a previous study suggested that P2Y purinoreceptor 12 (P2Y12) receptors may additionally be highly expressed in endothelial cells of culprit coronary plaques (14). The use of the P2Y12 receptor antagonists clopidogrel or ticagrelor may improve endothelial dysfunction in patients with acute (15), and stable coronary artery disease (16,17) or pig models with coronary stent restenosis (18), suggesting that P2Y12 receptor blockers may be potential drugs for the treatment of AS. This hypothesis has been preliminarily verified by Preusch et al (19), which documented that treatment with ticagrelor induces a reduction in lesion size in the necrotic core area within the aortic sinus of apolipoprotein-E-deficient (ApoE−/−) mice (an AS animal model). In vitro studies additionally demonstrated that ox-LDL uptake and induced apoptosis of RAW264.7 macrophages are decreased following incubation with ticagrelor (19). Furthermore, Ren et al (20) identified that clopidogrel inhibits the progression of AS in a rabbit model by reducing the ratio of Bcl-2/Bax in the vascular wall. However, the anti-AS effect of P2Y12 receptor blockers remains rarely investigated. In addition, whether a reduction in endothelial cell apoptosis is involved and the mechanism of action have not been examined.
The aim of the present study was to further investigate the anti-AS effects in ApoE−/− mice and analyze the anti-apoptotic effects in the endothelial cell line EAhy926 following treatment with ticagrelor, a novel P2Y12 receptor inhibitor. In a previous study (21), it was observed that increased expression of proprotein convertase subtilisin/kexin type 9 (PCSK9), encoding a neural apoptosis-regulated convertase 1, is associated with ox-LDL-induced apoptosis in EAhy926 cells. Furthermore, downregulation of PCSK9 by short hairpin (sh)RNA inhibits apoptosis of EAhy926 cells and decreases levels of apoptosis-associated proteins (Bax and caspase-3) and the mitogen-activated protein kinase (MAPK) signaling pathway (21). Accordingly, it was hypothesized that the inhibition of PCSK9 expression may be a potential mechanism for ticagrelor to exert anti-AS effects. This has not been reported previously, to the best of the authors' knowledge, and was investigated in the present study.
Materials and methods
Cell culture and grouping
The human umbilical vein endothelial cell line EAhy926 was provided by the Pathology Laboratory of Tianjin Medical University (Tianjin, China). EAhy926 cells were maintained in Dulbecco's modified Eagle's medium (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum (HyClone; GE Healthcare Life Sciences, Logan, UT, USA), 100 U/ml penicillin G and 100 µg/ml streptomycin and cultured at 37°C in a humidified incubator with 5% CO2.
EAhy926 cells were divided into four groups; control, ox-LDL, ox-LDL + 40 µmol/l ticagrelor (T40) and ox-LDL + 60 µmol/l ticagrelor (T60). Oxidized LDL (50 µg/ml; Beijing Xinyuan Jiahe Biotech Co., Ltd., Beijing, China) was used to induce endothelial dysfunction of cells, as previously described (20). Cells in each group were treated for 24 h.
Apoptosis assay
The cultured cells were detached by trypsinization and stained with Annexin V labeled with fluorescein isothiocyanate (BD Pharmingen; BD Biosciences, San Jose, CA, USA) and propidium iodide (1 mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in the dark for 15 min at room temperature. Cells were subsequently analyzed using a flow cytometer (FACSCalibur; BD Biosciences) with CellQuest software (Version 6.0; BD Biosciences).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from EAhy926 cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), followed by RT into cDNA using an M-MLV RT system (Takara Biotechnology Co., Ltd., Dalian, China) with the reaction parameters of incubation at 65°C for 10 min, 42°C for 30 min and 70°C for 10 min. qPCR was performed using an ABI-7500 Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.) with a SYBR-Green PCR kit [Biocentury Transgene (China) Co., Ltd., Shenzhen, China]. The primers used are listed in Table I. The PCR reaction parameters were incubation at 95°C for 10 sec, then 40 cycles of 95°C for 10 sec, 58°C for 30 sec and 72 for 30 sec. The relative expression of genes, using a housekeeping gene (GAPDH) as an internal standard, was calculated by the 2−∆∆Cq method (22). All measurements were performed in triplicate.
Table I.
Primers used in the present study.
| Gene | Primer | Annealing temperature, °C | Product size, bp |
|---|---|---|---|
| GAPDH | 58 | 163 | |
| F | 5′-CACATGGCCTCCAAGGAGTA-3 | ||
| R | 5′-TCCCCTCTTCAAGGGGTCTA-3′ | 58 | |
| PCSK9 | 58 | 140 | |
| F | 5′-TGGAACTCACTCACTCTGGG-3′ | ||
| R | 5′-AAGAATCCTGCCTCCTTGGT-3′ | 58 | |
| Bax | 58 | 119 | |
| F | 5′-TGATCAGAACCATCATGGGC-3′ | ||
| R | 5′-GGACATCAGTCGCTTCAGTG-3′ | 58 | |
| Bcl-2 | 58 | 156 | |
| F | 5′-GAAGAAGCCACCCTCAAGC-3′ | 58 | |
| R | 5′-AGCAAGGACACCCGCACTC-3′ | ||
| Caspase-3 | 58 | 106 | |
| F | 5′-GAGGCCGACTTCTTGTATGC-3′ | ||
| R | 5′-GTTTCAGCATGGCACAAAGC-3′ | 58 | |
| NF-κB | 58 | 186 | |
| F | 5′-AGACAAATGGGCTACACCGA-3′ | ||
| R | 5′-AAAGCTGAGTTTGCGGAAGG-3′ | 58 |
F, forward; R, reverse; PCSK9, proprotein convertase subtilisin/kexin type 9; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2 associated X, apoptosis regulator; NF-κB, nuclear factor-κB.
Western blotting
Proteins were extracted from EAhy926 cells using a radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Haimen, China). Protein concentrations were measured using a 2-D Quant kit (GE Healthcare Life Sciences, Little Chalfont, UK) and equal amounts of protein (30 µg) from each group were separated by SDS-PAGE on 10% gels prior to being transferred to polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). Subsequent to blocking with 5% non-fat milk at 4°C for 1 h, membranes were probed with anti-PCSK9, caspase-3, Bax, Bcl-2, p38, phosphorylated (p)-p38, extracellular signal-regulated kinase (ERK), p-ERK, c-Jun N-terminal kinases (JNK), p-JNK or β-actin primary antibodies at 4°C overnight. Membranes were subsequently incubated with horseradish peroxidase-conjugated secondary antibodies at room temperature for 1 h. The protein bands were visualized with an enhanced chemiluminescence reagent (EMD Millipore). β-actin was used as an internal control. Detailed information regarding the antibodies used is listed in Table II. Densitometry was performed using Image-Pro Plus software (Version 6.0; Media Cybernetics, Rockville, MD, USA).
Table II.
Antibody details.
| Primary antibody | Secondary antibody | |||||||
|---|---|---|---|---|---|---|---|---|
| Antibody | Supplier | Catalog no. | Dilution | Species raised | Supplier | Catalog no. | Dilution | Molecular weight, kDa |
| PCSK9 | Sigma-Aldrich | SAB1302902 | 1:500 | Rabbit | Abcam | ab191866 | 1:2,000 | 72 |
| Caspase-3 | Abcam | ab32042 | 1:100 | Rabbit | Abcam | ab191866 | 1:5,000 | 32 |
| Bax | Abcam | ab32503 | 1:5,000 | Rabbit | Abcam | ab191866 | 1:10,000 | 21 |
| Bcl-2 | Abcam | ab32124 | 1:1,000 | Rabbit | Abcam | ab191866 | 1:5,000 | 26 |
| p38 | Abcam | ab27986 | 1:1,000 | Rabbit | Abcam | ab191866 | 1:10,000 | 38 |
| p-p38 | Abcam | ab4822 | 1:1,000 | Rabbit | Abcam | ab191866 | 1:10,000 | 38 |
| ERK | Abcam | ab17942 | 1:1,000 | Rabbit | Abcam | ab191866 | 1:5,000 | 42-44 |
| p-ERK | Abcam | ab214362 | 1:1,000 | Rabbit | Abcam | ab191866 | 1:5,000 | 42-44 |
| JNK | Abcam | ab179461 | 1:1,000 | Rabbit | Abcam | ab191866 | 1:5,000 | 46 |
| p-JNK | Abcam | ab124956 | 1:1,000 | Rabbit | Abcam | ab191866 | 1:5,000 | 46 |
| β-actin | Abcam | ab8226 | 1:10,000 | Mouse | Abcam | ab131368 | 1:10,000 | 43 |
Sigma-Aldrich; Merck KGaA Darmstadt, Germany and Abcam, Cambridge, UK. PCSK9, proprotein convertase subtilisin/kexin type 9; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2 associated X, apoptosis regulator; p-, phosphorylated; ERK, extracellular signal-regulated kinases; JNK, c-Jun N-terminal kinases.
Animal model
All animal experiments conformed with the Regulation of Animal Care Management of the Ministry of Public Health, People's Republic of China and were approved by the Ethical Committee of Second Hospital of Tianjin Medical University (Tianjin, China).
Six-week-old male weighing 20–25 g C57BL/6 mice (n=10) and homozygous ApoE−/− mice (n=10) were purchased from Beijing University (Beijing, China). The mice were housed in a specific pathogen-free facility under controlled conditions (temperature, 22±2°C; relative humidity, 55±15%; noise, <60 dB; light/dark cycle, 12/12 h) and were used following one week of accommodation. All animals were provided with free access to water/food. ApoE−/− mice were fed with a high-fat diet containing 0.25% cholesterol and 15% cocoa butter (Hunan Huakang Biotech Inc., Hunan, China) to induce the formation of the atherosclerotic plaques. Normal C57BL/6 mice were given standard chow throughout the experiment to serve as a control. After 20 weeks of feeding, ApoE−/− mice were randomly divided into the model group (AS, n=5) and treatment group (ticagrelor, n=5). Mice in the treatment group received ticagrelor (100 mg/kg, corresponding to concentration T60 in vitro) through intragastric administration for 10 consecutive days, while an equal amount of saline was intragastrically administered to AS and control mice in the same period.
Biochemical analysis
Mice in each group were anesthetized with 5% pentobarbital sodium (50 mg/kg) by intraperitoneal injection and blood was collected through direct cardiac puncture. The samples were subsequently centrifuged at 1,200 × g for 10 min at room temperature to obtain the plasma for biochemical measurements. The plasma levels of triglyceride (TG), cholesterol (TC), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) were determined using an automatic biochemical analyzer (Olympus AU5400; Olympus Corporation, Tokyo, Japan).
Histological analysis
Following cardiac exsanguination, the aorta were quickly harvested, fixed in 4% paraformaldehyde at 4°C for 24 h, dehydrated in a graded series of alcohol (70, 80, 90, 95 and 100%, each 90 min), embedded in 5-µm-thick paraffin and stained with hematoxylin (5 min) & eosin (5 min) at room temperature. Images were captured using a light microscope (OLYMPUS X81; Olympus Corporation) at magnification, ×10 and ×40.
Immunohistochemical staining
The embedded tissue sections were incubated overnight at 4°C with a primary antibody against PCSK9 (1:200; Sigma-Aldrich; Merck KGaA) following a rehydration in a graded series of alcohol (100, 95, 80, and 70%, each 5 min), hydrogen peroxide-induced endogenous peroxidase activity inhibition, microwave-based antigen retrieval (121°C for 10 min) and non-specific binding blocked with 10% normal goat serum (Invitrogen; Thermo Fisher Scientific, Inc.; at room temperature, 10 min). Following three washes in sterile PBS, the sections were incubated with the secondary antibody (1:500; Sigma-Aldrich; Merck KGaA) at room temperature for 30 min. The immunohistochemical reaction color was developed with diaminobenzidine (Vector Laboratories, Inc., Burlingame, CA, USA; 2–8 min) and counterstained with hematoxylin (2 min) at room temperature. The expression of PCSK9 in the tissues was viewed under a light microscope (Olympus X81; Olympus Corporation) at magnification, ×10 and ×40 and semi-quantitatively measured using Image Pro Plus software (Version 6.0; Media Cybernetics, Inc.).
Statistical analysis
Statistical analyses were performed using SPSS software (Version 18.0; SPSS Inc., Chicago, IL, USA). Data are expressed as the mean ± standard error. of three independent repeats. The significant difference between two experimental groups was analyzed using Student's t-test; however, statistical significance of differences among three groups were assessed using one-way analysis of variance followed by the Least Significant Difference post hoc test for multiple comparisons. P<0.05 was considered to indicate a statistically significant difference.
Results
Ticagrelor inhibits ox-LDL-induced apoptosis in EAhy926 cells
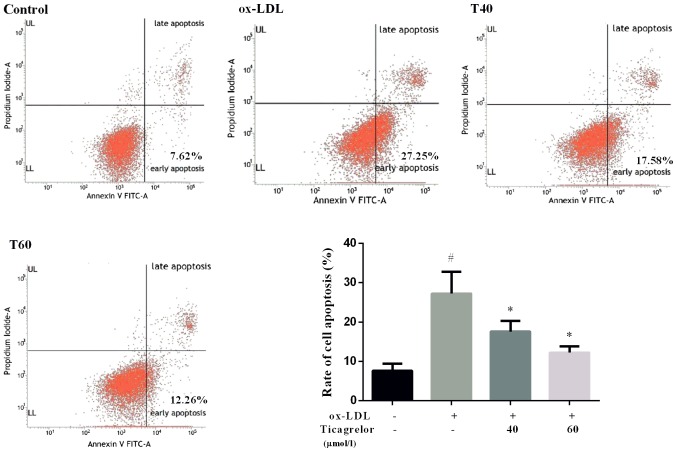
Damage in the vascular endothelium has been suggested as an initial step in the pathogenesis of AS (4). To determine whether ticagrelor alleviates AS, the effect of ticagrelor on ox-LDL-induced endothelial dysfunction was evaluated. The present data demonstrated that treatment with 50 µg/ml ox-LDL resulted in a significant increase in apoptosis of EAhy926 cells compared with the control (27.25±5.54 vs. 7.62±1.76%; P<0.05; Fig. 1). However, the addition of ticagrelor was able to decrease ox-LDL-induced apoptosis, particularly at a higher concentration (T40, 17.58±2.66%; T60, 12.26±1.54%; Fig. 1). Therefore, 60 µmol/l ticagrelor was used for subsequent experimentation.
Figure 1.
Apoptosis of human umbilical vein endothelial cell line EAhy926. *P<0.05 vs. ox-LDL; #P<0.05 vs. control. PI, propidium iodide; FITC, fluorescein isothiocyanate; ox-LDL, oxidized low-density lipoprotein; T40, 40 µmol/l ticagrelor; T60, 60 µmol/l ticagrelor; UL, upper left; LL, lower left.
Ticagrelor downregulates PCSK9 expression and downstream apoptosis pathways in EAhy926 cells
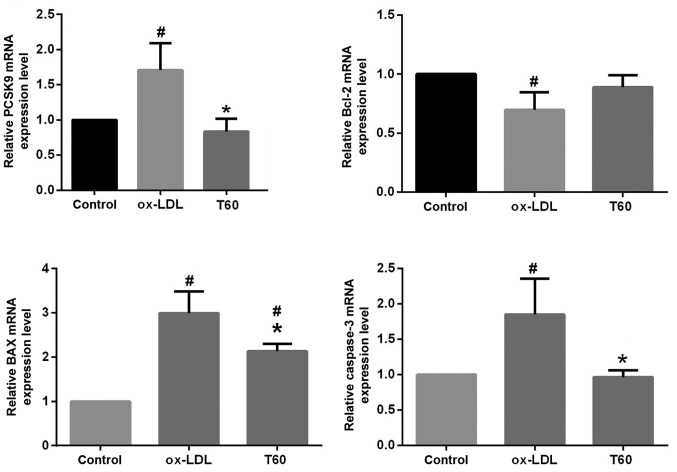
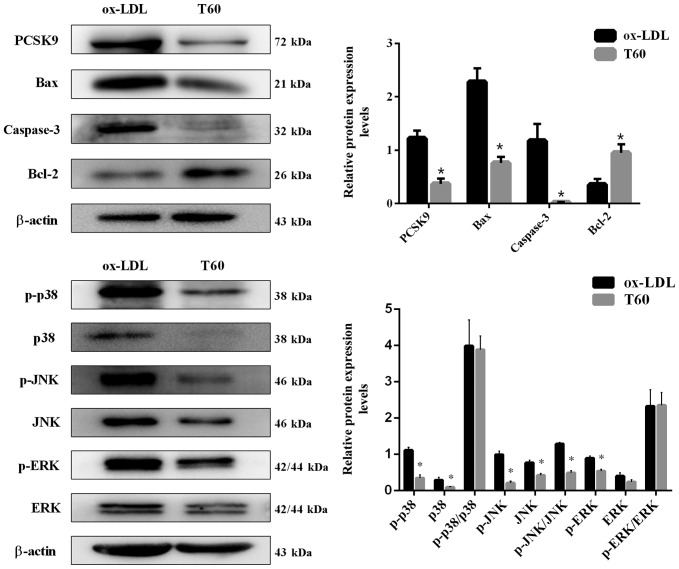
To elucidate the mechanisms by which ticagrelor inhibits endothelial apoptosis, the expression of PCSK9 and downstream apoptosis pathway genes were additionally evaluated. As expected, the mRNA expression level of PCSK9 determined by RT-qPCR was significantly decreased following treatment with ticagrelor (P<0.05; Fig. 2). The use of ticagrelor additionally upregulated mRNA expression of the anti-apoptotic factor Bcl-2 and significantly downregulated the expression of the pro-apoptotic factors Bax and caspase-3 (Fig. 2; P<0.05). This result was similarly observed in the analysis of protein expression levels by western blotting (Fig. 3). Furthermore, the expression levels of apoptosis pathway proteins, including p-p38, p-JNK, JNK and p-ERK, were significantly suppressed following treatment with ticagrelor; the JNK pathway may be of particular importance, as difference between the p-JNK/JNK ratios was statistically significant (P<0.05; Fig. 3). These results suggested that ticagrelor reduced ox-LDL-induced endothelial apoptosis by downregulating PCSK9 and downstream apoptosis pathways.
Figure 2.
Relative mRNA expression levels of PCSK9 and apoptosis-associated genes (Bax, Bcl-2 and caspase-3) detected by reverse-transcription quantitative polymerase chain reaction. All results are expressed as the mean ± standard error. *P<0.05 vs. respective ox-LDL; #P<0.05 vs. respective control. Ox-LDL, oxidized low-density lipoprotein; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2 associated X, apoptosis regulator; PCSK9, proprotein convertase subtilisin/kexin type 9; T40, 40 µmol/l ticagrelor; T60, 60 µmol/l ticagrelor.
Figure 3.
Protein expression levels of PCSK9, apoptosis-associated genes (Bax, Bcl-2 and caspase-3) and pathways (JNK, p38 and ERK) detected by western blotting. All results are expressed as the mean ± standard error. *P<0.05 vs. respective ox-LDL. Ox-LDL, oxidized low-density lipoprotein; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2 associated X, apoptosis regulator; PCSK9, proprotein convertase subtilisin/kexin type 9; T60, 60 µmol/l ticagrelor; p, phosphorylated; JNK, c-Jun N-terminal kinases; ERK, extracellular signal-regulated kinases.
Intragastric administration of ticagrelor reduces AS development in ApoE−/− mice
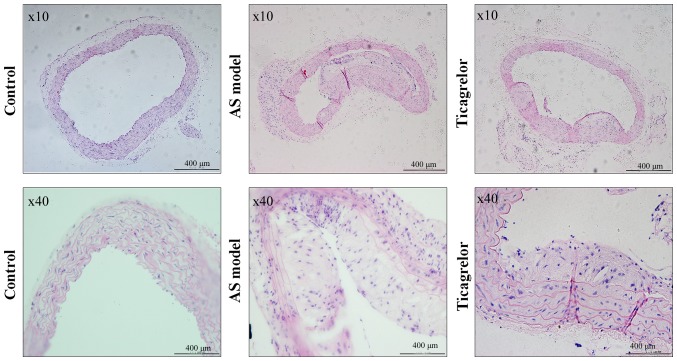
To further confirm the anti-atherosclerotic role and mechanisms of action of ticagrelor in vivo, an AS animal model, of ApoE−/− mice fed a high-fat diet, was constructed. The health condition of the majority of mice was excellent during the modeling, with no mortality observed in any group. Neither treatment affected food intake, and body weight increased in all groups after 20 weeks, particularly the AS and ticagrelor groups due to the high-fat diet provided (Table III). Unexpectedly, there was no significant difference in body weight between the AS and ticagrelor groups (Table III). Likewise, plasma concentrations of TC, HDL and LDL were equivalent in ApoE−/− mice irrespective of ticagrelor exposure (Table IV). These results suggested that ticagrelor had no effect on lipid metabolism. Histological analysis in the control group revealed that the vascular endothelium of the aorta was complete, smooth and arranged in order, without an increase in aortic arch inner membrane thickness and formation of plaques in the arteries (Fig. 4). However, the vascular endothelium was not intact and was even partly falling off in the AS model group. Additionally, abundant plaque formation and lipid deposition were observed in the lumen, accompanied by infiltration of numerous inflammatory cells (Fig. 4). Ticagrelor markedly decreased the atherosclerotic plaque area and increased the lumen area (Fig. 4), demonstrating the anti-AS effect of this drug.
Table III.
Body weight of control and apolipoprotein E-deficient mice.
| Group | 0 weeks, g | 20 weeks, g |
|---|---|---|
| Control (n=10) | 14.46±0.3779 | 29.19±0.5523a |
| AS model (n=5) | 14.84±0.6562 | 31.72±0.8672a,b |
| Ticagrelor (n=5) | 14.68±0.6160 | 31.04±0.4442a |
P<0.05 vs. respective 0 weeks
P<0.05 vs. control. AS, atherosclerosis.
Table IV.
Plasma lipids profile of control and apolipoprotein E-deficient mice.
| Group | TG, mmol/l | TC, mmol/l | HDL-C, mmol/l | HDL-L, mmol/l |
|---|---|---|---|---|
| Control (n=10) | 0.556±0.033 | 1.740±0.071 | 1.242±0.057 | 0.164±0.018 |
| AS model (n=5) | 1.220±0.129a | 19.56±1.892a | 2.860±0.246a | 6.210±0.560a |
| Ticagrelor (n=5) | 1.550±0.338a | 16.44±1.032a | 2.554±0.292a | 4.782±0.329a |
TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; AS, atherosclerosis.
P<0.05 vs. respective control.
Figure 4.
Hematoxylin-eosin staining of the cross section of aorta from control and ApoE−/− mice. Mice in the ticagrelor group received ticagrelor (100 mg/kg) through intragastric administration for 10 consecutive days. AS model was induced by feeding with a high-fat diet. AS, atherosclerosis; ApoE, apolipoprotein E.
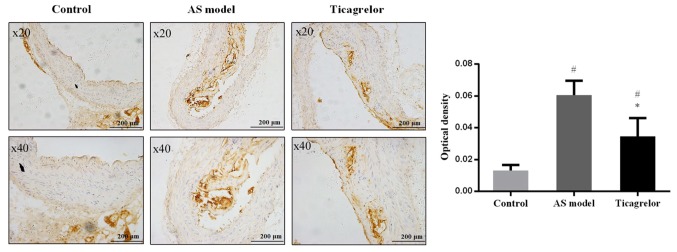
The aortas of the mice were additionally collected to analyze the expression of PCSK9 by immunohistochemistry to determine the association between PCSK9 and AS and the mechanism of action of ticagrelor. As anticipated, there were no brown-yellow particles (representing PCSK9 expression) around the blue nuclei in the control group. In contrast, PCSK9 was abundantly expressed in the aortic plaque of the AS group. Compared with the AS group, the expression level of PCSK9 was significantly decreased following treatment with ticagrelor (P<0.05; Fig. 5). These results further illustrated that downregulation of PCSK9 may be an important mechanism for ticagrelor to alleviate the formation and development of AS.
Figure 5.
Immunohistochemical analysis of proprotein convertase subtilisin/kexin type 9 protein in a cross-section of the aorta from control and apolipoprotein E-deficient mice. AS model was induced by feeding with a high-fat diet. Mice in the ticagrelor group received ticagrelor (100 mg/kg) through intragastric administration for consecutive 10 days. *P<0.05 vs. AS model; #P<0.05 vs. control. AS, atherosclerosis.
Discussion
Increasing evidence demonstrates that P2Y12 receptor antagonists may improve endothelial dysfunction in patients with acute (15), stable coronary artery disease (16,17) or pig models with coronary stent restenosis (18); however, the endothelial function in these studies was determined by measuring the vasomotor responses. The effects of P2Y12 receptor antagonists on endothelial cell apoptosis, a key event in the pathogenesis of AS, have not been investigated. In the present study, for the first time to the best of the authors' knowledge, the influence of ticagrelor, a relatively novel P2Y12 receptor inhibitor (23), on ox-LDL-induced apoptosis of the endothelial cell line EAhy926, was examined. As expected, the present results demonstrated that treatment with ticagrelor was able to decrease ox-LDL-induced apoptosis, particularly at higher doses (40 µmol/l vs. ox-LDL, 17.58±2.66 vs. 27.25±5.54%; 60 µmol/l vs. ox-LDL, 12.26±1.54 vs. 27.25±5.54%). This is in agreement with a previous study that demonstrated that clopidogrel reduces palmitic acid-induced apoptosis of human vascular endothelial cells (24). However, this finding may be attributed to the P2Y12 receptor expressed on endothelial cells (14) and to the interaction between the endothelium and platelets (25), as the P2Y12 receptor is primarily expressed in blood platelets (23). Ticagrelor may promote the degradation of adenine nucleotides released from platelets to adenosine (26) and thus inhibit ADP/ATP binding to endothelial P2Y receptors (13), blocking the production of pro-inflammatory factors (including nitric oxide and PGI2) (27,28) and the downregulation of nucleolin (29), which eventually suppresses cell cycle arrest in S phase and cell apoptosis and stimulates cell proliferation (29,30).
PCSK9 is a protein that was initially identified to be upregulated in apoptotic neural cells, and for this reason it was additionally termed neural apoptosis regulated convertase 1 (31,32). However, previous studies documented that PCSK9 is additionally involved in endothelial cell apoptosis. Knock-out of PCSK9 by small interfering RNA or shRNA inhibits ox-LDL-induced endothelial cell apoptosis and alleviates the formation of atherosclerotic plaques (21,33). Therefore, it was speculated that downregulation of PCSK9 may be an underlying mechanism for ticagrelor to inhibit endothelial cell apoptosis. This was demonstrated, for the first time to the best of the authors' knowledge, by the present results at the mRNA and protein expression level. Although the way in which ticagrelor regulates PCSK9 expression remains to be further elucidated, it is hypothesized it may serve as a link between PCSK9 and platelets (34). It has been demonstrated that increased PCSK9 serum levels are positively associated with the platelet count (r=0.218), plateletcrit (r=0.250) (35) and platelet reactivity (r=0.30) in patients with acute coronary syndrome (36). Therefore, the anti-platelet agent ticagrelor may influence PCSK9 expression by reducing platelets.
Caspase-3 and Bax are common genes that serve important roles in the process of apoptosis; whereas Bcl-2 exerts an anti-apoptotic effect via a mitochondria-dependent caspase-9 pathway. MAPKs are a group of associated serine-threonine protein kinases, which participate in the mediation of cell growth, apoptosis, proliferation and function. Therefore, these genes were predicted to be involved in ox-LDL-induced endothelial cell apoptosis and AS. This postulation has been confirmed by numerous studies. Chen et al (37) demonstrated that ox-LDL is able to induce endothelial cell apoptosis via a Bax-mitochondria-caspase protease pathway. Furthermore, Yu et al (38) demonstrated that ox-LDL activates the cell MAPKs, particularly JNK and p38. Therefore, inhibition of the expression of caspase-3, Bax and blocking of MAPK activation may protect endothelial cells against apoptosis. In our previous study, it was observed that shRNA-PCSK9 markedly inhibits the expression of pro-apoptotic proteins and promotes anti-apoptotic proteins accompanied with alteration in the phosphorylation of p38 and c-Jun N-terminal kinases (21). This was additionally concluded in the study conducted by Wu et al (33). Accordingly, it was hypothesized that suppression of these PCSK9-mediated apoptosis signaling pathways may be the inhibitory mechanism of ticagrelor for endothelial cell apoptosis and AS. Therefore, the expression levels of caspase-3, Bcl-2, Bax and MAPK were additionally analyzed following treatment with ticagrelor. In agreement with previous studies on anti-apoptosis drugs (6,38,39), the present results demonstrated that ticagrelor suppressed ox-LDL-induced p38MAPK, JNK, ERK activation (particularly JNK), caspase-3 and Bcl-2; however, upregulated Bax in EAhy926 cells.
In addition to in vitro experiments, an in vivo study was conducted. In accordance with previous studies, the present results demonstrated that the use of ticagrelor inhibited the progression of AS (20) and reduced the lesion size (19). Compared with the study of Preusch et al (19) who additionally used ApoE−/− mice, the present results may be of increased use to researchers as a high-fat diet was fed to the mice.
There are some limitations in the present study. First, further studies with quantitative assessment are still required to confirm the therapeutic effects of ticagrelor on the atherosclerotic plaque. Second, the MAPK agonists should be used to further validate the mechanisms of ticagrelor in AS.
In conclusion, the present results preliminarily demonstrated that ticagrelor may suppress ox-LDL-induced endothelial cell apoptosis and alleviate AS by downregulating PCSK9 and the downstream apoptotic MAPK signaling pathway. These results may provide a theoretical basis for the use of ticagrelor to treat patients with AS in a clinical setting.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant nos. 81570298 and 81700304), the Research Fund for Key Laboratory of Second Hospital of Tianjin Medical University (grant no. 2017ZDSYS02), and Youth Research Fund for Central Laboratory of Second Hospital of Tianjin Medical University (grant no. 2016ydey03).
Availability of data and materials
The datasets from current study are available from the corresponding author on reasonable request.
Authors' contributions
XX, XL and GL designed the study. XX and JL performed the experiments. SZ and JYL were involved in the statistical analyses. TL and MA participated in the interpretation of the data. XX, XL and GL wrote and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All animal experiments conformed with the Regulation of Animal Care Management of the Ministry of Public Health, People's Republic of China and were approved by the Ethical Committee of Second Hospital of Tianjin Medical University (Tianjin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Clarke R, Du H, Kurmi O, Parish S, Yang M, Arnold M, Guo Y, Bian Z, Wang L, Chen Y, et al. Burden of carotid artery atherosclerosis in Chinese adults: Implications for future risk of cardiovascular diseases. Eur J Prev Cardiol. 2017;24:647–656. doi: 10.1177/2047487317689973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barquera S, Pedroza-Tobías A, Medina C, Hernández-Barrera L, Bibbins-Domingo K, Lozano R, Moran AE. Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch Med Res. 2015;46:328–338. doi: 10.1016/j.arcmed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen M, Masaki T, Sawamura T. LOX-1, the receptor for oxidized low-density lipoprotein identified from endothelial cells: Implications in endothelial dysfunction and atherosclerosis. Pharmacol Ther. 2002;95:89–100. doi: 10.1016/S0163-7258(02)00236-X. [DOI] [PubMed] [Google Scholar]
- 5.Hong D, Bai YP, Gao HC, Wang X, Li LF, Zhang GG, Hu CP. Ox-LDL induces endothelial cell apoptosis via the LOX-1-dependent endoplasmic reticulum stress pathway. Atherosclerosis. 2014;235:310–317. doi: 10.1016/j.atherosclerosis.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 6.Bao MH, Zhang YW, Zhou HH. Paeonol suppresses oxidized low-density lipoprotein induced endothelial cell apoptosis via activation of LOX-1/p38MAPK/NF-κB pathway. J Ethnopharmacol. 2013;146:543–551. doi: 10.1016/j.jep.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Xu F, Sun Y, Chen Y, Sun Y, Li R, Liu C, Zhang C, Wang R, Zhang Y. Endothelial cell apoptosis is responsible for the formation of coronary thrombotic atherosclerotic plaques. Tohoku J Exp Med. 2009;218:25–33. doi: 10.1620/tjem.218.25. [DOI] [PubMed] [Google Scholar]
- 8.Durand E, Scoazec A, Lafont A, Boddaert J, Al Hajzen A, Addad F, Mirshahi M, Desnos M, Tedgui A, Mallat Z. In vivo induction of endothelial apoptosis leads to vessel thrombosis and endothelial denudation a clue to the understanding of the mechanisms of thrombotic plaque erosion. Circulation. 2004;109:2503–2506. doi: 10.1161/01.CIR.0000130172.62481.90. [DOI] [PubMed] [Google Scholar]
- 9.Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med. 2015;276:618–632. doi: 10.1111/joim.12296. [DOI] [PubMed] [Google Scholar]
- 10.Raskob GE, Angchaisuksiri P, Blanco AN, Büller H, Gallus A, Hunt BJ, Hylek EM, Kakkar TL, Konstantinides SV, Mccumber M, et al. Thrombosis: A major contributor to global disease burden. Semin Thromb Hemost. 2014;40:724–735. doi: 10.1055/s-0034-1390325. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Mu Q, Zhou Z, Song H, Zhang Y, Wu F, Jiang M, Wang F, Zhang W, Li L, et al. Protective effect of irisin on atherosclerosis via suppressing oxidized low density lipoprotein induced vascular inflammation and endothelial dysfunction. PLoS One. 2016;11:e0158038. doi: 10.1371/journal.pone.0158038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Hao Q, Wang YD, Wang WJ, Li DJ. Protective effects of dehydroepiandrosterone on atherosclerosis in ovariectomized rabbits via alleviating inflammatory injury in endothelial cells. Atherosclerosis. 2011;214:47–57. doi: 10.1016/j.atherosclerosis.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 13.Wang GF, Shi CG, Sun MZ, Wang L, Wu SX, Wang HF, Xu ZQ, Chen DM. Tetramethylpyrazine attenuates atherosclerosis development and protects endothelial cells from ox-LDL. Cardiovasc Drugs Ther. 2013;27:199–210. doi: 10.1007/s10557-013-6440-6. [DOI] [PubMed] [Google Scholar]
- 14.Lee CW, Hwang I, Park CS, Lee H, Park DW, Kang SJ, Lee SW, Kim YH Park SW, Park SJ. Comparison of differential expression of P2Y12 receptor in culprit coronary plaques in patients with acute myocardial infarction versus stable angina pectoris. Am J Cardiol. 2011;108:799–803. doi: 10.1016/j.amjcard.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Jeong HS, Hong SJ, Cho SA, Kim JH, Cho JY, Lee SH, Joo HJ, Park JH, Yu CW, Lim DS. Comparison of ticagrelor versus prasugrel for inflammation, vascular function, and circulating endothelial progenitor cells in diabetic patients with non-ST-segment elevation acute coronary syndrome requiring coronary stenting. JACC Cardiovasc Interv. 2017;10:1646–1658. doi: 10.1016/j.jcin.2017.05.064. [DOI] [PubMed] [Google Scholar]
- 16.Warnholtz A, Ostad MA, Velich N, Trautmann C, Schinzel R, Walter U, Munzel T. A single loading dose of clopidogrel causes dose-dependent improvement of endothelial dysfunction in patients with stable coronary artery disease: Results of a double-blind, randomized study. Atherosclerosis. 2008;196:689–695. doi: 10.1016/j.atherosclerosis.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Willoughby SR, Luu LJ, Cameron JD, Nelson AJ, Schultz CD, Worthley SG, Worthley MI. Clopidogrel improves microvascular endothelial function in subjects with stable coronary artery disease. Heart Lung Circ. 2014;23:534–541. doi: 10.1016/j.hlc.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Kim HK, Jeong MH, Lim KS, Kim JH, Lim HC, Kim MC, Hong YJ, Kim SS, Park KH, Chang KS. Effects of ticagrelor on neointimal hyperplasia and endothelial function, compared with clopidogrel and prasugrel, in a porcine coronary stent restenosis model. Int J Cardiol. 2017;240:326–331. doi: 10.1016/j.ijcard.2017.04.108. [DOI] [PubMed] [Google Scholar]
- 19.Preusch MR, Rusnak J, Staudacher K, Mogler C, Uhlmann L, Sievers P, Bea F, Katus HA, Blessing E, Staudacher I. Ticagrelor promotes atherosclerotic plaque stability in a mouse model of advanced atherosclerosis. Drug Des Devel Ther. 2016;10:2691–2699. doi: 10.2147/DDDT.S105718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren H, Li ML, Jiang J, Zhang Y, Zhang Y, Zhu X. Effects of clopidogrel on vascular proliferation and apoptosis in an atherosclerotic rabbit model. J Cardiovasc Pharmacol. 2010;55:617–624. doi: 10.1097/FJC.0b013e3181dc98dc. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Liang X, Wang Y, Xu Z, Li G. Investigation of highly expressed PCSK9 in atherosclerotic plaques and ox-LDL-induced endothelial cell apoptosis. Mol Med Rep. 2017;16:1817–1825. doi: 10.3892/mmr.2017.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Capodanno D, Dharmashankar K, Angiolillo DJ. Mechanism of action and clinical development of ticagrelor, a novel platelet ADP P2Yreceptor antagonist. Expert Rev Cardiovasc Ther. 2010;8:151–158. doi: 10.1586/erc.09.172. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Chen L, Li H, Yang J, Gong Z, Wang B, Zhao X. Clopidogrel reduces apoptosis and promotes proliferation of human vascular endothelial cells induced by palmitic acid via suppression of the long non-coding RNA HIF1A-AS1 in vitro. Mol Cell Biochem. 2015;404:203–210. doi: 10.1007/s11010-015-2379-1. [DOI] [PubMed] [Google Scholar]
- 25.Sachais BS. Platelet-endothelial interactions in atherosclerosis. Curr Atheroscler Rep. 2001;3:412–416. doi: 10.1007/s11883-001-0080-1. [DOI] [PubMed] [Google Scholar]
- 26.Nylander S, Femia EA, Scavone M, Berntsson P, Asztély AK, Nelander K, Löfgren L, Nilsson RG, Cattaneo M. Ticagrelor inhibits human platelet aggregation via adenosine in addition to P2Y12 antagonism. J Thromb Haemost. 2013;11:1867–1876. doi: 10.1111/jth.12360. [DOI] [PubMed] [Google Scholar]
- 27.Heitzer T, Rudolph V, Schwedhelm E, Karstens M, Sydow K, Ortak M, Tschentscher P, Meinertz T, Böger R, Baldus S. Clopidogrel improves systemic endothelial nitric oxide bioavailability in patients with coronary artery disease: Evidence for antioxidant and antiinflammatory effects. Arterioscler Thromb Vasc Biol. 2006;26:1648–1652. doi: 10.1161/01.ATV.0000225288.74170.dc. [DOI] [PubMed] [Google Scholar]
- 28.Gwozdz P, Csanyi G, Luzak B, Gajda M, Mateuszuk L, Chmura-Skirlinska A, Watala C, Chlopicki S. Endothelial dysfunction and circulating platelet activation in apoE/LDLR/-mice along the development of atherosclerosis. In: Conference on Frontiers in Cardiovascular Biology. Cardiovasc Res. 2010;87(Suppl):S94–S94. [Google Scholar]
- 29.Wang W, Luo J, Xiang F, Liu X, Jiang M, Liao L, Hu J. Nucleolin down-regulation is involved in ADP-induced cell cycle arrest in S phase and cell apoptosis in vascular endothelial cells. PLoS One. 2014;9:e110101. doi: 10.1371/journal.pone.0110101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coutinho-Silva R, Stahl L, Cheung KK, de Campos NE, de Oliveira Souza C, Ojcius DM, Burnstock G. P2X and P2Y purinergic receptors on human intestinal epithelial carcinoma cells: Effects of extracellular nucleotides on apoptosis and cell proliferation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1024–G1035. doi: 10.1152/ajpgi.00211.2004. [DOI] [PubMed] [Google Scholar]
- 31.Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S, Basak A, Prat A, Chrétien M. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): Liver regeneration and neuronal differentiation. Proc Natl Acad Sci USA. 2003;100:928–933. doi: 10.1073/pnas.0335507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kysenius K, Muggalla P, Mätlik K, Arumäe U, Huttunen HJ. PCSK9 regulates neuronal apoptosis by adjusting ApoER2 levels and signaling. Cell Mol Life Sci. 2012;69:1903–1906. doi: 10.1007/s00018-012-0977-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu CY, Tang ZH, Jiang L, Li XF, Jiang ZS, Liu LS. PCSK9 siRNA inhibits HUVEC apoptosis induced by ox-LDL via Bcl/Bax-caspase9-caspase3 pathway. Mol Cell Biochem. 2012;359:347–358. doi: 10.1007/s11010-011-1028-6. [DOI] [PubMed] [Google Scholar]
- 34.Gurbel PA, Navarese EP, Tantry US. Exploration of PCSK9 as a cardiovascular risk factor: Is there a link to the platelet? J Am Coll Cardiol. 2017;70:1463–1466. doi: 10.1016/j.jacc.2017.07.779. [DOI] [PubMed] [Google Scholar]
- 35.Li S, Zhu CG, Guo YL, Xu RX, Zhang Y, Sun J, Li JJ. The relationship between the plasma PCSK9 levels and platelet indices in patients with stable coronary artery disease. J Atheroscler Thromb. 2015;22:76–84. doi: 10.5551/jat.25841. [DOI] [PubMed] [Google Scholar]
- 36.Navarese EP, Kolodziejczak M, Winter MP, Alimohammadi A, Lang IM, Buffon A, Lip GY, Siller-Matula JM. Association of PCSK9 with platelet reactivity in patients with acute coronary syndrome treated with prasugrel or ticagrelor: The PCSK9-REACT study. Int J Cardiol. 2017;227:644–649. doi: 10.1016/j.ijcard.2016.10.084. [DOI] [PubMed] [Google Scholar]
- 37.Chen TG, Chen TL, Chang HC, Tai YT, Cherng YG, Chang YT, Chen RM. Oxidized low-density lipoprotein induces apoptotic insults to mouse cerebral endothelial cells via a Bax-mitochondria-caspase protease pathway. Toxicol Appl Pharmacol. 2007;219:42–53. doi: 10.1016/j.taap.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 38.Yu W, Ying H, Tong F, Zhang C, Quan Y, Zhang Y. Protective effect of the silkworm protein 30Kc6 on human vascular endothelial cells damaged by oxidized low density lipoprotein (Ox-LDL) PLoS One. 2013;8:e68746. doi: 10.1371/journal.pone.0068746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L, Jia YH, Zhao XS, Zhou FH, Pan YY, Wan Q, Cui XB, Sun XG, Chen YY, Zhang Y, Cheng SB. Trichosanatine alleviates oxidized low-density lipoprotein induced endothelial cells injury via inhibiting the LOX-1/p38 MAPK pathway. Am J Transl Res. 2016;8:5455–5464. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets from current study are available from the corresponding author on reasonable request.