Abstract
γδ T cells are a subset of unconventional T cells that serve a critical role in infectious diseases and various types of cancer. Cell therapy with genetically-modified γδ T cells is regarded as a promising tool for tumor treatment. However, since γδ T cells constitute a minority of T cells, their large-scale expansion is difficult to realize in an efficient and cost-effective manner. In the present study, based on previous studies, culture protocols for γδ T cells were tested using different combinations of isopentenyl pyrophosphate and interleukin 2 in order to satisfy different experimental purposes. One protocol was demonstrated to be the most suitable for lentiviral transduction. These results greatly reinforce the promising prospects of using γδ T cells in basic research and for clinical applications.
Keywords: γδ T cells, optimized culture, long-term culture, lentiviral transduction
Introduction
γδ T cells, a small population of unconventional or innate T cells, are characterized by the expression of γδ receptors (1). A variety of diseases such as tuberculosis (2), leprosy (3), typhoid fever (4), brucellosis (5), tularemia (6), ehrlichiosis (7), malaria (8) and toxoplasmosis (9) can stimulate the expansion of γδ T cells. γδ T cell receptors (TCRs) have various ligand-binding sites, allowing γδ T cells to recognize a broad range of pathogenic agents by common molecular patterns (10). They can kill target cells directly because of their cytotoxic activity or indirectly by influencing the activity of other immune cells (11). γδ T cells also exhibit antigen-presenting ability; in particular, blood Vγ9δ2 T cells are capable of responding to microbes, tumors as well as cluster of differentiation (CD)4+ and CD8+ T cells (12). Owing to these features shared by innate and adaptive immune cells, γδ T cells are defined as unconventional T cells (13). Vγ9δ2 T cells are a major subset in the peripheral blood (14), and can typically be activated by phosphomonoester antigen and expand in the blood of infected individuals (15). In previous studies, researchers have identified various characteristics of different γδ T cell subsets, particularly those of Vγ9δ2 T cells, which include immune defense capacity against tumors (16) and antiviral defense ability (17,18). In infectious diseases, Vγ9δ2 T cells are capable of recognizing phosphoantigens produced via the methylerythritol phosphate biochemical pathway of various bacteria, parasites and fungi, after which these cells become activated (13). Therefore, Vγ9δ2 T cells have been considered a promising candidate for immunotherapeutic drug development and represent a novel therapeutic tool (19). Consequently, a lot of research has focused on exploiting the potential of Vγ9δ2 T cells, by first trying to effectively expand this subset of cells. However, the initial proportion of γδ T cells in human peripheral blood mononuclear cells (PBMCs) is only 3–5% (20), making their expansion in vitro difficult, particularly during long-term culture.
There is still no widely accepted method for the specific expansion of γδ T cells. The different culture methods for γδ T cell expansion reported globally by different laboratories since 2000 are summarized in Table I. To yield abundant γδ T cells with high vitality, most researchers preferred to expand γδ T cells from PBMCs instead of purifying them prior to in vitro culture. This is reasonable because cell-cell contact is necessary (21) for the effective expansion of γδ T cells and less donor peripheral blood is required. It is well known that antigenic stimulant and cytokines are essential for γδ T cells (20). Multiple common antigens have been used to stimulate expansion of γδ T cells, including isopentenyl pyrophosphate (IPP) (22), (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP) (23), zoledronate (Zometa) (24) and bromohydrin pyrophosphate (BrHPP) (25). IPP is a natural antigen for Vγ9δ2 T cells, which can directly stimulate these cells in the absence of accessory antigen-presenting cells (22). Therefore, IPP has been chosen to expand γδ T cells in the present study and typical doses (Table I) were used. The other antigens can also effectively stimulate γδ T cells through various ways; for example, BrHPP is a synthetic analog of IPP and functions like IPP (26). Conversely, Zometa leads to the accumulation of IPP (1) and HMB-PP acts as an intermediate metabolite of microbial isoprenoid biosynthesis (1). These two agents are regarded as indirect or synthetic simulants of γδ T cells. In addition, interleukin 2 (IL-2) is an essential cytokine for maintaining T cell expansion and has been widely used in culture of γδ T cells (27). Considering the non-specific function of IL-2 in terms of T cell expansion, a step-wise increase in IL-2 concentration was tested for culture. Low dose IL-2 (100 U/ml) was used during the early stages of culture (0–5 days) until γδ T cells reached the logarithmic phase, allowing the other T cell subsets to die. Subsequently, the dose of IL-2 was increased to 1,000 U/ml to offer a better expansion environment for γδ T cells in the logarithmic phase. As a control, the effects of 1,000 U/ml IL-2 from start to finish were also detected. To understand the effects of various treatments on cell status, Vγ9δ2 T cell growth in different culture conditions was examined.
Table I.
Culture methods for γδ T cell expansion since 2000.
| Author, year | Cell source | Stimulant | Cytokines (concentration) | Relative increase in the number of cells (times) | Duration of culture (days) | Refs. |
|---|---|---|---|---|---|---|
| Duault et al, 2016 | PBMC | BrHPP | IL-2 (400 U/ml), IL-33 (100, 500 or 1,000 ng/ml) | 10-12 | 10 | (40) |
| Klimpel et al, 2003 | PBMC | None | IL-2 (100 U/ml), IL-15 (1,000 U/ml) | 10-12 | 8 | (41) |
| Kondo et al, 2011 | PBMC | Zoledronate (Zometa) | IL-2 (1,000 U/ml) | 12-16 | 14 | (24,42) |
| Sato et al, 2009 | ||||||
| Barcy et al, 2008 | PBMC | None | IL-2 (20 U/ml) IL-7 (100 U/ml) | 6-8 | 14 | (43) |
| Rincon-Orozco et al, 2005 | PBMC | IPP | IL-2 (100 U/ml) | – | 14 | (44) |
| Cabillic et al, 2010 | PBMC | BrHPP/Zoledronate (Zometa) | IL-2 (400 U/ml) | 12-14 | 14 | (45) |
| Casetti et al, 2009 | PBMC | IPP | IL-2 (6.5 U/ml), IL-15 (10 ng/ml), TNF-α (1.7 ng/ml) | 6-8 | 10 | (46) |
| Devilder et al, 2009 | PBMC | Phytohemagglutinin | None | 6-8 | – | (47) |
| Tsai et al 2015 | Magnetically isolated γδ T cells | None | None | – | – | (48) |
| McGill et al, 2016 | Magnetically isolated γδ T cells | None | IL-2 (10 U/ml) | 8-10 | – | (49) |
BrHPP, bromohydrin pyrophosphate; IL, interleukin; IPP, isopentenyl pyrophosphate; PBMC, peripheral blood mononuclear cell; TNF-α, tumor necrosis factor α.
Lentiviral transduction is a highly efficient method for genetic modification by integrating exogenous genes into host cells (28). Lentiviruses can infect non-dividing cells and are regarded as a powerful tool for basic research (28). HIV-1 pseudotypes with a protein coat of vesicular stomatitis virus glycoprotein, enables the lentivirus to be transduced into the majority of mammalian cells (29), regardless of cell cycle stage. However, artefacts caused by open reading frame (ORF) disruption or gene activation, can be introduced into the host cells, in knockdown studies or over-expression (28). Furthermore, depending on cell types the modulation efficiency of gene expression differs, particularly between primary cells and cell lines (28). Therefore, experiments should be carefully designed and a proper titration of the viral vector is necessary. Previously, given the low cell viability and the limitation of culturing time, transducing lentiviruses into primary T cells has been an issue for a number of researchers, especially in cell subsets that account for a small percentage of cells in PBMCs such as γδ T cells (28). To solve this problem, certain researchers have chosen to use CH-296 (30), a recombinant human fibronectin widely used in retroviral transduction, instead of lentiviruses. In order to achieve high transduction efficiency, the present study aimed to find the optimum transducing time and multiplicity of infection (MOI). Considering that the lentivirus generally needs 5–7 days to express completely, to guarantee that γδ T cells could withstand the damage caused by lentiviral transduction and remain active, cell growth was examined to find the appropriate time when the quantity and proportion of Vγ9δ2 T cells in PBMCs reached the highest level.
In the present study, optimal culture methods for Vγ9δ2 T cells were investigated in order to satisfy various experimental purposes. One protocol was confirmed to be suitable for genetic modification of Vγ9δ2 T cells by lentiviral transduction. The results provided effective and convenient methods to expand Vγ9δ2 T cells that fulfill various purposes for scientific and application studies.
Materials and methods
Donor samples
A total of 5 healthy volunteer donors without a history of autoimmune or other diseases were recruited from Nanfang Hospital between January 2016 and April 2018; their age ranged from 20–28 years old, and 3 of them were female and 2 male. The study was approved by the Ethics Committee of the Southern Medical University (Guangzhou, China). Prior to sample collection, written informed consent was obtained from all subjects. PBMCs were isolated from whole blood collected in K2 EDTA vacuum blood collection tubes (367525; BD Biosciences, Franklin Lakes, NJ, USA) using Ficoll-Hypaque (Axis-Shield Diagnostics Ltd., Dundee, UK) by density gradient centrifugation according to the manufacturer's protocols.
Flow cytometry
All cell numbers were accurately counted using flow cytometry according to the manufacturer's protocols. To analyze the proportion of Vγ9δ2 T cells, PBMCs were collected and stained at 4°C for 20 min in the dark with the following antibodies: Phycoerythrin (PE)-conjugated anti-γδ TCR antibody (12-9959-42; eBioscience; Thermo Fisher Scientific Inc., MA, USA) and allophycocyanin (APC)-conjugated anti-CD8 antibody (17-0087-42; eBioscience; Thermo Fisher Scientific Inc.). Briefly, the cells were first collected and counted. Then 1X PBS was used to wash the cells twice following which, they were incubated with 1,000-fold diluted antibody in 5% FBS PBS at 4°C for 20 min in the dark. The cells were then washed twice with 5% FBS PBS and resuspended in 1% paraformaldehyde (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), then immediately analyzed using flow cytometry (Attune NxT; Thermo Fisher Scientific Inc.) and FlowJo version 7.0 (FlowJo LLC, Ashland, OR, USA). As Vγ9δ2 T cells are CD4−CD8− T cells (27), Vγ9δ2 T cells were defined as γδ T+CD8− cells.
Cell lines and γδ T cells culture
293 cells (cat. no. CRL-11268) were purchased from the American Type Culture Collection (Manassas, VA, USA) and used for lentiviral packaging. The 293 cells were cultured in Dulbecco's modified Eagle's medium (Corning Incorporated, Corning, NY, USA) containing 10% fetal bovine serum (FBS; Corning Incorporated), 1% CTS™ GlutaMAX™-I (Gibco; Thermo Fisher Scientific Inc.) and 1% Minimum Essential Medium Non-Essential Amino Acids Solution (Gibco; Thermo Fisher Scientific Inc.) at 37°C with 5% CO2.
PBMCs were stimulated with different doses of IPP (Sigma-Aldrich; Merck KGaA) and recombinant human IL-2 (PeproTech, Inc., Rocky Hill, NJ, USA) in RPMI 1640 medium (Corning Incorporated) supplemented with 10% FBS at 37°C with 5% CO2. Briefly, PBMCs were isolated from whole blood of healthy donors and the proportion of Vγ9δ2 T cells was detected using flow cytometry. Subsequently, the cells were divided into four groups and exposed to different culture conditions, as described in Table II. During the 16 day culture period, the proportion of Vγ9δ2 T cells was monitored using flow cytometry and cell growth was observed under a fluorescence microscope (Nikon Corporation, Tokyo, Japan). γδ T cells were sorted from PBMCs using antiγδ TCR-labeled MACS magnetic beads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany).
Table II.
Expanding γδ T cells using different combinations of IPP and IL-2.
| IL-2 (U/ml) | |||
|---|---|---|---|
| Group | IPP (µg/ml) | Day 0–5 | Day 6–16 |
| a | 2 | 1,000 | 1,000 |
| b | 2 | 100 | 1,000 |
| c | 5 | 1,000 | 1,000 |
| d | 5 | 100 | 1,000 |
IL, interleukin; IPP, isopentenyl pyrophosphate.
Cell Counting Kit-8 (CCK-8) assay
Cell viability of Vγ9δ2 T cells in different culture conditions was assessed using the CCK-8 assay. Cells were seeded in 96-well plates (Shanghai ExCell Biology, Inc., Shanghai, China) at a density of 2.5×105 cells/100 µl/well and 10 µl/well CCK-8 solution (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added. Cells were incubated at 37°C with 5% CO2 for 4 h, then the supernatants were transferred to a new plate and the absorbance was measured at 450 and 630 nm using a Varioskan® Flash microplate reader (Thermo Fisher Scientific, Inc.). Cell viability was calculated as the optical density (OD) value at 450 nm divided by the OD value at the reference wavelength 630 nm.
Lentiviral packaging and transduction
The 293 cells were inoculated in T75 culture flasks (Nalge Nunc International, Penfield, NY, USA) at a density of 2×106 cells and allowed to reach 70–80% confluence the day prior to infection. The lentiviral plasmid pHAGE-fullEF1a-MCS-IZsGreen 6 µg, and packaging plasmids psPAX2 4.5 µg and pMD2.G 2.4 µg (all Invitrogen; Thermo Fisher Scientific Inc.) were transfected into 293 cells using X-tremeGENE™ HP DNA Transfection Reagent (Roche Applied Science, Mannheim, Germany) for 16 h at 37°C in 5% CO2, according to the manufacturer's protocol. Following 48–72 h, supernatants containing lentiviral particles were harvested and filtered through a 0.45-µm filter (EMD Millipore, Billerica, MA, USA) to remove cell debris. The supernatants were concentrated by ultracentrifugation at 50,000 × g at 4°C for 90 min, and the lentiviral particle pellet was resuspended in 100% FBS and stored at −80°C. The viral titers of concentrated lentiviral particles were measured by infecting 293 cells seeded at a density of 1×105 cells/well in a 12-well plate (Nalge Nunc International) with viral serial dilutions. Three days later, the green fluorescent protein (GFP) expression was detected using flow cytometry and the viral titer was calculated using the following equation: viral titer (Tu/µl) = (% GFP+ cells × number of cells transduced)/virus volume.
For lentiviral transduction, PBMCs stimulated with IPP and IL-2 were seeded at 1×106 cells/ml in 6-well plates (Nalge Nunc International), and the concentrated lentivirus was added at MOI=50 at 37°C and 5% CO2. The transduction was repeated 2–3 times a day. After 5–7 days culture, the cells were collected, and the expression of GFP and γδ TCR was detected using flow cytometry, as described above.
ELISA
Following lentiviral transduction, the cells were seeded into the 96-well plates (Nalge Nunc International, Penfield, NY, USA) for 18 h and the cell culture supernatant of lentivirus infected or uninfected Vγ9δ2 T cells was collected to detect secreted interferon gamma (IFN-γ) levels of using an ELISA kit (EH008-96; Shanghai ExCell Biology, Inc., Shanghai, China), according to the manufacturer's protocol.
Statistical analysis
All data are presented as the means ± standard deviation and are the results of 3 independent experiments. The IFN-γ secretion levels amongst different groups were analyzed using one-way analysis of variance followed by least significant difference post hoc test. All statistical analyses were performed using the SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). P-values were two-sided and P<0.05 was considered to indicate a statistically significant difference.
Results
Optimum culture conditions for γδ T cells
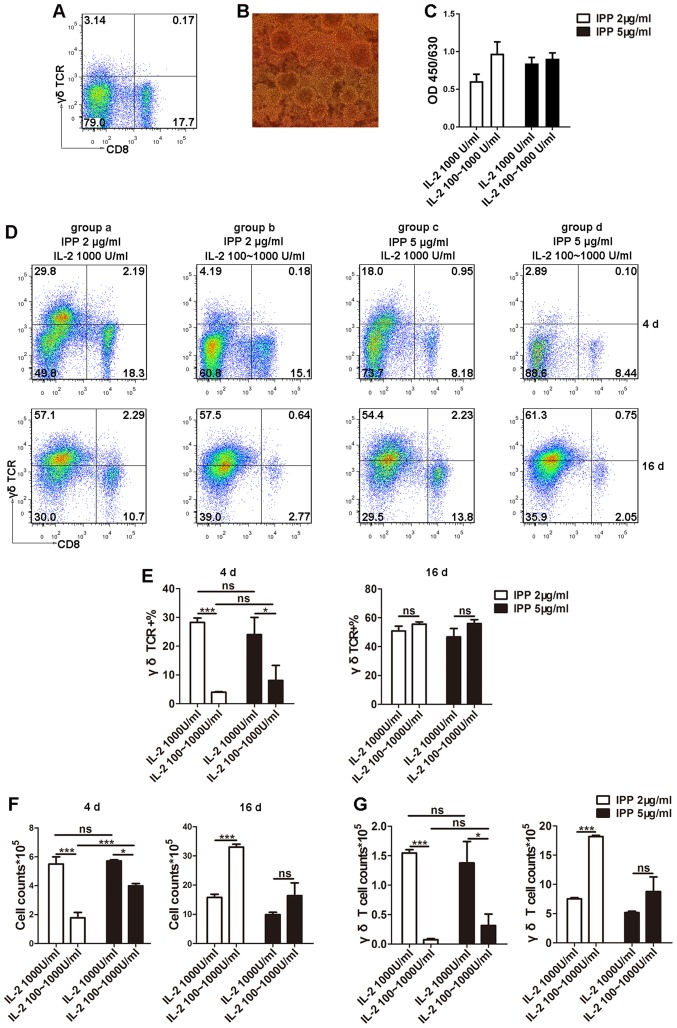
The primary proportion of Vγ9δ2 T cells in PBMCs (3.14%) was consistent with that observed in a previous study (26) using flow cytometry (Fig. 1A). Subsequently, different doses of IPP and IL-2 at separate stages (Table II) were tested in order to determine a suitable protocol for culturing Vγ9δ2 T cells. Following 3–6 days of culture, clonal clusters were observed (Fig. 1B), suggesting the start of the logarithmic growth phase. To quantify the viability of Vγ9δ2 cells, CCK-8 assays were performed and the results indicated no significant difference in cell viability amongst the four different groups (Fig. 1C). Furthermore, to determine whether different combinations of IPP and IL-2 could influence the culture conditions of γδ T cells during the early stages of culture and encourage entry into the logarithmic growth phase, the Vγ9δ2 T cell proportion was determined using flow cytometry (Fig. 1D and E) and the total number of cells in culture were counted (Fig. 1F). The results from day 4 revealed that groups a and c, which were exposed to high doses of IL-2 (1,000 U/ml) from the beginning of culture, possessed significantly higher total cell numbers and a higher proportion of Vγ9δ2 T cells compared to groups b and d. This in turn also resulted in a higher Vγ9δ2 T cell count (Fig. 1G). However, during the early stages of culture (0–5 days) there was no difference between high and low doses of IPP in terms of the proportion of Vγ9δ2 T cells (Fig. 1E). On day 16, the time period generally used for ex vivo T cell long-term culture (31,32), there was no difference in the proportion of Vγ9δ2 T cells amongst the four groups (Fig. 1E). However, group b, where cells were cultured in 2 µg/ml IPP plus 100 U/ml IL-2 at the early stage and 1,000 U/ml IL-2 at the later stage, demonstrated the highest total cell count at 16 days (Fig. 1F) and the highest number of Vγ9δ2 T cells (Fig. 1G; Table III). These results indicated that the group b protocol could maintain long-term culture of Vγ9δ2 T cells, which is necessary for genetic modification of T cells through lentiviral transduction because enough time is allowed for expression of exogenous genes. For this reason, the group b culture protocol was chosen to expand the Vγ9δ2 T cells for genetic modification.
Figure 1.
Optimization of culture conditions for γδ T cells. (A) Proportion of Vγ9δ2 T cells in PBMCs from healthy donors was determined using flow cytometry. (B) Following 3–6 days of culture, the clonal clusters of γδ T cells were observed using microscopy (magnification, ×20). (C) Cell viability was detected by Cell Counting Kit-8. (D and E) PBMCs were stimulated with different doses of IPP and IL-2, and the proportions of Vγ9δ2 T cells were detected using flow cytometry during the logarithmic growth phase (3–6 days) and the plateau phase (16 days). (F) Cell counts were determined to reflect the efficiency of γδ T cell expansion in different culture conditions. (G) From the total cell count and proportion of Vγ9δ2 T cells, the Vγ9δ2 T cell counts were calculated. Data are expressed as the mean ± standard deviation. *P<0.05, ***P<0.001. The experiments were repeated three times. CD, cluster of differentiation; IL-2, interleukin 2; IPP, isopentenyl pyrophosphate; ns, not significant; PBMC, peripheral blood mononuclear cell, TCR, T cell receptor.
Table III.
Summary of Vγ9δ2 T cells harvested under four different culture conditions and recommended application for the culture methods.
| Group | a | b | c | d |
|---|---|---|---|---|
| Initial seeding density (×105 cells) | 2.00 | 2.00 | 2.00 | 2.00 |
| Day 4 | ||||
| Total cell count (×105 cells) | 5.50±0.50 | 1.78±0.38 | 5.73±0.08 | 3.99±0.16 |
| Vγ9δ2 T cell (%) | 28.25±1.55 | 4.02±0.17 | 24±6.00 | 8.10±5.21 |
| Vγ9δ2 T cell count (×105 cells) | 1.55±0.06 | 0.07±0.02 | 1.38±0.33 | 0.31±0.19 |
| Day 16 | ||||
| Total cell count (×105 cells) | 15.8±1.10 | 33.00±1.00 | 9.90±0.80 | 16.40±4.40 |
| Vγ9δ2 T cell (%) | 47.75±6.76 | 55.15±4.39 | 52.50±18.09 | 53.25±4.40 |
| Vγ9δ2 T cell count (×105 cells) | 7.52±0.40 | 18.18±0.40 | 5.18±1.21 | 8.77±0.75 |
| Application | Short-term culture | Long-term culture | Unsuitable | Unsuitable |
Expansion of γδ T cells under optimum culture conditions for lentiviral infection
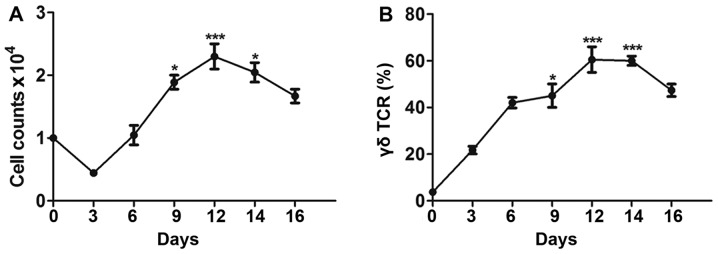
After determining the appropriate conditions for culturing Vγ9δ2 T cells, cell expansion was monitored in order to find the optimal time point for lentiviral transduction. Since a minimum of 5–7 days is required for expression of an exogenous gene using a recombinant lentivirus, long-term culture of Vγ9δ2 T cells is necessary. The culture condition with 2 µg/ml of IPP plus 100 U/ml IL-2 at the early stage and 1,000 U/ml IL-2 at the later stage (group b) was believed to be the most suitable for genetic modification. Therefore, the relative cell number and proportion of Vγ9δ2 T cells under this condition were determined every 3 days. As presented in Fig. 2A, during the first 3 days of culture, the number of PBMCs decreased greatly and only 50% of cells survived during this period. In line with this, a lot of cell debris was observed in the culture under the microscope. However, the proportion of Vγ9δ2 T cells increased gradually (Fig. 2B). On day 6, the cell count returned to the initial level, and the average proportion of Vγ9δ2 T cells reached ~40%. Clonal clusters were also observed under the microscope (data not shown). As the cells entered the logarithmic growth phase (6–12 days), both the cell count and the proportion of Vγ9δ2 T cells rapidly increased. During the mid-logarithmic growth phase (8–14 days), the culture contained the maximum cell count and highest proportion of Vγ9δ2 T cells, indicating the optimum time to perform subsequent experiments. Finally, during the later stages of culture (10–20 days), the plateau phase, the cell count and proportion of Vγ9δ2 T cells started to decrease.
Figure 2.
Expansion of γδ T cells under optimum culture conditions for lentiviral infection. (A) Total number of peripheral blood mononuclear cells was counted every 3 days and the highest number of total cells was observed between 8–14 days of culture. (B) Proportions of Vγ9δ2 T cells were determined using flow cytometry every 3 days and the highest proportion of Vγ9δ2 T cells was observed between 8–10 days. Data are expressed as mean ± standard deviation. *P<0.05, ***P<0.001 vs. Day 0. The experiments were repeated three times. TCR, T cell receptor.
Lentiviral transduction of γδ T cells
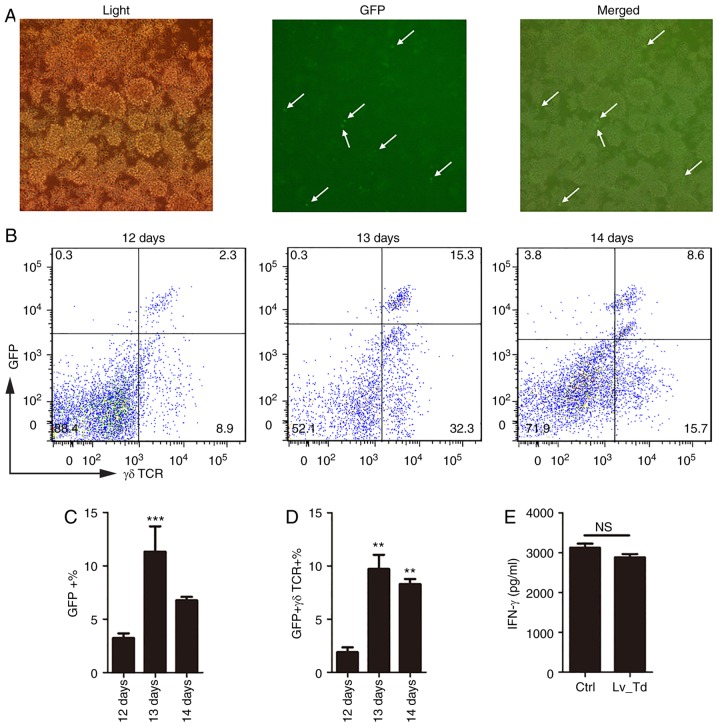
Lentiviral transduction is an efficient method for modulating gene expression that has been extensively used in life science research (28). However, the transduction protocol should be carefully designed to improve transduction efficiency, particularly for primary cells. According to the previous experiment, 8–10 days was selected as the optimum culture time for γδ T cells prior to treatment. Therefore, lentivirus was added to γδ T cells at this time point at an MOI=50, which has been demonstrated to be most suitable for primary T cells (33). The reporter GFP carried by the recombinant lentivirus was observed under a fluorescence microscope (Fig. 3A) and quantified using flow cytometry (Fig. 3B), 5–7 days following lentiviral transduction. The proportion of cells expressing both GFP and γδ TCR was daily detected between days 12–14 of culture. The results confirmed that 5 days following infection (the 13th day of the entire culturing period), there was a significantly higher expression of GFP (P<0.001) and γδ T cell expansion was influenced by the lentivirus but still maintained high proportion (Fig. 3B). To further investigate whether these γδ T cells were suitable for experimental purposes, γδ T cells were magnetically isolated and IFN-γ secretion was measured to confirm whether γδ T cells were able to retain their function following lentiviral transduction (Fig. 3E). According to the ELISA results (Fig. 3E), cells transfected with the lentivirus still secreted high levels of IFN-γ 5 days following lentiviral transduction, and there was no significant difference between the uninfected control group and the lentiviral transduction group. These results indicated that lentiviral transduction did not affect the IFN-γ secretion-ability of γδ T cells. Therefore, lentiviral infection did not appear to cause damage to cells, and the harvested cells may be used to perform subsequent functional experiments.
Figure 3.
Optimization of lentiviral transduction efficiency in γδ T cells. (A) GFP expression in cells was observed using a fluorescence microscope, 5–7 days following lentiviral vector transduction. (B-D) Proportions of γδ T cells expressing GFP were detected using flow cytometry, 5–7 days following lentiviral vector transduction. (C-D) The proportion of GFP positive, and GFP and γδ TCR double positive cells was used to select the most suitable time point for performing subsequent functional assays. (E) IFN-γ secretion was detected using ELISA to confirm functional activity of the cells. Data are expressed as mean ± standard deviation. **P<0.01, ***P<0.001 vs. 12 days. The experiments were repeated three times. GFP, green fluorescent protein; IFN-γ, interferon γ; Lv_Td, lentiviral transduction; NS, not significant; TCR, T cell receptor.
Discussion
Using different doses of IPP and IL-2, a set of methods for Vγ9δ2 T cell culture were established, for meeting different experimental requirements. A total of four different combinations of IPP and IL-2 tested in the present study could effectively stimulate the expansion of Vγ9δ2 T cells. Notably, following 16 days of culture, cell counts for the four different groups increased 3–10 fold and the proportion of Vγ9δ2 T cells was >50%. To quickly stimulate the expansion of Vγ9δ2 T cells or increase the rate of Vγ9δ2 T cells entering the logarithmic growth phase, a high dose of IL-2 (1,000 U/ml) was necessary, whereas 2 µg/ml IPP could be used for reasons of economy. The most suitable culture method for experiments requiring long-term culture of Vγ9δ2 T cells consisted of adding 2 µg/ml IPP plus 100 U/ml IL-2 at the early stage, and increasing to 1,000 U/ml IL-2 at the later stage. Under this culture condition, the largest number of Vγ9δ2 T cells could be harvested. This culture condition was optimal for long-term culture of Vγ9δ2 T cells, in order to satisfy the time restraints of lentiviral transduction. According to the growth pattern of Vγ9δ2 T cells under this culture condition, 8–10 days was considered the most appropriate timing to perform lentiviral transduction. Following 5 days of lentiviral transduction, the proportion of double-positive GFP and γδ TCR cells reached the highest level, and the harvested cells were still capable of secreting IFN-γ.
Effective expansion of Vγ9δ2 T cells is necessary in order to perform research on this subset of cells. IPP is regarded as a direct stimulant of Vγ9δ2 T cells and has been used in previous studies for the expansion of Vγ9δ2 T cells (Table I). In the present study, IPP was used to stimulate γδ T cells in PBMCs at a high (5 µg/ml) and low (2 µg/ml) dose, in combination with different concentrations of IL-2. Since Vγ9δ2 T cells are CD4−CD8− T cells (27), cells were labeled with CD8 fluorescent antibody to distinguish the Vγ9δ2 T cells from other subsets of γδ T cells. By comparing the four different culture conditions, it was observed that high doses of IL-2 at the early stage of γδ T cell culture led to an acceleration of γδ T cell expansion and increased the rate at which cells entered the logarithmic growth phase. The different doses of IPP did not result in any difference both in cell count and proportion of Vγ9δ2 T cells, but the combination of the high dose of IPP with low dose of IL-2 at the early stage was most effective at stimulating cell expansion. Studies on the basic function of Vγ9δ2 T cells, including cytotoxicity, cytokine and chemokine secretion, antigen presentation, and immunomodulatory activity (34–36) generally require quick and effective expansion of Vγ9δ2 T cells, suggesting that initial harvesting of sufficient Vγ9δ2 T cells is necessary. Therefore, as presented in Table III, the culture method in group a was regarded as the most suitable for experiments focusing on Vγ9δ2 T cell functional assays, as Vγ9δ2 T cells could expand 2–3 times during the 6–8-day culture period. The doses of IL-2 and IPP did not influence the final culture results in terms of Vγ9δ2 T cell proportion and T cell activity, indicating that all four methods could stimulate effective expansion of Vγ9δ2 T cells. However, in group b where 2 µg/ml IPP combined with 100 U/ml IL-2 was used at the early stage and 1,000 U/ml IL-2 at the later stage, more Vγ9δ2 T cells were harvested compared with the other groups. This may be because a low dose of IPP exerts less damage on the cells compared with a high IPP dose (37), while a low dose of IL-2 at the early stage causes other cell subsets to die, leading to less cell competition with γδ T cells. Studies investigating other aspects of Vγ9δ2 T cells, including Vγ9δ2 T cell growth and genetic modification, generally require a large number of cells or need longer incubation times. Therefore, for these experiments, the group b protocol is suitable, as the largest number of Vγ9δ2 T cells was harvested and long-term culture of cells was possible (Table III). Overall the results indicated two effective culture methods for Vγ9δ2 T cells expansion. For short-term expansion of Vγ9δ2 T cells (0–6 days), the most suitable culture method was adding 2 µg/ml IPP and 1,000 U/ml IL-2; while for long-term culture of Vγ9δ2 T cells to obtain a large number of cells, the most suitable culturing method was adding 2 µg/ml IPP and 100 U/ml IL-2 at the early stage and increasing the dose of IL-2 to 1,000 U/ml when the cells reached logarithmic growth phase (and clonal clusters could be observed).
Lentiviral transduction is the main method for genetic modification of primary cells and can be used in adoptive antigen-specific T cell therapy (38). A previous study demonstrated the anti-tumor effects of γδ T cells in various cancer types (39), leading to increasing interest in understanding the potential application of Vγ9δ2 T cells in adoptive T cell therapy, particularly in metastatic melanoma. Transferring adoptive T cells to cancer patients with tumor infiltrating lymphocytes expanded ex vivo may develop anti-tumor immune responses (36). γδ T cells with tumor-infiltrating ability are considered to be one of the most effective lymphocyte subsets because of their ability to kill tumor cells in various types of cancers, including leukemia, neuroblastoma and carcinomas (39). For these reasons, genetic modification of γδ T cells has become a promising research tool.
Unlike cell lines, primary cells, particularly γδ T cells, which account for a small percentage of PBMCs, are difficult to use for lentiviral transduction because of their sensitivity to cell death and limited proliferative capacity (28). Therefore, the experimental design should be carefully considered to improve transduction efficiency. In the present study, based on the different γδ T cell culture methods, an optimized lentiviral transduction protocol for γδ T cells was developed by investigating the optimum time for transduction. Since lentiviral transduction generally requires 5–7 days for expressing an exogenous gene, long-term culture of Vγ9δ2 T cells was necessary. Therefore, the group b culture method using 2 µg/ml IPP plus 100 U/ml IL-2, at the early stage and 1,000 U/ml IL-2 at the later stage was selected. Furthermore, by monitoring γδ T cells, it was identified that the mid-logarithmic growth phase (8–10 days) was when there was the greatest proportion of γδ T cells, indicating the most suitable time point to perform subsequent experiments. MOI=50 is suitable for T cell lentiviral transduction (35) because of the high transduction rate and relatively low toxicity. In the present study, the protocol for lentiviral transduction of γδ T cells resulted in a relatively high transduction ratio and low damage of cell function at the same time. On day 5 post-lentiviral transduction, the transduction rate reached the highest level, the proportion of Vγ9δ2 T cells remained high and the IFN-γ secretory function was unchanged.
In conclusion, Vγ9δ2 T cells exhibit distinct proliferation characteristics during ex vivo culture and studies using Vγ9δ2 T cells have to take this into account when designing experimental procedures. In the present study, two culture methods for expansion of Vγ9δ2 T cells were developed to satisfy various experimental purposes, using different combinations of IPP and IL-2 doses. However, these methods are only tailored for culturing these cells in the laboratory. If a larger scale culture of Vγ9δ2 T cells is required to meet the needs of clinical applications or bioengineering, further optimization of the methods is required. According to the long-term culture method and the growth of Vγ9δ2 T cells, lentiviral transduction was optimized for these cells. In the future, the genetic modification efficiency and functional effects of the modified cells may be explored, to better understand the application of these cells for biological therapy. The present study provided a set of optimized protocols for Vγ9δ2 T cell culture to fulfill various research purposes; therefore, it will greatly promote basic research and clinic application of γδ T cells.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural Science Foundation of China (grant nos. 81772150 and 81571951), Guangdong Natural Science Foundation (grant no. 2016A030311001), Science and Technology Project of Guangdong Province (grant no. 2017A020212007) and Science and Technology Project of Guangzhou (grant no. 201707010215).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
LM and RNW conceived and designed the experiments. WTH, JHY and RNW performed the experiments. RNW, QW, CYZ and WJX analyzed the data. LM provided reagents, materials and analysis tools. LM and RNW drafted the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Southern Medical University (Guangzhou, China). Prior to sample collection, written informed consent was obtained from all subjects.
Patient consent for publication
All healthy volunteers provided consent for publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Pang DJ, Neves JF, Sumaria N, Pennington DJ. Understanding the complexity of γδ T-cell subsets in mouse and human. Immunology. 2012;136:283–290. doi: 10.1111/j.1365-2567.2012.03582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balbi B, Moller DR, Kirby M, Holroyd KJ, Crystal RG. Increased numbers of T lymphocytes with gamma delta-positive antigen receptors in a subgroup of individuals with pulmonary sarcoidosis. J Clin Invest. 1990;85:1353–1361. doi: 10.1172/JCI114579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Modlin RL, Pirmez C, Hofman FM, Torigian V, Uyemura K, Rea TH, Bloom BR, Brenner MB. Lymphocytes bearing antigen-specific gamma delta T-cell receptors accumulate in human infectious disease lesions. Nature. 1989;339:544–548. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- 4.Hara T, Mizuno Y, Takaki K, Takada H, Akeda H, Aoki T, Nagata M, Ueda K, Matsuzaki G, Yoshikai Y. Predominant activation and expansion of V gamma 9-bearing gamma delta T cells in vivo as well as in vitro in Salmonella infection. J Clin Invest. 1992;90:204–210. doi: 10.1172/JCI115837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertotto A, Gerli R, Spinozzi F, Muscat C, Scalise F, Castellucci G, Sposito M, Candio F, Vaccaro R. Lymphocytes bearing the gamma delta T cell receptor in acute Brucella melitensis infection. Eur J Immunol. 1993;23:1177–1180. doi: 10.1002/eji.1830230531. [DOI] [PubMed] [Google Scholar]
- 6.Poquet Y, Kroca M, Halary F, Stenmark S, Peyrat MA, Bonneville M, Fournié JJ, Sjöstedt A. Expansion of Vgamma9 Vdelta2 T cells is triggered by Francisella tularensis-derived phosphoantigens in tularemia but not after tularemia vaccination. Infect Immun. 1998;66:2107–2114. doi: 10.1128/iai.66.5.2107-2114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caldwell CW, Everett ED, McDonald G, Yesus YW, Roland WE. Lymphocytosis of gamma/delta T cells in human ehrlichiosis. Am J Clin Pathol. 1995;103:761–766. doi: 10.1093/ajcp/103.6.761. [DOI] [PubMed] [Google Scholar]
- 8.Perera MK, Carter R, Goonewardene R, Mendis KN. Transient increase in circulating gamma/delta T cells during Plasmodium vivax malarial paroxysms. J Exp Med. 1994;179:311–315. doi: 10.1084/jem.179.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scalise F, Gerli R, Castellucci G, Spinozzi F, Fabietti GM, Crupi S, Sensi L, Britta R, Vaccaro R, Bertotto A. Lymphocytes bearing the gamma delta T-cell receptor in acute toxoplasmosis. Immunology. 1992;76:668–670. [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka Y, Sano S, Nieves E, De Libero G, Rosa D, Modlin RL, Brenner MB, Bloom BR, Morita CT. Nonpeptide ligands for human gamma delta T cells. Proc Natl Acad Sci USA. 1994;91:8175–8179. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viey E, Fromont G, Escudier B, Morel Y, Da Rocha S, Chouaib S, Caignard A. Phosphostim-activated gamma delta T cells kill autologous metastatic renal cell carcinoma. J Immunol. 2005;174:1338–1347. doi: 10.4049/jimmunol.174.3.1338. [DOI] [PubMed] [Google Scholar]
- 12.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science. 2005;309:264–268. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 13.Lawand M, Déchanet-Merville J, Dieu-Nosjean MC. Key features of gamma-delta T-cell subsets in human diseases and their immunotherapeutic implications. Front Immunol. 2017;8:761. doi: 10.3389/fimmu.2017.00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beetz S, Marischen L, Kabelitz D, Wesch D. Human gamma delta T cells: candidates for the development of immunotherapeutic strategies. Immunol Res. 2007;37:97–111. doi: 10.1007/BF02685893. [DOI] [PubMed] [Google Scholar]
- 15.Morita CT, Lee HK, Wang H, Li H, Mariuzza RA, Tanaka Y. Structural features of nonpeptide prenyl pyrophosphates that determine their antigenicity for human gamma delta T cells. J Immunol. 2001;167:36–41. doi: 10.4049/jimmunol.167.1.36. [DOI] [PubMed] [Google Scholar]
- 16.Kabelitz D, Wesch D, Pitters E, Zöller M. Potential of human gammadelta T lymphocytes for immunotherapy of cancer. Int J Cancer. 2004;112:727–732. doi: 10.1002/ijc.20445. [DOI] [PubMed] [Google Scholar]
- 17.Poccia F, Agrati C, Martini F, Capobianchi MR, Wallace M, Malkovsky M. Antiviral reactivities of gammadelta T cells. Microbes Infect. 2005;7:518–528. doi: 10.1016/j.micinf.2004.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poccia F, Battistini L, Cipriani B, Mancino G, Martini F, Gougeon ML, Colizzi V. Phosphoantigen-reactive Vgamma9Vdelta2 T lymphocytes suppress in vitro human immunodeficiency virus type 1 replication by cell-released antiviral factors including CC chemokines. J Infect Dis. 1999;180:858–861. doi: 10.1086/314925. [DOI] [PubMed] [Google Scholar]
- 19.Hiasa A, Nishikawa H, Hirayama M, Kitano S, Okamoto S, Chono H, Yu SS, Mineno J, Tanaka Y, Minato N, et al. Rapid alphabeta TCR-mediated responses in gammadelta T cells transduced with cancer-specific TCR genes. Gene Ther. 2009;16:620–628. doi: 10.1038/gt.2009.6. [DOI] [PubMed] [Google Scholar]
- 20.Puan KJ, Jin C, Wang H, Sarikonda G, Raker AM, Lee HK, Samuelson MI, Märker-Hermann E, Pasa-Tolic L, Nieves E, et al. Preferential recognition of a microbial metabolite by human Vgamma2Vdelta2 T cells. Int Immunol. 2007;19:657–673. doi: 10.1093/intimm/dxm031. [DOI] [PubMed] [Google Scholar]
- 21.Witkin AJ, Hsu J. Induction of gamma delta T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Retina. 2013;102:2310–2311. doi: 10.1182/blood-2003-05-1655. [DOI] [PubMed] [Google Scholar]
- 22.Poquet Y, Constant P, Halary F, Peyrat MA, Gilleron M, Davodeau F, Bonneville M, Fournié JJ. A novel nucleotide-containing antigen for human blood gamma delta T lymphocytes. Eur J Immunol. 1996;26:2344–2349. doi: 10.1002/eji.1830261011. [DOI] [PubMed] [Google Scholar]
- 23.Eberl M, Engel R, Beck E, Jomaa H. Differentiation of human gamma-delta T cells towards distinct memory phenotypes. Cell Immunol. 2002;218:1–6. doi: 10.1016/S0008-8749(02)00519-1. [DOI] [PubMed] [Google Scholar]
- 24.Kondo M, Izumi T, Fujieda N, Kondo A, Morishita T, Matsushita H, Kakimi K. Expansion of human peripheral blood γδ T cells using zoledronate. J Vis Exp. 2011;2:6–11. doi: 10.3791/3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Espinosa E, Belmant C, Pont F, Luciani B, Poupot R, Romagné F, Brailly H, Bonneville M, Fournié JJ. Chemical synthesis and biological activity of bromohydrin pyrophosphate, a potent stimulator of human gamma delta T cells. J Biol Chem. 2001;276:18337–18344. doi: 10.1074/jbc.M100495200. [DOI] [PubMed] [Google Scholar]
- 26.Gertner-Dardenne J, Bonnafous C, Bezombes C, Capietto AH, Scaglione V, Ingoure S, Cendron D, Gross E, Lepage JF, Quillet-Mary A, et al. Bromohydrin pyrophosphate enhances antibody-dependent cell-mediated cytotoxicity induced by therapeutic antibodies. Blood. 2009;113:4875–4884. doi: 10.1182/blood-2008-08-172296. [DOI] [PubMed] [Google Scholar]
- 27.Hintz M, Reichenberg A, Altincicek B, Bahr U, Gschwind RM, Kollas AK, Beck E, Wiesner J, Eberl M, Jomaa H. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human gammadelta T cells in Escherichia coli. FEBS Lett. 2001;509:317–322. doi: 10.1016/S0014-5793(01)03191-X. [DOI] [PubMed] [Google Scholar]
- 28.Shearer RF, Saunders DN. Experimental design for stable genetic manipulation in mammalian cell lines: Lentivirus and alternatives. Genes Cells. 2015;20:1–10. doi: 10.1111/gtc.12183. [DOI] [PubMed] [Google Scholar]
- 29.Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 30.Heemskerk MHM, Hagedoorn RS, van der Hoorn MAWG, van der Veken LT, Hoogeboom M, Kester MG, Willemze R, Falkenburg JH. Efficiency of T-cell receptor expression in dual-specific T cells is controlled by the intrinsic qualities of the TCR chains within the TCR-CD3 complex. Blood. 2007;109:235–243. doi: 10.1182/blood-2006-03-013318. [DOI] [PubMed] [Google Scholar]
- 31.Carlens S, Gilljam M, Chambers BJ, Aschan J, Guven H, Ljunggren HG, Christensson B, Dilber MS. A new method for in vitro expansion of cytotoxic human CD3-CD56+ natural killer cells. Hum Immunol. 2001;62:1092–1098. doi: 10.1016/S0198-8859(01)00313-5. [DOI] [PubMed] [Google Scholar]
- 32.da Silva RF, Petta CA, Derchain SF, Alici E, Guimarães F. Up-regulation of DNAM-1 and NKp30, associated with improvement of NK cells activation after long-term culture of mononuclear cells from patients with ovarian neoplasia. Hum Immunol. 2014;75:777–784. doi: 10.1016/j.humimm.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Zhou CY, Wen Q, Chen XJ, Wang RN, He WT, Zhang SM, Du XL, Ma L. Human CD8(+) T cells transduced with an additional receptor bispecific for both Mycobacterium tuberculosis and HIV-1 recognize both epitopes. J Cell Mol Med. 2016;20:1984–1998. doi: 10.1111/jcmm.12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eberl M, Hintz M, Reichenberg A, Kollas AK, Wiesner J, Jomaa H. Microbial isoprenoid biosynthesis and human gammadelta T cell activation. FEBS Lett. 2003;544:4–10. doi: 10.1016/S0014-5793(03)00483-6. [DOI] [PubMed] [Google Scholar]
- 38.van der Veken LT, Hagedoorn RS, van Loenen MM, Willemze R, Falkenburg JHF, Heemskerk MHM. Alphabeta T-cell receptor engineered gammadelta T cells mediate effective antileukemic reactivity. Cancer Res. 2006;66:3331–3337. doi: 10.1158/0008-5472.CAN-05-4190. [DOI] [PubMed] [Google Scholar]
- 39.Silva-Santos B, Serre K, Norell H. γδ T cells in cancer. Nat Rev Immunol. 2015;15:683–691. doi: 10.1038/nri3904. [DOI] [PubMed] [Google Scholar]
- 40.Duault C, Franchini DM, Familliades J, Cayrol C, Roga S, Girard JP, Fournié JJ, Poupot M. TCRVγ9 γδ T Cell Response to IL-33: A CD4 T Cell-Dependent Mechanism. J Immunol. 2016;196:493–502. doi: 10.4049/jimmunol.1500260. [DOI] [PubMed] [Google Scholar]
- 41.Klimpel GR, Matthias MA, Vinetz JM. Leptospira interrogans activation of human peripheral blood mononuclear cells: Preferential expansion of TCR gamma delta+ T cells vs TCR alpha beta+ T cells. J Immunol. 2003;171:1447–1455. doi: 10.4049/jimmunol.171.3.1447. [DOI] [PubMed] [Google Scholar]
- 42.Sato K, Kondo M, Sakuta K, Hosoi A, Noji S, Sugiura M, Yoshida Y, Kakimi K. Impact of culture medium on the expansion of T cells for immunotherapy. Cytotherapy. 2009;11:936–946. doi: 10.3109/14653240903219114. [DOI] [PubMed] [Google Scholar]
- 43.Barcy S, De Rosa SC, Vieira J, Diem K, Ikoma M, Casper C, Corey L. Gamma delta+ T cells involvement in viral immune control of chronic human herpesvirus 8 infection. J Immunol. 2008;180:3417–3425. doi: 10.4049/jimmunol.180.5.3417. [DOI] [PubMed] [Google Scholar]
- 44.Rincon-Orozco B, Kunzmann V, Wrobel P, Kabelitz D, Steinle A, Herrmann T. Activation of V gamma 9V delta 2 T cells by NKG2D. J Immunol. 2005;175:2144–2151. doi: 10.4049/jimmunol.175.4.2144. [DOI] [PubMed] [Google Scholar]
- 45.Cabillic F, Toutirais O, Lavoué V, de La Pintière CT, Daniel P, Rioux-Leclerc N, Turlin B, Mönkkönen H, Mönkkönen J, Boudjema K, et al. Aminobisphosphonate-pretreated dendritic cells trigger successful Vgamma9Vdelta2 T cell amplification for immunotherapy in advanced cancer patients. Cancer Immunol Immunother. 2010;59:1611–1619. doi: 10.1007/s00262-010-0887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Casetti R, Agrati C, Wallace M, Sacchi A, Martini F, Martino A, Rinaldi A, Malkovsky M. Cutting edge: TGF-beta1 and IL-15 Induce FOXP3+ gammadelta regulatory T cells in the presence of antigen stimulation. J Immunol. 2009;183:3574–3577. doi: 10.4049/jimmunol.0901334. [DOI] [PubMed] [Google Scholar]
- 47.Devilder MC, Allain S, Dousset C, Bonneville M, Scotet E. Early triggering of exclusive IFN-gamma responses of human Vgamma9Vdelta2 T cells by TLR-activated myeloid and plasmacytoid dendritic cells. J Immunol. 2009;183:3625–3633. doi: 10.4049/jimmunol.0901571. [DOI] [PubMed] [Google Scholar]
- 48.Tsai CY, Liong KH, Gunalan MG, Li N, Lim DS, Fisher DA, MacAry PA, Leo YS, Wong SC, Puan KJ, et al. Type I IFNs and IL-18 regulate the antiviral response of primary human γδ T cells against dendritic cells infected with Dengue virus. J Immunol. 2015;194:3890–3900. doi: 10.4049/jimmunol.1303343. [DOI] [PubMed] [Google Scholar]
- 49.McGill JL, Rusk RA, Guerra-Maupome M, Briggs RE, Sacco RE. Bovine gamma delta T Cells Contribute to exacerbated IL-17 production in response to co-infection with Bovine RSV and Mannheimia haemolytica. PLoS One. 2016;11:e0151083. doi: 10.1371/journal.pone.0151083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.