Abstract
The present study aimed to investigate the anti-arthritic effects of curculigoside isolated from the rhizome of Curculigo orchioides Gaertn in vivo and in vitro, as well as to determine the potential underlying mechanisms. A rat model of arthritis was induced with type II collagen. Arthritic rats were treated with curculigoside (50 mg/kg) and blood samples were collected to determine serum levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-10, IL-12 and IL-17A. Furthermore, indices of the thymus and spleen were determined. The anti-proliferative effects of curculigoside were detected with Cell Counting kit-8 assays in rheumatoid arthritis-derived fibroblast-like synoviocyte MH7A cells. In addition, expression levels of Janus kinase (JAK)1, JAK3, signal transducer and activator of transcription (STAT)3, nuclear factor (NF)-κB p65 and its inhibitor (IκB) were determined by western blotting. The results revealed that curculigoside inhibited paw swelling and arthritis scores in type II collagen-induced arthritic (CIA) rats. Additionally, curculigoside decreased serum levels of TNF-α, IL-1β, IL-6, IL-10, IL-12 and IL-17A in CIA rats. Curculigoside also significantly inhibited MH7A cell proliferation in a time and concentration-dependent manner. Furthermore, treatment downregulated the expression of JAK1, JAK3 and STAT3, and upregulated cytosolic nuclear factor (NF)-κB p65 and IκB. In conclusion, the results of the present study indicated that curculigoside exhibited significant anti-arthritic effects in vivo and in vitro, and the molecular mechanism may be associated with the JAK/STAT/NF-κB signaling pathway.
Keywords: curculigoside, anti-arthritic effects, type II collagen-induced arthritis rats, MH7A cells, Janus kinase/signal transducer and activator of transcription/nuclear factor-κB
Introduction
Curculiginis orchioides (C. orchioides) is the dried rhizome of the plant of Curculigo orchioides Gaertn., belonging to the Amaryllidaceae family (1). C. orchioides is predominantly found in the Sichuan, Guizhou, Yunnan and Guangxi provinces of China, and is a well-known traditional Chinese medicinal herb (2). It has long been used for the treatment of kidney disease, pain in the lumbar spine, frequent urination, arthralgia and myalgia (2). It has been reported that C. orchioides exhibits various pharmacological activities, including antioxidant, immunoenhancement and anti-osteoporotic effects, as well as promoting estrogen expression (3,4). In addition, it has been reported that C. orchioides contains a large number of chemical constituents, such as saponin, phenols and glycosides (2). Curculigoside (Fig. 1) is the main saponin in C. orchioides, and its content in C. orchioides varies from 0.11–0.35% (5). Numerous investigations have shown that curculigoside exerts significant antioxidant, anti-osteoporosis, antidepressant and neuroprotection effects (6). However, to the best of our knowledge, no studies have yet investigated the effects of curculigoside in rheumatoid arthritis (RA).
Figure 1.

Structure of curculigoside isolated from Curculigo orchioides.
RA is an autoimmune disease which results in chronic proliferative synovitis and inflammatory cell infiltration into the joint synovial tissue (7–9). Chronic inflammation in RA causes permanent joint destruction and deformity (10). Currently, RA treatment primarily includes a combination of patient education, rest and exercise, joint protection, medication and occasionally surgery (11). Medication for RA includes nonsteroidal anti-inflammatory and disease modifying antirheumatic drugs, as well as T-cell activation inhibitors, B-cell depleters, tumor necrosis factor (TNF)-α inhibitors, interleukin (IL)-6 inhibitors and Janus kinase (JAK) inhibitors.
Therefore, the present study was designed to systematically investigate the anti-inflammatory effects of curculigoside on rats with type II collagen-induced arthritis (CIA), and its antiproliferative effects against RA-derived fibroblast-like synoviocyte MH7A cells. Furthermore, its potential molecular effect mechanisms were explored, which may have significant value for further identifying useful agents from the plant of C. orchioides to treat diseases. Results from the present study may provide an important scientific basis for future studies on therapeutic applications.
Materials and methods
Chemicals and reagents
Bovine type II collagen (CII) was purchased from the Chondrex, Inc. (Redmond, WA, USA), while Complete Freund's Adjuvant (CFA) and Incomplete Freund's Adjuvant (IFA) were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Interleukin (IL)-1β, interleukin-6 (IL-6), IL-10, IL-12 and IL-17A ELISA kits were purchased from Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). RPMI-1640 medium and fetal bovine serum (FBS) and trypsinase were obtained from Gibco (Thermo Fisher Scientific, Inc.). A Cell Counting kit-8 kit, BCA protein assay reagent and horseradish-peroxidase (HRP)-conjugated secondary antibodies (cat. no. A0208) were purchased from Beyotime Institute of Biotechnology (Haimen, China). Tumor necrosis factor (TNF)-α (cat. no. RAB0477), dimethyl sulfoxide (DMSO) and IκB (cat. no. SAB1305978) were purchased from Sigma-Aldrich; Merck KGaA. JAK1 (cat. no. ab133666), JAK3 (cat. no. ab203611), STAT3 (cat. no. ab119352), nuclear factor (NF)-κB p65 (Cytosolic; cat. no. ab19870), and β-actin (cat. no. ab8226) antibodies were purchased from Abcam (Cambridge, MA, USA). All other reagents used were of analytical grade.
Animals
Male Wistar rats (5–6 weeks old, 170–180 g; n=40) were purchased from the Experimental Animal Center of Kunming Medical University (Kunming, China). Animals were housed at 21±1°C and 30–70% humidity under a 12 h light/dark cycle with free access to standard pellet food and water. All animal experiments in the present study were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (12), and the experimental protocols were approved by the Animal Care and Use Committee of the First Affiliated Hospital of Kunming Medical University (approval no. KMUH-2016023).
Cell culture
The RA-derived fibroblast-like synoviocyte MH7A cell line was obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). MH7A cells were cultured in RPMI-1640 medium with 10% FBS, 1% penicillin and 1% streptomycin in a 5% CO2 humidified atmosphere at 37°C.
Curculigoside extraction
Dried rhizomes of C. orchioides were purchased from Beijing Tongrentang (Beijing, China) and identified by the department of Traditional Chinese Medicine, The First Affiliated Hospital of Kunming Medical University (Kunming, China). According to previously published protocols (13,14), the rhizome of C. orchioides was powdered and then extracted using 75% aqueous ethanol by reflux three times. Following this, the filtrates were concentrated under 50°C in vacuum, and were partitioned continuously with petroleum ether, ethyl acetate, and n-butanol. The ethyl acetate fraction mentioned above was eluted via silica-gel (100–200 mesh) with petroleum ether-acetone (15:1, 10:1, 5:1, 2:1, 1:1) to obtain five sub-fractions (F1-F5). By using a series of chromatographic techniques, including silica gel column chromatography and Sephadex LH-20 chromatography (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), curculigoside was extracted from the F3 fraction (13). In addition, curculigoside (Fig. 1) was identified by 1H-nuclear magnetic resonance (NMR), 13C-NMR and previously reported NMR data according to methods described previously (13,14).
CIA animal model preparation
To investigate the potential anti-arthritic effects of curculigoside, a total of 40 rats were divided into the following four groups (n=10): Normal (not immunized and treated with 10 ml/kg/day saline), control (immunized and treated with 10 ml/kg/day saline), positive [immunized and treated with methotrexate (MTX); 1 mg/kg, three times a week] and curculigoside group (immunized and treated with 50 mg/kg curculigoside).
The CIA rat model was prepared according to previously reported methods, with minor modifications (15,16). Briefly, CII was dissolved in 0.1 mM acetic acid to achieve a final concentration of 4 mg/ml. Then, the CII solution was emulsified with an equal volume of CFA. Rats were initially immunized by subcutaneous injection of CII emulsion at the tail root (100 µl/rat). After 7 days, the rats were immunized by CII again, emulsified by an equal volume of IFA at the same location (100 µl/rat). After approximately 10 days from the initial immunization, rats evidently exhibited RA symptoms at the toe joint, including observable inflammatory reactions, erythema and swelling.
At 10 days after the initial immunization with CII, rats were orally treated with either saline, MTX or curculigoside (50 mg/kg/day). During the experiment, the rats body weight and paw volume were measured by using a PV-200 Plethysmometer (Paw Volume) Meter (Techman Soft, Chengdu, China) every 5 days. In addition, the arthritis indices of rats were measured every 3 days, using the following ordinal scale: 0, no obvious signs of arthritis; 1, one joint affected (swelling and erythema); 2, two joints affected; 3, three joints affected; 4, three joints affected and maximal erythema and swelling (17). After 30 days of drug treatment, rat weight was recorded (for the normal and curculigoside treatment groups, rats weighed 320–340 g; for the control and MTX groups, rats weighed 280–300 g), then rats were sacrificed by decapitation under aesthesia with sodium pentobarbital (35 mg/kg; intraperitoneal injection). Next, blood was collected from abdominal aorta. The spleen and thymus were dissected from each mouse to determine the ratio (mg/g) of thymus or spleen wet weight to body weight.
Determination of serum cytokines
Serum samples were prepared and centrifugation 15 min (1,800 × g) at 4°C, and were stored at −80°C until analysis. Then, serum TNF-α (cat. no. RAB0477), IL-1β (cat. no. BMS6002), IL-6 (cat. no. BMS603-2), IL-10 (cat. no. 88-7105-88), IL-12 (cat. no. BMS616) and IL-17A (cat. no. BMS6001) were detected by using commercial ELISA kits according to the manufacturer's protocol and instruction (Invitrogen; Thermo Fisher Scientific, Inc.).
Cell counting kit-8 (CCK-8) assay
Effects of curculigoside on cell viability were determined by Cell Counting kit-8 according to the manufacturer's protocol. MH7A cells (5×103 cells/well) were seeded in 96-well plates and incubated with various concentrations of curculigoside (1, 2, 4, 8, 16, 32 and 64 µg/ml) for 12, 24, 36, 48 or 72 h. CCK-8 solution was added to each well and incubated for another 1 h at 37°C. Optical density (OD) was measured at 450 nm using a 96-well plate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Results were reported as a percentage of DMSO control cells.
Western blotting
JAK1, JAK3, STAT3, NF-κB p65 (C) and IκB expression was measured in MH7A cells by western blotting. Following treatment with various concentrations of curculigoside (4 and 16 µg/ml) or vehicle (DMSO) in the presence of 10 ng/ml TNF-α for 36 h, Cells (5×106) were harvested and homogenized with lysis buffer for 10 min and centrifuged at 4°C for 5 min (10,000 × g). Total protein was extracted from cells using the cell lysis buffer for western blotting and IP (cat. no. P0013; Beyotime Institute of Biotechnology), in addition, the cytoplasmic protein was extracted by using NE-PER™ Nuclear and Cytoplasmic Extraction Reagents (cat. no. 78833; Thermo Fisher Scientific, Inc.). The protein concentration was determined with a bicinchoninic acid protein assay. Subsequently, 40 µg total proteins in each sample was separated by 12% SDS-PAGE and blotted onto polyvinylidene difluoride (PVDF) membranes. Membranes were blocked with 5% fat-free dry milk in 1X TBST (containing 0.1% Tween-20; Beyotime Institute of Biotechnology; cat. no. P0233) at room temperature for 2 h. Thereafter, proteins on the PVDF membranes were probed with JAK1 (dilution 1:1,000), JAK3 (dilution 1:1,000), STAT3 (dilution 1:1,000), NF-κB p65 (C) (dilution 1:1,000), IκB (dilution 1:1,000) and β-actin (dilution 1:2,000) antibodies at 4°C for 12 h, followed by incubation with corresponding horseradish peroxidase-conjugated secondary antibodies (1:1,000; cat. no. A0208) for 2 h at 37°C. Finally, immunoreactive bands were visualized with enhanced chemiluminescence detection reagents (Beyotime Institute of Biotechnology; cat. no. P0018A) and analyzed using the ImageQuant LAS 4000 Imaging system (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). Protein expression was normalized to β-actin.
Statistical analysis
All data are presented as the mean ± standard deviation of three independent experiments, which were performed in triplicate. Statistical analyses were performed via one-way ANOVA followed by Dunnett's test using SPSS 19.0 software package (IBM Corp., Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Determination of paw swelling and arthritis index in CIA rats
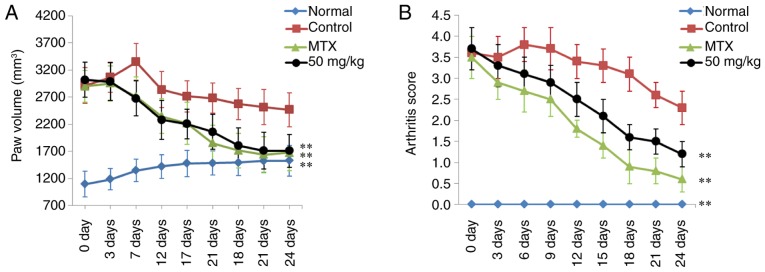
The paw swelling and arthritis score of rats in each treatment group was determined to evaluate the therapeutic effects of curculigoside on RA. As shown in Fig. 2, significant RA symptoms were observed in the control CIA rats when compared to normal rats at 24 days, including paw swelling (P<0.01) and higher arthritis score (P<0.01). Following treatment with MTX, paw swelling and arthritis scores were reduced significantly, compared with the control group (P<0.01). In addition, curculigoside (50 mg/kg) also markedly decreased paw swelling and arthritis scores of CIA rats (P<0.01), compared with control rats.
Figure 2.
Effects of curculigoside on (A) paw volume and (B) arthritis scores of type II collagen-induced arthritis rats. Data are presented as the mean ± standard deviation (n=10). **P<0.01 vs. control group.
Curculigoside reduces spleen and thymus indices in CIA rats
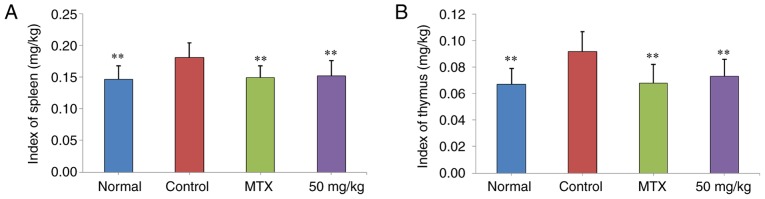
The effects of curculigoside treatment on spleen and thymus indices in CIA rats were presented in Fig. 3. It was demonstrated that spleen and thymus indices in the control group were significantly higher than those of normal rats (P<0.01). Following treatment with curculigoside (50 mg/kg), spleen and thymus indices were decreased (P<0.01), compared with the control group.
Figure 3.
Effects of curculigoside on index of the (A) spleen and (B) thymus in type II collagen-induced arthritis rats. Data are presented as the mean ± standard deviation (n=10). **P<0.01 vs. control group.
ELISA assay
The expression of TNF-α, IL-1β, IL-6, IL-10, IL-12 and IL-17A in serum following treatment were shown in Fig. 4. It was observed that TNF-α, IL-1β, IL-6, IL-10, IL-12 and IL-17A expression in control rats was significantly increased when compared to the normal group (P<0.01). The expression of these proteins in rat serum decreased significantly following treatment with 50 mg/kg curculigoside (P<0.01), compared with control CIA rats.
Figure 4.
Effects of curculigoside on the serum levels of TNF-α, IL-1β, IL-6, IL-10, IL-12 and IL-17A in type II collagen-induced arthritis rats. Data are presented as the mean ± standard deviation (n=10), **P<0.01, vs. control group. IL, interleukin; TNF-α, tumor necrosis factor-α.
Curculigoside reduces MH7A cell viability
The effect of curculigoside on MH7A cell viability was detected with CCK-8 assays. As presented in Fig. 5, curculigoside exerted significant inhibitory effects on MH7A cell viability between 1 and 64 µg/ml. In addition, our results also showed that curculigoside at the concentrations of 4, 8 and 16 µg/ml possessed inhibitory effects on MH7A cell viability within 72 h.
Figure 5.
Inhibitory effects of curculigoside on the proliferation of MH7A cells. (A) Cells were treated with curculigoside (1, 2, 4, 8, 16, 32 and 64 µg/ml) for 36 h, and then a CCK-8 assay was performed to determine the percentage of cell proliferation inhibition (n=4). (B) Cells were treated with curculigoside (4, 8 and 16 µg/ml) for 12, 24, 36, 48, and 72 h time intervals, and then a CCK-8 assay was performed to determine the percentage of cell proliferation inhibition (%) (n=4). CCK-8, Cell Counting kit-8.
Curculigoside decreases JAK1, JAK3 and STAT3 expression in TNF-α stimulated MH7A cells
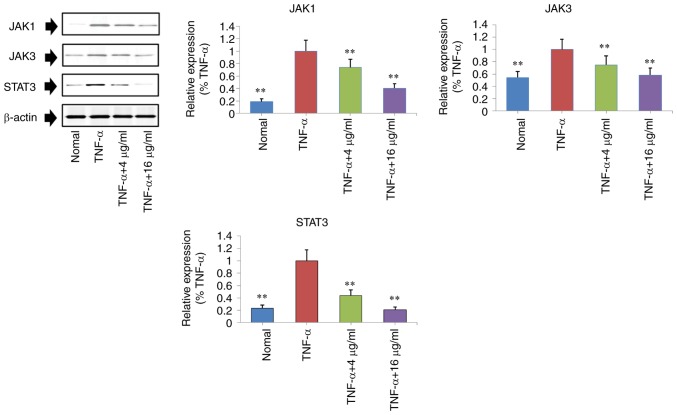
Protein expression levels of JAK1, JAK3 and STAT3 in TNF-α stimulated MH7A cells were measured by western blotting. Compared to the TNF-α group, the protein expression of JAK1, JAK3 and STAT3 were significantly downregulated in the curculigoside-treated groups (4 and 6 µg/ml) (P<0.01; Fig. 6).
Figure 6.
Regulatory effects of curculigoside on the expression levels of JAK1, JAK3 and STAT3 in TNF-α-stimulated MH7A cells were determined. Cells were treated with TNF-α (10 ng/ml) for 12 h, exposed to curculigoside (4 and 16 µg/ml) for a further 24 h and then subjected to western blotting assays to determine the expression levels of NF-κB and IκB (n=4). **P<0.01 vs. TNF-α group. JAK, Janus kinase; TNF-α, tumor necrosis factor-α; NF-κB, nuclear factor-κB; STAT3, signal transducer and activator of transcription 3.
Curculigoside increases IκB and cytosolic NF-κB p65 expression in TNF-α stimulated MH7A cells
Furthermore, the effects of curculigoside on NF-κB p65 (cytosolic) and IκB expression was determined by western blotting in TNF-α stimulated MH7A cells. As presented in Fig. 7, the expression of NF-κB p65 (cytosolic) and IκB in TNF-α stimulated MH7A cells was downregulated, compared with normal MH7A cells. Following treatment with curculigoside (4 and 6 µg/ml) for 24 h, the expression of NF-κB p65 (Cytosolic) and IκB was significantly upregulated (P<0.01).
Figure 7.
Regulatory effects of curculigoside on the expression levels of NF-κB p65 (C) and IκB in TNF-α-stimulated MH7A cells. Cells were treated with TNF-α (10 ng/ml) for 12 h, exposed to curculigoside (4 and 16 µg/ml) for a further 24 h and then subjected to western blotting assays to determine the expression levels of NF-κB and IκB (n=4). **P<0.01 vs. TNF-α group. NF-κB, nuclear factor-κB; TNF-α, tumor necrosis factor-α.
Discussion
Natural constituents isolated from plants or herbs may have some pharmacological activity, and their discovery will be useful in the development of novel drugs for treating RA and other similar diseases (18,19). In the present investigation, the anti-arthritic effect of curculigoside isolated from the C. rhizoma was investigated in CIA rats and fibroblast-like synoviocyte MH7A cells. The results indicated that curculigoside possessed significant anti-arthritic effects in vivo and in vitro, and this may be at least partially via regulation of the JAK/STAT/NF-κB signaling pathway.
It has been reported that RA is an immune-mediated disease with chronic progressive inflammation (20). Currently, the CIA and adjuvant-induced arthritis (AIA) models are two commonly used RA animal models (21). CIA is a well-known RA animal model, which induces immunological and pathological features similar to those in the RA in humans (22,23). In the present study, a CII-induced arthritis rat model was successfully established, and the anti-arthritic effects of curculigoside were evaluated. It was demonstrated that curculigoside decreased paw swelling and arthritis scores, suggesting that curculigoside may possess potential therapeutic effects in CIA.
To study the potential pharmacological mechanism, the effects of curculigoside on the release of TNF-α, IL-1β, IL-6, IL-10, IL-12 and IL-17A in rat serum were examined. Pro-inflammatory cytokines have been reported as potential therapeutic targets for RA, as these cytokines stimulate inflammatory responses in arthritic joints and synovial tissues (24–28). TNF-α is known to play a vital role in the inflammatory and immunological responses in RA progression and TNF-α is generally recognized as a promising target for anti-RA drug (15). IL-1β and IL-17A are other important pro-inflammatory cytokines in the development of RA (15). Furthermore, IL-6 and IL-12 also serve an important role in RA inflammation via activating inflammatory reactions (29). By contrast, IL-10 has been regarded as a potent anti-inflammatory cytokine through inhibiting the releases of pro-inflammatory cytokines (30). The results of the present study demonstrated that curculigoside decreased TNF-α, IL-1β, IL-6, IL-10, IL-12 and IL-17A release in the serum of CIA rats.
Synovial cell expansion is one of the main pathological events in the inflamed synovium of patients with RA (31). RA-derived fibroblast-like synoviocytes with tumor-like expansion are the predominant cell type in the hyperplastic synovium, and result in aggressive cartilage invasion (32). In the present study, the anti-proliferative effects of curculigoside on MH7A cells suggested that it may be useful in RA treatment.
The JAK/STAT signaling pathway is involved in cytokine signaling regulation. JAK/STAT signaling serves an important role in the pathogenesis and progression of RA, and JAK proteins can activate immune cells, induce proinflammatory cytokine expression and transmit cytokine signaling (33–35). JAK1 and JAK3 regulate cell signal transduction by binding with cytokines (36). Additionally, STAT3 is a key pathogenic factor in RA pathogenesis, and may inhibit fibroblasts apoptosis, promote angiogenesis and the expression of matrix metalloproteinase (MMP)-2 and MMP-9 (37). The results of the present study showed that curculigoside downregulated JAK1, JAK3 and STAT3 expression in TNF-α stimulated MH7A cells, indicating that curculigoside exerted anti-arthritic effects on MH7A cells, potentially via the JAK/STAT pathway.
The NF-κB pathway is a prototypical inflammatory and immune signaling pathway (38). NF-κB is a key coordinator of innate immunity and inflammation (39). p65, a NF-κB subunit, is commonly localized to the cytoplasm by its inhibitor IκB (40). Following stimulation, NF-κB p65 translocates to the nucleus and exerts its function as a transcription factor when IκB dissociates from NF-κB (41). In the present study, IκB and cytosolic NF-κB p65 protein expression levels could be upregulated by curculigoside, indicating that the anti-inflammatory effect of curculigoside may be associated with inhibition of the NF-κB signaling pathway.
Collectively, the present study demonstrated that curculigoside exhibited significant anti-arthritic activity in vivo and in vitro. This may be mediated by inhibition of pro-inflammatory cytokine release and downregulation of JAK/STAT signaling pathway proteins, as well as an increase in NF-κB and IκB expression. The results of the present study suggested that curculigoside could be regarded as a potential candidate drug for RA treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by Scientific Research Fund of Yunnan Provincial Education Department of China (grant no. 2016zzx009), Scientific Research Fund of Yunnan provincial of China (grant no. 2017FB108), the Fund of Yunnan Provincial Health Science and Technology Plan (grant nos. 2016NS052, 2016NS051, 2017NS051 and 2017NS052), National Natural Science Foundation of China (grant nos. 81460256, 81501406 and 81760296), Innovative Research Team of Kunming Medical University (grant no. CXTD201613), Yunnan Provincial Fund for Preparatory Young Leaders in Academia and Technology (grant no. 2015HB071), the Funding of Yunnan Provincial Department of Education (grant no. 2017FE467), Yunnan Applied Basic Research Projects-Union Foundation [grant no. 2017FE467 (−138)] and National Key R&D Program-Specialized Research in Precision Medicine (grant no. 2017YFC0907605).
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Authors' contributions
JX, AL, RC, RB, SLi, WL and GZ performed the measurements. SJ, SLiu and MZ analyzed and interpreted data. ST, JX and WW made substantial contributions to conception and design, and were involved in drafting, revising the manuscript and interpreting all data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All animal experiments in the present study were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, and the experimental protocols were approved by the Animal Care and Use Committee of the First Affiliated Hospital of Kunming Medical University (approval no. KMUH-2016023).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.People's Medical Publishing House; Beijing: 2015. Chinese Pharmacopoeia Commission: Pharmacopoeia of the People's Republic of China Part I; p. 102. [Google Scholar]
- 2.Huang YL. Research progress of Curculiginis orchioides. J Chin Med Mater. 2003;26:225–229. (In Chinese) [Google Scholar]
- 3.Zhang XJ, Sun YH, Wang HoY. Chemical constituents from Curculigo orchioids. Chin Tradit Pat Med. 2017;29:1869–1872. (In Chinese) [Google Scholar]
- 4.Fan PT, Zhang LM, Heng M, Liu BC, Xie X, Ning ZS, Xu H. Effects of curculigoside on expressions of Caspase-3, PARP-1 and estrogen receptor in hippocampus of model rats with vascular dementia. Chin J Neuroanat. 2017;33:453–458. (In Chinese) [Google Scholar]
- 5.Lu HW, Zhu BH, Liang YK. Determination of curculigoside in crude medicine curculigo orchioides by HPLC. Zhongguo Zhong Yao Za Zhi. 2002;27:192–194. (In Chinese) [PubMed] [Google Scholar]
- 6.Ooi J, Azmi NH, Imam MU, Alitheen NB, Ismail M. Curculigoside and polyphenol-rich ethyl acetate fraction of Molineria latifolia rhizome improved glucose uptake via potential mTOR/AKT activated GLUT4 translocation. J Food Drug Anal. 2018;26:1253–1264. doi: 10.1016/j.jfda.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pu J, Fang FF, Li XQ, Shu ZH, Jiang YP, Han T, Peng W, Zheng CJ. Matrine exerts a strong anti-arthritic effect on type II collagen-induced arthritis in rats by inhibiting inflammatory responses. Int J Mol Sci. 2016;17(pii):E1410. doi: 10.3390/ijms17091410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okada Y, Wu D, Trynka G, Raj T, Terao C, Ikari K, Kochi Y, Ohmura K, Suzuki A, Yoshida S, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014;506:376–381. doi: 10.1038/nature12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Q, Wang L, Wu L, Zhang M, Hu S, Wang R, Han Y, Wu Y, Zhang L, Wang X, et al. Paroxetine alleviates T lymphocyte activation and infiltration to joints of collagen-induced arthritis. Sci Rep. 2017;7:45364. doi: 10.1038/srep45364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Firestein GS, McInnes IB. Immunopathogenesis of rheumatoid arthritis. Immunity. 2017;46:183–196. doi: 10.1016/j.immuni.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie QC, Yang YP. Anti-proliferative of physcion 8-O-β-glucopyranoside isolated from Rumex japonicus Houtt. on A549 cell lines via inducing apoptosis and cell cycle arrest. BMC Complement Alter Med. 2014;14:377. doi: 10.1186/1472-6882-14-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institute of Health, USA. Public health service policy on humane care and use of laboratory animals. 2002 [Google Scholar]
- 13.Wu XY, Li JZ, Guo JZ, Hou BY. Ameliorative effects of curculigoside from Curculigo orchioides Gaertn on learning and memory in aged rats. Molecules. 2012;17:10108–10118. doi: 10.3390/molecules170910108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu DX, Lei GQ, Cheng XW, Chen JK, Zhou TS. Curculigoside C, a new phenolic glucoside from rhizomes of curculigo orchioides. Acta Bot Sin. 2004;46:621–624. [Google Scholar]
- 15.Peng W, Wang L, Qiu X, Jiang Y, Han T, Pan L, Jia X, Qin L, Zheng C. Therapeutic effects of Caragana pruinosa Kom. roots extract on type II collagen-induced arthritis in rats. J Ethnopharmacol. 2016;191:1–8. doi: 10.1016/j.jep.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 16.Wang F, Qiao YH, Niu HM, Zhao H. Anti-arthritic effect of total anthraquinone from Polygonum cuspidatum on type II collagen-induced arthritis in rats. Trop J Pharm Res. 2017;16:2453–2459. [Google Scholar]
- 17.Zheng CJ, Zhao XX, Ai HW, Lin B, Han T, Jiang YP, Xing X, Qin LP. Therapeutic effects of standardized Vitex negundo seeds extract on complete Freund's adjuvant induced arthritis in rats. Phytomedicine. 2014;21:838–846. doi: 10.1016/j.phymed.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Wang Q, Kuang H, Su Y, Sun Y, Feng J, Guo R, Chan K. Naturally derived anti-inflammatory compounds from Chinese medicinal plants. J Ethnopharmacol. 2013;146:9–39. doi: 10.1016/j.jep.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Peng W, Shen H, Lin B, Han P, Li CH, Zhang QY, Ye BZ, Rahman K, Xin HL, Qin LP, Han T. Docking study and antiosteoporosis effects of a dibenzylbutane lignan isolated from Litsea cubeba targeting Cathepsin K and MEK1. Med Chem Res. 2018;27:2062–2070. doi: 10.1007/s00044-018-2215-8. [DOI] [Google Scholar]
- 20.Majithia V, Geraci SA. Rheumatoid arthritis: Diagnosis and management. Am J Med. 2007;120:936–939. doi: 10.1016/j.amjmed.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 21.McNamee K, Williams R, Seed M. Animal models of rheumatoid arthritis: How informative are they? Eur J Pharmacol. 2015;759:278–286. doi: 10.1016/j.ejphar.2015.03.047. [DOI] [PubMed] [Google Scholar]
- 22.Luo Y, Liu M, Xia Y, Dai Y, Chou G, Wang Z. Therapeutic effect of norisoboldine, an alkaloid isolated from Radix Linderae, on collagen-induced arthritis in mice. Phytomedicine. 2010;17:726–731. doi: 10.1016/j.phymed.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Trentham DE, McCune WJ, Susman P, David JR. Autoimmunity to collagen in adjuvant arthritis of rats. J Clin Investig. 1980;66:1109–1117. doi: 10.1172/JCI109940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imboden JB. The immunopathogenesis of rheumatoid arthritis. Annu Rev Pathol Mech Dis. 2009;4:417–434. doi: 10.1146/annurev.pathol.4.110807.092254. [DOI] [PubMed] [Google Scholar]
- 25.Sennikov SV, Alshevskaya AA, Shkaruba NS, Chumasova OA, Sizikov AE, Lopatnikova JA. Expression of TNFα membrane-bound receptors in the peripheral blood mononuclear cells (PMBC) in rheumatoid arthritis patients. Cytokine. 2015;73:288–294. doi: 10.1016/j.cyto.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Kalden JR. Emerging role of anti-tumor necrosis factor therapy in rheumatic diseases. Arthritis Res. 2002;4(Suppl 2):S34–S40. doi: 10.1186/ar552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mariaselvam CM, Aoki M, Salah S, Boukouaci W, Moins-Teisserenc H, Charron D, Krishnamoorthy R, Tamouza R, Negi VS. Cytokine expression and cytokine-based T cell profiling in South Indian rheumatoid arthritis. Immunobiology. 2014;219:772–777. doi: 10.1016/j.imbio.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Roeleveld DM, Koenders MI. The role of the Th17 cytokines IL-17 and IL-22 in rheumatoid arthritis pathogenesis and developments in cytokine immunotherapy. Cytokine. 2015;74:101–107. doi: 10.1016/j.cyto.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Rossi D, Modena V, Sciascia S, Roccatello D. Rheumatoid arthritis: Biological therapy other than anti-TNF. Int Immunopharmacol. 2015;27:185–188. doi: 10.1016/j.intimp.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Conti P, Kempuraj D, Kandere K, Di Gioacchino M, Barbacane RC, Castellani ML, Felaco M, Boucher W, Letourneau R, Theoharides TC. IL-10, an inflammatory/inhibitory cytokine, but not always. Immunol Lett. 2003;86:123–129. doi: 10.1016/S0165-2478(03)00002-6. [DOI] [PubMed] [Google Scholar]
- 31.Tran CN, Lundy SK, Fox DA. Synovial biology and T cells in rheumatoid arthritis. Pathophysiology. 2005;12:183–189. doi: 10.1016/j.pathophys.2005.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia Y, Li X, Ou-Yang Z, Chen JW. Selective modulation of MAPKs contribute to the anti-proliferative and anti-inflammatory activities of 1,7-dihydroxy-3,4-dimethoxyxanthone in rheumatoid arthritis-derived fibroblast-like synoviocyte MH7A cells. J Ethnopharmacol. 2015;168:248–254. doi: 10.1016/j.jep.2015.03.069. [DOI] [PubMed] [Google Scholar]
- 33.O'Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory response. Immunity. 2008;28:477–487. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542–550. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Migita K, Izumi Y, Torigoshi T, Satomura K, Izumi M, Nishino Y, Jiuchi Y, Nakamura M, Kozuru H, Nonaka F, et al. Inhibition of Janus kinase/signal transducer and activator of transcription (JAK/STAT) signalling pathway in rheumatoid synovial fibroblasts using small molecule compounds. Clin Exp Immunol. 2013;174:356–363. doi: 10.1111/cei.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sweeney SE, Firestein GS. Signal transduction in rheumatoid arthritis. Curr Opin Rheumatol. 2004;16:231–237. doi: 10.1097/00002281-200405000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Dutzmann J, Daniel JM, Bauersachs J, Hilfiker-Kleiner D, Sedding DG. Emerging translational approaches to target STAT3 signalling and its impact on vascular dsease. Cardiovasc Res. 2015;106:365–374. doi: 10.1093/cvr/cvv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 40.Beg AA, Baldwin AS., Jr The I kappa B proteins: Multifunctional regulators of Rel/NF-kappa B transcription factors. Genes Dev. 1993;7:2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- 41.Lyss G, Knorre A, Schmidt TJ, Pahl HL, Merfort I. The anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-kappaB by directly targeting p65. J Biol Chem. 1998;273:33508–33516. doi: 10.1074/jbc.273.50.33508. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the present study are included in this published article.