Abstract
Gestational trophoblastic neoplasia (GTN) is a rare tumor that originates from pregnancy that includes invasive mole, choriocarcinoma (CCA), placental site trophoblastic tumor and epithelioid trophoblastic tumor (PSTT/ETT). GTN presents different degrees of proliferation, invasion and dissemination, but, if treated in reference centers, has high cure rates, even in multi-metastatic cases. The diagnosis of GTN following a hydatidiform molar pregnancy is made according to the International Federation of Gynecology and Obstetrics (FIGO) 2000 criteria: four or more plateaued human chorionic gonadotropin (hCG) concentrations over three weeks; rise in hCG for three consecutive weekly measurements over at least a period of 2 weeks or more; and an elevated but falling hCG concentrations six or more months after molar evacuation. However, the latter reason for treatment is no longer used by many centers. In addition, GTN is diagnosed with a pathological diagnosis of CCA or PSTT/ETT. For staging after a molar pregnancy, FIGO recommends pelvic-transvaginal Doppler ultrasound and chest X-ray. In cases of pulmonary metastases with more than 1 cm, the screening should be complemented with chest computed tomography and brain magnetic resonance image. Single agent chemotherapy, usually Methotrexate (MTX) or Actinomycin-D (Act-D), can cure about 70% of patients with FIGO/World Health Organization (WHO) prognosis risk score ≤ 6 (low risk), reserving multiple agent chemotherapy, such as EMA/CO (Etoposide, MTX, Act-D, Cyclophosphamide and Oncovin) for cases with FIGO/WHO prognosis risk score ≥ 7 (high risk) that is often metastatic. Best overall cure rates for low and high risk disease is close to 100% and > 95%, respectively. The management of PSTT/ETT differs and cure rates tend to be a bit lower. The early diagnosis of this disease and the appropriate treatment avoid maternal death, allow the healing and maintenance of the reproductive potential of these women.
Keywords: Gestational trophoblastic neoplasia, Chemotherapy, Chorionic gonadotropin, Invasive mole, Choriocarcinoma, Placental site trophoblastic tumor, Epithelioid trophoblastic tumor
Core tip: Gestational trophoblastic neoplasia is a cancer that originates from placental tissue, with potential for invasion and widespread metastasis. It secretes human chorionic gonadotrophin, which serves as a highly useful biomarker that contributes to the diagnosis, monitoring of therapeutic response, subsequent early detection of relapse and assessment of cure. Once the diagnosis is made, staging and International Federation of Gynecology and Obstetrics/World Health Organization prognostic risk score should be obtained, to initiate the treatment of choice – chemotherapy, which allows high cure rates, especially if the treatment occurs in Reference Centers, which has specialized staff in the treatment of this neoplasm.
INTRODUCTION
Gestational trophoblastic neoplasia (GTN) is a rare tumor that originates from pregnancy and, if treated in reference centers, has high cure rates, even in cases of multi-metastatic neoplasia[1,2]. GTN includes the following histopathological forms: Invasive mole (IM), choriocarcinoma (CCA), placental site trophoblastic tumor (PSTT) and epithelioid trophoblastic tumor (ETT), encompassing lesions that originate in the chorionic villi and the extravillous trophoblast, with different degrees of proliferation, invasion and dissemination[3]. About 50% of all cases of GTN occur after hydatidiform mole, 25% after abortions or ectopic pregnancies and 25%, after term or preterm deliveries. However, PSTT and ETT can arise after term deliveries or non-molar pregnancies in 95% of the cases[4].
Although GTN is a highly metastatic and lethal neoplasia, its natural history was modified in the 1950s, when Li et al[5] introduced Methotrexate (MTX) as an effective antineoplastic treatment to promote the systematic cure of women with non-metastatic disease. Further advances, combined multiple drugs, notably those with etoposide and cisplatin, allowed high remission rates, even in cases of disseminated neoplasia[6,7].
With the establishment of chemotherapy in the treatment of GTN, the systematization of the diagnosis and GTN staging proposed by the International Federation of Gynecology and Obstetrics (FIGO), held at the Washington meeting in 2000, represented a great advance in the treatment of women with GTN[8]. The FIGO 2000 guideline not only standardized the GTN classification, but also proposed well-established diagnostic and therapeutic criteria and standardized the risk factors for chemoresistance, highlighting patients who would benefit from initial treatment with a single agent or, on the contrary, signaling patients who should be initially treated with multiple agent chemotherapy[8]. However, it is important to note that the FIGO 2000 criteria should not be applied to the management of PSTT/ETT which be-have quite differently from the other forms of GTN.
After more than 15 years of the FIGO 2000 guideline implementation for the diagnosis and treatment of GTN, many questions arose as real challenges for the treatment of women with GTN[9]. The purpose of this editorial will be to discuss the situations that still limit the best treatment of GTN, as well as to reflect on alternatives to improve the treatment of women with this condition worldwide.
BASIC OF GTD PATHOLOGY
The commonest forms of GTD are complete and partial molar pregnancies. Their cytogenetic origin derives from an abnormal fertilization. In cases of complete hydatidiform mole, the oocyte loses its DNA, being fertilized by 1 spermatozoa with diploid genetic load, or by 2 haploid spermatozoa - generating a diploid parthenogenetic zygote. In the cases of partial hydatidiform mole, the oocyte has conserved its DNA, being fertilized by 1 spermatozoa with diploid genetic load, or by 2 haploid spermatozoa - generating an zygote with a diandrical triploidy. Women with complete hydatidiform mole may develop postmolar GTN about 20%-25%, while only 1%-5% of women with partial hydatidiform mole will present malignant lesions.
The presence of chorionic villi in the myometrium, with or without vascular invasion, characterizes the IM, the most common form of GTN. Usually its diagnosis is obtained through the uterine histopathology obtained by hysterectomy.
CCA is the most malignant and metastatic form of GTN. Although it’s primary lesion usually presents with great uterine invasion, in about 30% of the cases it crosses with distant metastases, notably in the lungs, liver and brain, by hematogenous dissemination.
Among the non-villous lesions that make up a GTN, PSTT and ETT are derived from the intermediate trophoblast. These clinical forms exhibit lower levels of hCG relative to invasive spring and CCA. In addition, the therapeutic response of PSTT and ETT to chemotherapy alone is limited, requiring hysterectomy to maximize cure rates.
HOT TOPICS ON GTN DIAGNOSIS AND STAGING
The FIGO 2000 guideline established the diagnostic criteria for GTN that would determine the immediate onset of chemotherapy[8]: (1) Four or more plateaued hCG concentrations over three weeks; (2) Increase of hCG concentrations for three or more consecutive measurements for at least two weeks; (3) If there is a histologic diagnosis of choriocarcinoma; and (4) Elevated hCG concentrations for six months or longer.
It should be highlighted that the fundamental pillar of the GTN diagnosis is the hormonal surveillance of serum hCG, the biological and tumor marker of this disease. However, two situations pointed out by FIGO 2000 guideline are currently being questioned. The first relates to whether chemotherapy needs to always be initiated for women with a histopathological diagnosis of CCA. The second concerns if chemotherapy is really needed for patients whose hCG remains raised but falling beyond the 6 mo after uterine evacuation of a molar pregnancy.
Prior literature unanimously suggests immediate onset of chemotherapy for patients with metastatic CCA or with elevated rising hCG. However, there are not infrequent cases of patients who arrive at referral centers with histological diagnosis of CCA and who have declining or even normal levels of hCG, without evidence of metastatic disease. This situation can happen because the histopathological diagnosis of CCA is not always given quickly and/or because the disease was completely resected at the time of diagnosis. A Brazilian retrospective cohort study that followed 47 women with a histopathological diagnosis of CCA managed expectantly, observed that only 44.7% received chemotherapy due to plateauing or rising hCG level after an initial follow up of 2-3 wk[10]. It is noteworthy that the expectant management initially adopted for patients with histological diagnosis of CCA when compared to patients immediately treated with chemotherapy did not worsen the prognosis of these patients, besides no cases of relapse or death were found in this population studied[10].
Regarding the FIGO 2000 recommendation to initiate chemotherapy for patients during postmolar follow-up with hCG raised but falling after 6 mo of uterine evacuation, FIGO itself is controversial, once retracted this opinion in 2012[11], but then resumed the recommendation in the FIGO Cancer Report in 2015[12]. Although this situation is uncommon, affecting about 1% of the women in the post-molar follow-up, expectant management has about 80% spontaneous remission, without the need for chemotherapy[13-15]. These results are more favorable, the lower the hCG levels. No woman developed relapsed disease and overall survival was 100%[13-15]. It is likely that the new FIGO Cancer Report due out in 2018 will recommend that automatic chemotherapy should not be started in this group of women and that continued hCG surveillance is reasonable.
Delaying the onset of chemotherapy, as recommended by the FIGO criteria, could lead to the occurrence of tumor chemoresistance or even metastatic disease and the need for multiple agent chemotherapy[15]. However, the data available shows continued surveillance avoids exposing women unnecessarily to potential toxicities of chemotherapy without increase the risk of resistance or more aggressive treatment later, if necessary[13-15]. In settings where patients with nonmetastatic CCA or with a raised but falling hCG beyond 6 mo from uterine can only be followed with periodic measurements of hCG and evaluation of metastatic disease, since the vast majority of these women will present spontaneous remission[15].
Despite the nearly universal acceptance of the FIGO 2000 criteria to initiate chemotherapy for patients with GTN[8], there is still a set of recommendations initially outlined by the Charing Cross Trophoblastic Disease Center (London, United Kingdom), which were adopted by the European Organization for the Treatment of Trophoblastic Disease (EOTTD)[3,16]. Although plateau or elevated hCG remains the most important diagnostic criteria for GTN, many countries worldwide consider immediate indication for chemotherapy serum hCG concentration of ≥ 20000 IU/L four weeks or more after uterine evacuation, due to the increased chance of such patients developing GTN and/or uterine perforation. Despite the United Kingdom recommendations[3,16], this indication for chemotherapy has not been adopted by FIGO[8].
A Brazilian study confirmed the increased risk for developing postmolar GTN in patients with an hCG ≥ 20000 IU/L four weeks after evacuation, about 80%[17]. However, this study did not report any uterine perforation or to an increase in the aggressiveness of chemotherapy when comparing the groups of women immediately treated with those in which an initial expectant treatment was adopted. In fact, maintaining hormonal surveillance among women with hCG levels higher than 20000 IU/L in the fourth week after molar evacuation would prevent unnecessary chemotherapy in 20% of women[17]. However, the study population was small and further validation work in a larger population would be desirable.
Once the clinical diagnosis of GTN has been made following a histopathological diagnosis of a molar pregnancy, repeat biopsies to confirm malignant progression are unnecessary and nearly always contraindicated because of the risk of promoting life-threatening hemorrhage. Indeed, as samples are usually taken from the uterus in women of reproductive age who can expect to be cured by chemotherapy, a biopsy might result in a hysterectomy or loss of life which is reprehensible[18]. Moreover, biopsies of metastatic sites where bleeding cannot be controlled such as the lungs and abdominal/pelvic organs may precipitate severe hemorrhage, resulting in death[18]. In addition, it should be always considered that the diagnosis of GTN, in almost all cases, is hormonal - by the evaluation of the hCG behavior[19].
Before initiating chemotherapy, staging of GTN is critical. And here are two differences that must be stressed. While in the United States, initial staging with brain and abdomen-pelvis magnetic resonance imaging (MRI), and chest computed tomography (CT) is recommended[20], FIGO/EOTTD recommends that only pelvic-transvaginal Doppler ultrasound and chest X-ray should be initially requested in patients with post-molar GTN. In cases of doubts regarding the normality of the chest X-ray or in the case of metastases with more than 1cm, the screening of metastases with chest CT and brain MRI should be complemented[3,8]. The major problem of using CT rather than chest X-ray for assessing the presence of pulmonary metastases following a molar pregnancy is the risk of including micrometastases < 1 cm. This will upstage and or increase the prognostic score for patients leading to more women starting on multi-agent chemotherapy than necessary. Indeed, several studies have shown that CT defined chest micro-metastasis as opposed to chest X-ray defined pulmonary metastases does not affect outcomes and should not influence staging/scoring or the selection of chemotherapy[21,22].
The role of positron emission tomography (PET), associated or not to CT in the evaluation of metastatic GTN, has not yet been well established[22]. The available information points out that the PET does not add anything to the GTN staging when compared to conventional imaging work-up that is less expensive and more widely available. PET may help to evaluate metastases in unusual sites or to differentiate active metastatic nodules from necrotic and/or hemorrhagic tissue following chemotherapy and in cases of chemoresistance or relapse, notably in patients with PSTT or ETT, for guiding surgical intervention[22,23]. Both false positive and negative results can occur with FDG-PET imaging so careful co-evaluation with other imaging modalities is desirable[24].
PERSPECTIVES OF THE TREATMENT ON GTN
Before discussing GTN treatment in detail, we will initially consider the use of prophylactic chemotherapy for cases of hydatidiform mole thought to be at high risk of developing GTN. The criteria for diagnosing such high risk moles varies and includes very high hCG at the time of evacuation and women who are unable to comply with an hCG surveillance programme following the molar evacuation. Although there is a clear reduction in the risk of development of postmolar GTN[25], the use of prophylactic chemotherapy may increase patients' morbidity (by the side effects of cytotoxic drugs), the risk of chemoresistance, and medical care costs, for the treatment of a neoplasm fully curable without the use of prophylactic chemotherapy[26]. While there is no clear scientific evidence about the benefits of using prophylactic chemotherapy for cases of high-risk hydatidiform mole, we agree that it is time to stop recommending prophylactic chemotherapy for these women[27].
Similarly, prophylactic hysterectomy for the treatment of high-risk hydatidiform mole, or even as primary GTN treatment, should only be considered in women that completed childbearing[28]. However, what we have observed in several settings across the world is that women frequently underwent hysterectomy as their main treatment for a suspected molar pregnancy. Apart from preventing such women from getting pregnant in the future, many fail to then adhere to hCG surveillance because they think they are cured after surgery[18]. This is a serious problem as a significant number will still end up needing chemotherapy due to growth of micrometastases outside the uterus. These patients will be diagnosed late if they are not on hCG surveillance and so worsen their prognosis.
It has also been pointed out that second curettage for some patients diagnosed with GTN can avoid the need for starting chemotherapy. Although the outcomes are controversial and the studies are either small, non-randomised[29] or retrospective in design[30,31], a reduction in the need for chemotherapy was observed between 9%-40% of the patients undergoing a second curettage. Nevertheless, whilst the efficacy of this procedure remains unclear, the benefit appears to be greatest only in patients with non-metastatic GTN and levels of hCG below 5000 IU/L[29-31].
The choice of chemotherapy treatment is based on the combination of the anatomic staging with the World Health Organization (WHO) scoring system based on risk factors[8] (Table 1). According to this scoring system, tumors are divided into two categories: Low-risk GTN, if the score is equal to or lower than 6; and high-risk, if the score is equal to or greater than 7. The score is associated with the risk of developing chemoresistance, and thus guides the choice of first line chemotherapy[8].
Table 1.
International Federation of Gynecology and Obstetrics/World Health Organization staging and classification of gestational trophoblastic disease
| GTN: FIGO staging and classification (Washington, 2000) | ||||
| FIGO anatomic staging | ||||
| Stage I: Disease confined to the uterus | ||||
| Stage II: GTN extends outside of the uterus, but is limited to the genital structures (adnexa, vagina, broad ligament) | ||||
| Stage III: GTN extends to the lungs, with or without known genital tract involvement | ||||
| Stage IV: All other metastatic sites | ||||
| Modified WHO prognostic scoring system as adapted by FIGO | ||||
| Prognostic factors | Score | |||
| 0 | 1 | 2 | 4 | |
| Age | < 40 | ≥ 40 | - | - |
| Antecedent gestation | Mole | Abortion | Term | - |
| Interval (mo) | < 4 | 4-6 | 7-12 | > 12 |
| Pretreatment serum hCG (IU/L) | < 103 | 103 to < 104 | 104 to < 105 | > 105 |
| Largest tumor size (including uterus) | < 3 | 3 to 4 | ≥ 5 | - |
| Site of metastases | Lung | spleen, kidney | gastro intestinal tract | brain, liver |
| Number of metastases | - | 1-4 | 5-8 | > 8 |
| Previous failed chemotherapy | - | - | single drug | 2 or more drugs |
Interval (in months) between the end of antecedent gestation (when known) and symptom onset. FIGO: International Federation of Gynecology and Obstetrics; WHO: World Health Organization; GTN: Gestational trophoblastic neoplasia; hCG: Human chorionic gonadotropin.
Low-risk GTN should be first treated with a single agent, either MTX or Actinomycin-D (ActD)[32,33]. Although a Cochrane review points to a superiority of Act-D over MTX[34], what we observe is that there are numerous chemotherapy regimens for either MTX (50 mg fixed dose or 50 mg/m2 or 1 mg/kg on days 1, 3, 5, 7, with or without folinic acid rescue, 0.4 mg/kg D1-5, 30-50 mg/m2 once weekly), and for Act-D (10-13 mcg/kg D1-5, 1.25 mg/m2 biweekly), making it impossible, with the data available, to actually evaluate the best initial treatment for low-risk GTN[32-34].
Although cases of low-risk GTN are widely cured with single agent chemotherapy[8,32,33], it has been observed that patients with GTN and with a FIGO score of 5-6 only have about a 35% chance of cure with MTX regimen. This indicates that these patients form an “intermediate-risk group”, for whom the MTX regimen might be considered to be relatively unlikely to achieve a cure[35]. For these patients, one could either start on a more aggressive chemotherapy regimen, or develop a new assessment which could be added to the existing scoring system to enable improved patient stratification to single verses multi-agent therapy. Recent work suggests that the uterine artery pulsatility index[36], might help to identify patients resistant to MTX treatment. However, it is still unclear how to incorporate the pulsatility index into the FIGO scoring system.
Indeed, there is an international scientific effort to validate the FIGO/WHO prognostic risk score[37]. Studies have shown that of the eight patients who had a pre-treatment hCG exceeding 10000 IU/L and 100000 IU/L, interval exceeding 7 mo since previous pregnancy and tumor size of over 5 cm were identified as being predictive of single-agent resistance[38]. Another perspective shows that no patient with pre-treatment hCG level higher than 400000 IU/L achieve remission under single agent chemotherapy treatment, regardless of the prognostic risk score[39].
Less commonly, patients reach referral centers for treatment with high-risk GTN and disseminated disease. These patients were usually treated with the regimen of choice for high-risk GTN[3,8,32]: EMA/CO (combining Etoposide, MTX, Act-D, Cyclophosphamide and Oncovin). Initial reports indicated a survival rate of about 86% with deaths occurring either early within 4 wk of admission due mainly to bleeding or metabolic upset from tumor lysis in patients with very advanced disease or late from drug resistant disease. In addition, some deaths were due to non-gestational tumors that histopathologically mimicked GTN[40]. To avoid these early deaths, high risk GTN patients with a FIGO score ≥ 13, with or without a higher number of metastases (> 6) and higher hCG (> 1000000 IU/L), seem to benefit from the use of induction low-dose Etoposide 100 mg/m2 and cisplatin 20 mg/m2 (EP; days 1 and 2 every 7 d) for one to three cycles until well enough to start EMA/CO[40].
Although more than 90% of patients with GTN are cured with chemotherapy regimens based on Etoposide and Cisplatin[3,8,32], there are some patients with chemoresistant neoplasia who present a major therapeutic challenge. In such cases, one must try to obtain tumor tissue to determine the genetic origin of CCA (gestational verses non-gestational) and to rule out the possibility of PSTT/ETT (where treatment necessarily includes surgery)[3,8,32]. Indeed, the management of PSTT/ETT is quite different reflecting its distinct biological behavior. The disease is slower growing, produces less hCG, remains confined to the uterus for longer, is more likely to involve local lymph nodes and is a little more resistant to chemotherapy than CCA[41]. It is now appreciated that all types of preceding pregnancy can give rise to PSTT/ETT and that the key poor prognostic factor is an interval more than 4 years from the last known or causative pregnancy[42]. Moreover, recent work has revealed that 10%-15% of women with atypical placental site nodules (APSN) may either have a co-existent or subsequently develop a PSTT/ETT so APSN can no longer be ignored[43]. Patients with histologically confirmed PSTT/ETT confined to the uterus are best managed with hysterectomy whilst those with metastatic disease will need combination agent chemotherapy followed by resection of residual disease sites. Patients with an interval more than 4 years from the causative pregnancy are unlikely to be cured with regular platinum and etoposide based chemotherapy regimens such as EP/EMA plus surgery and so should be considered for experimental systemic therapies regardless of stage[42]. Some GTN patients including those with PSTT/ETT who have disease with some sensitivity to platinum and etoposide may still be salvaged with high dose chemotherapy but other more effective and less toxic alternatives are needed[3]. In studying the immuno-expression of these tissues, it has been found that PD-L1 and its receptor PD-1 are strongly expressed by GTN, suggesting the ligand is involved in tumor-immune evasion[44]. Indeed, a few cases of multi drug-resistant GTN including PSTT/ETT have recently shown complete responses to the anti-PD-1 agent Pembrolizumab with several women off treatment and well for 6-24 mo[45]. Therefore, it is tempting to speculate that such checkpoint immunotherapies may provide a new effective salvage treatment for women with GTN failing existing therapies and that this might replace the need for high dose chemotherapy.
Despite the great improvement observed in the treatment of women with GTN, especially in the methods of disease monitoring, more accurate metastasis screening and more effective treatments, even in multimetastatic cases, we believe that the most important key for survival of women affected by this disease is their treatment in Reference Centers. Brewer was the first to report that both the morbidity and mortality of patients with GTN was nine times lower at a center staffed by physicians experienced in the management of this neoplasia than with the “occasional” physician treating this entity[46]. Moreover, the UK experience of centralized care within a national health system has provided an exemplar of what can be achieved with the UK specialized centers reporting the highest cure rates globally[47]. The Brazilian experience now also clearly shows that when these patients are followed in Reference Centers they demonstrate lower metastasis rate, lower median time interval between molar evacuation and chemotherapy onset shorter than those initially treated outside the Reference Centers[48].
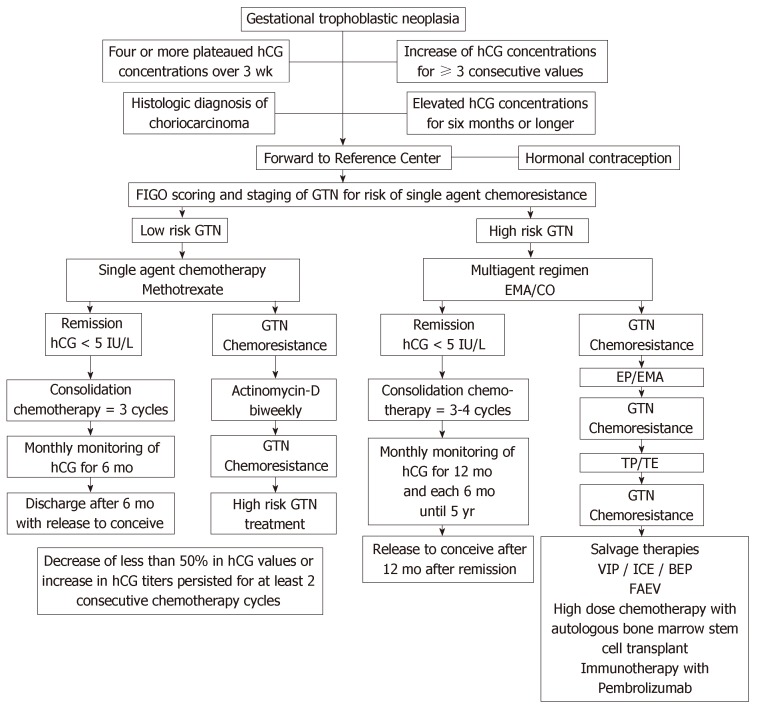
Between advances[49-51] and challenges[52], the truth is that GTN is still an unknown disease of many physicians in the world. When the obstetrician is unable to recognize this anomaly of pregnancy, postponing its diagnosis[53,54]; when the gynecologist does not understand the importance of hormonal vigilance and strict contraception during this period[55,56]; when the oncologist indicates unnecessary surgeries to treat women with GTN or uses incorrect chemotherapy regimens, our women with GTN will suffer, sometimes losing their uterus or even their lives. Figure 1 briefly illustrates the entire treatment of GTD.
Figure 1.
Algorithm summarizing the modern treatment of gestational trophoblastic neoplasia. GTN: Gestational trophoblastic neoplasia; hCG: Human chorionic gonadotropin.
It is important to highlight that GTN can arise from any pregnancy form (abortion, ectopic, term/preterm, and, of course after hydatidiform mole), and that it should be ruled out in cases of metastatic neoplasia in women during the menacme, with unknown primary site, especially if the clinical history reveals a recent gestational history. Finally, it is important to remember, that a simple hCG test may help provide the diagnosis of this neoplasia, monitor the treatment, confirm the cure and detect relapse early to enable effective salvage therapy.
Footnotes
Conflict-of-interest statement: The authors have no conflict of interest to declare.
Manuscript source: Invited manuscript
Peer-review started: August 1, 2018
First decision: August 31, 2018
Article in press: January 1, 2019
Specialty type: Oncology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kanat O, Kupeli S, Ortiz-Sanchez E S- Editor: Ji FF L- Editor: A E- Editor: Wu YXJ
Contributor Information
Antonio Braga, Postgraduate Program of Medical Sciences, Fluminense Federal University, Niterói 24033-900, Brazil; Department of Gynecology and Obstetrics, Faculty of Medicine, Rio de Janeiro Federal University, Postgraduate Program of Perinatal Health, Maternity School, Rio de Janeiro 22240-000, Brazil. bragamed@yahoo.com.br.
Paulo Mora, Postgraduate Program of Medical Sciences, Fluminense Federal University, Niterói 24033-900, Brazil; Brazilian National Cancer, Hospital do Câncer 2, Rio de Janeiro 20220-410, Brazil.
Andréia Cristina de Melo, Brazilian National Cancer, Hospital do Câncer 2, Rio de Janeiro 20220-410, Brazil.
Angélica Nogueira-Rodrigues, Department of Internal Medicine, Faculty of Medicine, Minas Gerais Federal University, Belo Horizonte 30130-100, Brazil.
Joffre Amim-Junior, Department of Gynecology and Obstetrics, Faculty of Medicine, Rio de Janeiro Federal University, Postgraduate Program of Perinatal Health, Maternity School, Rio de Janeiro 22240-000, Brazil.
Jorge Rezende-Filho, Department of Gynecology and Obstetrics, Faculty of Medicine, Rio de Janeiro Federal University, Postgraduate Program of Perinatal Health, Maternity School, Rio de Janeiro 22240-000, Brazil.
Michael J Seckl, Department of Medical Oncology, Charing Cross Gestational Trophoblastic Disease Centre, Charing Cross Hospital, Imperial College London, London W6 8RF, United Kingdom.
References
- 1.Biscaro A, Braga A, Berkowitz RS. Diagnosis, classification and treatment of gestational trophoblastic neoplasia. Rev Bras Ginecol Obstet. 2015;37:42–51. doi: 10.1590/SO100-720320140005198. [DOI] [PubMed] [Google Scholar]
- 2.Maestá I, Braga A. [Challenges of the treatment of patients with gestational trophoblastic disease] Rev Bras Ginecol Obstet. 2012;34:143–146. [PubMed] [Google Scholar]
- 3.Seckl MJ, Sebire NJ, Fisher RA, Golfier F, Massuger L, Sessa C ESMO Guidelines Working Group. Gestational trophoblastic disease: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi39–vi50. doi: 10.1093/annonc/mdt345. [DOI] [PubMed] [Google Scholar]
- 4.Mangili G, Lorusso D, Brown J, Pfisterer J, Massuger L, Vaughan M, Ngan HY, Golfier F, Sekharan PK, Charry RC, Poveda A, Kim JW, Xiang Y, Berkowtiz R, Seckl MJ. Trophoblastic disease review for diagnosis and management: a joint report from the International Society for the Study of Trophoblastic Disease, European Organisation for the Treatment of Trophoblastic Disease, and the Gynecologic Cancer InterGroup. Int J Gynecol Cancer. 2014;24:S109–S116. doi: 10.1097/IGC.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 5.Hertz R, Li MC, Spencer DB. Effect of methotrexate therapy upon choriocarcinoma and chorioadenoma. Proc Soc Exp Biol Med. 1956;93:361–366. doi: 10.3181/00379727-93-22757. [DOI] [PubMed] [Google Scholar]
- 6.Newlands ES, Bagshawe KD, Begent RH, Rustin GJ, Holden L. Results with the EMA/CO (etoposide, methotrexate, actinomycin D, cyclophosphamide, vincristine) regimen in high risk gestational trophoblastic tumours, 1979 to 1989. Br J Obstet Gynaecol. 1991;98:550–557. doi: 10.1111/j.1471-0528.1991.tb10369.x. [DOI] [PubMed] [Google Scholar]
- 7.Berkowitz RS, Goldstein DP. Current advances in the management of gestational trophoblastic disease. Gynecol Oncol. 2013;128:3–5. doi: 10.1016/j.ygyno.2012.07.116. [DOI] [PubMed] [Google Scholar]
- 8.FIGO Oncology Committee. FIGO staging for gestational trophoblastic neoplasia 2000. FIGO Oncology Committee. Int J Gynaecol Obstet. 2002;77:285–287. doi: 10.1016/s0020-7292(02)00063-2. [DOI] [PubMed] [Google Scholar]
- 9.Brown J, Naumann RW, Seckl MJ, Schink J. 15years of progress in gestational trophoblastic disease: Scoring, standardization, and salvage. Gynecol Oncol. 2017;144:200–207. doi: 10.1016/j.ygyno.2016.08.330. [DOI] [PubMed] [Google Scholar]
- 10.Braga A, Campos V, Filho JR, Lin LH, Sun SY, de Souza CB, da Silva RCAF, Leal EAS, Silveira E, Maestá I, Madi JM, Uberti EH, Viggiano M, Elias KM, Horowitz N, Berkowitz RS. Is chemotherapy always necessary for patients with nonmetastatic gestational trophoblastic neoplasia with histopathological diagnosis of choriocarcinoma? Gynecol Oncol. 2018;148:239–246. doi: 10.1016/j.ygyno.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Ngan HY, Kohorn EI, Cole LA, Kurman RJ, Kim SJ, Lurain JR, Seckl MJ, Sasaki S, Soper JT. Trophoblastic disease. Int J Gynaecol Obstet. 2012;119 Suppl 2:S130–S136. doi: 10.1016/S0020-7292(12)60026-5. [DOI] [PubMed] [Google Scholar]
- 12.Ngan HY, Seckl MJ, Berkowitz RS, Xiang Y, Golfier F, Sekharan PK, Lurain JR. Update on the diagnosis and management of gestational trophoblastic disease. Int J Gynaecol Obstet. 2015;131 Suppl 2:S123–S126. doi: 10.1016/j.ijgo.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Gillespie AM, Kumar S, Hancock BW. Treatment of persistent trophoblastic disease later than 6 months after diagnosis of molar pregnancy. Br J Cancer. 2000;82:1393–1395. doi: 10.1054/bjoc.1999.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal R, Teoh S, Short D, Harvey R, Savage PM, Seckl MJ. Chemotherapy and human chorionic gonadotropin concentrations 6 months after uterine evacuation of molar pregnancy: a retrospective cohort study. Lancet. 2012;379:130–135. doi: 10.1016/S0140-6736(11)61265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braga A, Torres B, Burlá M, Maestá I, Sun SY, Lin L, Madi JM, Uberti E, Viggiano M, Elias KM, Berkowitz RS. Is chemotherapy necessary for patients with molar pregnancy and human chorionic gonadotropin serum levels raised but falling at 6months after uterine evacuation? Gynecol Oncol. 2016;143:558–564. doi: 10.1016/j.ygyno.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Sarwar N, Newlands ES, Seckl MJ. Gestational trophoblastic neoplasia: the management of relapsing patients and other recent advances. Curr Oncol Rep. 2004;6:476–482. doi: 10.1007/s11912-004-0079-1. [DOI] [PubMed] [Google Scholar]
- 17.Braga A, Biscaro A, do Amaral Giordani JM, Viggiano M, Elias KM, Berkowitz RS, Seckl MJ. Does a human chorionic gonadotropin level of over 20,000 IU/L four weeks after uterine evacuation for complete hydatidiform mole constitute an indication for chemotherapy for gestational trophoblastic neoplasia? Eur J Obstet Gynecol Reprod Biol. 2018;223:50–55. doi: 10.1016/j.ejogrb.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Lima LLA, Padron L, Câmara R, Sun SY, Rezende J Filho, Braga A. The role of surgery in the management of women with gestational trophoblastic disease. Rev Col Bras Cir. 2017;44:94–101. doi: 10.1590/0100-69912017001009. [DOI] [PubMed] [Google Scholar]
- 19.Delmanto LRG, Maestá I, Braga A, Michelin OC, Passos JRS, Gaiotto FR, Rudge MVC. Are curves of human chorionic gonadotropin useful in the early diagnosis of post-molar trophoblastic neoplasia? Rev Bras Ginecol Obstet. 2007;29:506–510. [Google Scholar]
- 20.Committee on Practice Bulletins-Gynecology, American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #53. Diagnosis and treatment of gestational trophoblastic disease. Obstet Gynecol. 2004;103:1365–1377. doi: 10.1097/00006250-200406000-00051. [DOI] [PubMed] [Google Scholar]
- 21.Kani KK, Lee JH, Dighe M, Moshiri M, Kolokythas O, Dubinsky T. Gestatational trophoblastic disease: multimodality imaging assessment with special emphasis on spectrum of abnormalities and value of imaging in staging and management of disease. Curr Probl Diagn Radiol. 2012;41:1–10. doi: 10.1067/j.cpradiol.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Lima LL, Parente RC, Maestá I, Amim Junior J, de Rezende Filho JF, Montenegro CA, Braga A. Clinical and radiological correlations in patients with gestational trophoblastic disease. Radiol Bras. 2016;49:241–250. doi: 10.1590/0100-3984.2015.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mapelli P, Mangili G, Picchio M, Gentile C, Rabaiotti E, Giorgione V, Spinapolice EG, Gianolli L, Messa C, Candiani M. Role of 18F-FDG PET in the management of gestational trophoblastic neoplasia. Eur J Nucl Med Mol Imaging. 2013;40:505–513. doi: 10.1007/s00259-012-2324-4. [DOI] [PubMed] [Google Scholar]
- 24.Dhillon T, Palmieri C, Sebire NJ, Lindsay I, Newlands ES, Schmid P, Savage PM, Frank J, Seckl MJ. Value of whole body 18FDG-PET to identify the active site of gestational trophoblastic neoplasia. J Reprod Med. 2006;51:879–887. [PubMed] [Google Scholar]
- 25.Uberti EM, Fajardo Mdo C, da Cunha AG, Rosa MW, Ayub AC, Graudenz Mda S, Schmid H. Prevention of postmolar gestational trophoblastic neoplasia using prophylactic single bolus dose of actinomycin D in high-risk hydatidiform mole: a simple, effective, secure and low-cost approach without adverse effects on compliance to general follow-up or subsequent treatment. Gynecol Oncol. 2009;114:299–305. doi: 10.1016/j.ygyno.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Wang Q, Fu J, Hu L, Fang F, Xie L, Chen H, He F, Wu T, Lawrie TA. Prophylactic chemotherapy for hydatidiform mole to prevent gestational trophoblastic neoplasia. Cochrane Database Syst Rev. 2017;9:CD007289. doi: 10.1002/14651858.CD007289.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seckl M. Time to stop offering prophylactic chemotherapy after molar pregnancies? BJOG. 2014;121:1420. [PubMed] [Google Scholar]
- 28.Eysbouts YK, Massuger LFAG, IntHout J, Lok CAR, Sweep FCGJ, Ottevanger PB. The added value of hysterectomy in the management of gestational trophoblastic neoplasia. Gynecol Oncol. 2017;145:536–542. doi: 10.1016/j.ygyno.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Osborne RJ, Filiaci VL, Schink JC, Mannel RS, Behbakht K, Hoffman JS, Spirtos NM, Chan JK, Tidy JA, Miller DS. Second Curettage for Low-Risk Nonmetastatic Gestational Trophoblastic Neoplasia. Obstet Gynecol. 2016;128:535–542. doi: 10.1097/AOG.0000000000001554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Trommel NE, Massuger LF, Verheijen RH, Sweep FC, Thomas CM. The curative effect of a second curettage in persistent trophoblastic disease: a retrospective cohort survey. Gynecol Oncol. 2005;99:6–13. doi: 10.1016/j.ygyno.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 31.Pezeshki M, Hancock BW, Silcocks P, Everard JE, Coleman J, Gillespie AM, Tidy J, Coleman RE. The role of repeat uterine evacuation in the management of persistent gestational trophoblastic disease. Gynecol Oncol. 2004;95:423–429. doi: 10.1016/j.ygyno.2004.08.045. [DOI] [PubMed] [Google Scholar]
- 32.Lurain JR. Gestational trophoblastic disease II: classification and management of gestational trophoblastic neoplasia. Am J Obstet Gynecol. 2011;204:11–18. doi: 10.1016/j.ajog.2010.06.072. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein DP, Berkowitz RS, Horowitz NS. Optimal management of low-risk gestational trophoblastic neoplasia. Expert Rev Anticancer Ther. 2015;15:1293–1304. doi: 10.1586/14737140.2015.1088786. [DOI] [PubMed] [Google Scholar]
- 34.Lawrie TA, Alazzam M, Tidy J, Hancock BW, Osborne R. First-line chemotherapy in low-risk gestational trophoblastic neoplasia. Cochrane Database Syst Rev. 2016;(6):CD007102. doi: 10.1002/14651858.CD007102.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sita-Lumsden A, Short D, Lindsay I, Sebire NJ, Adjogatse D, Seckl MJ, Savage PM. Treatment outcomes for 618 women with gestational trophoblastic tumours following a molar pregnancy at the Charing Cross Hospital, 2000-2009. Br J Cancer. 2012;107:1810–1814. doi: 10.1038/bjc.2012.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sita-Lumsden A, Medani H, Fisher R, Harvey R, Short D, Sebire N, Savage P, Lim A, Seckl MJ, Agarwal R. Uterine artery pulsatility index improves prediction of methotrexate resistance in women with gestational trophoblastic neoplasia with FIGO score 5-6. BJOG. 2013;120:1012–1015. doi: 10.1111/1471-0528.12196. [DOI] [PubMed] [Google Scholar]
- 37.Jiang F, Wan XR, Xu T, Feng FZ, Ren T, Yang JJ, Zhao J, Yang T, Xiang Y. Evaluation and suggestions for improving the FIGO 2000 staging criteria for gestational trophoblastic neoplasia: A ten-year review of 1420 patients. Gynecol Oncol. 2018;149:539–544. doi: 10.1016/j.ygyno.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Eysbouts YK, Ottevanger PB, Massuger LFAG, IntHout J, Short D, Harvey R, Kaur B, Sebire NJ, Sarwar N, Sweep FCGJ, Seckl MJ. Can the FIGO 2000 scoring system for gestational trophoblastic neoplasia be simplified? A new retrospective analysis from a nationwide dataset. Ann Oncol. 2017;28:1856–1861. doi: 10.1093/annonc/mdx211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGrath S, Short D, Harvey R, Schmid P, Savage PM, Seckl MJ. The management and outcome of women with post-hydatidiform mole 'low-risk' gestational trophoblastic neoplasia, but hCG levels in excess of 100 000 IU l(-1) Br J Cancer. 2010;102:810–814. doi: 10.1038/sj.bjc.6605529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alifrangis C, Agarwal R, Short D, Fisher RA, Sebire NJ, Harvey R, Savage PM, Seckl MJ. EMA/CO for high-risk gestational trophoblastic neoplasia: good outcomes with induction low-dose etoposide-cisplatin and genetic analysis. J Clin Oncol. 2013;31:280–286. doi: 10.1200/JCO.2012.43.1817. [DOI] [PubMed] [Google Scholar]
- 41.Horowitz NS, Goldstein DP, Berkowitz RS. Placental site trophoblastic tumors and epithelioid trophoblastic tumors: Biology, natural history, and treatment modalities. Gynecol Oncol. 2017;144:208–214. doi: 10.1016/j.ygyno.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 42.Schmid P, Nagai Y, Agarwal R, Hancock B, Savage PM, Sebire NJ, Lindsay I, Wells M, Fisher RA, Short D, Newlands ES, Wischnewsky MB, Seckl MJ. Prognostic markers and long-term outcome of placental-site trophoblastic tumours: a retrospective observational study. Lancet. 2009;374:48–55. doi: 10.1016/S0140-6736(09)60618-8. [DOI] [PubMed] [Google Scholar]
- 43.Kaur B, Short D, Fisher RA, Savage PM, Seckl MJ, Sebire NJ. Atypical placental site nodule (APSN) and association with malignant gestational trophoblastic disease; a clinicopathologic study of 21 cases. Int J Gynecol Pathol. 2015;34:152–158. doi: 10.1097/PGP.0000000000000128. [DOI] [PubMed] [Google Scholar]
- 44.Bolze PA, Patrier S, Massardier J, Hajri T, Abbas F, Schott AM, Allias F, Devouassoux-Shisheboran M, Freyer G, Golfier F, You B. PD-L1 Expression in Premalignant and Malignant Trophoblasts From Gestational Trophoblastic Diseases Is Ubiquitous and Independent of Clinical Outcomes. Int J Gynecol Cancer. 2017;27:554–561. doi: 10.1097/IGC.0000000000000892. [DOI] [PubMed] [Google Scholar]
- 45.Ghorani E, Kaur B, Fisher RA, Short D, Joneborg U, Carlson JW, Akarca A, Marafioti T, Quezada SA, Sarwar N, Seckl MJ. Pembrolizumab is effective for drug-resistant gestational trophoblastic neoplasia. Lancet. 2017;390:2343–2345. doi: 10.1016/S0140-6736(17)32894-5. [DOI] [PubMed] [Google Scholar]
- 46.Brewer JI, Eckman TR, Dolkart RE, Torok EE, Webster A. Gestational trophoblastic disease. A comparative study of the results of therapy in patients with invasive mole and with choriocarcinoma. Am J Obstet Gynecol. 1971;109:335–340. [PubMed] [Google Scholar]
- 47.Seckl MJ, Sebire NJ, Berkowitz RS. Gestational trophoblastic disease. Lancet. 2010;376:717–729. doi: 10.1016/S0140-6736(10)60280-2. [DOI] [PubMed] [Google Scholar]
- 48.Dantas PR, Maestá I, Cortés-Charry R, Growdon WB, Braga A, Rudge MV, Berkowitz RS. Influence of hydatidiform mole follow-up setting on postmolar gestational trophoblastic neoplasia outcomes: a cohort study. J Reprod Med. 2012;57:305–309. [PubMed] [Google Scholar]
- 49.Bagshawe KD. Trophoblastic reminiscences. J Reprod Med. 2006;51:849–854. [PubMed] [Google Scholar]
- 50.Braga A, Uberti EM, Fajardo Mdo C, Viggiano M, Sun SY, Grillo BM, Padilha SL, de Andrade JM, de Souza CB, Madi JM, Maestá I, Silveira E. Epidemiological report on the treatment of patients with gestational trophoblastic disease in 10 Brazilian referral centers: results after 12 years since International FIGO 2000 Consensus. J Reprod Med. 2014;59:241–247. [PubMed] [Google Scholar]
- 51.Braga A, Burlá M, Freitas F, Uberti E, Viggiano M, Sun SY, Maestá I, Elias KM, Berkowitz RS Brazilian Network for Gestational Trophoblastic Disease Study Group. Centralized Coordination of Decentralized Assistance for Patients with Gestational Trophoblastic Disease in Brazil: A Viable Strategy for Developing Countries. J Reprod Med. 2016;61:224–229. [PubMed] [Google Scholar]
- 52.Kohorn E. Regional centers for trophoblastic disease. Am J Obstet Gynecol. 2007;196:95–96. doi: 10.1016/j.ajog.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Sun SY, Goldstein DP, Bernstein MR, Horowitz NS, Mattar R, Maestá I, Braga A, Berkowitz RS. Maternal Near Miss According to World Health Organization Classification Among Women with a Hydatidiform Mole: Experience at the New England Trophoblastic Disease Center, 1994-2013. J Reprod Med. 2016;61:210–214. [PubMed] [Google Scholar]
- 54.Braga A, Moraes V, Maestá I, Amim Júnior J, Rezende-Filho Jd, Elias K, Berkowitz R. Changing Trends in the Clinical Presentation and Management of Complete Hydatidiform Mole Among Brazilian Women. Int J Gynecol Cancer. 2016;26:984–990. doi: 10.1097/IGC.0000000000000682. [DOI] [PubMed] [Google Scholar]
- 55.Braga A, Maestá I, Short D, Savage P, Harvey R, Seckl MJ. Hormonal contraceptive use before hCG remission does not increase the risk of gestational trophoblastic neoplasia following complete hydatidiform mole: a historical database review. BJOG. 2016;123:1330–1335. doi: 10.1111/1471-0528.13617. [DOI] [PubMed] [Google Scholar]
- 56.Dantas PRS, Maestá I, Filho JR, Junior JA, Elias KM, Howoritz N, Braga A, Berkowitz RS. Does hormonal contraception during molar pregnancy follow-up influence the risk and clinical aggressiveness of gestational trophoblastic neoplasia after controlling for risk factors? Gynecol Oncol. 2017;147:364–370. doi: 10.1016/j.ygyno.2017.09.007. [DOI] [PubMed] [Google Scholar]