Abstract
Background: Acyl-CoA: cholesterol acyltransferases (ACAT) is the only enzyme that catalyzes the synthesis of cholesterol esters (CE) from free cholesterol and long-chain fatty acyl-CoA and plays a critical role in cellular cholesterol homeostasis. In the present study, our primary objective was to explore whether the single-nucleotide polymorphisms (SNPs) in ACAT-2 gene were associated with coronary artery disease (CAD) in Uygur subjects, in Xinjiang, China.
Methods: We designed a case–control study including 516 CAD patients and 318 age- and sex-matched control subjects. Using the improved multiplex ligation detection reaction (iMLDR) method, we genotyped two SNPs (rs28765985 and rs7308390) of ACAT-2 gene in all subjects.
Results: We found that the genotypes, the dominant model (CC + CT vs TT) and over-dominant model (CT vs CC + TT) of rs28765985 were significantly different between CAD patients and the controls (P=0.027, P=0.012 and P=0.035, respectively). The rs28765985 C allele was associated with a significantly elevated CAD risk [CC/CT vs TT: odds ratio (OR) = 1.48, 95% confidence interval (CI) = 1.02–2.16, P=0.04] after adjustment for confounders. The TC and LDL-C levels were significantly higher in rs28765985 CC/CT genotypes than that in TT genotypes (P<0.05).
Conclusions: Rs28765985 of ACAT-2 gene are associated with CAD in Uygur subjects. Subjects with CC/CT genotype or C allele of rs28765985 were associated with an increased risk of CAD.
Keywords: ACAT-2 gene, coronary artery disease, polymorphism, susceptibility
Introduction
Coronary artery disease (CAD) is a common cardiovascular disease and has been the leading cause of death in the world wide [1]. CAD is recognized as a complex disease and is caused by multiple risk factors, including smoking, chronic inflammation, immune diseases, hypertension, diabetes and hyperlipidemia [2–6]. In the past decades, with the development of genome technology, genome wide association study (GWAS) and the next-generation sequencing (NGS) were more and more used in etiology research and a number of genetic susceptibility genes associated with the risk of CAD have been discovered [7–9].
Acyl-CoA: cholesterol acyltransferases (ACATs), known as sterol O-acyltransferases (SOATs), are microsomal proteins responsible for catalyzing the synthesis of intracellular cholesterol ester (CE) from free cholesterol and long-chain fatty acyl-CoA and thus play an important role in cellular cholesterol homeostasis [10]. There exist two subtypes of ACAT, ACAT-1 is ubiquitously expressed in a variety of tissues while ACAT-2 is expressed in a species-specific manner and exclusively expressed in enterocyte of the intestine and hepatocyte of the liver [10]. One of the important roles of ACAT-2 is to provide CEs for lipoprotein assemblies, including chylomicrons and very low-density lipoproteins (VLDL) [11].
Previous studies have found that deletion of ACAT-2 may delay the development of atherosclerosis in animal models. Researchers found that both global restricted and tissue-restricted ACAT-2 gene deletions mice had significantly lower levels of intestinal cholesterol absorption and reduced percentages of hole sterol palmitate and cholesterol oleate in LDL CE. Further histological experiment also showed that deletion of ACAT-2 gene in LDLr/ mice significantly delayed the development of atherosclerosis [12–14]. All these suggested that ACAT-2 play an important role in cholesterol metabolism and genetic variations in the ACAT-2 gene may be associated with the individual’s susceptibility to CAD.
Genetic factors may affect CAD via distinct mechanisms and an individual with a certain gene polymorphism may be more susceptible to CAD [15,16]. Therefore, gene polymorphism might be not only an independent risk factor of CAD but also biomarkers for the diagnosis, treatment and prognosis of CAD in precision medicine. However, little is known about the association between ACAT-2 polymorphisms and CAD. The present case–control study aimed to explore the association of ACAT-2 gene polymorphisms with CAD in a Uygur population.
Methods
Ethical approval of the study protocol
The present study was approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University (Xinjiang, China). It was conducted according to the standards of the Declaration of Helsinki. All of the patients provided written informed consent and explicitly provided permission for DNA analyses, as well as for the collection of relevant clinical data.
Subjects
The analyses were carried out in a case–control study design. A total of 834 subjects (516 diagnosed CAD cases and 318 healthy controls) of Uygur were recruited from the First Affiliated Hospital of Xinjiang Medical University between August 2010 and October 2016. CAD was defined as presence of at least one significant coronary artery stenosis of >50% luminal diameter based on the coronary angiography. In the present study, we only collected patients with stable angina as CAD group, and patients with acute coronary syndrome were excluded. Patients with concomitant valvar heart disease, congenital heart disease and/or no ischemic cardiomyopathy were also excluded. All control subjects were selected from volunteers who had angiographically normal coronary arteries and had no history of CAD [17]. Coronary angiography in the control individuals was performed for the evaluation of chest pain. Individuals were excluded from the present study if they had: a history of CAD; electrocardiographic signs of CAD; regional wall motion abnormalities; relevant valvar abnormalities in echocardiograms and/or carotid atherosclerosis. Hypertension was defined as a systolic blood pressure ≥ 140 mmHg and/or a diastolic blood pressure ≥ 90 mmHg at least on two distinct occasions. Diabetes mellitus was defined as two fasting plasma glucose level ≥ 7.0 mmol/l. The following information was collected: age, gender, hypertension, diabetes, total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C).
Genotyping
Fasting blood samples drawn via venipuncture in the catheter room were taken from all participants before cardiac catheterization. The blood samples were drawn into a 5-ml ethylene diamine tetraacetic acid (EDTA) tube and centrifuged at 4000 × g for 5 min to separate the plasma content. Genomic DNA was extracted from the peripheral leukocytes using the standard phenol–chloroform method. The DNA samples were stored at −80°C until use. For use, the DNA was diluted to a concentration of 50 ng/μl. Using Haploview 4.2 software and International HapMap Project website phase I &II database (http:// www.hapmap.org), we obtained four tag SNPs of ACAT-2: SNP1 (rs28765985) and SNP2 (rs7308390), by using minor allele frequency (MAF) ≥0.05 and linkage disequilibrium patterns with r2 ≥ 0.8 as a cutoff. The SNP genotyping was performed using an improved multiplex ligation detection reaction (iMLDR) technique (Genesky Biotechnologies Inc., Shanghai, China). The primers for the polymerase chain reaction (PCR) and the probes for the LDR were listed in Table 1. Genotyping was performed in a blinded fashion without knowledge of the patients’ clinical data, and a total of 10% of the genotyped samples were duplicated to monitor genotyping quality.
Table 1. The primer sequences for each SNP.
| SNPs | Primers or probes | Sequences |
|---|---|---|
| rs28765985 | Upstream primer | CCCCAGAGTAGCTTCTGTTGTAATAGC |
| Downstream primer | GACAGGATTTCTCCATATTGGTCAGG | |
| RC | TCTCTCGGGTCAATTCGTCCTTTCAGGCTGGTCTCAAACTCACG | |
| RP | ACCTCAGGTGATCTGCCCGTTTTTTTTTTTT | |
| RT | TGTTCGTGGGCCGGATTAGTTCAGGCTGGTCTCAAACTCACA | |
| rs7308390 | Upstream primer | |
| Downstream primer | ||
| RC | TTCCGCGTTCGGACTGATATCACATCTGCCTCAGGGCAAGTTG | |
| RP | TCATTGAGAAGCATTTGAGARTGGCTTTTTTTTTTTTTTTTT | |
| RT | TACGGTTATTCGGGCTCCTGTCACATCTGCCTCAGGGCAAGCTA |
Statistical analysis
The data analysis was performed using SPSS version 17.0 for Windows (SPSS Inc., Chicago, IL, U.S.A.). The Hardy–Weinberg equilibrium was assessed via Chi-square analysis. The measurement data are shown as the means ± SD, and the differences between the CAD subjects and the control subjects were assessed using an independent-sample t-test. Differences in the enumeration data, such as the frequencies of smoking, drinking, hypertension and ACAT-2 genotypes between the CAD patients and the control subjects, were analyzed using the Chi-square test. Additionally, logistic regression analyses with effect ratios (odds ratio [OR] and 95% CI) were used to assess the contribution of the major risk factors. The dominant model is defined as heterozygote+homozygote variant vs. homozygote wild; the recessive mode is defined as homozygote variant vs. heterozygote+homozygote wild; the overdominant model is defined as heterozygote vs. homozygote wild and homozygote variant vs. homozygote wild respectively; the codominant model is defined as heterozygote vs. homozygote variant +homozygote wild. A P value < 0.05 was considered to be statistically significant
Result
Characteristics of subjects
The baseline characteristics of 516 CAD patients and 318 control subjects were shown in Table 2. The mean age, BMI, TG levels and the prevalence of female, smoking and drinking were similar between CAD patients and the controls (all P>0.05). The CAD patients and controls differed significantly with regards to hypertension (P=0.002), diabetes mellitus (DM) (P=0.009), TC (P=0.002), HDL-C (P=0.014), LDL-C (P=0.001) and glucose (P=0.001).
Table 2. Clinical and metabolic characteristics of subjects.
| Characteristics | Control (n=318) | CAD (n=516) | χ2 or t | P value |
|---|---|---|---|---|
| Age, mean (SD) | 55.98 ± 10.02 | 56.85 ± 10.05 | 1.219 | 0.223 |
| Sex, female (%) | 108(34.0) | 155(30.0) | 1.403 | 0.236 |
| Hypertension, n (%) | 123(38.7) | 256(49.6) | 9.486 | 0.002 |
| Diabetes, n (%) | 6(1.9) | 29(5.6) | 6.821 | 0.009 |
| Smoking, n (%) | 85(26.7) | 166(32.2) | 2.769 | 0.096 |
| Drinking, n (%) | 55(17.3) | 104(20.2) | 1.043 | 0.307 |
| BMI, mean (SD) | 26.71 ± 3.99 | 26.76 ± 3.59 | 0.22 | 0.826 |
| TG, mean (SD) | 1.82 ± 1.23 | 1.95 ± 1.08 | 1.546 | 0.123 |
| TC, mean (SD) | 4.09 ± 1.30 | 4.39 ± 1.32 | 3.119 | 0.002 |
| HDL-C, mean (SD) | 0.96 ± 0.27 | 0.90 ± 0.33 | 2.453 | 0.014 |
| LDL-C, mean (SD) | 2.70 ± 0.81 | 2.94 ± 1.07 | 3.49 | 0.001 |
Continuous variables are expressed as mean ± SD. Categori cal variables are expressed as percentages.
The P value of the continuous variables was calculated by the independent samples t test. The P value of the categorical variables was calculated by χ2 test.
Abbreviations: BMI, body mass index; HDL-C, high-density lipoprotein-cholesterol; LDL-C low-density lipoprotein-cholesterol; TC, total cholesterol; TG, triglyceride.
Distributions of genotype and allele in CAD patients and controls
Table 3 shows the distribution of genotypes and alleles for the two SNPs (rs28765985 and rs7308390) of the ACAT-2 gene. The genotype distributions of the two SNPs were in accordance with the Hardy–Weinberg equilibrium in controls (all P>0.05). For rs28765985, the distribution of the genotypes, the dominant model (CC + CT vs TT), the overdominant model (CT vs CC + TT) showed significant differences between CAD patients and the controls (P=0.027, P=0.012 and P=0.035, respectively). Whereas there were no significant differences between CAD patients and controls in the distribution of rs7308390 genotypes, dominant model, recessive model and overdominant model (P=0.776, P=0.488, P=0.73 and P=0.545, respectively).
Table 3. Distribution of SNPs of ACAT-2 gene in CAD and controls.
| Genotype | Model | Case (n, %) | Control (n, %) | P | Crude OR (95% CI) | P | |
|---|---|---|---|---|---|---|---|
| rs28765985 | Codominant | TT | 396 (76.7) | 267 (84.0) | 0.027 | 1 | 0.031 |
| CT | 110 (21.3) | 49 (15.4) | 1.514 (1.045–2.193) | 0.028 | |||
| CC | 10 (1.9) | 2 (0.6) | 3.371 (0.733–15.508) | 0.119 | |||
| Dominant | TT | 396 (76.7) | 267 (84.0) | 0.012 | 1 | 0.013 | |
| CT+CC | 120 (23.3) | 51 (16.0) | 1.586 (1.104–2.280) | ||||
| Recessive | TT+CT | 506 (98.1) | 316 (99.4) | 0.123 | 1 | 0.143 | |
| CC | 10 (1.9) | 2 (0.6) | 3.123 (0.680–14.344) | ||||
| Overdominant | CC+TT | 406 (78.7) | 269 (84.6) | 0.035 | 1 | 0.036 | |
| CT | 110 (21.3) | 49 (15.4) | 1.487 (1.027–2.154) | ||||
| rs7308390 | Codominant | CC | 427 (82.8) | 269 (84.6) | 0.776 | 1 | 0.776 |
| CT | 81 (15.7) | 45 (14.2) | 1.134 (0.764–1.683) | 0.533 | |||
| TT | 8 (1.6) | 4 (1.3) | 1.260 (0.376–4.225) | 0.708 | |||
| Dominant | CC | 427 (82.8) | 269 (84.6) | 0.488 | 1 | 0.488 | |
| CT+TT | 89 (17.2) | 49 (15.4) | 1.144 (0.782–1.674) | ||||
| Recessive | CT+CC | 508 (98.4) | 314 (98.7) | 0.73 | 1 | 0.731 | |
| TT | 8 (1.6) | 4 (1.3) | 1.236 (0.369–4.139) | ||||
| Overdominant | CC+TT | 435 (84.3) | 273 (85.8) | 0.545 | 1 | 0.545 | |
| CT | 81 (15.7) | 45 (14.2) | 1.130 (0.761–1.676) |
Tables 4 and 5 show the multivariable logistic regression analyses of the major confounding factors for CAD. Following the multivariate adjustments for the confounders, such as age, sex, BMI, TG, TC, HDL-C, LDL-C and prevalence of hypertension, DM, smoking and drinking, rs28765985 is still an independent risk factor for CAD [CC/CT vs. TT: odds ratio (OR) = 1.48, 95% confidence interval (CI) = 1.02–2.16, P=0.04]. Whereas after adjustment for the confounders, rs7308390 is still not the independent risk factor for CAD (P=0.383).
Table 4. Results of logistic analysis (rs28765985).
| OR | 95% CI | P value | |
|---|---|---|---|
| rs28765985 (CT+CC vs. TT) | 1.483 | 1.018–2.161 | 0.04 |
| Gender | 1.189 | 0.855–1.654 | 0.304 |
| Age | 1.007 | 0.992–1.022 | 0.367 |
| BMI | 0.985 | 0.947–1.025 | 0.461 |
| Hypertension | 1.515 | 1.122–2.046 | 0.007 |
| TG | 1.063 | 0.932–1.213 | 0.364 |
| TC | 1.146 | 1.007–1.303 | 0.038 |
| HDL-C | 0.485 | 0.294–0.800 | 0.005 |
| LDL-C | 1.284 | 1.068–1.542 | 0.008 |
| Diabetes | 2.739 | 1.105–6.794 | 0.03 |
| Smoking | 1.294 | 0.869–1.925 | 0.204 |
Table 5. Results of logistic analysis (7308390).
| OR | 95% CI | P value | |
|---|---|---|---|
| rs7308390 (CT+TT vs. CC) | 1.193 | 0.803–1.774 | 0.383 |
| Gender | 1.181 | 0.849–1.642 | 0.322 |
| Age | 1.006 | 0.991–1.021 | 0.417 |
| BMI | 0.982 | 0.944–1.021 | 0.363 |
| Hypertension | 1.513 | 1.121–2.041 | 0.007 |
| TG | 1.066 | 0.934–1.217 | 0.342 |
| TC | 1.155 | 1.015–1.315 | 0.029 |
| HDL-C | 0.471 | 0.287–0.775 | 0.003 |
| LDL-C | 1.31 | 1.090–1.574 | 0.004 |
| Diabetes | 2.699 | 1.089–6.690 | 0.032 |
| Smoking | 1.273 | 0.856–1.891 | 0.233 |
Stratified analysis between ACAT-2 gene polymorphisms and CAD risk
Gender, hypertension, smoking and drinking are the most common risk factors of CAD. To further analyze the interaction between CAD and environment, we performed stratification analyses in terms of gender, hypertension, smoking and drinking status to evaluate how these variables modified the association between the SNPs (rs28765985 and rs7308390) and CAD risk (Table 6). For smoker, the dominant model (CC/CT vs. TT) of rs28765985 remains significantly associated with CAD (OR = 2.41, 95% CI = 1.06–5.48, P=0.036). For drinker, the dominant model (CC/CT vs. TT) of rs28765985 remains significantly associated with CAD (OR = 3.17, 95% CI = 1.06–9.45, P=0.036).
Table 6. Stratified analysis between ACAT gene polymorphisms and CAD risk.
| Case/control | rs28765985 (Genetype) | Adjusted OR (95% CI) | P value | rs7308390 (Genetype) | Adjusted OR (95% CI) | P value | |
|---|---|---|---|---|---|---|---|
| Gender | |||||||
| Male | 361/210 | CT+CC/TT | 1.524 (0.963–2.413) | 0.072 | CT+TT/CC | 1.583 (0.987–2.540) | 0.057 |
| Female | 155/108 | CT+CC/TT | 1.800 (0.878–3.689) | 0.108 | CT+TT/CC | 0.566 (0.243–1.318) | 0.187 |
| Hypertension | |||||||
| No | 260/195 | CT+CC/TT | 1.538 (0.939–2.520) | 0.088 | CT+TT/CC | 1.360 (0.798–2.317) | 0.59 |
| Yes | 256/123 | CT+CC/TT | 1.546 (0.844–2.829) | 0.158 | CT+TT/CC | 1.078 (0.588–1.979) | 0.807 |
| Smoking | |||||||
| No | 350/233 | CT+CC/TT | 1.342 (0.863–2.086) | 0.192 | CT+TT/CC | 0.914 (0.555–1.506) | 0.725 |
| Yes | 166/85 | CT+CC/TT | 2.406 (1.057–5.479) | 0.036 | CT+TT/CC | 2.027 (0.997–4.121) | 0.051 |
| Drinking | |||||||
| No | 412/263 | CT+CC/TT | 1.364 (0.904–2.057) | 0.139 | CT+TT/CC | 1.012 (0.645–1.588) | 0.96 |
| Yes | 104/55 | CT+CC/TT | 3.171 (1.064–9.445) | 0.038 | CT+TT/CC | 1.897 (0.793–4.539) | 0.15 |
Genotypes and serum lipid levels
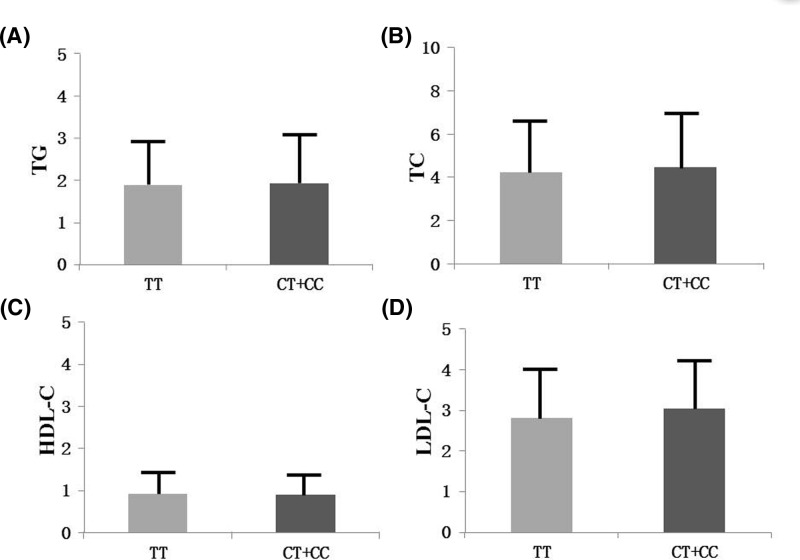
As shown in Figure 1, the TC and LDL-C levels were significantly higher in rs28765985 CC/CT genotypes than that in TT genotypes (P=0.028 and P=0.003 respectively).
Figure 1. Association between rs28765985 and lipid parameters.
(A) There exists no significant difference of TG between rs28765985 CC/CT genotypes and TT genotypes (P>0.05). (B) The TC levels were significantly higher in rs28765985 CC/CT genotypes than that in TT genotypes (P<0.05). (C) There exists no significant difference of HDL-C between rs28765985 CC/CT genotypes and TT genotypes (P>0.05). (D) The LDL-C levels were significantly higher in rs28765985 CC/CT genotypes than that in TT genotypes (P<0.05).
Discussion
In the present study, we investigated associations between two SNPs in the ACAT-2 gene and CAD risk in a Chinese Uygur population. Our results show that rs28765985 is significantly associated with CAD susceptibility. Our findings also demonstrated significant associations between rs28765985 of ACAT-2 gene and TC and LDL-C concentration.
ACAT is the only enzyme that catalyzes the synthesis of CE from free cholesterol and long-chain fatty acyl-CoA in cells and plays an important role in the absorption, transport and storage of lipids such as cholesterol and fatty acids [10]. ACAT has always been thought to be drug targets for therapeutic intervention of several diseases including atherosclerosis, cancer, Alzheimer and gallbladder disease [18–21]. There exist two subtypes of ACAT in mammalian, ACAT-1 and ACAT-2. These two subtypes are quite different with regard to gene chromosomal locations, tissue-specific expression and substrate specificity. ACAT-2 is specifically expressed in hepatocytes and enterocytes, where CE can be incorporated into very-low-density lipoprotein (VLDL), as well as cytoplasmic lipid droplets. Research have demonstrated that increased cholesteryl ester secretion occurs in livers of animals fed with cholesterol and is closely related to the occurrence and extent of coronary artery atherosclerosis. All these suggested that ACAT2-derived cholesteryl esters may promote arterial cholesterol accumulation during coronary artery atherosclerosis.
The development of atherosclerosis in ACAT-2 deficiency was first studied by Willner et al. [13]. They investigated the contribution of ACAT2-derived cholesterol esters in ACAT-2–/– /ApoE–/– mice model and ACAT-2+/+/ApoE–/– (control) mice model. Although ACAT-2–/– /ApoE–/– mice and ACAT-2+/+/ApoE–/– mice had similar elevated plasma apoB and total plasma lipids, the main components of the lipid are different. The primarily lipid cores of the apoB-containing lipoproteins were triglycerides, but not CE, in ACAT-2–/– /ApoE–/– mice. Further, ACAT-2–/– /ApoE–/– mice also had lower level of aortic atherosclerosis compared with controls. These dates suggested that the amount of CE exists in the core of lipoproteins is apparently more important in atherosclerosis. Whereas ACAT-2 deficiency in mice may restrict synthesis of CE and reduce the accumulation of CE in the plasma lipoproteins and atherosclerosis development in ACAT-2–/–/ApoE–/– mice. Lee et al. [12] got the similar conclusion in LDLr–/–/ mice. They found that ACAT-2−/− deficiency had a significant decrease of plasma CEs. ACAT-2−/− deficiency mice lead to not only a 78% reduce of aortic surface area as lesion, but also 88% reduce of CE deposited in atherosclerotic plaques.
Previous studies have also demonstrated an atherogenic potential of ACAT2-derived CE in humans. Ma et al. [22] performed a study on the association of plasma phospholipid and cholesterol ester fatty acid composition to carotid artery intima-media thickness in 2872 participants and found that the carotid intima-media thickness was significantly associated with ACAT-2-derived CE in lipoproteins. Furthermore, Warensjö et al. [23] performed a community-based prospective study with a maximum follow-up of 33 years and found that ACAT2-derived CE in lipoproteins was significantly associated with CVD mortality. To identify the association of plasma CE levels derived from the ACAT-2 and acute coronary syndrome, Miller et al. [24] performed a single-center prospective cohort study in America. They found that ACAT2-derived CE in lipoproteins have strong potential value in the prediction of acute coronary syndrome.
The association between ACAT-2 gene polymorphisms and CAD and serum lipid levels was poor. He et al. [25] performed a study in 809 CAD patients and 1304 controls from three distinct Singaporean ethnic groups (1228 Chinese, 367 Malays and 518 Indians). They found that the 734C>T variant in ACAT-2 gene were significantly associated with plasma concentrations of apoA-1, apoB and lipoprotein (a) in Indians and with apoA-1 in Malays and the 734T allele frequency was significantly lower in CAD than that in controls in Chinese. The 41A>G variant in ACAT-2 gene is associated with total cholesterol in Indians. Chen et al. [26] performed a study to analyzed relationship between plasma HDL-C levels and putatively functional SNPs in 42 genes in a Caucasian population. They found that rs2272296 in ACAT-2 gene were independent genetic determinants of plasma HDL-C levels. From the above example, we have considered that the association between ACAT-2 gene and CAD and lipid levels was different on account of polymorphisms of the ACAT-2 gene and ethnic differences.
In the present study, we genotyped polymorphisms of rs28765985 and rs7308390 SNPs in the ACAT-2 gene and found that rs28765985 was associated with CAD and lipid levels. The rs28765985 CC/CT genotype has a higher frequency in CAD patients than that in controls. After adjustments for several confounders, this association remained exist, indicating that the rs28765985 CC/CT were independent risk factors for CAD and the risk of CAD was increased in the subjects with the C allele in rs28765985. Furthermore, we found that C allele (CC/CT) carriers in rs28765985 have higher levels of TC and LDL-C when compared with C allele non-carriers. These results might provide convincing evidence for assuming subjects who carry C allele in rs28765985 may have increased susceptibility to CAD than that who carry T allele.
Despite the promising findings in the present study, several limitations should be mentioned. First of all, we only drawed conclusions based on the present observational association study, we failed to get a cause-and-effect relationship between risk factors and CAD. Second, the sample size of present study is relatively moderate. The association between rs28765985 and CAD should be confirmed by studies with larger sample size. Third, the present study lacked functional validation. Additional studies need to be undertaken to clarify the underlying molecular mechanism that associates the ACAT-2 gene polymorphisms with CAD.
Conclusion
The present study revealed that rs28765985 of ACAT-2 gene is associated with CAD in Uygur subjects. Subjects with CC/CT genotype or C allele of rs28765985 were associated with an increased risk of CAD. The CC/CT genotypes of rs28765985 were also associated with increased serum TC and LDL-C levels.
Availability to data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ACAT
Acyl-CoA: cholesterol acyltransferases
- CAD
coronary artery disease
- CE
cholesterol ester
- GWAS
genome wide association study
- HDL-C
high-density lipoprotein-cholesterol
- LDL-C
low-density lipoprotein-cholesterol
- SNP
single-nucleotide polymorphism
- TC
total cholesterol
- TG
triglyceride
- VLDL
very low-density lipoprotein
Author Contribution
Y.T.W. participated in the study design, data collection, analysis of data and wrote the manuscript. B.M. participated in the study design, data collection. Y.T.M. and Z.Y.F. carried out the study design. Y.N.Y. and X.M. participated in the data collection. X.M.L., F.L. and B.D.C. participated in the analysis of data. All authors read and approved the final manuscript.
Funding
This work was supported financially by The Science and Technology support project Xinjiang Uygur Autonomous Region [2017E0269].
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University (Xinjiang, China). It was conducted according to the standards of the Declaration of Helsinki. Written informed consent was obtained from each participants, who explicitly provided permission for all DNA analyses and the collection of relevant clinical data.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Go A.S., Mozaffarian D., Roger V.L., Benjamin E.J., Berry J.D., Blaha M.J.. et al. (2014) Heart disease and stroke statistics-2014 update: a report from the American Heart Association. Circulation 129, e28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammal F., Ezekowitz J.A., Norris C.M., Wild T.C., Finegan B.A. and APPROACH Investigators (2014) Smoking status and survival: impact on mortality of continuing to smoke one year after the angiographic diagnosis of coronary artery disease, a prospective cohort study. BMC Cardiovasc. Disord. 14, 133 10.1186/1471-2261-14-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strissel K.J., Denis G.V. and Nikolajczyk B.S. (2014) Immune regulators of inflammation in obesity-associated type 2 diabetes and coronary artery disease. Curr. Opin. Endocrinol. Diab. Obes. 21, 330–338 10.1097/MED.0000000000000085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsen S.B., Grove E.L., Würtz M., Neergaard-Petersen S., Hvas A.M. and Kristensen S.D. (2015) The influence of low-grade inflammation on platelets in patients with stable coronary artery disease. Thromb. Haemost. 114, 519–529 10.1160/TH14-12-1007 [DOI] [PubMed] [Google Scholar]
- 5.Aronson D. and Edelman E.R. (2014) Coronary artery disease and diabetes mellitus. Cardiol. Clin. 32, 439–455 10.1016/j.ccl.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakahara T., Dweck M.R., Narula N., Pisapia D., Narula J. and Strauss H.W. (2017) Coronary artery calcification: from mechanism to molecular imaging. JACC Cardiovasc. Imaging 10, 582–593 10.1016/j.jcmg.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 7.Dichgans M., Malik R., König I.R., Rosand J., Clarke R. and Gretarsdottir S. (2014) Shared genetic susceptibility to ischemic stroke and coronary artery disease: a genome-wide analysis of common variants. Stroke 45, 24–36 10.1161/STROKEAHA.113.002707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikpay M., Goel A., Won H.H., Hall L.M., Willenborg C., Kanoni S.. et al. (2015) A comprehensive 1000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat. Genet. 47, 1121–1130 10.1038/ng.3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girelli D., Piubelli C., Martinelli N., Corrocher R. and Olivieri O. (2017) A decade of progress on the genetic basis of coronary artery disease. Practical insights for the internist. Eur. J. Intern. Med. 41, 10–17 10.1016/j.ejim.2017.03.019 [DOI] [PubMed] [Google Scholar]
- 10.Chang T.Y., Li B.L., Chang C.C. and Urano Y. (2009) Acyl-coenzyme A:cholesterol acyltransferases. Am. J. Physiol. Endocrinol. Metab. 297, E1–E9 10.1152/ajpendo.90926.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedrelli M., Davoodpour1 P., Degirolamo C., Gomaraschi M., Graham M., Ossoli A.. et al. (2014) Hepatic ACAT2 knock down increases ABCA1 and modifies HDL metabolism in mice. PLoS One 9, e93552 10.1371/journal.pone.0093552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee R.G., Kelley K.L., Sawyer J.K., Farese R.V. Jr, Parks J.S. and Rudel L.L. (2004) Plasma cholesteryl esters provided by lecithin:cholesterol acyltransferase and acyl-coenzyme a:cholesterol acyltransferase 2 have opposite atherosclerotic potential. Circ. Res. 95, 998–1004 10.1161/01.RES.0000147558.15554.67 [DOI] [PubMed] [Google Scholar]
- 13.Willner E.L., Tow B., Buhman K.K., Wilson M., Sanan D.A., Rudel L.L.. et al. (2003) Deficiency of acyl CoA:cholesterol acyltransferase 2 prevents atherosclerosis in apolipoprotein E-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 100, 1262–1267 10.1073/pnas.0336398100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J., Sawyer J.K., Marshall S.M., Kelley K.L., Davis M.A., Wilson M.D.. et al. (2014) Cholesterol esters (CE) derived from hepatic sterol Oacyltransferase 2 (SOAT2) are associated with more atherosclerosis than CE from intestinal SOAT2. Circ. Res. 115, 826–833 10.1161/CIRCRESAHA.115.304378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webb T.R., Erdmann J., Stirrups K.E., Stitziel N.O., Masca N.G., Jansen H.. et al. (2017) Systematic evaluation of pleiotropy identifies 6 further loci associated with coronary artery disease. J. Am. Coll. Cardiol. 69, 823–836 10.1016/j.jacc.2016.11.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McPherson R. and Tybjaerg-Hansen A. (2016) Genetics of coronary artery disease. Circ. Res. 118, 10.1161/CIRCRESAHA.115.306566 [DOI] [PubMed] [Google Scholar]
- 17.Ruiz-Narvaez E.A., Yang Y., Nakanishi Y., Kirchdorfer J. and Campos H. (2005) APOC3/A5 haplotypes, lipid levels, and risk of myocardial infarction in the Central Valley of Costa Rica. J. Lipid Res. 46, 2605–2613 10.1194/jlr.M500040-JLR200 [DOI] [PubMed] [Google Scholar]
- 18.Rong J.X., Blachford C., Feig J.E., Bander I., Mayne J., Kusunoki J.. et al. (2013) ACAT inhibition reduces the progression of preexisting, advanced atherosclerotic mouse lesions without plaque or systemic toxicity. Arterioscler. Thromb. Vasc. Biol. 33, 4–12 10.1161/ATVBAHA.112.252056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lathe R., Sapronova A. and Kotelevtsev Y. (2014) Atherosclerosis and Alzheimer-diseases with a common cause? Inflammation, oxysterols, vasculature BMC Geriatr. 14, 36 10.1186/1471-2318-14-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers M.A., Liu J., Song B.L., Li B.L., Chang C.C. and Chang T.Y. (2015) Acyl-CoA:cholesterol acyltransferases (ACATs/SOATs): Enzymes with multiple sterols as substrates and as activators. J. Steroid Biochem. Mol. Biol. 151, 102–107 10.1016/j.jsbmb.2014.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shibuya Y., Chang C.C. and Chang T.Y. (2015) ACAT1/SOAT1 as a therapeutic target for Alzheimer’s disease. Future Med. Chem. 7, 2451–2467 10.4155/fmc.15.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma J., Folsom A.R., Lewis L. and Eckfeldt J.H. (1997) Relation of plasma phospholipid and cholesterol ester fatty acid composition to carotid artery intima-media thickness: the Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Clin. Nutr. 65, 551–559 10.1093/ajcn/65.2.551 [DOI] [PubMed] [Google Scholar]
- 23.Warensjo E., Sundstrom J., Vessby B., Cederholm T. and Riserus U. (2008) Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: a population-based prospective study. Am. J. Clin. Nutr. 88, 203–209 10.1093/ajcn/88.1.203 [DOI] [PubMed] [Google Scholar]
- 24.Miller C.D., Thomas M.J., Hiestand B., Samuel M.P., Wilson M.D., Sawyer J.. et al. (2012) Cholesteryl esters associated with acyl-CoA:cholesterol acyltransferase predict coronary artery disease in patients with symptoms of acute coronary syndrome. Acad. Emerg. Med. 19, 673–682 10.1111/j.1553-2712.2012.01378.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He X., Lu Y., Saha N., Yang H. and Heng C.K. (2005) Acyl-CoA: cholesterol acyltransferase-2 gene polymorphisms and their association with plasma lipids and coronary artery disease risks. Hum. Genet. 118, 393–403 10.1007/s00439-005-0055-3 [DOI] [PubMed] [Google Scholar]
- 26.Chen S.N., Cilingiroglu M., Todd J., Lombardi R., Willerson J.T., Gotto A.M. Jr. et al. (2009) Candidate genetic analysis of plasma high-density lipoprotein-cholesterol and severity of coronary atherosclerosis. BMC Med. Genet. 10, 111 10.1186/1471-2350-10-111 [DOI] [PMC free article] [PubMed] [Google Scholar]