Abstract
BACKGROUND:
Acute renal failure (ARF) is a serious disease characterised by a rapid loss of renal functions due to nephrotoxic drug or ischemic insult. The clinical treatment approach such as dialysis techniques and continuous renal enhancement have grown rapidly during past decades. However, there is yet no significant effect in improving renal function. Hypoxia-preconditioned mesenchymal stem cells (HP-MSCs) have positive effects on the in vitro survival and stemness, in addition to angiogenic potential.
AIM:
In this study, we aimed to analyse the effect of HP-MSCs administration in improving renal function, characterised by blood urea nitrogen (BUN) and creatinine level.
METHODS:
A group of 15 male Wistar rats weighing 250 g to 300 g were used in this study (n = 5 for each group). Rats were randomly distributed into 3 groups: Vehicle control (Veh) as a control group, HP-MSCs and normoxia MSCs (N-MSCs) as the treatment group. Renal function was evaluated based on the BUN and creatinine levels using the colourimetric method on day 5 and 13. The histological analysis using HE staining was performed on day 13.
RESULTS:
The result showed there is a significant decrease in BUN and creatinine level (p < 0.05). The histological analysis of renal tissue also showed a significant decrease between Veh and treatment group (p < 0.05).
CONCLUSION:
Based on this study, we conclude that HP-MSCs have a superior beneficial effect than N-MSCs in improving renal function in an animal model of gentamicin-induced ARF.
Keywords: HP-MSCs, N-MSCs, ARF, BUN, Creatinin
Introduction
Acute renal failure (ARF) is a serious disease characterised by a rapid loss of renal functions due to the renal damage by the nephrotoxic drug (cisplatin, cyclosporine A, gentamicin) and renal ischemia [1]. Depending on the severity and duration of renal dysfunction, ARF disease has high morbidity and mortality rate caused by massive retention of the nitrogenous compounds that indicated by the increase of blood urea nitrogen (BUN) and creatinine level [2]. The clinical treatment approach of ARF such as dialysis techniques and continuous renal enhancement have grown rapidly during past decades [3]. However there is yet no significant effect in improving renal function [4]. Therefore, the alternative treatments for ARF disease remain a major issue.
Stem cells transplantation has become an alternative and promising strategy in recent years in regenerating tissue damage including renal dysfunction of ARF. Among many kinds of stem cell, mesenchymal stem cells (MSCs) have several advantages distinguished to the others, such as their ease of isolation and harvest, no immunogenicity, abundant distribution and low tumour formation risk [5]. MSCs are defined as a stromal cell that expresses specific markers such as CD44, CD73, CD90, CD105, and lack the expression of express hematopoietic markers, such as CD14 or CD11b, CD19 or CD79a, CD34, CD45 and HLA-DR [6]. Specifically, MSCs have multipotent differentiation capacity into various tissue lineages, including renal cells, in addition to self-renewal [7]. Several studies reported that transplanted-MSCs had been used to treat various types of injured tissues, including renal injury [8]. However, there are some limitations of MSCs transplantation [5], [6], [7] such as poor engraftment and low survival rate at injury area [9]. Therefore, it is critical to optimise the survival capacity of MSCs by modifying MSCs under certain stimulation.
HP-MSCs have positive effects on the in vitro stemness and survival capacity, in addition to angiogenic potential [10] that is mediated by hypoxia-inducible factors (HIFs), such as HIF-1α and HIF-2α [10], [11], [12]. HP-MSCs have a role in increased migration and engraftment capacities after transplantation into the injury area compared to N-MSCs as well as the extended lifespan [13], [14]. However, the role of HP-MSCs condition to improve the renal function of ARF remains unclear.
In this study, we aimed to analyse the effect of HP-MSCs administration in improving BUN and creatinine level (renal function marker) as well as histological appearances in an animal model of gentamicin-induced ARF.
Material and Methods
ARF Animal Model and Experimental Design
Fifteen male Wistar rats, weighing about 250-300 g, were used in this study (n = 5 for each group). Rats were housed in a 12h light-dark cycle cages at 24°C, with water and food ad-libitum. To generate ARF rat model, the rats were induced by gentamicin 140 mg/kg/day for 10 days, i.p., Rats were randomly distributed into 3 groups: Vehicle control (Veh) receiving NaCl injection, Hypoxia preconditioned-MSCs (HP-MSCs) receiving injection of 1 x 106 cell, and Normoxic-MSCs (N-MSCs) receiving injection of 1 x 106 cell intraperitoneally as treatment group respectively.
HUC-MSCs Isolation and Cultivation
Ethical concern was acquired by the institutional review board of the committee ethic of the medical faculty, Sultan Agung Islamic University of Semarang, Indonesia. Human umbilical cord-MSCs (hUC-MSCs) were isolated from umbilical cords obtained from donors with written informed consent. The isolation and expansion of hUC-MSCs were performed as described previously [15]. Briefly, the cords were chopped into small pieces. Then, cord pieces were cultured in Dulbecco`s Modified Eagle Media (DMEM) (Sigma-Aldrich, Louis St, MO) supplemented with 10% Fetal Bovine Serum (FBS) (Gibco™ Invitrogen, NY, USA) and 1% antibiotic/antimycotic (Gibco™ Invitrogen, NY, USA) at 37°C and 5% CO2. The medium was renewed every 3 days. The cells were passaged with trypsin- EDTA after 80% confluence. The fourth passage cells were used for experiments.
Characterzation of UC-MSCs
HUC-MSCs-like were fixed with Cytofix™ fixation buffer (554655, BD Biosciences, Franklin Lakes, NJ, USA), and washed twice with stain buffer (554657, BD Biosciences). For the phenotype markers analysis, the cells were stained with phycoerythrin (PE) mouse anti-human CD44 (Clone G44-26, 555479; BD Biosciences), allophycocyanin (APC) mouse anti-human CD73 (Clone AD2, 560847; BD Biosciences), fluorescein isothiocyanate (FITC) mouse anti-human CD90 (Clone 5E10, 561969 BD Biosciences) and PerCP-Cy5.5.1 mouse anti-human CD105 (Clone 266, 560819, BD Biosciences) antibodies. Cells were stained with specific antibody for 30 minutes at room temperature, washed twice with stain buffer (554657, BD Biosciences) and examined with a BD FACSAria™ II flow cytometer (BD Biosciences) and BD FACSDiva™ software (BD Biosciences).
Differentiation Assay
HUC-MSCs-like at passage 4 were trypsinised and seeded at a concentration of 7.5 x 104 in a 24-well culture plate with standard medium. After 12 h incubation, hUC-MSCs were cultured in the medium of osteogenic differentiation containing DMEM (Sigma-Aldrich, Louis St, MO), supplemented with 10% FBS (Gibco™ Invitrogen, NY, USA), 10-7 mol/L/ 0.1 μM dexamethasone, 10 mmol/L β glycerophosphate, and 50 μmol/L ascorbate-2-phosphate (Sigma-Aldrich, Louis St, MO) in 5% CO2 and at 37°C for 21 day incubation. The osteogenic differentiation analysis, cells were washed with PBS, and with Alizarin Red staining (Sigma-Aldrich Corp., St. Louis, MO, USA).
HP-MSCs Preparation
To prepare HP-MSCs, hUC-MSCs were cultivated under standard culture condition at fourth passage. After reached 80% confluence, the cells were transferred into a hypoxic chamber containing 5% O2 and incubated for 24h at 37°C CO2 5%, then collected for the following experiment.
Histological Analysis
Rats were sacrificed by cervical dislocation at day 13 after transplantation. Renal samples were collected after surgery and fixed in 4% paraformaldehyde. Sections (4 μm thick) were prepared from each group of renal tissue with standard protocols and processed by hematoxylin and eosin (H & E) staining. Then, the slides were analysed and scored with a semi-quantitative scale by an experienced technician evaluating changes found in ARF as the reference [16]. By previous reports [17], the tubular injury was assessed based on the level of tubular dilatation, loss of brush border, tubular necrosis and cast formation. The average histological score was used to analyse the renal tissue morphology.
Evaluation of renal function
Renal function was evaluated based on the BUN and creatinine levels using the colourimetric method (Quanti Chrom TM assay kits). Blood samples were collected from each rat (n = 5 for each group) on day 5 and 13 to compare the serum chemistry profile. Measurement of BUN and creatinine level was performed with the standard protocol.
Data Analysis
Data were presented as the means ± SD. All calculations were carried out using IBM SPSS 22.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. The statistical significance of the differences between the groups was assessed using one way-ANOVA and continued with Duncan post-hoc analysis. P values: **, P < 0.001.
Result
MSC characteristic
At the end of the expansion, the cells were arranged in monolayers with spindle-shaped and fibroblast-like cells. The surface markers of hUC-MSCs-like were analysed using Flow cytometry and revealed that these cells expressed high levels of CD44 (92.4%), CD73 (94.9%), CD90 (86.6%) and CD105 (96.8%).
Figure 1.
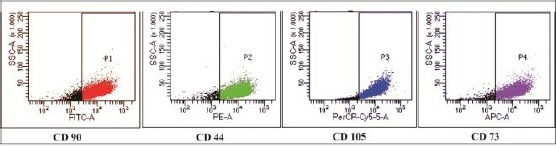
Immunophenotyping analysis of hUC-MSCs which was positive for CD44, CD73, CD90 and CD105
HUC-MSCs Isolation and Osteogenic Differentiation
HUC-MSCs isolation was carried out based on the plastic adherent capability under standard conditions (37°C, 5% CO2). The cells showed fibroblast-like (spindle shape) and peculiar morphology (Figure 2A). Osteocyte differentiations were determined using a standard protocol. The calcium deposits were confirmed by Alizarin Red. The positive osteogenic cells under the microscope were stained bright red.
Figure 2.
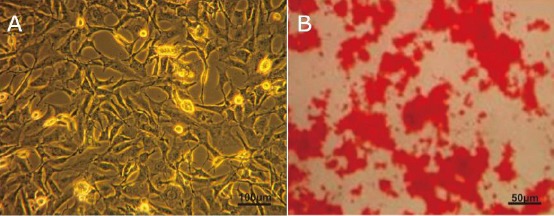
a) HUC-MSCs-like showed fibroblast-like or spindle-shaped characteristic (scale bar 100 μm); b) Osteogenic differentiation hUC-MSCs-like was evidenced by mineralised matrix formation using Alizarin red staining that showed by bright red colour (Scale bar 50 μm)
Renal Function Analysis
To ensure the ARF animal model, firstly we checked the level of BUN and creatinine of animal model before treatment for ARF confirmation. Then, after the treatment, the level of BUN and creatinine was analysed to compare the effect of HP-MSCs to N-MSCs in ARF animal model. The result showed that there was a significant decrease in BUN and creatinine level on day 5 and 13 between all treatment group than veh (p < 0.05). However, the result of HP-MSCs treatment is better than N-MSCs.
Figure 3.
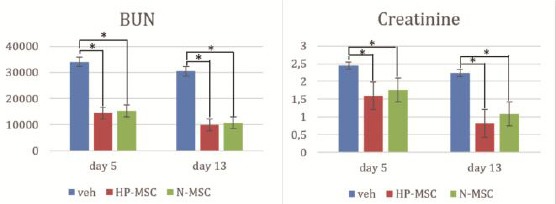
The result showed the highest decrease in BUN level is on HP-MSCs (9911.4 ± 1316.8) at day 13. The level of creatinine also significant decrease on day 13 in HP-MSCs (0.817 ± 0.038)
Histopathological analysis
The histological analysis was performed by HE staining. The average histological score was used to analyse the renal tissue morphology. The results showed that the histological features of renal tissues treated with HP-MSCs and N-MSCs groups showed normal renal cells compared to the vehicle group (Figure 4) in which HP-MSCs showed better renal cell repair.
Figure 4.
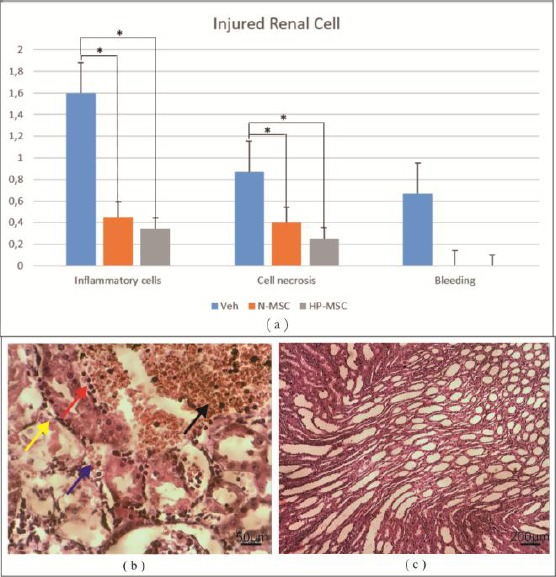
a) The histological analysis of renal tissue showed a significant decrease between and treatment group (p < 0.05). The HP-MCSs showed better result in decreasing inflammatory cells (0.333 ± 0.115) and cell necrosis (0.266 ± 0.305); b) Veh showed patchy necrosis and atrophy of distal and proximal tubules (yellow arrow), pyknotic nuclei (red arrow), bleeding (black arrow) and cell debris in the lumen (blue arrow); c) All treatment groups showed dense interstitial tissue infiltrate between tubules at corticomedullary junction
Discussion
ARF also was known as the acute renal injury is one of the serious renal diseases characterized by rapid deterioration of renal functions indicated by the accumulation of the nitrogenous compounds (urea and creatinine). Several studies reported that UC-MSCs as attractive candidates for renal repair of ARF [17], [18] due to the most of renal tissues structure such as nephrons are derived from mesenchymal that is similar source to MSCs, in addition to they have potential role for releasing the molecule differentiation signalling to both nephrons and collecting duct [18], [19]. However, the transplanted MSCs have poor engraftment and survivability in the injury area. To enhance their therapeutic capacity, the activated MSCs using hypoxic condition is needed to augment their homing and survival in renal injury sites. To analyse the effect of MSCs under hypoxic condition in ameliorating renal function of ARF we used gentamicin-injected rats as established ARF experimental animal model according to a previous protocol. In this study, we transplanted the HP-MSCs into ARF rat models intraperitoneally then analyse the BUN and creatinine level as well as histological finding on day 5 and 13. Our study is the first to administrate HP-MSCs to ARF animal model.
The biochemical result of pretreatment group showed marked impairment of the renal function of ARF that indicated by the plasma increase of BUN and creatinine level. The histological appearance also showed glomerular tuft shrinkage (damage) and haemorrhage; capsular space widened as well as pyknotic nuclei of the tubular cells (necrosis). These suggested that the all groups in this study were under ARF condition (BUN = 36.422 mg/dl and creatinine= 2.46 mg/dl).
In our study, we found that the level of BUN and creatinine were significantly decreasing at day 5 and 13 in all group (p < 0.05). These were supported by our morphology finding which shown that there is a significantly decrease in damage, necrosis, and hemorrhagic of the renal cell on day 13 in all treatment group (Figure 4). However, the BUN and creatinine level of HP-MSCs is lower compared to N-MSCs and morphological appearances as well. These suggested that the regeneration process of ARF that indicated by normal renal function has been well controlled in HP-MSCs group.
Active inflammation release several inflammatory molecules that triggering proteolytic degradation of the most growth factors of regeneration process [20] however they release several chemokine molecules for attracting the exogenous circulating HP-MSCs homing to damaged renal tissue of ARF. HP-MSCs homing to renal injury area involved specific attractant molecules such as VEGF, HGF, integrin α4 and integrin β1 forming very late antigen 4 (VLA-4), and stromal-derived factor-1 (SDF-1) [21] (Figure 5).
Figure 5.
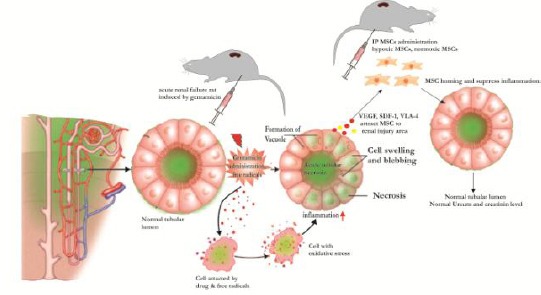
The homing mechanism of MSCs in renal failure. Model of oxidative stress-induced inflammation has been controlled post-HP-MSCs administration. The gentamicin induced-oxidative stress leads to inflammation in ARF. The interactions of ligands to MSCs receptor: (SDF-1 and HGF)-(CXCR4 and cMet) and (VCAM-1 and VEGF) – (VLA-4 and CD44) may trigger the binding of MSCs to target endothelial cells leading to the migration of MSCs to renal injury (homing phenomena). In this area, MSCs being activated and released anti-inflammatory molecules such as TGF-β, TSG-6, IL-10, IL-1ra, and PGE2 for controlling the inflammation process. Under-controlled inflammation may trigger the alteration of inflammatory to proliferation phase that characterised by the secretion of regeneration molecule (growth factor). These were correlated to the formation of renal cells (glomerulus and tubules) into a normal arrangement
HP-MSCs in renal tissue injury release anti-inflammatory molecule such as TGF-β and IL-10 for suppressing the inflammation process then accelerate the shifting of inflammation to the proliferation phase [22]. In this proliferation stage, HP-MSCs release several molecules such as VEGF, PDGF and HGF to actively induce the endogenous stem cells and cells surrounding to repair and regenerate the damaged tissue of ARF, known as a paracrine mechanism. On the other side, HP-MSCs also may differentiate and transdifferentiate into several renal cells as well as exosomes mechanism by donating their crucial vesicle to accelerate the regeneration of ARF.
HGF has a crucial role as a mitogenic molecule to stimulate the proliferation of renal epithelial cell lines [23], including a rat visceral glomerular and proximal tubular cell in damage tissue of ARF by nephrotoxic drug administration (cisplatin, cyclosporine A, gentamicin) and renal ischemia [24], [25]. VEGF as a potent angiogenic factor and concurrently to PDGF have an essential role as potent mitogens in cell growth and regeneration of ARF [26]. All these cytokines play a role in the initiation phase of renal cell regeneration and prevention of apoptosis through Akt or ERK1/2 pathway activation [27]. This study has a limitation in which we do not analyse the molecule released by MSCs whether in inflammation or proliferation phase. Hence, the molecule anti-inflammation and regeneration released by MSCs as well as homing mechanism still unclear. We also do not analyse another soluble factor that may affect to the regeneration process.
In conclusion, HP-MSCs have superior beneficial effect than N-MSCs in ameliorating renal function and regeneration of ARF in an animal model of gentamicin-induced ARF.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Albright RC., Jr Acute renal failure:a practical update. Mayo Clinic Proceedings. 2001;76(1):67–74. doi: 10.4065/76.1.67. https://doi.org/10.4065/76.1.67 PMid:11155415. [DOI] [PubMed] [Google Scholar]
- 2.Ciriano ME, Porta JP, de Vera Floristán CV, García SO, Lipe RÁ, de Vera Floristán JV. Morbimortalidad del fracaso renal agudo en la Unidad de Cuidados Críticos de un hospital comarcal. Revista Espa-ola de Anestesiología y Reanimación. 2018 doi: 10.1016/j.redar.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Demirjian SG. Renal Replacement Therapy for Acute Renal Injury:We Need Better Therapy. 2011;174:242–51. doi: 10.1159/000329402. [DOI] [PubMed] [Google Scholar]
- 4.Ta M, Choi YO, Atouf FO, Heol C, Park H, Lumelsky NA. The Defined Combination of Growth Factors Controls Generation of Long-Term-Replicating Islet Progenitor-Like Cells from Cultures of Adult Mouse Pancreas. Stem Cells. 2006;24:1738–49. doi: 10.1634/stemcells.2005-0367. https://doi.org/10.1634/stemcells.2005-0367 PMid:16556710. [DOI] [PubMed] [Google Scholar]
- 5.Lameire N, Van Biesen W, Vanholder R. The changing epidemiology of acute renal failure. Nat Clin Pract Nephrol. 2006;2(7):364–77. doi: 10.1038/ncpneph0218. https://doi.org/10.1038/ncpneph0218 PMid:16932465. [DOI] [PubMed] [Google Scholar]
- 6.Ren M, Peng W, Yang Z, Sun X, Zhang S, Wang Z, et al. Allogeneic Adipose-Derived Stem Cells With Low Immunogenicity Constructing Tissue-Engineered Bone for Repairing Bone Defects in Pigs. 2012;21:2711–21. doi: 10.3727/096368912X654966. [DOI] [PubMed] [Google Scholar]
- 7.Dominici M, Blanc K Le, Mueller I, Marini FC, Krause DS, Deans RJ, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. https://doi.org/10.1080/14653240600855905 PMid:16923606. [DOI] [PubMed] [Google Scholar]
- 8.Sarugaser R, Hanoun L, Keating A, Stanford WL, Davies JE. Human Mesenchymal Stem Cells Self-Renew and Differentiate According to a Deterministic Hierarchy. 2009; 4(8) doi: 10.1371/journal.pone.0006498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee MJ, Kim J, Lee K Il, Shin JM, Chae J Il, Chung HM. Enhancement of wound healing by secretory factors of endothelial precursor cells derived from human embryonic stem cells. Cytotherapy. 2011;13(2):165–78. doi: 10.3109/14653249.2010.512632. https://doi.org/10.3109/14653249.2010.512632 PMid:21235296. [DOI] [PubMed] [Google Scholar]
- 10.Pattappa G, Thorpe SD, Jegard NC, Heywood HK, de Bruijn JD, Lee DA. Continuous and uninterrupted oxygen tension influences the colony formation and oxidative metabolism of human mesenchymal stem cells. Tissue Engineering Part C:Methods. 2012;19(1):68–79. doi: 10.1089/ten.TEC.2011.0734. https://doi.org/10.1089/ten.tec.2011.0734 PMid:22731854. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Chiang C, Hung S, Chian C. Hypoxia-preconditioned mesenchymal stem cells ameliorate ischemia/reperfusion-induced lung injury. 2017:1–20. doi: 10.1371/journal.pone.0187637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Molecular cell. 2010;40(2):294–309. doi: 10.1016/j.molcel.2010.09.022. https://doi.org/10.1016/j.molcel.2010.09.022 PMid:20965423 PMCid:PMC3143508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hung SC, Pochampally RR, Hsu SC, Sanchez C, Chen SC, Spees J, Prockop DJ. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PloS one. 2007;2(5):e416. doi: 10.1371/journal.pone.0000416. https://doi.org/10.1371/journal.pone.0000416 PMid:17476338 PMCid:PMC1855077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nugraha A, Putra A. Tumor necrosis factor-α-activated mesenchymal stem cells accelerate wound healing through vascular endothelial growth factor regulation in rats. 2018;37(2):125–32. [Google Scholar]
- 15.Lu L, Zhao Q, Wang X, Xu Z, Lu Y, Chen Z, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials Lu-Lu. Hematol J. 2006;91(8) [PubMed] [Google Scholar]
- 16.Chen Y-T, Sun C-K, Lin Y-C, Chang L-T, Chen Y-L, Tsai T-H, et al. Adipose-Derived Mesenchymal Stem Cell Protects Kidneys against Ischemia-Reperfusion Injury through Suppressing Oxidative Stress and Inflammatory Reaction. J Transl Med. 2011;9(51) doi: 10.1186/1479-5876-9-51. https://doi.org/10.1186/1479-5876-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sa AE, A HA, A SM, A FA, Soliman R. Bone Marrow Derived Mesenchymal Stem Cell Therapy in Induced Acute Renal Injury in Adult Male Albino Rats. J Cytol Histol. 2017;8(2) [Google Scholar]
- 18.Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, Prockop DJ. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proceedings of the National Academy of Sciences. 2006;103(46):17438–43. doi: 10.1073/pnas.0608249103. https://doi.org/10.1073/pnas.0608249103 PMid:17088535 PMCid:PMC1634835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell transplantation. 2011;20(1):5–14. doi: 10.3727/096368910X. https://doi.org/10.3727/096368910X PMid:21396235. [DOI] [PubMed] [Google Scholar]
- 20.Cybulsky A V, Mctavish AJ, Papillon J, Takano T. Role of Extracellular Matrix and Ras in Regulation of Glomerular Epithelial Cell Proliferation. 1999; 154(3):899–908. doi: 10.1016/S0002-9440(10)65337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haque N, Rahman MT, KAsim NHA, Alabsi AM. Hypoxic culture conditions as a solution for mesenchymal stem cell based regenerative therapy. Scientific World Journal. 2013;2013 doi: 10.1155/2013/632972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Putra A, Ridwan FB, Putridewi AI, Kustiyah AR, Wirastuti K, Sadyah NA, Rosdiana I, Munir D. The Role of TNF-αinduced MSCs on Suppressive Inflammation by Increasing TGF-βand IL-10. Open Access Maced J Med Sci. 2018;6(10):1779. doi: 10.3889/oamjms.2018.404. https://doi.org/10.3889/oamjms.2018.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumoto K, Nakamura T. Hepatocyte growth factor:Renotropic role and potential therapeutics for renal diseases. Kidney Int. 2001;59(6):2023–38. doi: 10.1046/j.1523-1755.2001.00717.x. https://doi.org/10.1046/j.1523-1755.2001.00717.x PMid:11380804. [DOI] [PubMed] [Google Scholar]
- 24.Faubel S, Lewis EC, Reznikov L, Ljubanovic D, Hoke TS, Somerset H, et al. Cisplatin-Induced Acute Renal Failure Is Associated with an Increase in the Cytokines Interleukin (IL) -1 beta, IL-18, IL-6, and Neutrophil Infiltration in the Kidney. Pharmacology. 2007;322(1):8–15. doi: 10.1124/jpet.107.119792. [DOI] [PubMed] [Google Scholar]
- 25.Selby NM, Shaw S, Woodier N, Fluck RJ, Kolhe N V. Gentamicin-associated acute kidney injury. Qjm. 2009;102(12):873–80. doi: 10.1093/qjmed/hcp143. https://doi.org/10.1093/qjmed/hcp143 PMid:19820138. [DOI] [PubMed] [Google Scholar]
- 26.Beckermann B, Kallifatidis G, Groth A, Frommhold D, Apel A, Mattern J, et al. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer. 2008;99:622–31. doi: 10.1038/sj.bjc.6604508. https://doi.org/10.1038/sj.bjc.6604508 PMid:18665180 PMCid:PMC2527820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang Y, Brekken RA, Hyder SM. Vascular endothelial growth factor induces proliferation of breast cancer cells and inhibits the anti-proliferative activity of anti-hormones. Endocr Relat Cancer. 2006;13(3):905–19. doi: 10.1677/erc.1.01221. https://doi.org/10.1677/erc.1.01221 PMid:16954439. [DOI] [PubMed] [Google Scholar]


