Abstract
BACKGROUND
Neurotrophins [nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4)] and glial cell line-derived neurotrophic factor (GDNF) are soluble polypeptide growth factors that are widely recognized for their roles in promoting cell growth, survival and differentiation in several classes of neurons. Outside the nervous system, neurotrophin (NT) and GDNF signaling events have substantial roles in various non-neural tissues, including the ovary.
OBJECTIVE AND RATIONALE
The molecular mechanisms that promote and regulate follicular development and oocyte maturation have been extensively investigated. However, most information has been obtained from animal models. Even though the fundamental process is highly similar across species, the paracrine regulation of ovarian function in humans remains poorly characterized. Therefore, this review aims to summarize the expression and functional roles of NTs and GDNF in human ovarian biology and disorders, and to describe and propose the development of novel strategies for diagnosing, treating and preventing related abnormalities.
SEARCH METHODS
Relevant literature in the English language from 1990 to 2018 describing the role of NTs and GDNF in mammalian ovarian biology and phenotypes was comprehensively selected using PubMed, MEDLINE and Google Scholar.
OUTCOMES
Studies have shown that the neurotrophins NGF, BDNF, NT-3 and NT-4 as well as GDNF and their functional receptors are expressed in the human ovary. Recently, gathered experimental data suggest putative roles for NT and GDNF signaling in the direct control of ovarian function, including follicle assembly, activation of the primordial follicles, follicular growth and development, oocyte maturation, steroidogenesis, ovulation and corpus luteum formation. Additionally, crosstalk occurs between these ovarian regulators and the endocrine signaling system. Dysregulation of the NT system may negatively affect ovarian function, leading to reproductive pathology (decreased ovarian reserve, polycystic ovary syndrome and endometriosis), female infertility and even epithelial ovarian cancers.
WIDER IMPLICATIONS
A comprehensive understanding of the expression, actions and underlying molecular mechanisms of the NT/GDNF system in the human ovary is essential for novel approaches to therapeutic and diagnostic interventions in ovarian diseases and to develop more safe, effective methods of inducing ovulation in ART in the treatment of female infertility.
Keywords: nerve growth factor, brain-derived neurotrophic factor, neurotrophins, glial cell line-derived neurotrophic factor, follicular development, diminished ovarian reserve, polycystic ovary syndrome, endometriosis, female infertility
Introduction
The mammalian ovary is a highly innervated organ that contains sympathetic and sensory nervous system fibers. These nerve fibers can reach most of the intraovarian compartments, including the ovarian artery, interstitial tissue and ovarian follicle (Malamed et al., 1992). A rat ovary that was transplanted to an ectopic site was promptly reinnervated by both sympathetic and sensory nerve fibers (Lara et al., 1991), indicating that the ovary could produce certain substances that promote the rapid ingrowth of such nerve fibers from the surrounding tissues. Indeed, evidence supported by animal studies showed that the innervated rat ovary was able to produce and secrete nerve growth factor (NGF), which was stimulated and regulated by the innervation (Lara et al., 1990). In the rhesus monkey ovary, a two-fold increase in the density of ovarian innervation occurred during prepubertal development, suggesting that NGF exerts its trophic effects on the development of ovarian sympathetic neurons that contribute to ovarian development and reproductive capacity (Schultea et al., 1992). During mammalian development, the tissue-derived neurotrophic and neural guidance factors are essential to determine the proper directionality of the related tissues and organs, including heart, intestine and airways (Keast, 2013). Dysregulation of these neurotrophic and neural systems can cause defects of innervation that have been associated with various human neurological diseases, including Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, neuroblastoma, multiple sclerosis-like model of experimental allergic encephalomyelitis, peripheral neuropathies and even motion sickness (Calissano et al., 2010).
Neurotrophic factors are divided into the following main families: neurotrophins (NTs) and glial cell line-derived neurotrophic factor (GDNF; Airaksinen and Saarma, 2002; Chao, 2003). NTs were initially discovered as molecules that can promote the survival of developing neurons that newly innervate or terminate in their target peripheral tissues; these molecules constitute a class of polypeptide growth factors that are critical for the development, maintenance and regeneration of the central and peripheral nervous system (Hamburger, 1952). Since then, neurotrophic factors and their putative receptors have also been found to play substantial roles in various peripheral tissues, including the highly innervated ovary. Furthermore, experimental data gathered from clinical samples have demonstrated that these neurotrophic factors, including brain-derived neurotrophic factor (BDNF) and GDNF, are highly expressed in the human granulosa cells (GCs) in the absence of innervation (Zhao et al., 2011). Similar to the bidirectional communication between neurons and glial cells, NTs, together with other hormones and growth factors, exert a considerable effect on both germ cells and somatic cells during the developmental stages of folliculogenesis (Davies and Wright, 1995; Dissen et al., 2009). Over the past few decades, the effects of NTs on growing ovarian follicles have been well studied with regard to follicular assembly, growth, survival and apoptosis (for reviews, see Ojeda et al., 2000; Dissen et al., 2002, 2009). Furthermore, the involvement of NTs and GDNF has been demonstrated in the regulation of the final stage of folliculogenesis, which is characterized by oocyte maturation, steroidogenesis, ovulation and corpus luteum formation; the end point of this process is the acquisition of oocyte competence for embryo development following fertilization (for reviews, see Linher-Melville and Li, 2013). However, the functional roles of NTs and GDNF in the regulation of ovarian function remain poorly defined in humans because most information has been obtained from animal models, even though the fundamental process of ovarian folliculogenesis and oocyte maturation is highly similar across mammalian species. Therefore, in this review, we have summarized the functional roles of NTs and GDNF in human ovarian biology and ovarian disorders. Furthermore, we described and proposed the development of novel strategies for diagnosing, treating and preventing related abnormalities.
Methods
Comprehensive literature searches using either PubMed, MEDLINE or Google Scholar were performed to review articles written in English focusing on the role of NTs and GDNF in follicle assembly, primordial follicle activation, follicular growth and development, oocyte maturation, steroidogenesis, ovulation and corpus luteum formation, as well as ovarian disorders. The search included all relevant mammals, especially human studies published before August 2018.
NTs and functional receptors
The NTs are a family of soluble polypeptide growth factors that comprises five members, including NGF, BDNF, neurotrophin-3 (NT-3), neurotrophin-4 (NT-4, also known as NT-4/5 or NT-5) and neurotrophin 6 (NT-6), which share approximately 50% amino acid sequence identity (Skaper, 2018). To date, NT-6 has been identified only in non-mammalian species, such as fish (Götz et al., 1994). Initially synthesized as pre-pro-proteins in the endoplasmic reticulum, NT precursors are cleaved off the signal peptide and converted into pro-NTs. Prior to their release from the cell, pro-NTs are proteolytically processed by proprotein convertase subtilisin/kexin (PCSK) enzymes that dimerize to their mature forms in the trans-Golgi network (Lessmann and Brigadski, 2009). In certain tissues, the extracellular pro-BDNF can be cleaved by plasmin, which is activated by tissue plasminogen activator (Pang et al., 2004). Mature NTs are non-covalently linked homodimers that are formed by two identical peptide chains of approximately 120 amino acids. Each monomer contains six highly conserved cysteine residues that form a cysteine knot and stabilize the conformation of the proteins (McDonald et al., 1991).
Both pro- and mature NTs initiate their biological actions by binding to the following principal receptor types: high-affinity transmembrane tropomyosin receptor kinase (Trk) receptors and a low-affinity p75 neurotrophin receptor (p75NTR). High-affinity Trk receptors are encoded by members of the Trk proto-oncogene family that exhibit ligand-dependent activation of tyrosine kinase receptor activity, thus functioning as signaling receptors (Barbacid, 1994; Longo and Massa, 2013). Trk receptors contain a tyrosine kinase intracellular domain and extracellular domain (with a leucine- and cysteine-rich motif) that confers ligand binding (Yano and Chao, 2000). The intracellular action specificity of NTs is determined by the selective interaction with their cognate Trk receptors, TrkA (also known as NTRK1) for NGF, TrkB (also known as NTRK2) for BDNF and NT-4, and TrkC (also known as NTRK3) for NT-3 (Yancopoulos et al., 1990; Barbacid et al., 1991; Raffioni et al., 1993; Chao, 2003) (Table I). Notably, NT-3 can also bind to non-cognate Trk receptors (TrkA or TrkB), although the physiological role of this ligand-receptor interaction is not clear (Benedetti et al., 1993) (Table I).
Table I.
Ligand preferences of neurotrophin/glial cell line-derived neurotrophic factor receptors.
| Receptor | Ligand | References |
|---|---|---|
| TrkA | NGF, NT-3a | Yancopoulos et al. (1990), Benedetti et al. (1993) |
| TrkB | BDNF, NT-4, NT-3a | Yancopoulos et al. (1990), Benedetti et al. (1993) |
| TrkC | NT-3 | Yancopoulos et al. (1990) |
| p75NTR | NGF, BDNF, NT-3, NT-4 | Chao (2003) |
| GFRα1 | GDNF | Takahashi (2001) |
| RET | GDNF, NRTN, ARTN, PSPN | Takahashi (2001), Airaksinen et al. (1999) |
aLigand has lower affinity or is less commonly important for receptor activation in vivo.
Trk, transmembrane tropomyosin kinase; NGF, nerve growth factor; NT-3, neurotrophin-3, BDNF, brain-derived neurotrophic factor; NT-4, neurotrophin-4; p75NTR, p75 neurotrophin receptor; GDNF, glial cell line-derived neurotrophic factor; GFRα1, GDNF receptor-α1; NRTN, neurturin; ARTN, artemin; PSPN, persephin.
In contrast to the Trk family of receptors, the low-affinity NT receptor p75NTR (also known as the pan-neurotrophin receptor) binds all NTs with equal affinity (Chao, 2003; Kashyap et al., 2018) (Table I). The p75NTR is a type I transmembrane glycoprotein receptor that is structurally related to the tumor necrosis factor (TNF) receptor family (Chao, 2003). Unlike Trk receptors, p75NTR lacks intrinsic enzyme activity but instead recruits signal adaptors that modulate Trk signaling (Longo and Massa, 2013). Ligand preferences of NT receptors are listed in Table I.
GDNF and functional receptors
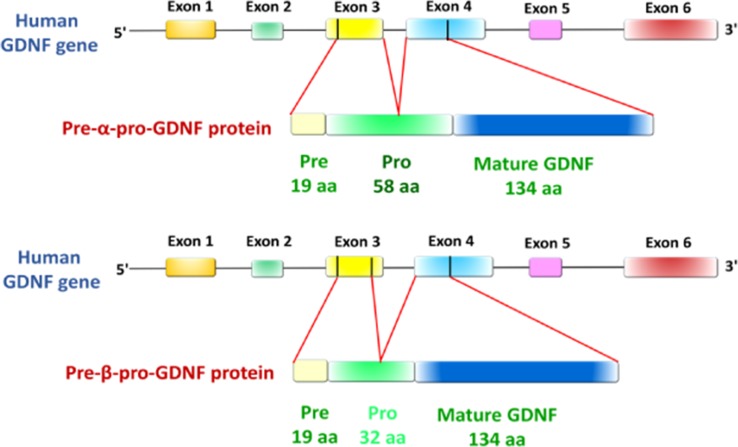
First identified as a trophic factor that promotes the survival of midbrain dopaminergic neurons during embryonic development, GDNF is another neurotrophic factor that has been shown to play an essential role in the development and maintenance of the central and peripheral nervous system (Lin et al., 1993; Tomac et al., 1995). As distant relatives of the transforming growth factor β (TGF-β) superfamily, the GDNF family comprises four structurally related members, including GDNF, neurturin, artemin and persephin (Airaksinen and Saarma, 2002). Despite low amino acid sequence identity (<20%), GDNF and members of the TGF-β superfamily have similar structural conformations that belong to the cysteine-knot protein family and function as homodimers (Ibáñez, 1998). Similar to NTs, GDNF is initially produced in the form of a precursor, pre-pro-GDNF, in which the signal sequence is cleaved on secretion, and activation of pro-GDNF most likely occurs through proteolytic cleavage by PCSK enzymes (specifically PCSK3, also known as furin; Airaksinen and Saarma, 2002). Animal studies have shown that GDNF can bind heparan sulfate proteoglycans of the extracellular matrix, which increases its local concentration by restricting diffusion in the tissue (Hamilton et al., 2001). The human GDNF gene contains six exons and generates two conserved isoforms by alternative splicing of exon 3, including a full-length pre-α-pro-GDNF (α-GDNF) and the shorter pre-β-pro-GDNF (β-GDNF) (Grimm et al., 1998) (Fig. 1). Compared to the full-length isoform, the short isoform lacks 26 amino acids in the pro-region (Airavaara et al., 2011) (Fig. 1). However, the lack of amino acids does not affect the proteolytic cleavage site, with both isoforms cleaved into mature GDNF. Recent studies have shown that both GDNF isoforms have a comparable biological effect and efficacy in the central nervous system (Penttinen et al., 2018).
Figure 1.
Schematic representation of the human GDNF gene and two splicing protein isoforms. The human glial cell line-derived neurotrophic factor (GDNF) gene has six exons (color boxes represent exons, although not to scale) that encode the pre- α-pro-GDNF isoform with a full-length 58 amino acid pro-region and the pre-β-pro-GDNF isoform with a shorter 32 amino acid pro-region. Adapted from (Penttinen et al., 2018).
In vitro studies using overexpression approaches revealed that both GDNF isoforms are secreted from cells in two different ways. α-GDNF and its corresponding mature GDNF proteins are secreted constitutively, whereas the secretion of the β-GDNF (and its related mature GDNF) proteins appears to be activity-dependent (Lonka-Nevalaita et al., 2010). Despite the different secretory patterns, the two major isoforms of GDNF are usually expressed in the same tissues but in varying proportions (Suter-Crazzolara and Unsicker, 1994). In the mammalian ovary, the LH surge dramatically induces the expression and secretion of GDNF, which promotes maturation of the oocyte (Zhao et al., 2011). Furthermore, the treatment of human GCs with hCG alone or combined with FSH significantly increased GDNF mRNA levels in vivo and in vitro, indicating the positive regulatory role of LH and hCG in the production of GDNF (Zhao et al., 2011). However, our most recent studies have shown a potential physiological function exerted by an intraovarian growth factor; bone morphogenetic protein 2 (BMP2), which negatively regulates the expression of GDNF in human granulosa-lutein cells (Zhang et al., 2018).
GDNF signals through a unique multicomponent receptor complex that consists of the GDNF family receptor-α1 (GFRα1) and RET receptor tyrosine kinase (Takahashi, 2001). GFRα1 receptor is usually linked to the plasma membrane by a glycosyl phosphatidylinositol anchor; however, it can be cleaved by an unknown phospholipase or protease that produces soluble forms of these coreceptors (Paratcha et al., 2001). GDNF binds to GFRα1 and then forms a complex with RET as a signaling component (Table I). First discovered as a proto-oncogene, RET is a single-pass transmembrane protein that contains two main cellular portions (Takahashi, 2001). The extracellular portion consists of four cadherin-like repeats, a cysteine-rich domain and a calcium-binding site, while the intracellular portion comprises a characteristic structural feature of tyrosine kinase domain (Airaksinen and Saarma, 2002).
NT signal transduction pathways
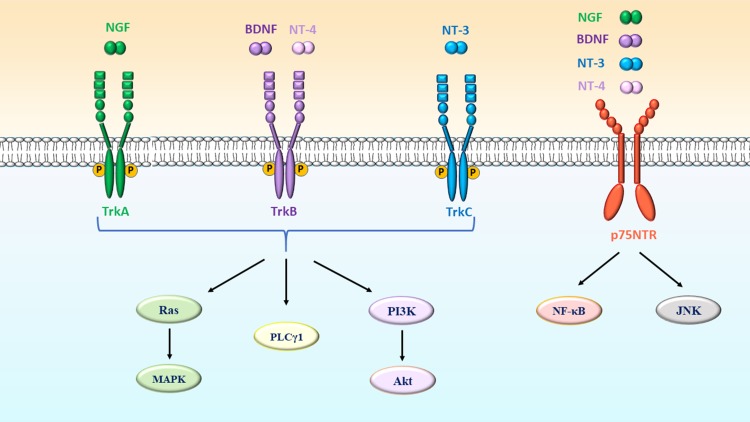
Upon NT binding, Trk receptors are tyrosine kinase receptors that subsequently trigger the activation of several intracellular signaling pathways, including Ras/mitogen-activated protein kinase (MAPK), phosphoinositide-specific phospholipase C-γ1 (PLCγ1) and phosphoinositide 3-kinase/protein kinase B-mammalian target of rapamycin (PI3K/Akt-mTOR) pathways (Chao, 2003) (Fig. 2). Additionally, all four NTs bind to low-affinity p75NTR, which belongs to a family of transmembrane molecules that include receptors for the TNF family (Barbacid, 1994; Bibel et al., 1999) (Fig. 2).
Figure 2.
Neurotrophin receptors and their downstream signaling pathways. NGF binds to TrkA, BDNF and NT-4 bind to TrkB, and NT-3 binds to TrkC, which further induces the activation of Ras/MAPK, PLCγ1 and PI3K/Akt pathways. All neurotrophins (NGF, BDNF, NT-3 and NT-4) also bind to p75NTR receptor, which initiates the recruitment of various adaptors that further activate NF-κB and JNK pathways. BDNF, brain-derived neurotrophic factor; JNK, Jun kinase; MAPK, mitogen-activated protein kinase; NGF, nerve growth factor; NT-3, neurotrophin-3; NT-4, neurotrophin-4; NF-κB, nuclear factor-κB; PI3K, Phosphatidylinositol-3-kinase; PLCγ1, phospholipase Cγ1.
The Ras/MAPK pathway
Following the binding of NTs to their corresponding Trk receptors, the activation of Ras protein occurs, which is an initial response required for cell survival and differentiation. The signal activation cascade is mediated by the binding of an adaptor molecule, Shc, to the phosphorylated tyrosine 490 where the phosphorylated Shc recruits an adaptor protein growth factor receptor-bound protein 2 (Grb2) and a guanine nucleotide exchange factor son of sevenless (SOS; Nimnual et al., 1998; Robinson et al., 2005). The Grb2-SOS complex further increases Ras-GTP levels by catalyzing nucleotide exchange on Ras (York et al., 2000). This activated Ras molecule can stimulate a signaling cascade through various cytoplasmic kinase proteins, including a kinase specific for serine/threonine (Raf), MAPK kinase activator, MAPK and extracellular signal-regulated kinase 1/2 (ERK1/2; Wood et al., 1992; Xing et al., 1998). The activation of ERK1/2, which further phosphorylates and activates a variety of kinases and nuclear transcription factors, is critical for many Ras-induced cellular responses (Liebmann, 2001). Indeed, the Ras/MAPK pathway mediates various NT-induced regulations of ovarian function in humans (Dissen et al., 2009; Julio-Pieper et al., 2009).
The PLCγ1 pathways
In addition to the Ras/MAPK pathway, one of the main mechanisms that mediates NT/Trk receptor interactions is driven by PLCγ1, which is an intracellular messenger that transmits membrane receptor signaling to several molecules (Reichardt, 2006). The activation of PLCγ1 leads to cleavage of the membrane lipid phosphatidylinositol 4,5-bisphosphate to form inositol trisphosphate (IP3) and diacylglycerol (DAG), two key secondary messengers used in signal transduction in most cells (Reichardt, 2006). IP3 is a soluble molecule and is capable of diffusing to the rough endoplasmic reticulum where IP3 can bind to its receptor and trigger the release of Ca2+ into the cytoplasm (Taylor and Tovey, 2010). The increase in intracellular Ca2+ or DAG concentration may stimulate the activity of protein kinase C, which plays essential roles in several signal transduction cascades (Wilson et al., 2015; Ali et al., 2016).
The PI3K/Akt-mTOR pathway
The PI3K/Akt-mTOR signaling pathway integrates both extracellular and intracellular signals that serve as a principal regulator of various cell functions, including cell growth, proliferation, metabolism and survival (Laplante and Sabatini, 2009). In the mammalian ovary, this signaling pathway is the most studied pathway that governs the activation of primordial follicles (Adhikari and Liu, 2009). Recent studies have provided evidence showing that the PI3K/Akt-mTOR signaling pathway and KIT ligand play indispensable roles in the co-ordination of GCs and oocytes, which initiate the activation of primordial follicles in adult ovaries (Zhang and Liu, 2015). Notably, the achievement of this regulation over the reproductive lifespan involves several extracellular intraovarian factors, including NTs, KIT ligand, BMP4, BMP7, vascular endothelial growth factor (VEGF) and other molecules (Adhikari and Liu, 2009; Hsueh, 2014).
In different subsets of tissues, PI3K is activated through Ras-dependent and Ras-independent signaling pathways (Reichardt, 2006). In most neuronal cells, the Ras-dependent activation of PI3K initiates the major signaling pathways that produce several cell survival signals (Vaillant et al., 1999). Additionally, the activation of PI3K can be induced through the Shc-Grb2-Gab1 (Grb2-associated binding protein-1) cascade. Specifically, this Ras-independent signaling pathway occurs when Shc is phosphorylated by TrkA and is associated with Grb2 to produce a complex, which further phosphorylates Gab1 adaptor protein and activates PI3K (Holgado-Madruga et al., 1997). In addition, the phosphorylation of Trk receptors activates PI3K by promoting the phosphorylation of insulin receptor substrate-1 in some neurons (Yamada et al., 1997). Once activated, either by a Ras-dependent or Ras-independent pathway, PI3K may phosphorylate several downstream effectors that are essential for NT-mediated cell function. Akt kinase is regarded as the most important intracellular protein that modulates the activity of certain transcription factors related to cell survival and apoptosis (Brunet et al., 2001) (Fig. 2). One of the major regulators of cellular growth and metabolism activated by the PI3K/Akt downstream pathway is the mechanistic target rapamycin complex 1 or 2 (mTORC1 or mTORC2) (Dibble and Cantley, 2015).
The p75NTR-mediated signaling pathway
All NTs bind to p75NTR with relatively low affinity (Fig. 2). Following the binding of NTs to p75NTR, several signaling pathways that are mediated through the binding of p75NTR to several adaptor proteins may be activated (Reichardt, 2006). One of the key pathways activated by NT-engaged p75NTR receptor binding is the Jun kinase signaling cascade, which leads to the activation of p53 and enhancement of cell apoptosis (Reichardt, 2006) (Fig. 2). Additionally, the binding of NTs to p75NTR may induce the activation of nuclear factor-κB (NF-κB), which contributes to NF-κB-dependent neuronal survival (Hamanoue et al., 1999) (Fig. 2). Furthermore, the interaction of NTs with p75NTR can activate acidic sphingomyelinase and induce the formation of ceramide (Dobrowsky et al., 1994). Ceramide is responsible for the p75NTR-mediated amplification or inhibition effects on Trk-mediated cellular responses because ceramide modulates several signaling pathways, including ERK/MAPK, PI3K/Akt, Jun kinase and NF-κB, as well as the activity of the TrkA receptor in a cell type-dependent manner (MacPhee and Barker, 1997; Müller et al., 1998; Zundel et al., 2000).
GDNF signal transduction pathway
Similar to other members of the GDNF ligand family, GDNF signals through two functional receptors, GFRα1 and RET (for reviews, see Takahashi, 2001). The GDNF homodimer first binds to GFRα1 (either a monomer or dimer), which then interacts with the extracellular domain of two RET molecules and induces their homodimerization and tyrosine autophosphorylation (Airaksinen et al., 1999). Upon phosphorylation, the intracellular domain of the activated RET acts as high-affinity binding sites for several intracellular signaling proteins, including PI3K, MAPK and PLC-γ (for reviews, see Airaksinen and Saarma, 2002). However, there is evidence for RET-independent signaling mechanisms, as many tissues express GDNF and GFRα1, but not RET (Trupp et al., 1999). Recent studies have revealed that in the absence of RET, GDNF can interact with other receptors, such as neural cell adhesion molecules and integrins (for reviews, see Fielder et al., 2018).
The GDNF signaling pathway may participate in crosstalk with other growth factors and receptors. In the peripheral nervous system, the endogenous TGF-β system is required as a cofactor for the exogenous GDNF-induced neuroprotective effect (Airaksinen et al., 1999). Furthermore, TGF-β can induce cell responsiveness to GDNF by recruiting GFRα1 to the plasma membrane during several critical steps of signal transduction (Peterziel et al., 2002). Similar co-operation has been shown between GDNF and NT signaling, as both GDNF and BDNF are required for tissue survival in sensory neurons (Erickson et al., 2001). Indeed, a novel communication pathway between RET and NGF signaling has been demonstrated by using TrkA receptor (Tsui-Pierchala et al., 2002). In the mammalian ovary, these synergistic, co-operative mechanisms seem to be of great importance for the development and maintenance of female reproductive function as these growth factors (TGF-β, NTs and GDNF), and their functional receptors are highly expressed in ovarian follicles (Roy and Kole, 1998; Abir et al., 2005; Farhi et al., 2010).
Expression and localization in the human ovary
NTs and GDNF, as well as their cognate receptors, are expressed in preantral and antral follicles of the mammalian ovary. Additionally, data from clinical samples have shown that these neurotrophic factors are detectable in follicular fluid (FF; Seifer et al., 2002, 2002; Kawamura et al., 2008; Palumbo et al., 2013). Furthermore, an imbalance of any of these intraovarian growth factors may contribute to abnormal follicular development and ovarian dysfunction or pathology in humans. The expression and localization of the NT and GDNF system in the mammalian ovary has been reviewed in detail elsewhere (Streiter et al., 2016). In this review, we focused on the expression and potential function of these neurotrophic factors in the human ovary and their clinical connection to pathological conditions.
In the human ovary, primordial follicles undergo a series of histological and functional changes during follicular development. Under the stimulation of multiple intraovarian factors, the primordial follicle activates and changes into primary and, later, into secondary follicles, followed by transitions to tertiary (antral) follicles (Hsueh et al., 2015). The development and growth of antral follicles is dependent on gonadotrophins, especially FSH (Mihm and Bleach, 2003). To discuss the expression and localization of neurotrophic factors and their receptors in the human ovary, we divided the ovarian follicles into the preantral stage (primordial, primary and secondary) and antral stage (Table II).
Table II.
Localization of neurotrophins/GDNF and receptors in the human ovary.
| Ligands/Receptors | Localization | Expression | Detection method | References |
|---|---|---|---|---|
| NGF | Preantral | |||
| Follicles | ||||
| Oocytes | Protein | IHC | Abir et al. (2005) | |
| GCs | Protein | IHC | Abir et al. (2005), Salas et al. (2006) | |
| Antral | ||||
| Follicles | ||||
| GCs | mRNA and Protein | RT-qPCR, IHC | Salas et al. (2006) | |
| CCs | Protein | IHC | Seifer et al. (2006) | |
| TCs | Protein | IHC | Salas et al. (2006) | |
| FF | Protein | ELISA | Palumbo et al. (2013) | |
| (213.76 pg/ml, ranged from 100 to 360 pg/ml) | ||||
| BDNF | Preantral | |||
| Follicles | ||||
| Oocytes | Protein | IHC | Harel et al. (2006) | |
| GCs | Protein | IHC | Harel et al. (2006) | |
| Antral | ||||
| Follicles | ||||
| GCs | mRNA and Protein | RT-qPCR, IHC | Zhao et al. (2011) | |
| CCs | Protein | IHC | Seifer et al. (2006) | |
| FF | Protein | ELISA | Seifer et al. (2002) | |
| (645.2 ± 23.6 pg/ml)a | ||||
| NT-3 | Preantral | |||
| Follicles | ||||
| Oocytes | Protein | IHC | Oron et al. (2011) | |
| GCs | Protein | IHC | Oron et al. (2011) | |
| Antral | ||||
| Follicles | ||||
| GCs | Protein | IHC | Seifer et al. (2006) | |
| CCs | Protein | IHC | Seifer et al. (2006) | |
| FF | Protein | ELISA | Seifer et al. (2002) | |
| (509 ± 97 pg/ml)a | ||||
| NT-4 | Preantral | |||
| Follicles | ||||
| Oocytes | mRNA and protein | ISH, IHC | Anderson et al. (2002), Harel et al. (2006) | |
| Pre-GCs | mRNA and protein | ISH, IHC | Anderson et al. (2002) | |
| GC | Protein | IHC | Harel et al. (2006) | |
| Antral | ||||
| Follicles | ||||
| CCs | Protein | IHC | Seifer et al. (2006) | |
| FF | Protein | ELISA | Seifer et al. (2002) | |
| (397 ± 71 pg/ml)a | ||||
| TrkA | Preantral | |||
| Follicles | ||||
| Oocytes | Protein | IHC | Abir et al. (2005) | |
| GCs | Protein | IHC | Abir et al. (2005), Salas et al. (2006) | |
| Antral | ||||
| Follicles | ||||
| Oocytes | Protein | IHC | Seifer et al. (2006) | |
| GCs | mRNA and Protein | RT-qPCR, IHC | Salas et al. (2006) | |
| CCs | Protein | IHC | Seifer et al. (2006) | |
| TCs | Protein | IHC | Salas et al. (2006) | |
| TrkB | Preantral | |||
| Follicles | ||||
| Oocytes | Protein | IHC | Anderson et al. (2002), Harel et al. (2006) | |
| Pre-GCs | Protein | IHC | Anderson et al. (2002) | |
| GC | Protein | IHC | Harel et al. (2006) | |
| Antral | ||||
| Follicles | ||||
| Oocytes | Protein | IHC | Seifer et al. (2006) | |
| CCs | Protein | IHC | Seifer et al. (2006) | |
| TrkC | Preantral | |||
| Follicles | ||||
| Oocytes | mRNA and Protein | ISH, IHC | Oron et al. (2011) | |
| GCs | mRNA and Protein | ISH, IHC | Oron et al. (2011) | |
| Antral | ||||
| Follicles | ||||
| Oocytes | Protein | IHC | Seifer et al. (2006) | |
| CCs | Protein | IHC | Seifer et al. (2006) | |
| p75NTR | Preantral | |||
| Follicles | ||||
| Stroma | Protein | IHC | Anderson et al. (2002), Abir et al. (2005) | |
| Antral | ||||
| Follicles | ||||
| TCs | Protein | IHC | Anesetti et al. (2001) | |
| GDNF | Preantral | |||
| Follicles | ||||
| Oocytes | Protein | IHC | Farhi et al. (2010) | |
| GCs | Protein | IHC | Farhi et al. (2010) | |
| Stroma | Protein | IHC | Farhi et al. (2010) | |
| Antral | ||||
| Follicles | ||||
| Oocytes | Protein | IHC | Farhi et al. (2010) | |
| GCs | mRNA and Protein | RT-qPCR, IHC | Farhi et al. (2010), Zhao et al. (2011) | |
| FF | Protein | ELISA | Kawamura et al. (2008) | |
| (0.4–10.8 ng/ml) | ||||
| GFRα1 | Preantral | |||
| Follicles | ||||
| Oocytes | mRNA and Protein | ISH, IHC | Farhi et al. (2010) | |
| GCs | mRNA and Protein | ISH, IHC | Farhi et al. (2010) | |
| Stroma | Protein | IHC | Farhi et al. (2010) | |
| Antral | ||||
| Follicles | ||||
| Oocytes | Protein | IHC | Farhi et al. (2010) | |
| GCs | Protein | IHC | Farhi et al. (2010) | |
| CCs | mRNA | RT-qPCR | Cui et al. (2018) | |
| RET | Preantral | |||
| Follicles | ||||
| Oocytes | Protein | IHC | Farhi et al. (2010) | |
| GCs | Protein | IHC | Farhi et al. (2010) | |
| Stroma | Protein | IHC | Farhi et al. (2010) | |
| Antral | ||||
| Follicles | ||||
| CCs | mRNA | RT-qPCR | Cui et al. (2018) |
CCs, Cumulus cells; GCs, Granulosa cells; TCs, Theca cells; RT-qPCR, Quantitative real-time PCR; FF, Follicular fluid; ISH, in situ hybridization; IHC, Immunohistochemistry.
aData are expressed as the mean ± SD.
Expression of NTs and functional receptors in the human ovary
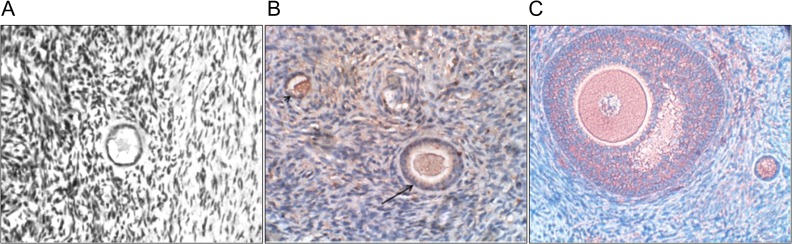
NGF mRNA transcripts and protein are expressed in both fetal and adult human ovaries; they are especially localized to the oocytes and GCs of preantral follicles (mainly from primordial to secondary follicles) (Anderson et al., 2002; Abir et al., 2005; Salas et al., 2006) (Fig. 3A). NGF protein has also been identified in both GCs (especially cumulus cells) and theca cells (TCs) of antral follicles (Salas et al., 2006; Seifer et al., 2006). Clinical samples obtained from infertile women undergoing IVF showed that NGF levels in FF are much higher than those in the serum (213.76 pg/ml vs. 46.47 pg/ml), indicating that NGF is locally produced in the ovarian follicle (Palumbo et al., 2013) (Table II).
Figure 3.
Immunohistochemical staining of NGF, BDNF and GDNF in the human ovary. All sections were stained with the immunoperoxidase staining and then counterstained with Mayer’s haematoxylin. (A) Section of a human ovary from a 38-year-old woman. NGF is expressed in granulosa cells of the primordial follicle, and there is partial staining (with positive nuclear staining) in the oocyte. Original magnification X400. Reprinted with permissions from Abir et al. (2005). (B) Section of a human ovary from a 16-year-old girl. BDNF is expressed in the secondary (arrow) and primordial (arrow head) follicles. Original magnification X400. Reprinted with permissions from Streiter et al. (2016). (C) Section of a human ovary from a 22-year-old woman. GDNF is expressed in the oocytes and a portion of the granulosa cells and stroma cells. Original magnification X400. Reprinted with permissions from Farhi et al. (2010).
BDNF is predominantly expressed in the somatic cells of primordial follicles in the human fetal ovary from 9 to 12 weeks gestation (Childs et al., 2010). BDNF is also detected in the oocytes and GCs (only in a minority of samples) of fetal and adult ovaries (Harel et al., 2006; Streiter et al., 2016) (Fig. 3B). Immunohistochemistry showed that BDNF was detected in cumulus cells (CCs) but at very low levels in the mural GCs of antral follicles (Seifer et al., 2006; Zhao et al., 2011; Streiter et al., 2016) (Fig. 3B). In mice, antral follicular levels of BDNF concomitantly increased with the LH surge during the preovulatory stage (Kawamura et al., 2005). In humans, the administration of LH or hCG increased the secretion of BDNF (up to a 4- or 14-fold increase, respectively) in the conditioned medium of cultured CCs obtained from IVF patients, indicating that BDNF is produced by CCs and is positively regulated by gonadotrophins (Feng et al., 2003). The mean FF (obtained from patients undergoing IVF) concentration of BDNF was 645.2 ± 23.6 pg/ml (Table II).
Transcripts of NT-4 have been identified in the extracts of all ovarian tissues obtained from fetuses and women (Harel et al., 2006). Similar to BDNF, NT-4 was primarily identified in oocytes and some GCs in 21-week-old fetal ovaries (Harel et al., 2006). Likewise, NT-4 has been detected in FF (mean concentration: 397 ± 71 pg/ml) obtained from women undergoing IVF (Seifer et al., 2002). NT-3 protein was identified in the oocytes and GCs of ovarian preantral follicles from fetuses, girls and women (Oron et al., 2011). In the antral follicles, NT-3 protein was identified by immunohistochemistry in GCs and CCs (Seifer et al., 2006), and the mature form of NT-3 protein was detected in the FF (mean concentration: 509 ± 97 pg/ml) (Seifer et al., 2002) (Table II).
In the preantral follicles of the human ovary, TrkA protein has been detected mainly in oocytes, some stroma cells and GCs of ovarian samples obtained from fetuses, girls and women (Abir et al., 2005). In the majority of fetal oocytes and oogonia TrkA was located in the cytoplasm but not in the nucleus (Abir et al., 2005). Additionally, TrkA mRNA transcripts were identified in all fetal and adult ovaries examined (Abir et al., 2005). Similarly, TrkB was mainly expressed in the oocytes/oogonia and some GCs of preantral follicles of the human fetal ovary (Anderson et al., 2002; Harel et al., 2006; Childs et al., 2010). TrkC protein was expressed in the oocytes and GCs of preantral follicles in ovarian samples from fetuses (at 19–33 weeks of gestation), girls and women (Oron et al., 2011). Using in situ hybridization, the same study showed that mRNA transcripts for full-length TrkC were detected in the oocytes of all samples examined and in GCs only in ovarian samples from girls and women (Oron et al., 2011). The expression of p75NTR in mammals is species-dependent. In the preantral follicles of human ovaries, the mRNA and protein of pan-specific p75NTR were detected in stroma cells and TCs of ovarian samples from young fetuses (at 19–21 weeks of gestation) but not in those from older fetuses, girls and women (Anesetti et al., 2001; Abir et al., 2005).
All of the NT receptors, including TrkA, TrkB, TrkC and p75NTR, have been detected in the antral follicles of human ovaries (Anesetti et al., 2001; Seifer et al., 2006). Many studies have demonstrated the expression of TrkA in the major compartments of human antral follicles, including oocytes, GCs and TCs (Shibayama and Koizumi, 1996; Salas et al., 2006; Seifer et al., 2006). Interestingly, the addition of hCG to cultured rat antral follicles rapidly increased the expression of TrkA, indicating that the increase in expression of TrkA during the preovulatory phase is LH dependent (Dissen et al., 1995). In contrast to the expression pattern in rodents (in oocytes only), TrkB was detected in oocytes and CCs of antral follicles in humans (Seifer et al., 2006). Similarly, TrkC protein has been identified in both oocytes and GCs in human antral follicles (Shibayama and Koizumi, 1996; Seifer et al., 2006). Using immunohistochemisty, p75NTR protein was identified in the TCs of human antral follicles (Anesetti et al., 2001) (Table II).
Expression of GDNF and functional receptors in the human ovary
In human preantral follicles, GDNF has been observed in the oocytes of all ovarian samples from fetuses and adult women, and in the GCs from half of all fetal samples and all adult samples (Farhi et al., 2010) (Fig. 3C). Additionally, GDNF mRNA was identified in human GCs of antral follicles and the expression can be upregulated by the addition of hCG alone or in combination with FSH (Zhao et al., 2011). Moreover, mature GDNF protein was detectable in human FF and ranged from 0.4 to 10.8 ng/ml, indicating a paracrine function in the development of ovarian follicles (Kawamura et al., 2008) (Table II).
The two functional receptors for GDNF, GFRα1 and RET, have been detected in the oocytes and GCs of human preantral and antral follicles (Farhi et al., 2010). In particular, the mRNAs of GFRα1 and two RET isoforms were found in the oocytes and GCs of all ovarian samples from fetuses, girls and women. Our recent studies also showed that the mRNAs of GFRα1 and RET were detected in the CCs of mature follicles obtained from patients undergoing IVM (Cui et al., 2018) (Table II).
Regulation of ovarian follicle development
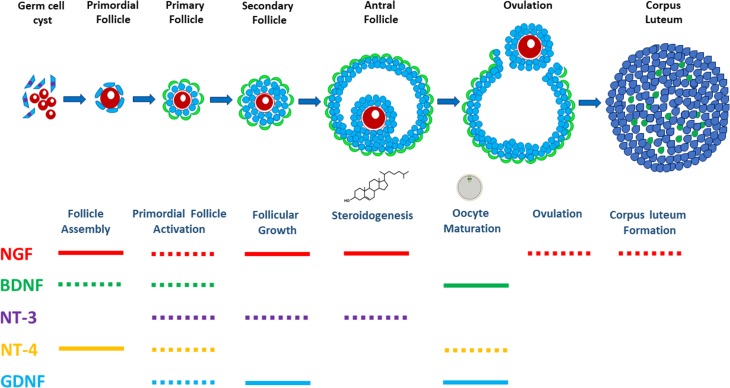
The detection of NTs, GDNF and their cognate receptors in the principal cells (oocytes, GCs and TCs) throughout the different follicular stages of the human ovary may suggest that these intraovarian growth factors are involved in various stages of human folliculogenesis. Indeed, recent studies using animal models, clinical samples and in vitro cell culture have indicated that neurotrophic factors play indispensable roles in the central and peripheral nervous system, as well as in various tissues, including the ovary under normal and pathological conditions (Streiter et al., 2016; Skaper, 2018). Recent studies suggest an unexpected diversity of NT and GDNF actions in the regulation of ovarian and follicular development through nervous system stimuli and cell–cell interactions in the mammalian ovary (Chaves et al., 2013; Streiter et al., 2016) (Fig. 4).
Figure 4.
Schematic showing the neurotrophic factors involved in regulation of ovarian folliculogenesis. Neurotrophins, including NGF (in red), BDNF (in green), NT-3 (in purple), NT-4 (in yellow-brown), as well as GDNF (in blue), participate in and regulate follicular development at each of the defined stages throughout folliculogenesis, including follicle assembly, primordial follicle activation, follicular growth, steroidogenesis, oocyte maturation, ovulation and corpus luteum formation. Solid lines following each neurotrophic factor represent data obtained from studies in humans, while dashed lines represent data obtained from animal models.
Ovarian follicle assembly
The assembly of primordial follicles in the human ovary is a complex developmental process that involves the extensive co-ordination of many growth factors; among these factors, NTs are the major players (Maheshwari and Fowler, 2008; Grive and Freiman, 2015; Streiter et al., 2016). The involvement of NTs in ovarian follicle assembly was supported by the identification of NTs and pan-specific p75NTR in the human fetal ovarian cortex during this process (Anderson et al., 2002; Abir et al., 2005). In particular, both NGF mRNA and protein are expressed in the human ovary prior to formation of the first primordial follicle (Abir et al., 2005). NT-4 localizes to oocytes and pre-GCs in the fetal ovaries of humans from 13 weeks until the completion of gestation (Anderson et al., 2002; Harel et al., 2006). Furthermore, p75NTR mRNA and proteins were detected in the pre-GCs that surround oocytes only in fetal ovaries at gestational ages 13–21 weeks, representing the time before and during human follicle assembly (Anderson et al., 2002; Abir et al., 2005). Compared to the addition of neutralizing antibodies against NT-4, the addition of NT-4 to cultured human fetal ovaries enhanced follicle assembly in vitro (Farhi et al., 2011). Animal studies also support a critical role of NTs in regulating ovarian follicle assembly. Depletion of both Bdnf and Nt-4 in mice led to impaired follicular organization, increased oocyte death and grossly abnormal ovaries (Ojeda et al., 2000; Spears et al., 2003) (Table III). Mice lacking Ngf or Trka had a phenotype of reduced primordial follicle number (Kerr et al., 2009) (Table III). Trkb knockout mice exhibited an increased number of non-encapsulated oocytes (Kerr et al., 2009) (Table III). Similarly, the addition of NGF to the cultured ovaries of Ngf-null mice resulted in an increased number of primordial follicles (Lee et al., 1992; Ojeda et al., 2000). Collectively, these results indicate that ovarian follicle assembly is most likely induced by the synergistic and complementary effects of these NTs and their corresponding receptors (Fig. 4).
Table III.
Genetic mutations in the neurotrophin/GDNF system that cause ovary-specific phenotypes.
| Type of mutation | Ovarian phenotype | References | |
|---|---|---|---|
| NGF knockout mice | Targeted deletion of the Ngf gene | Reduced population of primordial, primary and secondary follicles, oocytes failed to be incorporated into follicular structure | Dissen et al. (2001) |
| NGF knockin mice | Transgenic overexpression of the Ngf gene | Reproductive and metabolic alterations characteristic of PCOS, arrested follicular development and increased apoptosis of antral follicles | Wilson et al. (2014), Dissen et al. (2009) |
| NT-4 knockout mice | Targeted depletion of the Nt-4 gene | Normal ovarian folliculogenesis | Ojeda et al. (2000) |
| Double NT-4/BDNF knockout mice | Target depletion of both Nt-4 and Bdnf gene | Impaired follicular organization, increased oocyte death and grossly abnormal ovaries | Ojeda et al. (2000), Spears et al. (2003) |
| GDNF knockout mice | Targeted depletion of the Gdnf gene | Reversal in the orientation of the ovary in relation to the abdominal viscera | Moore et al. (1996) |
| TrkA knockout mice | Targeted depletion of the Trka gene | Reduced number of primordial follicles, reduced number of FSH receptors | Kerr et al. (2009) |
| TrkB knockout mice | Targeted depletion of the Trkb gene | Reduced number of primordial follicles, reduced number of FSH receptors, an increased number of non-encapsulated oocytes | Kerr et al. (2009) |
PCOS: polcystic ovary syndrome.
Activation of primordial follicles
Following follicle assembly, the human ovary contains the maximum number of primordial follicles at birth (a few days after birth in rodents), which is defined as the ovarian reserve (Findlay et al., 2015). These primordial follicles remain in a quiescent state in a primordial pool for decades until activation by certain stimuli in the surrounding microenvironment. Upon activation, the primordial follicle transforms into the primary follicle along with oocyte growth, TC recruitment and GC differentiation (from flat to cuboid shape) (Kerr et al., 2013). Among the members of the NT family, NGF seems to be involved in the activation of primordial follicles as NGF is expressed in rat follicles at the primordial stage. Knockout mice lacking NGF had a reduced number of both primary and secondary follicles (Dissen et al., 2001, 2002). Moreover, a functional study confirmed that the addition of NGF to cultured neonatal ovaries enhanced the activation rate of primordial follicles in mice (Paredes et al., 2004). NT-3 activates the progression of primordial follicles to growing follicles in cultured rat ovaries (Nilsson et al., 2009). NT-3 promotes the primordial to primary follicle transition by upregulating several target genes related to formation of the cytoskeleton and plasma membrane (Nilsson et al., 2009). In sheep, GDNF improves the activation rate of primordial follicles in cultured ovaries (Esmaielzadeh et al., 2013).
Recent studies using transgenic mouse models have indicated that the intraovarian PI3K/Akt-mTOR pathway is the key signaling pathway that directs the transition from primordial to primary follicles as mice lacking several genes [v-akt murine thymoma viral oncogene homolog 1 (Akt1), forkhead box O3 (Foxo3), phosphatase and tensin homolog (Pten), ribosomal protein S6 (RpS6) or tuberous sclerosis complex 1/tuberous sclerosis complex 2 (Tsc1/Tsc2)] related to this pathway had a phenotype of premature depletion of primordial follicles (Adhikari and Liu, 2009). A recent study using a rodent model demonstrated that localized ovarian trauma or ovulation can induce the activation of primordial follicles, which was completely blocked by the mTOR inhibitor rapamycin (He et al., 2017). A trauma-induced transient increase in locally produced NGF was proposed to trigger the activation of dormant primordial follicles through the PI3K/Akt-mTOR signaling pathway from pre-GCs to oocytes (He et al., 2017). This proposal may provide a molecular mechanism by which the signaling changes in the surrounding intraovarian environment affect the selective recruitment of a cohort of primordial follicles during the menstrual period. Indeed, the injury-induced activation and development of follicles can be used to explain the beneficial effects of ovarian wedge resection and laparoscopic ovarian diathermy drilling for treating women with polycystic ovary syndrome (PCOS; Lebbi et al., 2015).
Ovarian follicular growth and development
NTs are crucial growth factors for the survival, maintenance, and development of neurons in the central and peripheral nervous system (Huang and Reichardt, 2001). These neuronal growth factors also provide sympathetic innervation and trophic support for the ovary, which is essential for follicular development (Dissen et al., 2002). In humans, the addition of Trk receptor blockers to fetal ovaries in vitro (obtained at early gestation: 13–16 weeks) decreased oogonia survival, indicating an important role for NTs in maintaining germ cell survival (Spears et al., 2003). In vitro studies have demonstrated that treatment with NGF decreased cell apoptosis and increased the follicular growth of mouse ovaries by downregulating apoptosis-related genes (Roh and Pi, 2013). Additionally, NGF promoted follicular development and increased cell responses to FSH (and the formation of cAMP) by upregulating FSH receptors in neonatal rat ovaries, indicating crosstalk between this NT and the endocrine signaling system (Romero et al., 2002). Similarly, NGF also upregulated the expression of the FSH receptor in human GCs in vitro (Salas et al., 2006). In addition to the effects on germ cells and GCs, NGF has been shown to participate in promoting TC proliferation. In an in vitro culture of TCs from antral follicles, transfection with TrkA-expressing plasmids in the presence of NGF resulted in an increase in the bovine TC proliferation rate (Dissen et al., 2000). The addition of NT-3 to the culture medium also increased the cell proliferation rate in bovine TCs in vitro (Dissen et al., 2000).
GDNF and its cognate receptors are expressed in human fetal and adult ovaries and promote follicular development in rat ovaries (Dole et al., 2008; Farhi et al., 2010). Rat ovaries cultured in the presence of GDNF showed an increased number of developing follicles without an increased total number of ovarian follicles (Dole et al., 2008). Additionally, the combined treatment of cultured ovaries with GDNF, epidermal growth factor (EGF), kit ligand, and fibroblast growth factor enhanced the differentiation and viability of primordial follicles in sheep (Esmaielzadeh et al., 2013). Moreover, the most recent studies have shown that GDNF inhibited cell apoptosis in human CCs in vitro (Cui et al., 2018).
Ovarian steroidogenesis
Ovarian steroidogenesis products are the key components for maintaining normal ovarian function, including follicular development, oocyte maturation, ovulation and embryo implantation (Chang et al., 2016a). In mature human follicles, NGF is apparently involved in the modulation of steroid hormone production. For instance, NGF inhibited the differentiation of GCs into luteal cells by suppressing the production of progesterone in isolated human GCs (Salas et al., 2006). Alternatively, NGF directly promoted the production of estradiol and indirectly enhanced the cell response to FSH (FSH increases the production of estradiol in GCs) by upregulating expression of the FSH receptor in human GCs, which occurred through the activation of high-affinity tyrosine kinase NGF receptor TrkA (Romero et al., 2002; Salas et al., 2006). In contrast, the addition of antibodies blocking NGF reversed the inducing effect of NGF on estradiol secretion (Salas et al., 2006). In bovine antral follicles, NGF increased the production of both androgen and progesterone in freshly plated TCs through the TrkA receptor-mediated signaling pathway (Dissen et al., 2000). NT-3 is another intragonadal molecule that is involved in promoting follicular steroidogenesis (Waraksa et al., 1995). When NT-3 (at 100 ng/ml but not higher doses) was added to hamster follicles in vitro, NT-3 induced a two-fold increase in estradiol production (Waraksa et al., 1995).
Oocyte maturation
Oocyte maturation is a critical reproductive event that involves a series of functional and morphological changes, including nuclear maturation, cytoplasmic reorganization, meiotic resumption, cytoskeletal dynamics and gamete-somatic cell interactions (Coticchio et al., 2015). Oocyte nuclear maturation is characterized by germinal vesicle breakdown and extrusion of the first polar body (PBI), whereas oocyte cytoplasmic maturation is characterized by cytoplasmic changes essential for monospermic fertilization, and the subsequent preparation for development into preimplantation embryos (Eppig, 1996).
In addition to the endocrine system driven by the hypothalamic-pituitary-ovarian axis, oocyte maturation relies on several locally produced intraovarian factors (Hsueh et al., 2015; Chang et al., 2016b). NTs (especially BDNF and NT-4) and GDNF appear to be the crucial intrafollicular molecules that play a supporting role in oocyte maturation. In mice, oocytes cultured in the presence of BDNF showed increased oocyte maturation and PBI extrusion (an indicator of oocyte nuclear maturation) rates, which improved the competence of oocytes to complete preimplantation development (Seifer et al., 2002; Kawamura et al., 2005). Similarly, in vitro culture of mouse ovaries with a high concentration (100 ng/ml) of NT-4 resulted in an increased rate of PBI extrusion (Seifer et al., 2002). In humans, BDNF FF levels obtained from women undergoing IVF are positively correlated with the number of mature oocytes (Wang et al., 2011). Additionally, combined treatment with BDNF, EGF and insulin-like growth factor-1 (IGF-1) improved the maturation rates of human oocytes and subsequent embryo development (Yu et al., 2012).
GDNF has been identified in the GCs/CCs of antral follicles in several species, including mice, pigs and humans (Linher et al., 2007; Dole et al., 2008; Zhao et al., 2011). In addition, the mature form of GDNF protein was highly detectable in porcine and human FF (Linher et al., 2007; Zhao et al., 2011). Animal studies using porcine oocytes have shown that GDNF significantly enhanced CC expansion and increased the extrusion of the PBI (Linher et al., 2007). In humans, functional studies have demonstrated the role of GDNF in enhancing the developmental competence of oocytes (Zhao et al., 2011). Consistent with these results, our recent studies showed that exogenous GDNF promoted the maturation in vitro of cultured immature oocytes to metaphase II (MII) stage (Cui et al., 2018). Human microRNA (miRNA) analyses revealed that the addition of GDNF to culture medium induced several differentially downregulated miRNAs among in vitro matured CCs, which most likely mediated GDNF-induced maintenance of CC viability and enhancement of oocyte maturation (Cui et al., 2018). Intriguingly, mice lacking Gdnf had an abnormal ovarian topology that these transgenic mice exhibited a reversal in orientation of the ovary in relation to their abdominal viscera (Moore et al., 1996) (Table III).
Ovulation
Based on the mechanisms that initiate the ovulatory cascade, mammals are classified as either spontaneous (e.g. mice, pigs, horses and cattle) or induced (e.g. cats, rabbits, koala and camelids) ovulators (Silva et al., 2015). In spontaneous ovulators, ovulation is regularly triggered by an increase in the circulating concentration of estradiol, whereas in induced ovulators, ovulation is induced by stimulation of the female genital tract during the copulation (Silva et al., 2015). Animal studies have provided evidence that NGF is involved in the process of mammalian ovulation. In rats, both NGF and Trka receptor are dramatically increased in the cells of the follicular wall and interstitial tissue of the ovary, reaching their peak levels ~5 h before ovulation (Dissen et al., 1996). In vivo administration of antibodies against NGF attenuated the release of ovarian prostaglandin E2 and subsequently suppressed ovulation, indicating the role of NGF in triggering rodent ovulation (Dissen et al., 1996). Furthermore, NGF-induced activation of TrkA receptors can disrupt cell–cell communication by affecting the integrity of the connexin 43-based gap junctions, which further induced cellular dissociation of the follicular wall that precedes ovulatory rupture in rats (Mayerhofer et al., 1996).
NGF (especially β-NGF) has been regarded as the ovulation-inducing factor that is present in the seminal plasma of some male mammals (Ratto et al., 2012). Indeed, intramuscular, intravenous or intrauterine administration of NGF consistently induced ovulation in a dose-dependent manner in several induced ovulatory mammals, such as llamas, camelids and alpacas (Ratto et al., 2005; Silva et al., 2015; El Allali et al., 2017). A recent study using a camel model proposed that NGF in the seminal plasma was absorbed through the endometrium to the bloodstream and transferred to the hypothalamus where this growth factor stimulates neurons to produce kisspeptin (a potent stimulator of GnRH), which eventually regulates the preovulatory LH surge (El Allali et al., 2017). At present, the functional role of NTs in the regulation of ovulation in humans remains unknown.
Corpus luteum formation
In addition to the functional role of NGF in regulating ovulation, NGF was associated with enhanced tissue vascularization during the early stage of corpus luteum development in llamas (Ulloa-Leal et al., 2014). Several studies using animal models have revealed that the preovulatory LH surge induced by NGF can be sustained remarkably longer than that induced by GnRH administration, indicating that the luteotrophic effect is most likely triggered by NGF due to prolonged LH secretion (Adams et al., 2005; Silva et al., 2011; Ulloa-Leal et al., 2014). Subsequent studies have confirmed the luteotrophic role of NGF in regulating corpus luteum function through direct or indirect effects on GnRH neurons in the hypothalamus (Silva et al., 2011; Fernández et al., 2014; El Allali et al., 2017).
Roles for NTs in ovarian pathology and diseases
Given the important roles for NTs in regulating follicular development and oocyte competence, we can speculate that dysregulation of the NT system may negatively affect ovarian function, leading to reproductive pathology or female infertility. Clinical data obtained from fertile women revealed a cyclic change in plasma BDNF levels, which were positively correlated with estradiol and progesterone and negatively correlated with age (Begliuomini et al., 2007; Pluchino et al., 2009). Additionally, the concentrations of BDNF steadily declined after menopause (Begliuomini et al., 2007). Interestingly, in menopausal women, hormone replacement therapy restored their plasma BDNF to levels comparable to those in premenopausal women (Begliuomini et al., 2007). In mice, chronic unpredictable stress decreased the expression of BDNF in the antral follicles and reduced the number of mature oocytes and formation of blastocyst embryos, which was reversed by exogenous treatment with BDNF (Wu et al., 2012). In three goat breeds, a single nucleotide polymorphism (SNP; A705G) that was associated with larger litter size was detected in the NGF gene, indicating that NGF could potentially be used as a marker for goat litter size (An et al., 2013).
Diminished ovarian reserve
Altered FF levels of NTs have been identified in women with different etiologies of infertility undergoing IVF treatment, indicating the association of dysregulated NTs with infertility (Buyuk and Seifer, 2008). Compared to women with normal ovarian function, women with diminished ovarian reserve have higher FF levels of NGF and lower (although not statistically significant) FF levels of BDNF (Buyuk and Seifer, 2008). Lower expression levels of BDNF in women with diminished ovarian reserve may reflect their ovarian ages and a gradual decline in follicle quantity. Although many studies have demonstrated the decline in BDNF levels with advancing chronological age in women (Begliuomini et al., 2007; Ziegenhorn et al., 2007; Buyuk and Seifer, 2008), BDNF (unlike anti-Müllerian hormone) does not reflect ovarian function or serve as an indicator for cancer in women receiving chemotherapy (Aslam et al., 2011).
In humans, NGF levels were significantly higher (~4- to 5-fold) in FF than in serum, indicating local production of NGF in the ovarian follicle (Palumbo et al., 2013). Since NGF is expressed in the oocytes and GCs from primordial to secondary follicles in human fetal and adult ovaries, higher expression levels of NGF may accelerate the activation of primordial follicles that eventually leads to poor ovarian reserve. Another study using clinical samples obtained from women undergoing IVF treatment also showed that an increased FF level of NGF was correlated with a lower ovary response (especially in older women) (Palumbo et al., 2013). However, the high expression levels of NGF may be the byproduct of a lower response of germ cells or follicular cells to NGF receptors. Indeed, an in vitro study demonstrated that decreased expression levels of the NT receptors TrkA and p75NTR in human CCs were associated with diminished ovarian reserve in women undergoing ART (Buyuk et al., 2011). At present, studies using genetic approaches have not detected any naturally occurring mutations in genes of NTs or their receptors that are associated with women with primary ovarian insufficiency.
PCOS
NGF and BDNF are two neurotrophic factors that are most likely involved in the pathogenesis of PCOS. The BDNF levels in both FF and plasma were higher in women with PCOS than in healthy women (Russo et al., 2012). In human GCs, the addition of hCG to culture medium increased the levels of BDNF up to 3- to 4-fold, indicating that locally produced BDNF is mainly regulated by LH or hCG (Feng et al., 2003; Zhao et al., 2011). Therefore, the high levels of LH in women with PCOS likely contribute to increased expression of BDNF in GCs, resulting in increased secretion of BDNF in the FF and plasma (Russo et al., 2012). These findings suggest that BDNF could be a candidate neurotrophic factor capable of promoting follicular development. Indeed, the levels of BDNF in FF and plasma have been identified as potential predictors of IVF outcomes (Monteleone et al., 2007; Wang et al., 2011).
In rats, exogenous administration of steroids induced an increase in intraovarian synthesis of NGF and p75NTR, which led to polycystic ovaries that were characterized by the appearance of multiple follicular cysts (Lara et al., 2000). In mice, transgenic overexpression of Ngf targeted to the ovary caused an ovarian cystic morphology that represented both the reproductive and metabolic features of human PCOS (Dissen et al., 2009; Wilson et al., 2014) (Table III). Morphological evaluation of the ovaries in these mice showed that overproduction of NGF led to arrested follicular development and increased apoptosis of antral follicles (Dissen et al., 2009). Collectively, these findings support the hypothesis that sympathetic neuron hyperactivity of the ovary may contribute to the development and progression of PCOS (Wilson et al., 2014). Indeed, clinical data obtained from patients undergoing IVF revealed that women with PCOS had an approximate 2-fold increase in the FF level of NGF and a 6-fold increase in NGF expression in GCs compared with those in women without PCOS (Dissen et al., 2009). In contrast, another clinical study did not show a similar result regarding the increased FF levels of NGF in patients with PCOS (Buyuk and Seifer, 2008).
Endometriosis
Affecting ~10% of reproductive-aged women, endometriosis is a chronic gynecological disease that is defined as the appearance of endometrial fragments outside the uterine cavity (Vigano et al., 2004). Despite extensive investigations, the precise pathogenesis and detailed molecular mechanisms of this common disease remain elusive (Bulun et al., 2015). Emerging evidence has suggested a critical role for NTs in uterine physiology and endometrial pathology (Barcena de Arellano et al., 2013; Wessels et al., 2014). The immunointensity of NGF and TrkA was higher in both the endometriotic stroma and epithelium obtained from women with endometriosis and deep dyspareunia than that in those obtained from women with endometriosis without deep dyspareunia, indicating that NGF signaling may play an important role in endometriosis-associated sexual pain (Peng et al., 2018). A clinical study demonstrated that women with endometriosis had higher levels of NGF in their FF than did patients with male factor infertility (Buyuk and Seifer, 2008).
Proteomic analyses have identified the presence of both BDNF and NT-4 in the eutopic endometrium of women with endometriosis (Browne et al., 2012). Additionally, estrogen receptor, BDNF and TrkB mRNA and protein were detected in the ectopic endometrium of women with ovarian endometriosis (Yu et al., 2016). Furthermore, the estrogen-induced upregulation of BDNF and TrkB that enhances the proliferation of endometrial cells in uterine tissue has been proposed to explain the pathogenesis of estrogen-dependent diseases, including endometriosis (Wessels et al., 2015; Dong et al., 2017). Indeed, recent clinical studies have reported that plasma concentrations of BDNF were significantly higher in women with endometriosis, especially in Stages I and II disease, indicating that BDNF is a potential clinical marker for this painful disorder during its early stage (Wessels et al., 2016; Rocha et al., 2017; Perricos et al., 2018). Targeted genetic association analysis of a case-control study including 425 patients with endometriosis revealed the presence of a BDNF SNP that was linked to an increased severity of endometriosis (Stage III and Stage IV) (Zhang et al., 2012). Interestingly, higher FF levels of BDNF were detected in women with unexplained infertility than in those with known causes of infertility (Sadeu et al., 2012). Future studies will be of great interest to determine whether NTs are involved in the pathogenesis of endometriosis or whether they are merely disease-related byproducts.
Epithelial ovarian cancer
Epithelial ovarian cancer (EOC) represents approximately 90% of total ovarian cancer cases; EOC is a deadly cancer type with a low 5-year survival rate (Siegel et al., 2011). In mammals, NGF and its receptor TrkA might be involved in the regulation of morphological changes in ovarian surface epithelium during the breeding season (Bao et al., 2014). In humans, NGF and TrkA are highly expressed in EOC and are associated with the processes that are crucial for EOC development, including cancer cell proliferation, migration, invasion and angiogenesis (Vera et al., 2014). TrkA-mediated NGF-induced angiogenesis in endothelial cells promotes the development and progression of EOC (Julio-Pieper et al., 2006; Vera et al., 2014). In vitro studies have demonstrated that NGF upregulated the expression of VEGF, which contributes to the metastatic potential of a tumor (Julio-Pieper et al., 2006; Tapia et al., 2011). In addition to VEGF, other molecules regulated by NGF that are involved in the crucial process required for the progression of EOC include calreticulin (CRT), cyclo-oxygenase-2, disintegrin, metalloproteinase domain-containing protein 17 and several miRNAs (for reviews see (Retamales-Ortega et al., 2017)). These studies suggest that NGF may be applied as a biomarker for ovarian cancer.
Clinical applications and therapeutic potential
In many mammals, including humans, NGF, BDNF and GDNF are associated with oocyte maturation and embryo development (Seifer et al., 2002; Kawamura et al., 2005; Wang et al., 2011; Linher-Melville and Li, 2013; Cui et al., 2018). In clinical application, both FF and plasma levels of BDNF are reportedly potential predictors of pregnancy outcome during IVF/ICSI treatment (Monteleone et al., 2007; Wang et al., 2011). Furthermore, the addition of BDNF and GDNF to cultured immature human oocytes and human CCs promoted total yields of MII oocytes and CC viability, respectively (Zhao et al., 2011; Cui et al., 2018). Similarly, combined treatment with BDNF, EGF and IGF-1 improved the maturation rates of human oocytes and subsequent embryo development (Yu et al., 2012). Collectively, these findings have provided insights into the roles of NTs and GDNF in the regulation of oocyte maturation and CC viability. Supplementation of the IVM culture medium with these intraovarian growth factors (alone or in combination) may eventually be applied to enhance the developmental potential of immature oocytes obtained from patients undergoing ART.
Given the indispensable role of NGF-induced PI3K/Akt-mTOR signaling in the activation of dormant primordial follicles, a potential novel infertility therapy has been proposed by using PTEN inhibitors and PI3K stimulators to promote the PI3K/Akt-mTOR-mediated activation of residual follicles in patients with primary ovarian insufficiency (Kawamura et al., 2013). Based on animal studies showing that treatment with the mTOR inhibitor rapamycin can preserve the size of rat follicles (Adhikari et al., 2013), we speculate that blocking agents targeting ovary-specific mTOR signaling or NGF function could be a good option in the future for fertility preservation in women, who now have an extended life expectancy. However, future studies on NGF and human primordial follicle activation are needed.
Data obtained from clinical samples showing that plasma concentrations of BDNF were significantly higher in women with endometriosis (Stages I and II) (Wessels et al., 2016; Rocha et al., 2017; Perricos et al., 2018) may indicate the potential use of BDNF in diagnosing this chronic disease in its early stage. The participation of NGF signaling in the development of ovarian cancer and the findings that NGF and TrkA are highly expressed in women with EOC suggest that NGF may be applied as a biomarker for ovarian cancer. In human ovarian surface epithelium and EOC cell lines, NGF increased the expression of chaperone protein CRT, which was reversed by the addition of a specific TrkA receptor inhibitor (Vera et al., 2012), suggesting that blocking NGF activity could potentially be used as a therapeutic approach or adjuvant therapy in treating patients with EOC.
Conclusion
Recently, there has been substantial interest in locally produced growth factors and intrafollicular signaling between the oocyte and its supporting follicle cells. Evidence supported by animal studies and clinical trials in humans has suggested that intraovarian NTs and GDNF play crucial roles in regulating ovarian function, ranging from ovarian follicle assembly, primordial follicle activation, follicular growth and development, to steroidogenesis, oocyte maturation, ovulation and corpus luteum formation (Fig. 4). Normal ovarian function and follicular development rely on the synergistic and complementary effects of these neurotrophic factors and their corresponding receptors. Dysregulation of these intraovarian growth factors may lead to the development of infertility or ovarian diseases, such as diminished ovarian reserve, PCOS, endometriosis and even ovarian cancer. As our understanding of the expression, actions and underlying molecular mechanisms of these neurotrophic factors in the human ovary expands, the advancement of new diagnostic and therapeutic applications for the management of patients with infertility and ovarian pathology, as well as improvement in oocyte quality, will become plausible.
Authors’ roles
H.M.C. collected the information, designed the pictures and wrote the manuscript. H.C.W. collected the information and designed the pictures. Z.G.S. collected the information and wrote the manuscript. F.L collected the information and critically revised the manuscript. P.C.K.L. collected the information, designed the pictures and critically revised the manuscript. All the authors have seen and approved the final version.
Funding
This work was supported by the Foundation Scheme Grant FDN-143317 from the Canadian Institutes of Health Research to P.C.K.L. This work was also supported by the National Natural Science Foundation of China (Grant no. 81774355) to F.L.
Conflict of interest
We declare that H.C., H.W., Z.S., F.L. and P.C.L. have no conflict of interest with the contents of this manuscript.
References
- Abir R, Fisch B, Jin S, Barnnet M, Ben-Haroush A, Felz C, Kessler-Icekson G, Feldberg D, Nitke S, Ao A. Presence of NGF and its receptors in ovaries from human fetuses and adults. Mol Hum Reprod 2005;11:229–236. [DOI] [PubMed] [Google Scholar]
- Adams GP, Ratto MH, Huanca W, Singh J. Ovulation-inducing factor in the seminal plasma of alpacas and llamas. Biol Reprod 2005;73:452–457. [DOI] [PubMed] [Google Scholar]
- Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev 2009;30:438–464. [DOI] [PubMed] [Google Scholar]
- Adhikari D, Risal S, Liu K, Shen Y. Pharmacological inhibition of mTORC1 prevents over-activation of the primordial follicle pool in response to elevated PI3K signaling. PLoS One 2013;8:e53810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 2002;3:383–394. [DOI] [PubMed] [Google Scholar]
- Airaksinen MS, Titievsky A, Saarma M. GDNF family neurotrophic factor signaling: four masters, one servant? Mol Cell Neurosci 1999;13:313–325. [DOI] [PubMed] [Google Scholar]
- Airavaara M, Pletnikova O, Doyle ME, Zhang YE, Troncoso JC, Liu QR. Identification of novel GDNF isoforms and cis-antisense GDNFOS gene and their regulation in human middle temporal gyrus of Alzheimer disease. J Biol Chem 2011;286:45093–45102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali ES, Hua J, Wilson CH, Tallis GA, Zhou FH, Rychkov GY, Barritt GJ. The glucagon-like peptide-1 analogue exendin-4 reverses impaired intracellular Ca(2+) signalling in steatotic hepatocytes. Biochim Biophys Acta 2016;1863:2135–2146. [DOI] [PubMed] [Google Scholar]
- An X, Bai L, Hou J, Zhao H, Peng J, Song Y, Wang J, Cao B. Molecular cloning, tissue expression and SNP analysis in the goat nerve growth factor gene. Mol Biol Rep 2013;40:857–863. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Robinson LL, Brooks J, Spears N. Neurotropins and their receptors are expressed in the human fetal ovary. J Clin Endocrinol Metab 2002;87:890–897. [DOI] [PubMed] [Google Scholar]
- Anesetti G, Lombide P, D’Albora H, Ojeda SR. Intrinsic neurons in the human ovary. Cell Tissue Res 2001;306:231–237. [DOI] [PubMed] [Google Scholar]
- Aslam MF, Merhi ZO, Ahmed S, Kuzbari O, Seifer DB, Minkoff H. Changes in plasma müllerian-inhibiting substance and brain-derived neurotrophic factor after chemotherapy in premenopausal women. Fertil Steril 2011;95:1790–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Li Q, Liu Y, Li B, Sheng X, Han Y, Weng Q. Immunolocalization of NGF and its receptors in ovarian surface epithelium of the wild ground squirrel during the breeding and nonbreeding seasons. Eur J Histochem 2014;58:2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol 1994;25:1386–1403. [DOI] [PubMed] [Google Scholar]
- Barbacid M, Lamballe F, Pulido D, Klein R. The trk family of tyrosine protein kinase receptors. Biochim Biophys Acta 1991;1072:115–127. [DOI] [PubMed] [Google Scholar]
- Barcena de Arellano ML, Arnold J, Lang H, Vercellino GF, Chiantera V, Schneider A, Mechsner S. Evidence of neurotrophic events due to peritoneal endometriotic lesions. Cytokine 2013;62:253–261. [DOI] [PubMed] [Google Scholar]
- Begliuomini S, Casarosa E, Pluchino N, Lenzi E, Centofanti M, Freschi L, Pieri M, Genazzani AD, Luisi S, Genazzani AR. Influence of endogenous and exogenous sex hormones on plasma brain-derived neurotrophic factor. Hum Reprod 2007;22:995–1002. [DOI] [PubMed] [Google Scholar]
- Benedetti M, Levi A, Chao MV. Differential expression of nerve growth factor receptors leads to altered binding affinity and neurotrophin responsiveness. Proc Natl Acad Sci U S A 1993;90:7859–7863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibel M, Hoppe E, Barde YA. Biochemical and functional interactions between the neurotrophin receptors trk and p75NTR. EMBO J 1999;18:616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne AS, Yu J, Huang RP, Francisco AM, Sidell N, Taylor RN. Proteomic identification of neurotrophins in the eutopic endometrium of women with endometriosis. Fertil Steril 2012;98:713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol 2001;11:297–305. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Monsivais D, Kakinuma T, Furukawa Y, Bernardi L, Pavone ME, Dyson M. Molecular biology of endometriosis: from aromatase to genomic abnormalities. Semin Reprod Med 2015;33:220–224. [DOI] [PubMed] [Google Scholar]
- Buyuk E, Santoro N, Cohen HW, Charron MJ, Jindal S. Reduced neurotrophin receptor tropomyosin-related kinase A expression in human granulosa cells: a novel marker of diminishing ovarian reserve. Fertil Steril 2011;96:474–478.e474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyuk E, Seifer DB. Follicular-fluid neurotrophin levels in women undergoing assisted reproductive technology for different etiologies of infertility. Fertil Steril 2008;90:1611–1615. [DOI] [PubMed] [Google Scholar]
- Calissano P, Matrone C, Amadoro G. Nerve growth factor as a paradigm of neurotrophins related to Alzheimer’s disease. Dev Neurobiol 2010;70:372–383. [DOI] [PubMed] [Google Scholar]
- Chang HM, Fang L, Cheng JC, Taylor EL, Sun YP, Leung PC. Effects of growth differentiation factor 8 on steroidogenesis in human granulosa-lutein cells. Fertil Steril 2016. a;105:520–528. [DOI] [PubMed] [Google Scholar]
- Chang HM, Qiao J, Leung PC. Oocyte-somatic cell interactions in the human ovary-novel role of bone morphogenetic proteins and growth differentiation factors. Hum Reprod Update 2016. b;23:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 2003;4:299–309. [DOI] [PubMed] [Google Scholar]
- Chaves RN, Alves AM, Lima LF, Matos HM, Rodrigues AP, Figueiredo JR. Role of nerve growth factor (NGF) and its receptors in folliculogenesis. Zygote 2013;21:187–197. [DOI] [PubMed] [Google Scholar]
- Childs AJ, Bayne RA, Murray AA, Martins Da Silva SJ, Collins CS, Spears N, Anderson RA. Differential expression and regulation by activin of the neurotrophins BDNF and NT4 during human and mouse ovarian development. Dev Dyn 2010;239:1211–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coticchio G, Dal Canto M, Mignini Renzini M, Guglielmo MC, Brambillasca F, Turchi D, Novara PV, Fadini R. Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum Reprod Update 2015;21:427–454. [DOI] [PubMed] [Google Scholar]
- Cui L, Fang L, Mao X, Chang HM, Leung PCK, Ye Y. GDNF-induced down-regulation of miR-145-5p enhances human oocyte maturation and cumulus cell viability. J Clin Endocrinol Metab 2018;103:2510–2521. [DOI] [PubMed] [Google Scholar]
- Davies AM, Wright EM. Neurotrophic factors. Neurotrophin autocrine loops. Curr Biol 1995;5:723–726. [DOI] [PubMed] [Google Scholar]
- Dibble CC, Cantley LC. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol 2015;25:545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissen GA, Garcia-Rudaz C, Ojeda SR. Role of neurotrophic factors in early ovarian development. Semin Reprod Med 2009. a;27:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissen GA, Garcia-Rudaz C, Paredes A, Mayer C, Mayerhofer A, Ojeda SR. Excessive ovarian production of nerve growth factor facilitates development of cystic ovarian morphology in mice and is a feature of polycystic ovarian syndrome in humans. Endocrinology 2009. b;150:2906–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissen GA, Hill DF, Costa ME, Les Dees CW, Lara HE, Ojeda SR. A role for trkA nerve growth factor receptors in mammalian ovulation. Endocrinology 1996;137:198–209. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Hirshfield AN, Malamed S, Ojeda SR. Expression of neurotrophins and their receptors in the mammalian ovary is developmentally regulated: changes at the time of folliculogenesis. Endocrinology 1995;136:4681–4692. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Parrott JA, Skinner MK, Hill DF, Costa ME, Ojeda SR. Direct effects of nerve growth factor on thecal cells from antral ovarian follicles. Endocrinology 2000;141:4736–4750. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Romero C, Hirshfield AN, Ojeda SR. Nerve growth factor is required for early follicular development in the mammalian ovary. Endocrinology 2001;142:2078–2086. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Romero C, Paredes A, Ojeda SR. Neurotrophic control of ovarian development. Microsc Res Tech 2002;59:509–515. [DOI] [PubMed] [Google Scholar]
- Dobrowsky RT, Werner MH, Castellino AM, Chao MV, Hannun YA. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science 1994;265:1596–1599. [DOI] [PubMed] [Google Scholar]
- Dole G, Nilsson EE, Skinner MK. Glial-derived neurotrophic factor promotes ovarian primordial follicle development and cell-cell interactions during folliculogenesis. Reproduction 2008;135:671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong F, Zhang Q, Kong W, Chen J, Ma J, Wang L, Wang Y, Liu Y, Li Y, Wen J. Regulation of endometrial cell proliferation by estrogen-induced BDNF signaling pathway. Gynecol Endocrinol 2017;33:485–489. [DOI] [PubMed] [Google Scholar]
- El Allali K, El Bousmaki N, Ainani H, Simonneaux V. Effect of the Camelid’s seminal plasma ovulation-inducing factor/β-NGF: a Kisspeptin target hypothesis. Front Vet Sci 2017;4:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig JJ. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Dev 1996;8:485–489. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Brosenitsch TA, Katz DM. Brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor are required simultaneously for survival of dopaminergic primary sensory neurons in vivo. J Neurosci 2001;21:581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaielzadeh F, Hosseini SM, Nasiri Z, Hajian M, Chamani M, Gourabi H, Shahverdi AH, Vosough AD, Nasr-Esfahani MH. Kit ligand and glial-derived neurotrophic factor as alternative supplements for activation and development of ovine preantral follicles in vitro. Mol Reprod Dev 2013;80:35–47. [DOI] [PubMed] [Google Scholar]
- Farhi J, Ao A, Fisch B, Zhang XY, Garor R, Abir R. Glial cell line-derived neurotrophic factor (GDNF) and its receptors in human ovaries from fetuses, girls, and women. Fertil Steril 2010;93:2565–2571. [DOI] [PubMed] [Google Scholar]
- Farhi J, Fisch B, Garor R, Peled Y, Pinkas H, Abir R. Neurotrophin 4 enhances in vitro follicular assembly in human fetal ovaries. Fertil Steril 2011;95:1267–1271. [DOI] [PubMed] [Google Scholar]
- Feng B, Chen S, Shelden RM, Seifer DB. Effect of gonadotropins on brain-derived neurotrophic factor secretion by human follicular cumulus cells. Fertil Steril 2003;80:658–659. [DOI] [PubMed] [Google Scholar]
- Fernández A, Ulloa-Leal C, Silva M, Norambuena C, Adams GP, Guerra M, Ratto MH. The effect of repeated administrations of llama ovulation-inducing factor (OIF/NGF) during the peri-ovulatory period on corpus luteum development and function in llamas. Anim Reprod Sci 2014;149:345–352. [DOI] [PubMed] [Google Scholar]
- Fielder GC, Yang TW, Razdan M, Li Y, Lu J, Perry JK, Lobie PE, Liu DX. The GDNF family: a role in cancer? Neoplasia 2018;20:99–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay JK, Hutt KJ, Hickey M, Anderson RA. How is the number of primordial follicles in the ovarian reserve established? Biol Reprod 2015;93:111. [DOI] [PubMed] [Google Scholar]
- Grimm L, Holinski-Feder E, Teodoridis J, Scheffer B, Schindelhauer D, Meitinger T, Ueffing M. Analysis of the human GDNF gene reveals an inducible promoter, three exons, a triplet repeat within the 3′-UTR and alternative splice products. Hum Mol Genet 1998;7:1873–1886. [DOI] [PubMed] [Google Scholar]
- Grive KJ, Freiman RN. The developmental origins of the mammalian ovarian reserve. Development 2015;142:2554–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz R, Köster R, Winkler C, Raulf F, Lottspeich F, Schartl M, Thoenen H. Neurotrophin-6 is a new member of the nerve growth factor family. Nature 1994;372:266–269. [DOI] [PubMed] [Google Scholar]
- Hamanoue M, Middleton G, Wyatt S, Jaffray E, Hay RT, Davies AM. p75-mediated NF-kappaB activation enhances the survival response of developing sensory neurons to nerve growth factor. Mol Cell Neurosci 1999;14:28–40. [DOI] [PubMed] [Google Scholar]
- Hamburger V. Development of the nervous system. Ann N Y Acad Sci 1952;55:117–132. [DOI] [PubMed] [Google Scholar]
- Hamilton JF, Morrison PF, Chen MY, Harvey-White J, Pernaute RS, Phillips H, Oldfield E, Bankiewicz KS. Heparin coinfusion during convection-enhanced delivery (CED) increases the distribution of the glial-derived neurotrophic factor (GDNF) ligand family in rat striatum and enhances the pharmacological activity of neurturin. Exp Neurol 2001;168:155–161. [DOI] [PubMed] [Google Scholar]
- Harel S, Jin S, Fisch B, Feldberg D, Krissi H, Felz C, Freimann S, Tan SL, Ao A, Abir R. Tyrosine kinase B receptor and its activated neurotrophins in ovaries from human fetuses and adults. Mol Hum Reprod 2006;12:357–365. [DOI] [PubMed] [Google Scholar]
- He Y, Peng X, Wu T, Yang W, Liu W, Zhang J, Su Y, Kong F, Dou X, Li J. Restricting the induction of NGF in ovarian stroma engenders selective follicular activation through the mTOR signaling pathway. Cell Death Dis 2017;8:e2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgado-Madruga M, Moscatello DK, Emlet DR, Dieterich R, Wong AJ. Grb2-associated binder-1 mediates phosphatidylinositol 3-kinase activation and the promotion of cell survival by nerve growth factor. Proc Natl Acad Sci USA 1997;94:12419–12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh AJ. Fertility: the role of mTOR signaling and KIT ligand. Curr Biol 2014;24:R1040–R1042. [DOI] [PubMed] [Google Scholar]
- Hsueh AJ, Kawamura K, Cheng Y, Fauser BC. Intraovarian control of early folliculogenesis. Endocr Rev 2015;36:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 2001;24:677–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez CF. Emerging themes in structural biology of neurotrophic factors. Trends Neurosci 1998;21:438–444. [DOI] [PubMed] [Google Scholar]
- Julio-Pieper M, Lara HE, Bravo JA, Romero C. Effects of nerve growth factor (NGF) on blood vessels area and expression of the angiogenic factors VEGF and TGFbeta1 in the rat ovary. Reprod Biol Endocrinol 2006;4:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julio-Pieper M, Lozada P, Tapia V, Vega M, Miranda C, Vantman D, Ojeda SR, Romero C. Nerve growth factor induces vascular endothelial growth factor expression in granulosa cells via a trkA receptor/mitogen-activated protein kinase-extracellularly regulated kinase 2-dependent pathway. J Clin Endocrinol Metab 2009;94:3065–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap MP, Roberts C, Waseem M, Tyagi P. Drug targets in neurotrophin signaling in the central and peripheral nervous system. Mol Neurobiol 2018;55:6939–6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, Ho CH, Kawamura N, Tamura M, Hashimoto S et al. . Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci USA 2013;110:17474–17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K, Kawamura N, Mulders SM, Sollewijn Gelpke MD, Hsueh AJ. Ovarian brain-derived neurotrophic factor (BDNF) promotes the development of oocytes into preimplantation embryos. Proc Natl Acad Sci USA 2005;102:9206–9211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K, Ye Y, Kawamura N, Jing L, Groenen P, Gelpke MS, Rauch R, Hsueh AJ, Tanaka T. Completion of Meiosis I of preovulatory oocytes and facilitation of preimplantation embryo development by glial cell line-derived neurotrophic factor. Dev Biol 2008;315:189–202. [DOI] [PubMed] [Google Scholar]
- Keast JR. Innervation of developing and adult organs. Organogenesis 2013;9:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr B, Garcia-Rudaz C, Dorfman M, Paredes A, Ojeda SR. NTRK1 and NTRK2 receptors facilitate follicle assembly and early follicular development in the mouse ovary. Reproduction 2009;138:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JB, Myers M, Anderson RA. The dynamics of the primordial follicle reserve. Reproduction 2013;146:R205–R215. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci 2009;122:3589–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara HE, Dees WL, Hiney JK, Dissen GA, Rivier C, Ojeda SR. Functional recovery of the developing rat ovary after transplantation: contribution of the extrinsic innervation. Endocrinology 1991;129:1849–1860. [DOI] [PubMed] [Google Scholar]
- Lara HE, Dissen GA, Leyton V, Paredes A, Fuenzalida H, Fiedler JL, Ojeda SR. An increased intraovarian synthesis of nerve growth factor and its low affinity receptor is a principal component of steroid-induced polycystic ovary in the rat. Endocrinology 2000;141:1059–1072. [DOI] [PubMed] [Google Scholar]
- Lara HE, Hill DF, Katz KH, Ojeda SR. The gene encoding nerve growth factor is expressed in the immature rat ovary: effect of denervation and hormonal treatment. Endocrinology 1990;126:357–363. [DOI] [PubMed] [Google Scholar]
- Lebbi I, Ben Temime R, Fadhlaoui A, Feki A. Ovarian drilling in PCOS: is it really useful? Front Surg 2015;2:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KF, Li E, Huber LJ, Landis SC, Sharpe AH, Chao MV, Jaenisch R. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell 1992;69:737–749. [DOI] [PubMed] [Google Scholar]
- Lessmann V, Brigadski T. Mechanisms, locations, and kinetics of synaptic BDNF secretion: an update. Neurosci Res 2009;65:11–22. [DOI] [PubMed] [Google Scholar]
- Liebmann C. Regulation of MAP kinase activity by peptide receptor signalling pathway: paradigms of multiplicity. Cell Signal 2001;13:777–785. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993;260:1130–1132. [DOI] [PubMed] [Google Scholar]
- Linher K, Wu D, Li J. Glial cell line-derived neurotrophic factor: an intraovarian factor that enhances oocyte developmental competence in vitro. Endocrinology 2007;148:4292–4301. [DOI] [PubMed] [Google Scholar]
- Linher-Melville K, Li J. The roles of glial cell line-derived neurotrophic factor, brain-derived neurotrophic factor and nerve growth factor during the final stage of folliculogenesis: a focus on oocyte maturation. Reproduction 2013;145:R43–R54. [DOI] [PubMed] [Google Scholar]
- Longo FM, Massa SM. Small-molecule modulation of neurotrophin receptors: a strategy for the treatment of neurological disease. Nat Rev Drug Discov 2013;12:507–525. [DOI] [PubMed] [Google Scholar]
- Lonka-Nevalaita L, Lume M, Leppänen S, Jokitalo E, Peränen J, Saarma M. Characterization of the intracellular localization, processing, and secretion of two glial cell line-derived neurotrophic factor splice isoforms. J Neurosci 2010;30:11403–11413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPhee IJ, Barker PA. Brain-derived neurotrophic factor binding to the p75 neurotrophin receptor reduces TrkA signaling while increasing serine phosphorylation in the TrkA intracellular domain. J Biol Chem 1997;272:23547–23551. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Fowler PA. Primordial follicular assembly in humans--revisited. Zygote 2008;16:285–296. [DOI] [PubMed] [Google Scholar]
- Malamed S, Gibney JA, Ojeda SR. Ovarian innervation develops before initiation of folliculogenesis in the rat. Cell Tissue Res 1992;270:87–93. [DOI] [PubMed] [Google Scholar]
- Mayerhofer A, Dissen GA, Parrott JA, Hill DF, Mayerhofer D, Garfield RE, Costa ME, Skinner MK, Ojeda SR. Involvement of nerve growth factor in the ovulatory cascade: trkA receptor activation inhibits gap junctional communication between thecal cells. Endocrinology 1996;137:5662–5670. [DOI] [PubMed] [Google Scholar]
- McDonald NQ, Lapatto R, Murray-Rust J, Gunning J, Wlodawer A, Blundell TL. New protein fold revealed by a 2.3-A resolution crystal structure of nerve growth factor. Nature 1991;354:411–414. [DOI] [PubMed] [Google Scholar]
- Mihm M, Bleach EC. Endocrine regulation of ovarian antral follicle development in cattle. Anim Reprod Sci 2003;78:217–237. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Artini PG, Simi G, Cela V, Casarosa E, Begliuomini S, Ninni F, Pluchino N, Luisi M, Genazzani AR. Brain derived neurotrophic factor circulating levels in patients undergoing IVF. J Assist Reprod Genet 2007;24:477–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MW, Klein RD, Fariñas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature 1996;382:76–79. [DOI] [PubMed] [Google Scholar]
- Müller G, Storz P, Bourteele S, Döppler H, Pfizenmaier K, Mischak H, Philipp A, Kaiser C, Kolch W. Regulation of Raf-1 kinase by TNF via its second messenger ceramide and cross-talk with mitogenic signalling. EMBO J 1998;17:732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson E, Dole G, Skinner MK. Neurotrophin NT3 promotes ovarian primordial to primary follicle transition. Reproduction 2009;138:697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimnual AS, Yatsula BA, Bar-Sagi D. Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science 1998;279:560–563. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Romero C, Tapia V, Dissen GA. Neurotrophic and cell-cell dependent control of early follicular development. Mol Cell Endocrinol 2000;163:67–71. [DOI] [PubMed] [Google Scholar]
- Oron G, Ao A, Friedman O, Fisch B, Zhang XY, Ben-Haroush A, Peled Y, Abir R. Expression of neurotrophin 3 and its tropomyosin-related kinase receptor C in human preantral follicles. Fertil Steril 2011;95:2056–2062. [DOI] [PubMed] [Google Scholar]
- Palumbo MA, Giuffrida E, Gulino FA, Leonardi E, Cantarella G, Bernardini R. Nerve growth factor (NGF) levels in follicular fluid of infertile patients undergoing to in vitro fertilization (IVF) cycle. Gynecol Endocrinol 2013;29:1002–1004. [DOI] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science 2004;306:487–491. [DOI] [PubMed] [Google Scholar]
- Paratcha G, Ledda F, Baars L, Coulpier M, Besset V, Anders J, Scott R, Ibáñez CF. Released GFRalpha1 potentiates downstream signaling, neuronal survival, and differentiation via a novel mechanism of recruitment of c-Ret to lipid rafts. Neuron 2001;29:171–184. [DOI] [PubMed] [Google Scholar]
- Paredes A, Romero C, Dissen GA, DeChiara TM, Reichardt L, Cornea A, Ojeda SR, Xu B. TrkB receptors are required for follicular growth and oocyte survival in the mammalian ovary. Dev Biol 2004;267:430–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng B, Zhan H, Alotaibi F, Alkusayer GM, Bedaiwy MA, Yong PJ. Nerve growth factor is associated with sexual pain in women with endometriosis. Reprod Sci 2018;25:540–549. [DOI] [PubMed] [Google Scholar]
- Penttinen AM, Parkkinen I, Voutilainen MH, Koskela M, Bäck S, Their A, Richie CT, Domanskyi A, Harvey BK, Tuominen RK et al. . Pre-α-pro-GDNF and pre-β-pro-GDNF isoforms are neuroprotective in the 6-hydroxydopamine rat model of Parkinson’s disease. Front Neurol 2018;9:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perricos A, Ashjaei K, Husslein H, Proestling K, Kuessel L, Obwegeser R, Wenzl R, Yotova I. Increased serum levels of mBDNF in women with minimal and mild endometriosis have no predictive power for the disease. Exp Biol Med (Maywood) 2018;243:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterziel H, Unsicker K, Krieglstein K. TGFbeta induces GDNF responsiveness in neurons by recruitment of GFRalpha1 to the plasma membrane. J Cell Biol 2002;159:157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino N, Cubeddu A, Begliuomini S, Merlini S, Giannini A, Bucci F, Casarosa E, Luisi M, Cela V, Genazzani AR. Daily variation of brain-derived neurotrophic factor and cortisol in women with normal menstrual cycles, undergoing oral contraception and in postmenopause. Hum Reprod 2009;24:2303–2309. [DOI] [PubMed] [Google Scholar]
- Raffioni S, Bradshaw RA, Buxser SE. The receptors for nerve growth factor and other neurotrophins. Annu Rev Biochem 1993;62:823–850. [DOI] [PubMed] [Google Scholar]
- Ratto MH, Huanca W, Singh J, Adams GP. Local versus systemic effect of ovulation-inducing factor in the seminal plasma of alpacas. Reprod Biol Endocrinol 2005;3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratto MH, Leduc YA, Valderrama XP, van Straaten KE, Delbaere LT, Pierson RA, Adams GP. The nerve of ovulation-inducing factor in semen. Proc Natl Acad Sci USA 2012;109:15042–15047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci 2006;361:1545–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retamales-Ortega R, Oróstica L, Vera C, Cuevas P, Hernández A, Hurtado I, Vega M, Romero C. Role of Nerve Growth Factor (NGF) and miRNAs in epithelial ovarian cancer. Int J Mol Sci 2017;18:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KN, Manto K, Buchsbaum RJ, MacDonald JI, Meakin SO. Neurotrophin-dependent tyrosine phosphorylation of Ras guanine-releasing factor 1 and associated neurite outgrowth is dependent on the HIKE domain of TrkA. J Biol Chem 2005;280:225–235. [DOI] [PubMed] [Google Scholar]
- Rocha AL, Vieira EL, Ferreira MC, Maia LM, Teixeira AL, Reis FM. Plasma brain-derived neurotrophic factor in women with pelvic pain: a potential biomarker for endometriosis? Biomark Med 2017;11:313–317. [DOI] [PubMed] [Google Scholar]
- Roh J, Pi W. Regulation of follicle growth and apoptosis in cultured mouse ovaries by NGF and oxygen. Gynecol Endocrinol 2013;29:574–579. [DOI] [PubMed] [Google Scholar]
- Romero C, Paredes A, Dissen GA, Ojeda SR. Nerve growth factor induces the expression of functional FSH receptors in newly formed follicles of the rat ovary. Endocrinology 2002;143:1485–1494. [DOI] [PubMed] [Google Scholar]
- Roy SK, Kole AR. Ovarian transforming growth factor-beta (TGF-beta) receptors: in-vitro effects of follicle stimulating hormone, epidermal growth factor and TGF-beta on receptor expression in human preantral follicles. Mol Hum Reprod 1998;4:207–214. [DOI] [PubMed] [Google Scholar]
- Russo N, Russo M, Daino D, Bucci F, Pluchino N, Casarosa E, Artini PG, Cela V, Luisi M, Genazzani AR. Polycystic ovary syndrome: brain-derived neurotrophic factor (BDNF) plasma and follicular fluid levels. Gynecol Endocrinol 2012;28:241–244. [DOI] [PubMed] [Google Scholar]
- Sadeu JC, Doedée AM, Neal MS, Hughes EG, Foster WG. Neurotrophins (BDNF and NGF) in follicular fluid of women with different infertility diagnoses. Reprod Biomed Online 2012;24:174–179. [DOI] [PubMed] [Google Scholar]
- Salas C, Julio-Pieper M, Valladares M, Pommer R, Vega M, Mastronardi C, Kerr B, Ojeda SR, Lara HE, Romero C. Nerve growth factor-dependent activation of trkA receptors in the human ovary results in synthesis of follicle-stimulating hormone receptors and estrogen secretion. J Clin Endocrinol Metab 2006;91:2396–2403. [DOI] [PubMed] [Google Scholar]
- Schultea TD, Dees WL, Ojeda SR. Postnatal development of sympathetic and sensory innervation of the rhesus monkey ovary. Biol Reprod 1992;47:760–767. [DOI] [PubMed] [Google Scholar]
- Seifer DB, Feng B, Shelden RM. Immunocytochemical evidence for the presence and location of the neurotrophin-Trk receptor family in adult human preovulatory ovarian follicles. Am J Obstet Gynecol 2006;194:1129–1134. discussion 1134–1126. [DOI] [PubMed] [Google Scholar]
- Seifer DB, Feng B, Shelden RM, Chen S, Dreyfus CF. Brain-derived neurotrophic factor: a novel human ovarian follicular protein. J Clin Endocrinol Metab 2002. a;87:655–659. [DOI] [PubMed] [Google Scholar]
- Seifer DB, Feng B, Shelden RM, Chen S, Dreyfus CF. Neurotrophin-4/5 and neurotrophin-3 are present within the human ovarian follicle but appear to have different paracrine/autocrine functions. J Clin Endocrinol Metab 2002. b;87:4569–4571. [DOI] [PubMed] [Google Scholar]
- Shibayama E, Koizumi H. Cellular localization of the Trk neurotrophin receptor family in human non-neuronal tissues. Am J Pathol 1996;148:1807–1818. [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011;61:212–236. [DOI] [PubMed] [Google Scholar]
- Silva M, Fernández A, Ulloa-Leal C, Adams GP, Berland MA, Ratto MH. LH release and ovulatory response after intramuscular, intravenous, and intrauterine administration of β-nerve growth factor of seminal plasma origin in female llamas. Theriogenology 2015;84:1096–1102. [DOI] [PubMed] [Google Scholar]
- Silva ME, Smulders JP, Guerra M, Valderrama XP, Letelier C, Adams GP, Ratto MH. Cetrorelix suppresses the preovulatory LH surge and ovulation induced by ovulation-inducing factor (OIF) present in llama seminal plasma. Reprod Biol Endocrinol 2011;9:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper SD. Neurotrophic Factors: An Overview. Methods Mol Biol 2018;1727:1–17. [DOI] [PubMed] [Google Scholar]
- Spears N, Molinek MD, Robinson LL, Fulton N, Cameron H, Shimoda K, Telfer EE, Anderson RA, Price DJ. The role of neurotrophin receptors in female germ-cell survival in mouse and human. Development 2003;130:5481–5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streiter S, Fisch B, Sabbah B, Ao A, Abir R. The importance of neuronal growth factors in the ovary. Mol Hum Reprod 2016;22:3–17. [DOI] [PubMed] [Google Scholar]
- Suter-Crazzolara C, Unsicker K. GDNF is expressed in two forms in many tissues outside the CNS. Neuroreport 1994;5:2486–2488. [DOI] [PubMed] [Google Scholar]
- Takahashi M. The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev 2001;12:361–373. [DOI] [PubMed] [Google Scholar]
- Tapia V, Gabler F, Muñoz M, Yazigi R, Paredes A, Selman A, Vega M, Romero C. Tyrosine kinase A receptor (trkA): a potential marker in epithelial ovarian cancer. Gynecol Oncol 2011;121:13–23. [DOI] [PubMed] [Google Scholar]
- Taylor CW, Tovey SC. IP(3) receptors: toward understanding their activation. Cold Spring Harb Perspect Biol 2010;2:a004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ, Olson L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature 1995;373:335–339. [DOI] [PubMed] [Google Scholar]
- Trupp M, Scott R, Whittemore SR, Ibáñez CF. Ret-dependent and -independent mechanisms of glial cell line-derived neurotrophic factor signaling in neuronal cells. J Biol Chem 1999;274:20885–20894. [DOI] [PubMed] [Google Scholar]
- Tsui-Pierchala BA, Milbrandt J, Johnson EM. NGF utilizes c-Ret via a novel GFL-independent, inter-RTK signaling mechanism to maintain the trophic status of mature sympathetic neurons. Neuron 2002;33:261–273. [DOI] [PubMed] [Google Scholar]
- Ulloa-Leal C, Bogle OA, Adams GP, Ratto MH. Luteotrophic effect of ovulation-inducing factor/nerve growth factor present in the seminal plasma of llamas. Theriogenology 2014;81:1101–1107.e1101. [DOI] [PubMed] [Google Scholar]
- Vaillant AR, Mazzoni I, Tudan C, Boudreau M, Kaplan DR, Miller FD. Depolarization and neurotrophins converge on the phosphatidylinositol 3-kinase-Akt pathway to synergistically regulate neuronal survival. J Cell Biol 1999;146:955–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera C, Tapia V, Kohan K, Gabler F, Ferreira A, Selman A, Vega M, Romero C. Nerve growth factor induces the expression of chaperone protein calreticulin in human epithelial ovarian cells. Horm Metab Res 2012;44:639–643. [DOI] [PubMed] [Google Scholar]
- Vera C, Tapia V, Vega M, Romero C. Role of nerve growth factor and its TRKA receptor in normal ovarian and epithelial ovarian cancer angiogenesis. J Ovarian Res 2014;7:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigano P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol 2004;18:177–200. [DOI] [PubMed] [Google Scholar]
- Wang X, Sun Z, Zhen J, Yu Q. Brain-derived neurotrophic factor from follicular fluid is positively associated with rate of mature ooocytes collected and cleavage rate in intracytoplasmic sperm injection patients. J Assist Reprod Genet 2011;28:1053–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waraksa JA, Lindsay RM, Ip NY, Hutz RJ. Neurotrophin-3 augments steroid secretion by hamster ovarian follicles in vitro. Zoolog Sci 1995;12:499–502. [DOI] [PubMed] [Google Scholar]
- Wessels JM, Kay VR, Leyland NA, Agarwal SK, Foster WG. Assessing brain-derived neurotrophic factor as a novel clinical marker of endometriosis. Fertil Steril 2016;105:119–128.e111–115. [DOI] [PubMed] [Google Scholar]
- Wessels JM, Leyland NA, Agarwal SK, Foster WG. Estrogen induced changes in uterine brain-derived neurotrophic factor and its receptors. Hum Reprod 2015;30:925–936. [DOI] [PubMed] [Google Scholar]
- Wessels JM, Wu L, Leyland NA, Wang H, Foster WG. The brain-uterus connection: brain derived neurotrophic factor (BDNF) and its receptor (Ntrk2) are conserved in the mammalian uterus. PLoS One 2014;9:e94036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CH, Ali ES, Scrimgeour N, Martin AM, Hua J, Tallis GA, Rychkov GY, Barritt GJ. Steatosis inhibits liver cell store-operated Ca2+ entry and reduces ER Ca2+; through a protein kinase C-dependent mechanism. Biochem J 2015;466:379–390. [DOI] [PubMed] [Google Scholar]
- Wilson JL, Chen W, Dissen GA, Ojeda SR, Cowley MA, Garcia-Rudaz C, Enriori PJ. Excess of nerve growth factor in the ovary causes a polycystic ovary-like syndrome in mice, which closely resembles both reproductive and metabolic aspects of the human syndrome. Endocrinology 2014;155:4494–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KW, Sarnecki C, Roberts TM, Blenis J. ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, Raf-1, and RSK. Cell 1992;68:1041–1050. [DOI] [PubMed] [Google Scholar]
- Wu LM, Hu MH, Tong XH, Han H, Shen N, Jin RT, Wang W, Zhou GX, He GP, Liu YS. Chronic unpredictable stress decreases expression of brain-derived neurotrophic factor (BDNF) in mouse ovaries: relationship to oocytes developmental potential. PLoS One 2012;7:e52331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Kornhauser JM, Xia Z, Thiele EA, Greenberg ME. Nerve growth factor activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Mol Cell Biol 1998;18:1946–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Ohnishi H, Sano S, Nakatani A, Ikeuchi T, Hatanaka H. Insulin receptor substrate (IRS)-1 and IRS-2 are tyrosine-phosphorylated and associated with phosphatidylinositol 3-kinase in response to brain-derived neurotrophic factor in cultured cerebral cortical neurons. J Biol Chem 1997;272:30334–30339. [DOI] [PubMed] [Google Scholar]
- Yancopoulos GD, Maisonpierre PC, Ip NY, Aldrich TH, Belluscio L, Boulton TG, Cobb MH, Squinto SP, Furth ME. Neurotrophic factors, their receptors, and the signal transduction pathways they activate. Cold Spring Harb Symp Quant Biol 1990;55:371–379. [DOI] [PubMed] [Google Scholar]
- Yano H, Chao MV. Neurotrophin receptor structure and interactions. Pharm Acta Helv 2000;74:253–260. [DOI] [PubMed] [Google Scholar]
- York RD, Molliver DC, Grewal SS, Stenberg PE, McCleskey EW, Stork PJ. Role of phosphoinositide 3-kinase and endocytosis in nerve growth factor-induced extracellular signal-regulated kinase activation via Ras and Rap1. Mol Cell Biol 2000;20:8069–8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Ren H, Liu T, Yong M, Zhong H. Expression and significance of ERβ and TrkB in endometriosis. Clin Exp Obstet Gynecol 2016;43:75–81. [PubMed] [Google Scholar]
- Yu Y, Yan J, Li M, Yan L, Zhao Y, Lian Y, Li R, Liu P, Qiao J. Effects of combined epidermal growth factor, brain-derived neurotrophic factor and insulin-like growth factor-1 on human oocyte maturation and early fertilized and cloned embryo development. Hum Reprod 2012;27:2146–2159. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Chang HM, Taylor EL, Leung PCK, Liu RZ. BMP6 down-regulates GDNF expression through SMAD1/5 and ERK1/2 signaling pathways in human granulosa-lutein cells. Endocrinology 2018;159:2926–2938. [DOI] [PubMed] [Google Scholar]
- Zhang QY, Guan Q, Wang Y, Feng X, Sun W, Kong FY, Wen J, Cui W, Yu Y, Chen ZY. BDNF Val66Met polymorphism is associated with Stage III-IV endometriosis and poor in vitro fertilization outcome. Hum Reprod 2012;27:1668–1675. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu K. Cellular and molecular regulation of the activation of mammalian primordial follicles: somatic cells initiate follicle activation in adulthood. Hum Reprod Update 2015;21:779–786. [DOI] [PubMed] [Google Scholar]
- Zhao P, Qiao J, Huang S, Zhang Y, Liu S, Yan LY, Hsueh AJ, Duan EK. Gonadotrophin-induced paracrine regulation of human oocyte maturation by BDNF and GDNF secreted by granulosa cells. Hum Reprod 2011;26:695–702. [DOI] [PubMed] [Google Scholar]
- Ziegenhorn AA, Schulte-Herbrüggen O, Danker-Hopfe H, Malbranc M, Hartung HD, Anders D, Lang UE, Steinhagen-Thiessen E, Schaub RT, Hellweg R. Serum neurotrophins--a study on the time course and influencing factors in a large old age sample. Neurobiol Aging 2007;28:1436–1445. [DOI] [PubMed] [Google Scholar]
- Zundel W, Swiersz LM, Giaccia A. Caveolin 1-mediated regulation of receptor tyrosine kinase-associated phosphatidylinositol 3-kinase activity by ceramide. Mol Cell Biol 2000;20:1507–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]