Short abstract
Background
There are over 100 published studies of a therapy called Emotional Freedom Techniques (EFT). This popular form of energy psychology combines elements of established methods like cognitive therapy with acupressure. Our group reported the first evidence of its mechanisms of action at the molecular level, showing that it can influence levels of the stress hormone cortisol.
Objectives
Given recent advances in molecular genomics that have identified noncoding ribonucleic acid (RNA) molecules as important regulators of gene expression, the aim of this study is to explore the possibility that microRNAs play a role in mediating the effects of EFT.
Methods
We measured microRNA levels in stored blood samples from our previous study in which veterans were randomized into an EFT group receiving EFT and treatment as usual throughout a 10-week intervention period, and a control group receiving only treatment as usual during the intervention period and then receiving EFT. A broad panel of 800 microRNAs was probed using a multiplexed, direct hybridization, and detection system.
Results
All of the microRNA targets were expressed at low levels and most were below thresholds established by negative control probes. Baseline variability was determined using samples collected from the control group at the start and end of the intervention period, and used to filter out targets that were too noisy under control conditions to be able to distinguish a response to treatment. Analysis of the remaining viable targets found a general trend of reduced expression following EFT, compared to expression levels in samples from the control group during the intervention period. The most notable decreases in expression levels were found for 2 microRNAs: let-7b and let-7c, although no significance was found after adjusting for multiple comparisons.
Conclusions
These preliminary data support the feasibility of measuring microRNA expression level changes that correlate with effective EFT therapy.
Keywords: epigenetics, Emotional Freedom Techniques, posttraumatic stress disorder, energy medicine, microRNA
Introduction
Background
The term “energy psychology” refers to a group of therapeutic modalities that combine somatic stimulation such as eye movements and acupressure with psychological techniques. The most widely practiced form of energy psychology is Emotional Freedom Techniques (EFT), with an estimated 20 million users worldwide.1 EFT is a noninvasive therapy that pairs the recall of emotional disturbances with manual stimulation of specific acupuncture points on the body, as identified by Traditional Chinese Medicine. Evidence supporting the efficacy of EFT has been reported in over 100 studies published peer-reviewed biomedical journals.2
This literature encompasses a variety of psychological and physiological conditions. Notably, a systematic review and meta-analysis of randomized, controlled trials of EFT for anxiety found 14 studies (n = 658).3 This review reported that EFT treatment demonstrated a significant decrease in anxiety scores, even when accounting for the effect size of control treatments. The pre-post effect size for the EFT treatment group was 1.23, while the effect size for combined controls was 0.41. A systematic review and meta-analysis of randomized controlled trials of EFT for reducing depression found similarly compelling results.4 Nelms and Castel reported a large effect size (1.85) in the treatment of depression in 12 randomized controlled trials (n = 398), which is larger than the effect size measured in prior meta-analyses of antidepressant drug trials and psychotherapy studies.5,6
Our team conducted the first randomized controlled trial of EFT evaluating a physiological biomarker. We measured salivary cortisol levels in healthy volunteers before and after a single session of EFT and found positive effects.7 Cortisol is a hormone associated with high stress levels and several pathophysiological conditions that have been associated with its dysregulation.8 In our study, 83 participants were randomly assigned to a 1-time hour-long session of 3 different experimental conditions: EFT, talk therapy in the form of a supportive interview, and rest. The results showed a significantly lower level of cortisol in the EFT group (24%) when compared to the 2 other therapy groups, which showed a decline of only an average of 14%. The decline in this primary stress hormone was also positively associated with a reduction in a range of psychological symptoms, including anxiety and depression. Given the robust physiological and clinical impact reported for EFT, it is likely that genomic mechanisms play a role in its effects on the body.
A link between cortisol signaling and genomic mechanisms can be found at the level of the glucocorticoid receptor. Cortisol diffuses through the cell membrane and binds to the glucocorticoid receptor in the cytoplasm of the cell. One of the primary activities of the activated hormone-receptor complex is to move into the cell nucleus where it binds to glucocorticoid response elements in the promoter region of target nucleic acid sequences and influences their expression. These target sequences include microRNA, a type of noncoding RNA molecule.9 Numerous studies have shown that the activated glucocorticoid receptor can regulate microRNA expression either at the transcriptional level or through posttranscriptional maturation by interacting with microRNA processing factors.10 Thus, our prior empirical demonstration that EFT can influence cortisol levels7 suggests a theoretical pathway between EFT and the regulation of microRNA expression. We earlier argued that successful psychotherapeutic techniques such as EFT should be considered epigenetic interventions that produce change on the molecular level11,12 and conducted a pilot study of messenger RNA (mRNA) expression levels in blood samples collected from war veterans experiencing relief from symptoms of posttraumatic stress disorder (PTSD) following EFT.13 In this study, we analyze frozen blood samples collected from our previously published mRNA study to look “upstream” of the molecular signaling pathway at microRNA expression levels.
Methods
Published mRNA Study
The previous mRNA study recruited a small group of war veterans experiencing symptoms of PTSD and offered them a 10-week EFT program.13 The study was approved by the Institutional Review Board of the American Association for Acupuncture and Bioenergetic Medicine, and posted on ClinicalTrials.gov (NCT01250431). The design of the study met the quality criteria of the Task Force on Empirically Validated Treatments of Division 12 (Clinical Psychology) of the American Psychological Association14 as well as CONSORT standards for clinical trials. Validated self-report questionnaires were administered to participants to quantify the efficacy of the EFT intervention. The Symptom Assessment 45 (SA-45)15 was used to assess psychological symptoms. This instrument has 2 general scales: one measuring the severity of symptoms (GSI; Global Severity Index) and the other measuring the breadth (PST; Positive Symptom Total). PTSD was assessed using the Posttraumatic Checklist-Military (PCL-M),16 which is based on the diagnostic criteria of the Diagnostic and Statistical Manual (DSM-IV) published by the American Psychiatric Association.17
Participants were randomized into either an EFT group or a treatment as usual (TAU) control group using permuted block randomization (randomizer.org). After completion of a 10-week waiting period, TAU participants received the EFT intervention. Participants were assessed on intake, before and after treatment, and at 3 and 6 months. Posttreatment results for psychological symptoms from the TAU group were paired with those of the EFT group to provide maximum statistical power. One blood sample was drawn from each subject before and after the treatment period for the EFT group. For the TAU group, blood samples were collected before and after the waiting period, to assess baseline variability, and also after they received their post-wait EFT treatment. Blood samples were processed using the PAXgene RNA stabilization system (PreAnalytix, Doncaster, VIC).
Participant Characteristics
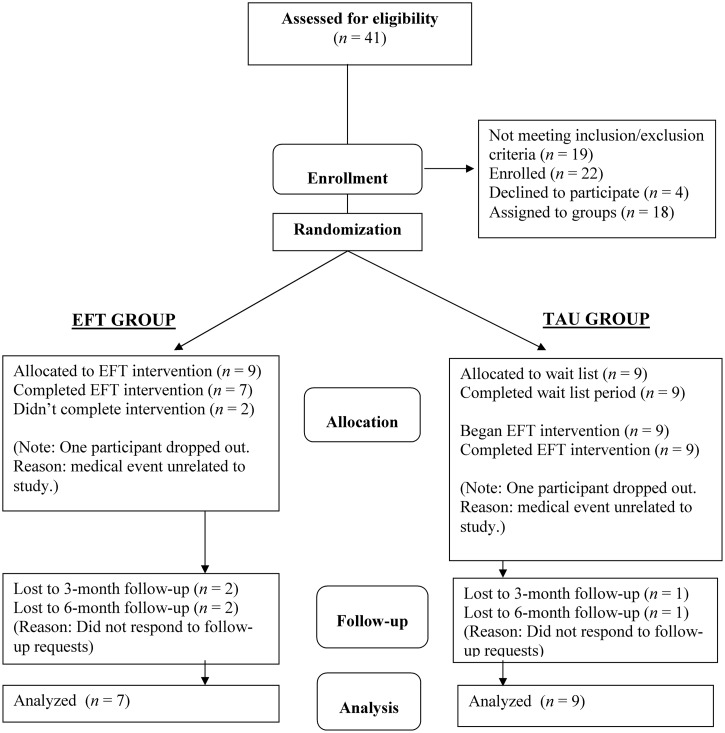
We made initial contact with 124 veterans, of whom 41 consented to be assessed for eligibility. Of these, 19 were excluded based on the inclusion/exclusion criteria and 22 enrolled. Four of those enrolling subsequently decided not to participate and 18 were randomly assigned to 1 of the 2 groups. After beginning EFT treatment, 2 participants dropped out for medical reasons unrelated to the study, resulting in 16 participants completing the 10-week EFT program. Analysis was performed on data from the 16 participants (11 males and 5 females) who completed treatment. The mean age of participants was 59.5 years (standard deviation [SD] = 8.32). The flow of participants through the study is illustrated in the CONSORT diagram in Figure 1.
Figure 1.
CONSORT Flow Chart. EFT, Emotional Freedom Technique; TAU, treatment as usual.
To make the results as generalizable as possible, the sole inclusion criterion was a score of >50 on the PCL-M.16 A score of 35 or greater represents heightened PTSD risk in a military population18 and a score of 50 or more indicates the likelihood of a clinical PTSD diagnosis.16 Whether in the TAU or EFT group, participants were required to remain under the care of a primary care provider. The characteristics of usual care (whether in the group receiving TAU alone or the group receiving EFT supplementary to TAU) were as follows: 6 (38%) were under the primary care of the Veterans Administration while 10 (62%) were also enrolled in private health-care plans. Twelve (75%) reported being under the care of a mental health professional in addition to their primary care physician. Thirteen (81%) had previously received a positive PTSD diagnosis while 3 had not. Pharmaceutical drug use was reported by 8 (50%), with the mean number of drugs being 2, primarily analgesics. Seven (44%) reported using complementary medicine techniques, including the following: acupuncture, qigong, tai chi, yoga, and herbs. One reported use of a transcutaneous electrical nerve stimulation unit for pain.
Energy Psychology Intervention
EFT was delivered to the participants according to The EFT Manual1,19 for approximately 1 hour, once per week for 10 weeks. Participants were given exercises to practice at home between sessions with EFT practitioners. All practitioners were certified in Clinical EFT (EFT Universe, Santa Rosa, CA), a manualized, evidence-based form of the EFT method. Treatment sessions followed the protocol described in The EFT Manual: participants compiled lists of traumatic memories in summary form, then rated their degree of emotional distress on a Likert scale ranging from 0 (no distress) to 10 (maximum distress). With the guidance of the practitioner, they then focused on each aspect of the memory while stimulating 1 of the 12 acupressure points described in The EFT Manual by tapping with their fingertips. When their self-reported emotional distress was zero or a low number, they moved on to the next memory in their list and repeated the process.
Noncoding RNA Expression Level Measurement
We probed a broad panel of 800 microRNAs using the nCounter® microRNA Expression Assay (Nanostring, Seattle, WA). The microRNA expression values were initially normalized by first subtracting the mean plus 2 SDs of the negative controls from all counts. This was followed by scaling to the geometric means of the synthetic positive controls to account for differences in hybridization efficiency. Changes in expression levels were calculated by taking the log transform of the ratio of expression levels with the initial time point as the denominator and the later time point in the numerator. All statistical analyses were performed using log-transformed ratios.
Control points were analyzed to assess baseline variability, and significant biases were noted in the overall expression levels. Although there are no well-established universal housekeeping microRNAs, situational normalization procedures have been successfully applied to normalize microRNA expression profiles. An iterative procedure was used by first using the sum of all microRNA counts as a coarse measure, and then selecting the top 5 microRNA targets that had the least variability while maintaining relatively small fold-changes in control as well as treatment outcomes (<10%). The geometric mean of these targets (microRNA 942, microRNA 25, microRNA 342, microRNA 151a, microRNA 182) were used to normalize the data.
Results
Previous Results Probing mRNA Levels
For our previously published study,13 analyses focused on the changes in the questionnaire responses and mRNA expression levels in the blood samples. There was no significant difference in PCL-M scores between the 2 groups on intake, and no significant change in scores in the TAU group between the start and end of the wait period. Symptom severity (GSI) scores on the SA-45 ranged between 43 and 81, with a mean of 68.87 and a SD of 9.486. Symptom breadth (PST) also ranged between 43 and 81, with a mean of 68.27 and SD of 8.762. For all SA-45 subscales and general scales, 60 indicates clinical symptom levels, and the lowest possible score is either 41 or 42 depending on gender and condition.
A test of significance comparing psychological symptom scores for the combined data pretest and after 10 EFT sessions was conducted using repeated measures 1-way ANOVA. PCL-M scores decreased by 25.63 points on average. This decrease was highly significant statistically. To determine whether participants maintained their gains, 3- and 6-month follow-up assessments were analyzed using repeated measures 1-way ANOVA. No significant change was found between posttreatment results and follow-up on any parameter, indicating that treatment results held over time. Paranoia, depression, and hostility dropped below the clinical cutoff after treatment and remained subclinical at 6 month follow-up, with no significant difference between posttreatment and follow-up results.
Significant differential expression of mRNA levels for 6 genes was found when comparing expression levels before and after the intervention period in participants receiving EFT: Chemokine Receptor 3 (CXCR3), Interleukin 18 (IL18), Interleukin 10 Receptor Beta (IL10RB), Tumor Necrosis Factor Alpha-Induced Protein 6 (TNFAIP6), Leukocyte-Endothelial Cell Adhesion Molecule 1 (SELL), and Interferon Induced Transmembrane Protein 1 (IFITM1). These genes are generally known to be involved in the regulation of cellular immunity and inflammation and are associated with stress. These findings were consistent with prior studies by Hollifield et al.20 and Logue et al.21 that found evidence for differential baseline expression of genes responsive to glucocorticoid signaling and inflammatory pathways in a cohort of trauma-exposed male veterans with PTSD.
Probing Noncoding RNA Levels
When we probed the same blood samples for expression levels of microRNAs in this study, we found that most of the microRNA targets were expressed at very low levels. After normalization, strict quality control measures were applied to the data using MATLAB Statistical Toolbox (Mathworks, Natick, MA). A conservative cutoff for signal strength was applied (15 normalized counts as determined from negative controls) based on guidelines provided by the manufacturer of the expression platform (Nanostring, Seattle, WA), resulting in the elimination of 742 (∼93%) of the target microRNAs from further analyses and leaving a set of 56 potential microRNA targets. As part of the experimental design, the expression levels in the TAU group during the waiting time period were used to assess baseline variability and identify targets too noisy under control conditions to be able to distinguish a response to the treatment condition. ANOVA and fold-change comparisons were used as filtering strategies based on published methods.22–24 As a prelude for this and further parametric statistical analysis, data for each group were evaluated for normal distribution and homoscedasticity by Lilliefors test and 2-sample F test, respectively.
To ensure adequate signal to noise ratios, an additional 8 (∼14% of potential set) targets were eliminated because they had moderate significant fold changes in the TAU group by Student’s t test (P < .15) and another 10 (∼18%) due to mean fold changes in excess of 15%. A comparison was also made in the magnitude of the response in the EFT group as compared to the magnitude of the change in the TAU group, eliminating 7 more targets (∼13%) in the remaining set for which apparent responses in the EFT group were not greater than changes in expression levels seen in the TAU group. Taken together, 25 out of the 56 targets were filtered out with these 3 quality measures leveraged from the control data (∼45%) leaving 31 viable targets to evaluate.
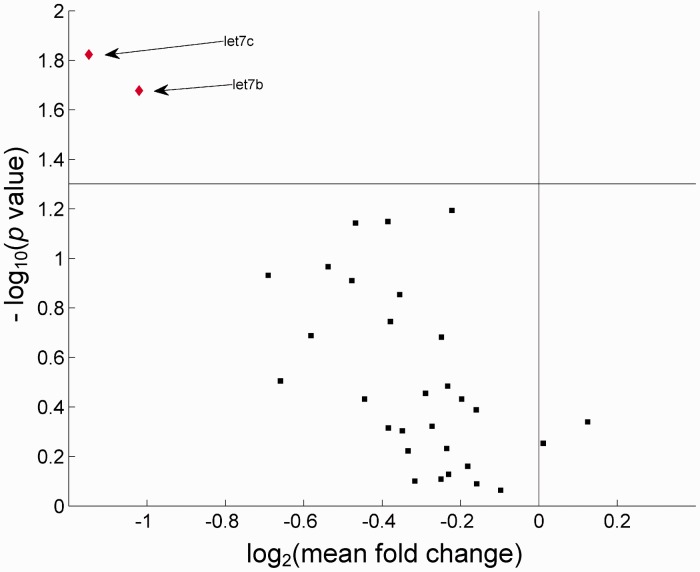
A Student’s t test was performed on the data from microRNA targets with a robust signal to noise ratio (31 microRNAs), comparing expression levels before and after the intervention period between the EFT and TAU groups. Notable decreases in expression levels were found for 2 microRNAs: microRNA let-7b (mean fold-change = −2.028; P = .021) and microRNA let-7c (mean fold-change = −2.216; P = .015), although no significance was found after adjusting for multiple comparisons using Bonferroni or Benjamini and Hochberg correction methods. Figure 2 presents a Volcano plot illustrating a general trend of downregulation of microRNA targets following EFT, along with the 2 let7 microRNAs that were downregulated most dramatically. Table 1 shows the descriptive statistics for the comparison.
Figure 2.
Volcano Plot of Measurements Obtained From the 31 Viable MicroRNA Targets, Highlighting the Differential Expression of MicroRNAs Let 7b and Let 7c (Diamonds). The x-axis is the log2 value of the mean fold change of the expression levels of the EFT group (posttherapy/pretherapy). The y-axis is the −log10 value of the P value from the Student’s t test between the fold changes of the EFT group against the fold changes of the TAU group (control), with expression levels at comparable time points. The horizontal reference line is at P = .05.
Table 1.
Test of Significance Comparing Changes in Expression Levels.
|
TAU (n = 9) |
EFT (n = 7) |
||
|---|---|---|---|
| miRNA | Mean Fold Change | Mean Fold Change | P |
| hsa_let_7c_5p | −1.03 | −2.216 | .015 |
| hsa_let_7b_5p | −1.05 | −2.028 | .021 |
| hsa_miR_342_3p | 1.113 | −1.166 | .064 |
| hsa_miR_296_5p | 1.007 | −1.306 | .071 |
| hsa_miR_652_3p | −1.046 | −1.383 | .072 |
| hsa_let_7d_5p | −1.089 | −1.452 | .108 |
| hsa_miR_185_5p | −1.101 | −1.614 | .117 |
| hsa_miR_125b_5p | −1.013 | −1.392 | .123 |
| hsa_miR_664a_3p | 1.043 | −1.279 | .14 |
| hsa_miR_425_5p | −1.049 | −1.3 | .18 |
| hsa_miR_140_3p | −1.06 | −1.496 | .205 |
| hsa_miR_423_5p | 1.12 | −1.188 | .208 |
| hsa_miR_19b_3p | −1.048 | −1.579 | .313 |
| hsa_miR_1180_3p | −1.019 | −1.175 | .328 |
| hsa_miR_182_5p | 1.044 | −1.222 | .351 |
| hsa_let_7a_5p | −1.108 | −1.361 | .37 |
| hsa_miR_151a_5p | 1.051 | −1.146 | .37 |
| hsa_miR_331_3p | 1.048 | −1.117 | .409 |
| hsa_miR_23a_3p | 1.011 | 1.09 | .457 |
| hsa_miR_194_5p | −1.058 | −1.208 | .476 |
| hsa_miR_16_5p | −1.038 | −1.305 | .484 |
| hsa_miR_22_3p | −1.129 | −1.273 | .497 |
| hsa_miR_25_3p | −1.005 | 1.008 | .558 |
| hsa_miR_2110 | −1.049 | −1.177 | .586 |
| hsa_miR_363_3p | −1.093 | −1.26 | .599 |
| hsa_miR_361_3p | −1.082 | −1.134 | .691 |
| hsa_miR_4454_hsa_miR_7975 | −1.02 | −1.173 | .745 |
| hsa_miR_148b_3p | −1.123 | −1.189 | .779 |
| hsa_miR_26b_5p | −1.146 | −1.245 | .793 |
| hsa_miR_451a | −1.061 | −1.116 | .813 |
| hsa_miR_223_3p | −1.037 | −1.07 | .863 |
Abbreviations: EFT, Emotional Freedom Technique; TAU, treatment as usual.
Secondary analyses were conducted to assess whether differential microRNA expression was correlated with the clinical phenotypes reported by the participants on the questionnaires. A correlation analysis was performed on the log-transformed ratios and the 3 comprehensive psychological symptoms scales: PCL-M, GSI, and PST. Pooled data from all participants were considered to attain the highest statistical strength. The differences in clinical parameters pre- and posttreatment were used for this analysis. Pearson product–moment correlation coefficients were calculated using the log-transformed ratios from the pooled treatment group. Three microRNAs had fold changes significantly correlated with PTSD symptoms: microRNA 30d, microRNA 532, microRNA 194; 5 with GSI: microRNA 181a, microRNA 378i, microRNA 222, microRNA 125a, microRNA 19b, and 1 with PST: microRNA 222.
Discussion
Here, we present preliminary data indicating that the remediation by EFT of psychological conditions, including depression and PTSD, is associated with a trend of reduced expression levels of microRNAs. MicroRNAs are short noncoding RNA molecules of 20 to 25 nucleotides in length found in animals and plants. More than 3000 human microRNAs have been identified, and many of these have been shown to regulate the expression of protein-encoding genes. MicroRNAs regulate gene expression at the posttranscriptional level, which means that they alter the process of gene expression at the point after genetic information is transcribed from DNA to mRNA and before the mRNA is translated into a protein. They generally bind to the 3-UTR (untranslated region) of their target mRNAs and repress protein production by destabilizing the mRNA and translational silencing.25 Due to their ability to bind with imperfect complementarity, microRNAs are able to regulate more than 1 target mRNA sequence. This molecular promiscuity enables each microRNA to affect the regulation of many different target genes. Together, microRNAs can affect a third of all cellular mRNAs and thus have wide-ranging influence in biological processes, including embryonic development, cell proliferation, and metabolic homeostasis.26,27 Notable in the context of PTSD, 2 studies found evidence that traumatic brain injury can alter microRNA levels in blood plasma.28,29
The 2 microRNAs we identified as being most robustly downregulated following EFT treatment, microRNAs let-7b and let-7c, belong to a family of microRNAs that was the first to be discovered in humans with roles in neurogenesis and synapse formation,30 which may underlie the neurological basis of PTSD maintenance.
Our results with microRNAs let-7b and let-7c corroborate a recent report of decreased expression for these same 2 microRNAs being associated with decreases in depression following electroconvulsive shock therapy (ECT).31 Researchers conducting the depression study compared microRNA expression levels in blood cells collected from healthy controls (n = 20) and before and after ECT from patients diagnosed with treatment-resistant depression (n = 40). As we found in our study, the largest effects following therapy were the downregulation of microRNAs let-7b and let-7c, which was correlated with decreases in depression. The finding that these 2 divergent therapeutic approaches that both result in reduced depression were associated with the same microRNA changes provides strong rationale for future studies of these microRNAs as potential mediators of the observed therapeutic effects.
The notion that microRNAs let-7b and let-7c could mediate neurological changes resulting in relief from depression and other PTSD symptoms is further supported by bioinformatic analysis revealing that both these microRNAs can regulate the expression genes in the PI3k-Akt-mTOR signaling pathway.31 The PI3k-Akt-mTOR pathway is an important component in the subcellular integration of synaptic neurotransmission and neuronal cell plasticity and has been linked to PTSD in numerous animal models. For example, pharmacological inhibition of the PI3k-Akt-mTOR pathway in a rat model of PTSD appears to reduce the emotional strength of established, traumatic memories.32 Another study found an association between the PI3k-Akt-mTOR pathway and PTSD-induced neuronal apoptosis.33 Particularly relevant because of the acupuncture point stimulation component of EFT, a study administering acupuncture to rats found evidence that acupuncture exerts antidepressant and anxiolytic effects on PTSD-related symptoms via the PI3k-Akt-mTOR pathway.34
The design of future studies will need to address the limitations of this pilot study, including the absence of an active control group receiving a treatment of demonstrated efficacy such as cognitive processing therapy. However, there is no evidence in the literature that the nonspecific effects of therapy can remediate PTSD35 and our previous cortisol study comparing a single session of EFT to a supportive interview found more than double the reduction in psychological symptoms in the EFT group.13 Another limitation of the current pilot study is the fact that no significance was found after adjusting for multiple comparisons. In support of designing a follow-up study focusing on let-7b and let-7c, we performed a power analysis to predict how an increase in sample size would enhance statistical significance when comparing responses between separate treatment and control groups. The R package pwr (version 1.2) was used, which utilizes Cohen’s d effect size to determine sample size. Based on the underlying variability of the control and treatment groups and the calculated changes in expression levels, these preliminary data predict that a sample size of 22 (11 in each group) would yield log ratios significantly different from 0 (P < .05) with 80% power for these 2 microRNAs. For 90% statistical power, a sample size of 28 (14 in each group) would be sufficient.
Conclusions
Results of this pilot study support the hypothesis that microRNAs play a role in mediating the physiological effects of EFT and demonstrate the feasibility of testing it formally with a larger clinical population. Notably, the 2 microRNAs we identified as being most robustly downregulated following EFT treatment, let-7b and let-7c, have also been shown to be downregulated following ECT-induced decreases in depression.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Soldaten und Veteranen Stiftung, Germany (Soldier and Veterans Foundation).
References
- 1.Church D. The EFT manual. 3rd ed Santa Rosa, CA: Energy Psychology Press; 2013. [Google Scholar]
- 2.Research.EFTuniverse.com
- 3.Clond M. Emotional freedom techniques for anxiety: a systematic review with meta-analysis. J Nerv Ment Dis. 2016; 204:388–395. [DOI] [PubMed] [Google Scholar]
- 4.Nelms JA, Castel L. A systematic review and meta-analysis of randomized and nonrandomized trials of clinical emotional freedom techniques (EFT) for the treatment of depression. Explore (NY). 2016; 12(6):416–426. [DOI] [PubMed] [Google Scholar]
- 5.Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. J Am Med Assoc. 2010; 303:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuijpers P, van Straten A, Bohlmeijer E, Hollon SD, Andersson G. The effects of psychotherapy for adult depression are overestimated: a meta-analysis of study quality and effect size. Psychol Med. 2010; 40:211–223. [DOI] [PubMed] [Google Scholar]
- 7.Church D, Brooks AJ, Yount G. The effect of emotional freedom techniques on stress biochemistry: a randomized controlled trial . J Nerv Ment Dis. 2012; 200(10):891–896. [DOI] [PubMed] [Google Scholar]
- 8.Spiegel D. Mind matters in cancer survival. Psycho-oncology. 2012; 21(6):588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bentwich I, Avniel A, Karov Y, et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nature Genet. 2005; 37:766–770. [DOI] [PubMed] [Google Scholar]
- 10.Yang Z, Wang L. Regulation of microRNA expression and function by nuclear receptor signaling. Cell Biosci. 2011; 1:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yount G. Energy healing at the frontier of genomics. Energy Psychol Theory Res Treat. 2013; 5(2):35–40. [Google Scholar]
- 12.Feinstein D, Church D. Modulating gene expression through psychotherapy: the contribution of non-invasive somatic interventions. Rev Gen Psychol. 2010; 14(4):283–295. [Google Scholar]
- 13.Church D, Yount G, Rachlin K, Fox L, Nelms J. Epigenetic effects of PTSD remediation in veterans using clinical EFT: a randomized controlled pilot study. Am J Health Promot. 2018; 32(1):112–122. [DOI] [PubMed] [Google Scholar]
- 14.Chambless DL, Hollon SD. Defining empirically supported therapies. J Consult Clin Psychol. 1998; 66(1):7–18. [DOI] [PubMed] [Google Scholar]
- 15.Davison ML, Bershadsky B, Bieber J, Silversmith D, Maruish ME, Kane RL. Development of a brief, multidimensional, self-report instrument for treatment outcomes assessment in psychiatric settings: preliminary findings. Assessment. 1997; 4(3):259–276. [DOI] [PubMed] [Google Scholar]
- 16.Weathers F, Huska J, Keane T. The PTSD Checklist Military Version (PCL-M). Boston, MA: National Center for PTSD; 1991. [Google Scholar]
- 17.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 18.Bliese PD, Wright KM, Adler AB, Cabrera O, Castro CA, Hoge CW. Validating the primary care posttraumatic stress disorder screen and the posttraumatic stress disorder checklist with soldiers returning from combat. J Consult Clin Psychol. 2008; 76(2):272–281. [DOI] [PubMed] [Google Scholar]
- 19.Church D. Clinical EFT as an evidence-based practice for the treatment of psychological and physiological conditions. Psychology. 2013; 4(8):645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollifield M, Moore D, Yount G. Gene expression analysis in combat veterans with and without post-traumatic stress disorder. Mol Med Rep. 2013; 8(1):238–244. [DOI] [PubMed] [Google Scholar]
- 21.Logue MW, Smith AK, Baldwin C, et al. An analysis of gene expression in PTSD implicates genes involved in the glucocorticoid receptor pathway and neural responses to stress. Psychoneuroendocrinology. 2015; 57:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woo HI, Lim SW, Myung W, Kim DK, Lee SY. Differentially expressed genes related to major depressive disorder and antidepressant response: genome-wide gene expression analysis. Exp Mol Med. 2018; 50:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casares FA. Simple method for optimization of reference gene identification and normalization in DNA microarray analysis. Med Sci Monit Basic Res. 2016; 22:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boedigheimer MJ, Wolfinger RD, Bass MB, et al. Sources of variation in baseline gene expression levels from toxicogenomics study control animals across multiple laboratories. BMC Genomics. 2008; 9:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cannell IG, Kong YW, Bushell M. How do microRNAs regulate gene expression? Biochemical Soc Trans. 2008; 36(6):1224–1231. [DOI] [PubMed] [Google Scholar]
- 26.Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol. 2008; 9:219–230. [DOI] [PubMed] [Google Scholar]
- 27.Rossbach M. Small non-coding RNAs as novel therapeutics. Curr Mol Med. 2010; 10(4):361–368. [DOI] [PubMed] [Google Scholar]
- 28.Redell JB, Moore AN, Ward NH, Hergenroeder GW, Dash PK. Human traumatic brain injury alters plasma microRNA levels. J Neurotrauma. 2010; 27(12):2147–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balakathiresan N, Bhomia M, Chandran R, Chavko M, McCarron RM, Maheshwari RK. MicroRNA let-7i is a promising serum biomarker for blast-induced traumatic brain injury. J Neurotrauma. 2012; 29(7):1379–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rehfeld F, Rohde AM, Nguyen DT, Wulczyn FG. Lin28 and let-7: ancient milestones on the road from pluripotency to neurogenesis. Cell Tissue Res. 2015; 359(1):145–160. [DOI] [PubMed] [Google Scholar]
- 31.Gururajan A, Naughton ME, Scott KA, et al. MicroRNAs as biomarkers for major depression: a role for let-7b and let-7c. Transl Psychiatry. 2016; 6:e862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacCallum PE, Hebert M, Adamec RE, Blundell J. Systemic inhibition of mTOR kinase via rapamycin disrupts consolidation and reconsolidation of auditory fear memory. Neurobiol Learn Mem. 2014; 112:176–185. [DOI] [PubMed] [Google Scholar]
- 33.Sui ZX, Liu H, Wang HT, et al. Alteration of apoptosis and Akt/mTOR signal pathway in hippocampal neurons of rat with post-traumatic stress. Sichuan Da Xue Xue Bao Yi Xue Ban. 2014; 45(2):221–224. [PubMed] [Google Scholar]
- 34.Oh JY, Kim YK, Kim SN, et al. Acupuncture modulates stress response by the mTOR signaling pathway in a rat post-traumatic stress disorder model. Sci Rep. 2018; 8(1):11864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradley R, Greene J, Russ E, Dutra L, Western D. A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry. 2005; 162:214–227. [DOI] [PubMed] [Google Scholar]