Abstract
Real-world management decisions for acute cough in children in primary care practice are not well understood. This study is an analysis of 560 encounters for children with cough, 19 days to 18 years of age, seen in a predominantly suburban academic pediatric practice, over 1 year. Past history, cough duration, and cough characteristics significantly affected treatment decisions. Children with cough frequently had a history of preterm birth, allergies, asthma, and neurological conditions. Most common therapies were bronchodilators, antibiotics, and oral corticosteroids. Children prescribed antibiotics were older, more likely to have a wet or productive cough, history of sinusitis, pneumonia or dysphagia, and longer cough duration. Children prescribed oral corticosteroids were younger, less likely to be wet or productive and more likely to have history of asthma or dysphagia. Children prescribed bronchodilators were more likely to have fever, nasal congestion, and wheezing and history of previous asthma, pneumonia, or dysphagia.
Keywords: acute cough, antibiotics, steroids, bronchodilators
Background
Cough is one of the most common reasons for primary care visits,1,2 and often frustrating to treat.3 Since acute cough usually resolves in days to a few weeks,4-6 diagnostic tests are rarely performed, and empiric management based on clinical assessment is the rule.7-9 There are a variety of therapeutic options, including expectorants, cough suppressants, bronchodilators, inhaled and oral corticosteroids, antibiotics, antihistamines, and decongestants. Cough medications are often ineffective or even potentially harmful.10-12 Physicians may feel significant pressure to prescribe something, including antibiotics,13 despite knowing they may not be useful.14 Bronchodilators and oral corticosteroids may help, but the diagnosis of asthma may be uncertain, particularly in young children.15 The relationships between signs and symptoms and use of bronchodilators, antibiotics, and oral corticosteroids by primary care pediatricians for coughing children are not well described.
Objectives
To clinically characterize children with cough when they present to their primary care pediatrician and better understand the relationships between patient characteristics and treatment with bronchodilators, antibiotics, and oral corticosteroids. The hypotheses were that children with a cough characterized as wet or productive were more likely to receive antibiotics and less likely to be treated with oral corticosteroids.
Design and Methods
In this cross-sectional retrospective study, demographic information, past medical history, history of present illness, medications, assessments, and treatments were extracted from the electronic health record (EHR) of children with a chief complaint of cough who presented to a predominantly suburban, academic pediatric faculty practice, over 1 year. This study was limited to encounters with children for which an EHR cough template was completed. Univariate analyses were performed to identify risk factors for receiving bronchodilators, antibiotics, or oral corticosteroids. Chi-square analysis was performed with calculation of odds ratios and 95% confidence intervals for clinical characteristics. Characteristics found to be significant in the univariate analysis with 2-tailed P values <.20 were further evaluated for significance using multivariate logistic regression analysis, with 2-tailed P values <.05 considered significant. Data were analyzed using STATA, version 15.1 (StataCorp LLC, College Station, TX). The institutional review board approved this study. Data confidentiality was preserved, and the study conformed to the Helsinki Declaration of Bioethics (2013).
Results
Five hundred and sixty patient encounters were included in this analysis. Age ranged from 19 days to 18 years, mean (SD): 6.6 (4.8). Eighteen percent were <2 years of age and 41% were 2 to 5 years of age. Forty-seven percent were female, 86% had private insurance, and 19% had previous MD encounters during the illness. Past medical histories are summarized in Table 1. Fifty-six percent had prior encounters for cough in the past year. Many had underlying conditions that might predispose children to cough. Twenty-one percent were born preterm, 42% had a history of allergies, and 41% had a history of asthma. Twelve percent had chronic respiratory disorders other than asthma, primarily bronchopulmonary dysplasia or airway disorders, of which 78% also had a diagnosis of asthma. Ten percent had chronic neurological or neuromuscular conditions. Twenty-six percent of the encounters were with patients also followed by pediatric subspecialists.
Table 1.
Past Medical History of Children With Cough.
| Total N = 560 | n | Proportion (95% CI) |
|---|---|---|
| History of previous cough within the past year | 316 | 56% (53% to 61%) |
| Preterm birth | 116 | 21% (18% to 24%) |
| Allergies | 236 | 42% (38% to 46%) |
| Asthma | 232 | 41% (36% to 44%) |
| Chronic respiratory disease other than asthmaa | 68 | 12% (10% to 15%) |
| Previous sinus infections | 125 | 22% (19% to 26%) |
| Previous pneumonia | 126 | 23% (19% to 26%) |
| Dysphagia | 30 | 5% (4% to 8%) |
| Gastroesophageal reflux disease | 91 | 16% (13% to 20%) |
| Chronic neurological or neuromuscular conditions | 58 | 10% (8% to 13%) |
Abbreviation: CI, confidence interval.
Fifty-four of the 68 subjects with chronic respiratory disease were also diagnosed with asthma.
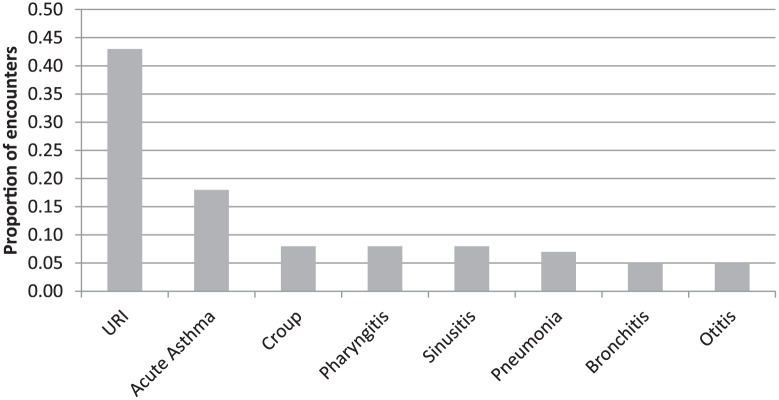
The assessments of providers at the time of the encounter are displayed in Figure 1. The most common were upper respiratory tract infection (URI) for 43% of the encounters, followed by acute asthma for 18%. Croup, acute pharyngitis, sinusitis, pneumonia, bronchitis, and otitis were each diagnosed during <10% of the encounters.
Figure 1.
Assessments of children with cough.
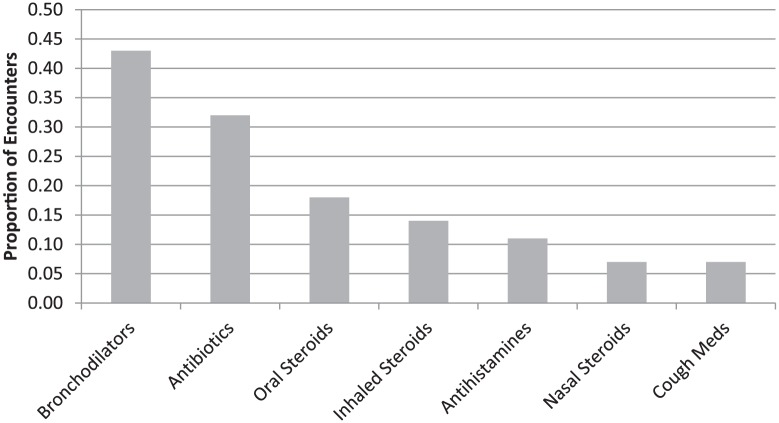
Treatment categories are displayed in Figure 2. Some subjects were prescribed more than one therapy. The most common forms of therapy for cough were, in order, bronchodilators, antibiotics, oral steroids, and antihistamines. Nasal steroids and a variety of cough medications, including both expectorants and suppressants, were prescribed in <10% of encounters.
Figure 2.
Treatment of children with cough.
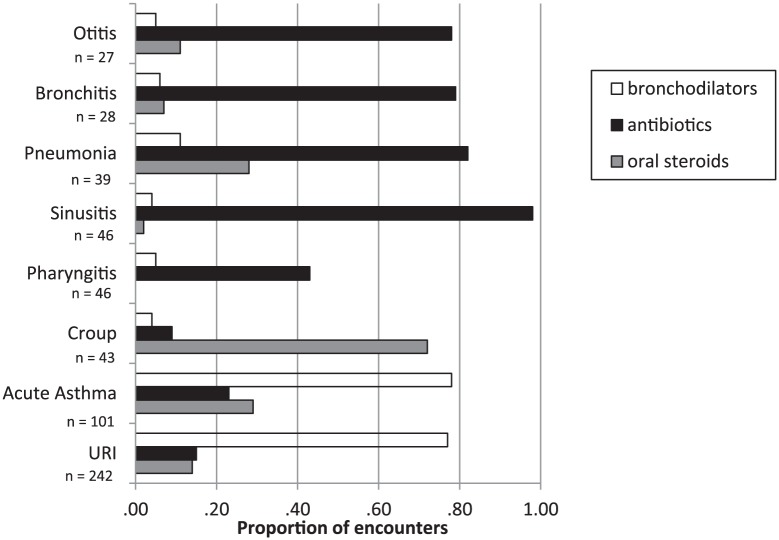
The relationships between assessments made at the time of the encounter by primary care providers and the use of bronchodilators, antibiotics, and oral steroids are described in Figure 3. Bronchodilators, antibiotics, and oral steroids were prescribed for 77%, 15%, and 14%, respectively, for children with URI; and 78%, 23%, and 29%, respectively, for children with acute asthma. Antibiotics were usually prescribed for sinusitis, pneumonia, bronchitis, and otitis. Bronchodilators and oral steroids were rarely prescribed for those encounters. The encounters in which oral steroids were commonly prescribed were acute asthma, pneumonia, and croup.
Figure 3.
Relationship between assessments and treatment.
The relationships between patient characteristics and the use of bronchodilators, antibiotics, and oral steroids are described in Table 2. Children prescribed bronchodilators were more likely to have a history of previous cough encounter within the past year, history of asthma, pneumonia, or dysphagia, and more likely to have fever, P < .001; nasal congestion, P < .004; and wheezing, P < .001. There was no relationship between the use of bronchodilators and cough characteristics or duration of coughing.
Table 2.
Comparison of Children With Acute Cough Treated With or Without Bronchodilators, Antibiotics, or Oral Steroidsa.
| Total | Bronchodilators | Antibiotics | Oral Steroids | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 560 | Yes (.43) | No (.57) | P | Yes (.32) | No (.68) | P | Yes (.18) | No (.82) | P | |
| Demographics and past medical history | ||||||||||
| Age, mean (SD) | 6.6 (4.8) | 6.5 (4.7) | 7.0 (4.5) | .137 | 6.8 (5.2) | 6.0 (4.5) | <.001 | 5.2 (4.5) | 6.9 (4.9) | <.001 |
| Age <2 years | .18 | .14 | .19 | .753 | .14 | .19 | .153 | .25 | .16 | .028 |
| Female | .47 | .45 | .49 | .427 | .48 | .47 | .745 | .45 | .48 | .698 |
| History of previous visit for cough in past year | .57 | .63 | .52 | .011 | .62 | .55 | .358 | .65 | .56 | .110 |
| Preterm birth | .21 | .23 | .20 | .310 | .15 | .23 | .039 | .26 | .19 | .254 |
| History of asthma | .42 | .67 | .22 | <.001 | .47 | .39 | .309 | .62 | .37 | <.001 |
| History of sinus infection | .23 | .21 | .23 | .663 | .33 | .18 | <.001 | .15 | .24 | .072 |
| History of pneumonia | .23 | .28 | .18 | .006 | .34 | .17 | <.001 | .24 | .22 | .784 |
| Dysphagia | .05 | .09 | .03 | .002 | .09 | .03 | .007 | .12 | .04 | .003 |
| Signs, symptoms, and physical examination | ||||||||||
| Cough duration >7 days | .23 | .27 | .27 | .920 | .41 | .15 | <.001 | .21 | .24 | .440 |
| Barking cough | .14 | .14 | .14 | 1.000 | .08 | .17 | .005 | .38 | .09 | <.001 |
| Dry cough | .21 | .20 | .21 | .624 | .14 | .24 | .012 | .24 | .20 | .499 |
| Wet or productive cough | .52 | .50 | .52 | .680 | .64 | .47 | <.001 | .37 | .56 | .001 |
| Fever | .28 | .08 | .02 | <.001 | .30 | .27 | .596 | .32 | .27 | .318 |
| Nasal congestion | .66 | .60 | .71 | .004 | .68 | .66 | .621 | .60 | .68 | .221 |
| Rhinorrhea | .32 | .29 | .34 | .233 | .24 | .36 | .004 | .38 | .31 | .161 |
| Wheezing | .13 | .25 | .05 | <.001 | .14 | .13 | .719 | .26 | .10 | <.001 |
All values expressed as proportions.
Children prescribed antibiotics were older, more likely to have a history of sinus infections, pneumonia, or dysphagia, and less likely to have a history of preterm birth. These children had longer durations of cough prior to presentation, P < .001; were more likely to have a wet or productive cough, P < .001; and their cough was less likely to be barking, P = .006; or dry, P = .013. On multivariate analysis (Table 3), age, history of prematurity, history of sinus infections, history of pneumonia, dysphagia, duration of cough >7 days, barking cough, dry cough, wet or productive cough, and rhinorrhea were included in the analysis. Of these, history of prematurity, history of pneumonia, longer duration of cough, and the presence of a wet or productive cough remained significantly associated with prescribing antibiotics.
Table 3.
Univariate and Multivariate Analysis for Prescribing Antibiotics.
| Odds Ratio (95% CI) | P | Adjusted Odds Ratio (95% CI) | P | |
|---|---|---|---|---|
| Demographics and past medical history | ||||
| Age <2 years | 0.68 (0.41-1.12) | .153 | 1.40 (0.77-2.60) | .284 |
| Preterm | 0.59 (0.36-0.95) | .039 | 0.52 (0.30-0.92) | .024 |
| History of sinus infection | 2.36 (1.56-3.56) | <.001 | 1.54 (0.91-2.59) | .109 |
| History of pneumonia | 2.41 (1.60-3.64) | <.001 | 2.15 (1.28-3.59) | .004 |
| Dysphagia | 2.88 (1.36-6.06) | .007 | 2.41 (0.79-7.37) | .123 |
| Signs, symptoms, and physical examination | ||||
| Cough duration >7 days | 3.84 (2.54-5.79) | <.001 | 4.10 (2.49-6.76) | <.001 |
| Barking cough | 0.41 (0.23-0.76) | .005 | 0.71 (0.33-1.51) | .371 |
| Dry cough | 0.53 (0.33-0.86) | .012 | 1.08 (0.57-2.05) | .808 |
| Wet or productive cough | 2.08 (1.44-3.00) | <.001 | 1.81 (1.07-3.04) | .026 |
| Rhinorrhea | 0.55 (0.37-0.82) | .004 | 0.76 (0.46-1.25) | .278 |
Abbreviation: CI, confidence interval.
Children prescribed oral corticosteroids were younger and more likely to have a history of asthma or dysphagia. Their cough was more likely to be described as barking, P < .001; less likely to be wet or productive, P = .001; and they were more likely to be wheezing, P < .001 (Table 4). Multivariate analysis included age <2 years, history of prior visit for cough in the past year, history of asthma, sinus infection, and dysphagia, presence of barking and wet and/or productive cough, rhinorrhea, and wheezing. After multivariable analysis, a history of asthma, dysphagia, and the presence of a barking cough or wheezing were associated with prescribing oral steroids (Table 4), but there was no longer an independent relationship between oral steroids and the presence of a wet or productive cough.
Table 4.
Univariate and Multivariate Analysis for Prescribing Oral Corticosteroids.
| Odds Ratio (95% CI) | P | Adjusted Odds Ratio (95% CI) | P | |
|---|---|---|---|---|
| Demographics and past medical history | ||||
| Age <2 years | 1.83 (1.10-3.06) | .028 | 1.34 (0.69-2.59) | .387 |
| History of prior visit for cough in past year | 1.50 (0.94-2.38) | .110 | 1.24 (0.72-2.14) | .439 |
| History of asthma | 2.70 (1.73-4.20) | <.001 | 2.32 (1.33-4.06) | .003 |
| History of sinus infection | 0.56 (0.31-1.01) | .072 | 0.54 (0.27-1.10) | .089 |
| Dysphagia | 3.33 (1.55-7.16) | .003 | 2.89 (1.05-7.96) | .040 |
| Signs, symptoms, and physical examination | ||||
| Barking cough | 6.85 (4.08-11.51) | <.001 | 8.24 (4.20-16.19) | <.001 |
| Wet or productive cough | 0.47 (0.30-0.74) | .001 | 1.13 (0.63-2.05) | .682 |
| Rhinorrhea | 1.42 (0.90-2.22) | .161 | 1.62 (0.94-2.80) | .085 |
| Wheezing | 3.14 (1.84-5.36) | <.001 | 2.20 (1.15-4.18) | .018 |
Abbreviation: CI, confidence interval.
Discussion
This study demonstrates that bronchodilators, antibiotics, and oral steroids are commonly prescribed for children who present to their primary care provider with a chief complaint of cough. The emotional and economic costs of acute and chronic cough in children and adults are considerable.16-18 While acute cough is most commonly related to a viral URI and will quickly resolve, coughing is disruptive, often keeping children from school, parents from work, and results in missed sleep for children and their parents. In one study, cough associated with URIs in children resulted in disturbed sleep in 88% of children and 72% of parents.19 Coughing is frightening, can trigger vomiting, and until the illness is resolved, parents will lay awake worrying there is something more serious going on. It is estimated that the economic burden of noninfluenza-related viral respiratory tract infections (all ages) approaches $40 billion per year.20
In this study, a significant proportion of children who present with cough to their primary care provider had a history of asthma, chronic lung disease, preterm birth, or encounters for previous coughing. This may be reflective of an academic pediatric practice and not generalizable. Most patients had coughing <21 days prior to their visit, so this is a study of how pediatricians assess and manage acute cough in children. Chronic cough is commonly defined as lasting >8 weeks in adults,21-23 and >4 weeks in children.23 The intermediate group (between 4 and 8 weeks) has been referred to as “prolonged acute cough.”24 The presumption is that acute cough resolves without specific therapy,4,5 and therefore, prescription medications are not needed. Despite the likelihood that acute cough will resolve within a few weeks, pediatricians prescribed medications for about two thirds of children. Cough medications, including expectorants and suppressants, were infrequently recommended. Pediatricians were more likely to prescribe antibiotics and asthma medications including oral steroids.
Bronchodilators were prescribed for 43% of encounters, though acute asthma was included in only 18%. This is not surprising since 41% of the patients enrolled in this study had a history of asthma. There was no relationship between the duration of coughing or the description of the cough, that is, dry, barking, wet, or productive, and the use of bronchodilators.
Antibiotics were prescribed during 32% of encounters. Risk factors for prescribing antibiotics were preterm birth, history of pneumonia, cough duration of >7 days, and coughing characterized as wet or productive. It is not known why wet or productive coughing was more likely to be treated with antibiotics. Wet coughs are often a sign of bronchitis, which is almost always viral in children.25 Bacterial sinusitis might benefit from antibiotics but generally triggers a dry hacking cough related to postnasal drip, and less likely to trigger a chesty or wet cough.26 Providers may be concerned about pneumonia, though there is no evidence that the nature of coughing is helpful in distinguishing bacterial from viral pneumonia.27 Data on which specific antibiotics were chosen for children with a diagnosis of pneumonia are not available, so it is not possible to deduce if providers prescribing antibiotics for patients with a diagnosis of pneumonia were considering Mycoplasma pneumoniae or other atypical infections.28
There has been much written about the adverse effects of overprescribing of antibiotics.29-31 In recent years, the concept of “Protracted Bacterial Bronchitis” has been recognized,32 and antibiotics have been shown to have some efficacy in children with persistent wet cough,26,33 though this is generally limited to coughing lasting more than 4 weeks. Providers and parents are understandably concerned that antibiotics will help prevent more serious complications or secondary infections.34 In a recent prospective study of over 8000 children presenting with acute cough (≤28 days) characteristics found to be independently associated with hospital admission included the following: age <2 years, current asthma, short illness duration, parent-reported moderate or severe vomiting in the previous 24 hours, parent-reported severe fever in the previous 24 hours, clinician-reported retractions, and wheeze.35
Oral steroids were prescribed for 18% of encounters, particularly for patients with acute asthma or croup. Interestingly, oral steroids were prescribed for 28% of the encounters in which the assessment included pneumonia. On multivariable analysis, risk factors for receiving oral steroids were a history of asthma, dysphagia, barking cough, and wheezing. Children with wet or productive coughs were less likely to receive oral steroids on univariate analysis, but this relationship did not hold up when confounding variables were taken into account.
There are a number of limitations to this study. It is retrospective and limited to data entered into an EHR. It is further limited to those patients for whom the practitioner chose to use the cough template in the EHR. Other children may have been coughing but other signs and symptoms were more prominent. There is no follow-up data, so it is not possible to venture any opinions on whether specific assessments or chosen treatments were effective. This study is limited to one suburban academic pediatric practice so the results cannot be generalized to other types of practices or locations.
Conclusions
Children with cough referred to an academic pediatric practice frequently have predisposing underlying conditions, which might help explain the frequent use of bronchodilators, antibiotics, and oral corticosteroids. Though acute asthma was the presumptive diagnosis in less than a fifth of children, bronchodilators were commonly prescribed. Antibiotics were more likely to be prescribed when the cough was described as wet or productive for children and in those with a history of previous pneumonia or cough duration of >7 days. Oral steroids were most commonly used when patients had croup or a barking cough, when wheezing was heard, or when there was a history of asthma. Cough expectorants and/or suppressants were rarely prescribed.
Acknowledgments
The authors wish to acknowledge the following who extracted data from the electronic health records: Lisa Fitzgerald, LPN, RN, Armando Ramirez, LPN, and Christine Bereth.
Footnotes
Author Contributions: SK: Contributed to conception and design; contributed to analysis; drafted the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
VI: Contributed to conception and design; contributed to analysis; drafted the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
JW: Contributed to conception and design; contributed to analysis; drafted the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
MMG: Contributed to conception and design; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
TN: Contributed to conception and design; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
AJD: Contributed to conception and design; contributed to analysis; drafted the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was partially funded by the Children’s Health and Research Foundation.
ORCID iD: Sankaran Krishnan  https://orcid.org/0000-0003-1449-127X
https://orcid.org/0000-0003-1449-127X
References
- 1. Rui P, Hing E, Okeyode T. National Ambulatory Medical Care Survey: 2014 state and national summary tables. https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2014_namcs_web_tables.pdf. Accessed April 1, 2018.
- 2. Cherry DK, Burt CW, Woodwell DA. National Ambulatory Medical Care Survey: 2001 summary. Adv Data. 2003;11:1-44. [PubMed] [Google Scholar]
- 3. Dicpinigaitis PV, Colice GL, Goolsby MJ, Rogg GI, Spector SL, Winther B. Acute cough: a diagnostic and therapeutic challenge. Cough. 2009;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pappas DE, Hendley JO, Hayden FG, Winther B. Symptom profile of common colds in school-aged children. Pediatr Infect Dis J. 2008;27:8-11. [DOI] [PubMed] [Google Scholar]
- 5. Thompson M, Vodicka T, Blair PS, Buckley DI, Heneghan C, Hay AD; TARGET Programme Team. Duration of symptoms of respiratory tract infections in children: systemic review. BMJ. 2013;347:f7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hay AD, Wilson AD. The natural history of acute cough in children aged 0 to 4 years in primary care: a systematic review. Br J Gen Pract. 2005;52:401-409. [PMC free article] [PubMed] [Google Scholar]
- 7. Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 suppl):1S-23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shields MD, Bush A, Everard ML, McKenzie S, Primhak R; British Thoracic Society Cough Guidelines Group. BTS guidelines: recommendations for the assessment and management of cough in children. Thorax. 2008;63(suppl 3):iii1-iii15. [DOI] [PubMed] [Google Scholar]
- 9. Pratter MR. Cough and the common cold: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:72S-74S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Malesker MA, Callahan-Lyon P, Ireland B, Irwin RS; CHEST Expert Cough Panel. Pharmacologic and nonpharmacologic treatment for acute cough associated with the common cold: CHEST Expert Panel Report. Chest. 2017;152:1021-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith SM, Schroeder K, Fahey T. Over-the-counter medications for acute cough in children and adults in ambulatory settings. Cochrane Database Sys Rev. 2008;(1):CD001831. [DOI] [PubMed] [Google Scholar]
- 12. Bolser DC. Cough suppressant and pharmacologic protussive therapy. ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 suppl):238S-249S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Butler CC, Rollnick S, Pill R, Maggs-Rapport F, Stott N. Understanding the culture of prescribing: qualitative study of general practitioners’ and patients’ perceptions of antibiotics for sore throats. BMJ. 1998;317:637-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zanasi A, Lanata L, Saibene F, et al. Prospective study of the efficacy of antibiotics versus antitussive drugs for the management of URTI-related acute cough in children. Multidiscip Respir Med. 2016;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Speight AN. Is childhood asthma being underdiagnosed and undertreated? BMJ. 1978;2:331-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marchant JM, Newcombe PA, Juniper EF, Sheffield JK, Stathis SL, Change AB. What is the burden of chronic cough for families? Chest. 2008;134:303-309. [DOI] [PubMed] [Google Scholar]
- 17. Hollinghurst S, Gorst C, Fahey T, Hay AD. Measuring the financial burden of acute cough in pre-school children: a cost of illness study. BMC Fam Pact. 2008;9:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lambert SB, Allen KM, Carter RC, Nolan TM. The cost of community-managed viral respiratory illnesses in a cohort of healthy preschool-aged children. Respir Res. 2008;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Blasio F, Dicpinigaitis PV, Rubin BK, De Daniele G, Lanata L, Zanasi A. An observational study on cough in children: epidemiology, impact on quality of sleep and treatment outcome. Cough. 2012;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non-influenza–related viral respiratory tract infection in the United States. Arch Intern Med. 2003;163:487-494. [DOI] [PubMed] [Google Scholar]
- 21. Chang AB, Glomb WB. Guidelines for evaluating chronic cough in pediatrics: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 suppl):260S-283S. [DOI] [PubMed] [Google Scholar]
- 22. Chang AB, Oppenheimer JJ, Weinberger M, Grant CC, Rubin BK, Irwin RS; CHEST Expert Cough Panel. Etiologies of chronic cough in pediatric cohorts: CHEST Guideline and Expert Panel Report. Chest. 2017;152:607-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chang AB, Oppenheimer JJ, Weinberger MM, et al. Use of management pathways or algorithms in children with chronic cough: CHEST Guideline and Expert Cough Panel Report. Chest. 2017;151:875-883. [DOI] [PubMed] [Google Scholar]
- 24. Shields MD, Thavagnanam S. The difficult coughing child: prolonged acute cough in children. Cough. 2013;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carolan PL. Pediatric bronchitis. http://emedicine.medscape.com/article/1001332-overview. Accessed March 31, 2018.
- 26. Wald ER, Applegate KE, Bordley C, et al. ; American Academy of Pediatrics. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics. 2013;132:e262-e280. [DOI] [PubMed] [Google Scholar]
- 27. Korppi M, Don M, Valent F, Canciani M. The value of clinical features in differentiating between viral, pneumococcal and atypical bacterial pneumonia in children. Acta Paediatr. 2008;97:943-947. [DOI] [PubMed] [Google Scholar]
- 28. Parrott GL, Kinjo T, Fujita J. A compendium for mycoplasma pneumoniae. Front Microbiol. 2016;7:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. The White House Washington. National strategy for combating antibiotic resistant bacteria. http://www.cdc.gov/drugresistance/pdf/carb_national_strategy.pdf. Published September 2014. Accessed April 1, 2018.
- 30. Cabral C, Lucas PJ, Ingram J, Hay AD, Horwood J. “It’s safer to . . . ” parent consulting and clinician antibiotic prescribing decisions for children with respiratory tract infections: an analysis across four qualitative studies. Soc Sci Med. 2015;136-137:156-164. [DOI] [PubMed] [Google Scholar]
- 31. Lucas PJ, Cabral C, Hay AD, Horwood J. A systematic review of parent and clinician views and perceptions that influence prescribing decisions in relation to acute childhood infections in primary care. Scand J Prim Health Care. 2015;33:11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Craven V, Everard ML. Protracted bacterial bronchitis: reinventing an old disease. Arch Dis Child. 2013;98:72-76. [DOI] [PubMed] [Google Scholar]
- 33. Chang AB, Oppenheimer JJ, Weinberger M, Rubin BK, Irwin RS. Children with chronic wet or productive cough—treatment and investigations: a systematic review. Chest. 2016;149:120-142. [DOI] [PubMed] [Google Scholar]
- 34. Petersen I, Johnson AM, Islam A, Duckworth G, Livermore DM, Hayward AC. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ. 2007;335:982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hay AD, Redmond NM, Turnbull S, et al. Development and internal validation of a clinical rule to improve antibiotic use in children presenting to primary care with acute respiratory tract infection and cough: a prognostic cohort study. Lancet Respir Med. 2016;4:902-910. [DOI] [PMC free article] [PubMed] [Google Scholar]