Short abstract
Background
Pain is one of the most common and distressing symptoms suffered by patients with progression of cancer; however, the mechanisms responsible for hyperalgesia are not well understood. Since the midbrain periaqueductal gray is an important component of the descending inhibitory pathway controlling on central pain transmission, in this study, we examined the role for pro-inflammatory cytokines of the periaqueductal gray in regulating mechanical and thermal hyperalgesia evoked by bone cancer via phosphatidylinositide 3-kinase (PI3K)–mammalian target of rapamycin (mTOR) signals.
Methods
Breast sarcocarcinoma Walker 256 cells were implanted into the tibia bone cavity of rats to induce mechanical and thermal hyperalgesia. Western blot analysis and ELISA were used to examine PI3K/protein kinase B (Akt)/mTOR and pro-inflammatory cytokine receptors and the levels of interleukin (IL)-1β, IL-6, and tumor necrosis factor-alpha (TNF-α).
Results
Protein expression levels of p-PI3K/p-Akt/p-mTOR were amplified in the periaqueductal gray of bone cancer rats, and blocking PI3K–mTOR pathways in the periaqueductal gray attenuated hyperalgesia responses. In addition, IL-1β, IL-6, and TNF-α were elevated in the periaqueductal gray of bone cancer rats, and expression of their respective receptors (namely, IL-1R, IL-6R, and tumor necrosis factor receptor (TNFR) subtype TNFR1) was upregulated. Inhibition of IL-1R, IL-6R, and TNFR1 alleviated mechanical and thermal hyperalgesia in bone cancer rats, accompanied with downregulated PI3K–mTOR.
Conclusions
Our data suggest that upregulation of pro-inflammatory cytokine signal in the periaqueductal gray of cancer rats amplifies PI3K–mTOR signal in this brain region and alters the descending pathways in regulating pain transmission, and this thereby contributes to the development of bone cancer-induced pain.
Keywords: Bone cancer, mechanical hyperalgesia, thermal hyperalgesia, cytokines, mTOR
Introduction
Pain is one of the most common and distressing symptoms suffered by patients with progression of cancer.1,2 Cancer pain mainly arises from a tumor compressing or infiltrating tissue and from nerve and other changes caused by a hormone imbalance or immune response, and so on.3 Of note, cancerous cells can originate in a number of different tissues such as prostate, breast, and lung. Many types of cancers have a propensity to metastasize to the bone microenvironment.3,4 Tumor burden within the bone causes excruciating breakthrough pain with properties of ongoing pain that is inadequately managed with current analgesics. Treatment options for bone cancer pain have been limited partly due to our poor understanding of the underlying mechanisms responsible for pain.
Mammalian target of rapamycin (mTOR) is a serine threonine protein kinase. Activation of mTOR, in particular, mTOR complex 1 that is more sensitive to rapamycin, leads to promotion of the phosphorylation of downstream effectors, that is, p70 ribosomal S6 protein kinase (p70S6K), and this further governs mRNA translation and plays a critical role in regulating protein synthesis and growth.5–7 Importantly, mTOR plays an important role in the modulation of long-term neuronal plasticity.8,9 Particularly, mTOR and its downstream effectors have been identified in the sensory neurons and spinal cord dorsal horn in the process of nociception, and studies further suggest that mTOR contributes to transmission and modulation of nociceptive information.10 For example, intrathecal administration of rapamycin, a specific inhibitor of mTOR, produces antinociception in models of inflammation.10–12 Local perfusion of rapamycin into the spinal cord attenuates formalin-induced neuronal hyperexcitability in the dorsal horn.13 These findings indicate that mTOR and its downstream effectors are likely activated and play an important role in the development of central sensitization under persistent pain conditions.
Nonetheless, the role played by mTOR signal in the central nervous system in regulating bone cancer pain needs to be studied. Note that the phosphatidylinositide 3-kinase (PI3K) is an upstream mediator of mTOR, and PI3K/protein kinase B (Akt)/mTOR are signal transduction pathways involved in the regulation of cellular functions including cell proliferation, survival, differentiation, adhesion, motility and invasion.14
As a component of the descending pain modulatory network, the midbrain periaqueductal gray (PAG) has an inhibitory or excitatory control on pain transmission via the rostral ventromedial medulla, projecting to the spinal dorsal horn.15–17 Thus, in this study, we examined the underlying mechanisms by which the changes in PI3K–mTOR signal in the PAG are engaged in mechanical and thermal hyperalgesia. We suspected that PI3K–mTOR of the PAG is a key player for the induction and maintenance of bone cancer pain.
Moreover, chronic neuroinflammation is one of the hallmarks in regulating neuropathic pain.18 Studies in neuropathic pain of human patients and experimental animals show that activation of glial cells and elevation of pro-inflammatory cytokines (PICs; i.e., interleukin (IL)-1β, IL-6 and tumor necrosis factor-alpha (TNF-α)) are common features of neuropathic pain.19,20 The releases of PICs by stimulated astrocytes and microglia lead to the exacerbation of neuronal cells in the pain regulation-related brain regions.19,20 Infiltration and accumulated immune cells from the periphery are also identified in and around the affected brain regions of animal models with neuropathic pain.21
Therefore, in this study, we used a rat model of bone cancer induced by implanting breast sarcocarcinoma Walker 256 cells into the tibia bone cavity to determine involvement of PICs in bone cancer pain via central PI3K/Akt/mTOR signal pathway. We hypothesized that bone cancer increases the protein expression of PI3K/Akt/mTOR signals in the PAG, resulting in mechanical pain and thermal hypersensitivity. It was anticipated that blocking PI3K–mTOR pathways attenuates pain responses. We also examined the levels of IL-1β, IL-6, and TNF-α and their receptors expression (IL-1R, IL-6R, and tumor necrosis factor receptor 1 (TNFR1)) in the PAG of control rats and bone cancer rats. We hypothesized that the levels of PICs and protein expression of PIC receptors are upregulated in the PAG after the development of bone cancer, and blocking PIC signals in the PAG attenuates mechanical and thermal hyperalgesia in bone cancer rats via PI3K–mTOR pathways. In addition, in order to assess the effects of inhibiting PIC signals on expression of mTOR, downstream mediator of mTOR such as phosphorylated p70 ribosomal S6 protein kinase 1 (p-S6K1) was also examined after using PIC receptor antagonists.
Materials and methods
Animals
All animal protocols were in accordance with the guidelines of the International Association for the Study of Pain and approved by the Institutional Animal Care and Use Committee of Jilin University. Adult male Wistar rats (200–250 g) were housed in individual cages with free access to food and water and were kept in a temperature-controlled room (25°C) on a 12/12 h light/dark cycle.
A model of bone cancer pain
Wister rat breast sarcocarcinoma Walker 256 cells were prepared as described previously.22 Briefly, Walker 256 cells (2 × 107 in 0.5 ml) were injected into the abdominal cavity of the rats. Seven to 14 days later, the produced ascites (approximately 50–150 ml) were collected and centrifuged at 1000 g for 5 min. The cells in the ascites were washed three times with 10 ml D-Hank’s solution and diluted to a final concentration of 2 × 107 cells/ml. The cells were then kept on ice before being used.
The bone cancer pain model was established by inoculating Walker 256 cells to the tibia of the rats as described previously.22 Briefly, the rats were anesthetized with sodium pentobarbital (45 mg/kg, intraperitoneally (i.p.)) and the lower one-third of the tibia was exposed. Each of rats was injected with 1 × 105 Walker 256 cells in 5 μl Hank’s solution into the right tibia of the hind paw, and the injection site was closed using bone wax to prevent cell leakage. Rats that underwent the same surgical procedures and received the same volume of vehicle served as the sham controls.
PAG cannulation and drug infusion
One day was allowed before the experiments. Rats were implanted with a stainless steel guide cannula (0.8 mm outside diameter [o.d.]) with sodium pentobarbital (60 mg/kg, i.p.), and then the guide cannula was secured to the skull. Stereotaxic coordinates for the dorsolateral PAG (dl-PAG) were 7.6 mm posterior to the bregma, 0.65 mm lateral to the midline, and 4.2 mm ventral to the brain surface.23
Following this, cannula was connected to an osmotic minipump (Alzet pump brain infusion kit; DURECT Inc., Cupertino, CA) with polycarbonate tubing. The pumps were placed subcutaneously between the scapulae and loaded with vehicle (artificial cerebrospinal fluid (aCSF)) as control or rapamycin (mTOR inhibitor; Sigma) and LY294002 (PI3K inhibitor; Sigma). In the similar way, each of PIC receptor antagonists (Tocris Co., Ellisville, MO), namely, IL-1Ra (IL-1β receptor antagonist), SC144 (gp130 antagonist to block IL-6R), and etanercept (TNF-α receptor antagonist), was loaded to the pumps, respectively. Rapamycin and LY294002 in 100 µM of concentration and the PIC receptor antagonists in 10 µM of concentration were delivered at 0.25 μl per hour (Alzet Model 1001D/1 day-delivery; DURECT Inc.). This intervention allowed animals to receive continuous PAG infusion via the osmotic minipumps before the experiments, and brain tissues were taken out. Note that all drugs were dissolved in aCSF as a final concentration.
Behavioral test
To quantify the mechanical sensitivity of the hindpaw, rats were placed in individual plastic boxes and allowed to acclimate for >30 min. Mechanical paw withdrawal threshold (PWT) of rat hindpaw in response to the stimulation of von Frey filaments was determined. A series of calibrated von Frey filaments (ranging from 0.4 to 15.0 g) were applied perpendicularly to the plantar surface of the hindpaw with a sufficient force to bend the filaments or until paw withdrew. In the presence of a response, the filament of next lower force was applied. In the absence of a response, the filament of next greater force was applied. To avoid injury during tests, the cutoff strength of the von Frey filament was 15 g. The tactile stimulus producing a 50% likelihood of withdrawal was determined using the “up-down” method. Each trial was repeated two times, and the mean value was used as the force produced a withdrawal response.
To determine thermal hyperalgesia, rat paw withdrawal latency (PWL) to a radiant heat was measured. Rats were placed individually in plastic cages on an elevated glass platform and allowed for 30-min acclimation. Each hind paw received three stimuli with a 10-min interval, and the mean of the three withdrawal latencies was defined as PWL. The heat was maintained at a constant intensity. To prevent tissue damage, the cutoff latency was set at 20 s. To prevent experimental bias, in all the behavioral tests, the experimenter had no knowledge (blinded) about the treatments that the rats had received.
No significant mechanical and thermal hyperalgesia were observed in control rats. As compared with controls, significant mechanical and thermal hyperalgesia were developed within a week after implantation of Walker 256 cells into the tibial canal of rats and lasted for four weeks. Previous studies also showed that the tumor occupies >90% of the intramedullary space on the day 14 following inoculation.12,13,22 Accordingly, the rats were subjected to the experiments in the current report two weeks after inoculation of cancer cells.
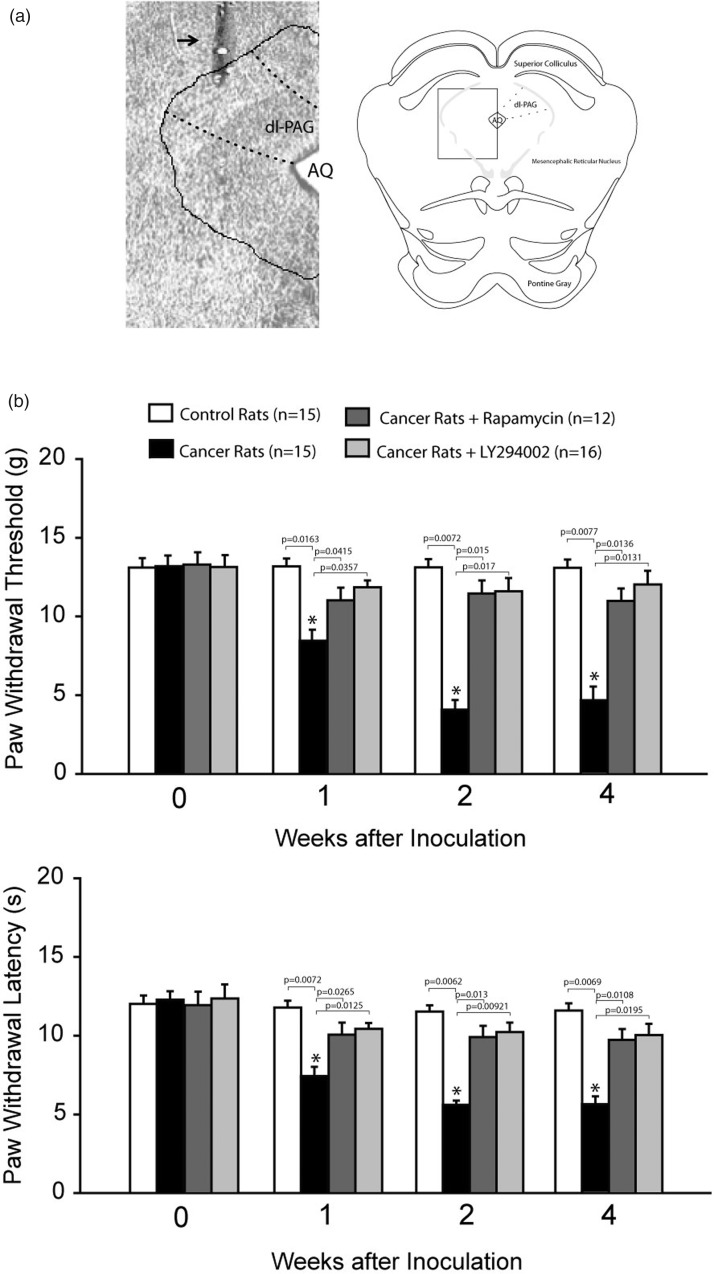
At the end of the experiments, 2% Evans blue in 0.25 μl was infused through the cannula. Then, the animals were anesthetized by sodium pentobarbital and intracardiacally perfused with physiological saline followed by 4% of paraformaldehyde solution. The midbrain was sectioned, and the location of injection sites was verified by histological examination of blue dye according to the atlas of Swanson.23 A histological section showing the location of the injection cannula was presented in Figure 1(a).
Figure 1.
Location of injection cannula track. (a) Left panel: a histological section showing the location of injection cannula track. Arrow indicates cannula track. AQ: cerebral aqueduct. Right panel showing a rectangle area selected for the photograph of a histological section. (b) Mechanical and thermal sensitivity in control rats and bone cancer rats. With the development of bone cancer, PWT and PWL were decreased as compared with control rats. Significant mechanical and thermal hyperalgesia appeared seven days after inoculation of cancer cells. The effects of blocking PI3K–mTOR in the dl-PAG on pain responses to mechanical and thermal stimulation were further determined. Blocking respective mTOR and PI3K by infusion of rapamycin and LY294002 into the PAG attenuated hypersensitive pain responses in bone cancer rats. *P < 0.05 versus control rats and bone cancer rats that received infusion of inhibitors. P values are shown. The number of animals is also indicated. dl-PAG: dorsolateral PAG.
ELISA measurements
The rats were first euthanized by overdose sodium pentobarbital (120 mg/kg, i.p.). The brain was quickly removed and placed in artificial cerebral spinal fluid (at 5°C). A tissue block containing the midbrain PAG was cut from the brain. Then, coronal sections (approximately 500 μm) containing the midbrain PAG were dissected from the tissue block, and the dl-PAG was dissected under an anatomical microscope. This approach allowed us to obtain the dl-PAG sample appropriately. Total protein of the dl-PAG tissue was then extracted by homogenizing sample in ice-cold radioimmunoprecipitation assay (RIPA) buffer with protease inhibitor cocktail kit. The lysates were centrifuged, and the supernatants were collected for measurements of protein concentrations using a bicinchoninic acid assay reagent kit. The levels of IL-1β, IL-6, and TNF-α were examined using an ELISA assay kit (Promega Corp and Wuhan Fine Biotech) corresponding to the provided description and modification. Briefly, polystyrene 96-well microtitel immunoplates were coated with affinity-purified polyclonal rabbit anti-IL-1β, anti-IL-6, and anti-TNF-α antibodies. Parallel wells were coated with purified rabbit immunoglobulin G (IgG) for evaluation of nonspecificity. After overnight incubation, the diluted samples and the PICs standard solutions were distributed in each plate. The plates were washed and incubated with anti-IL-1β, anti-IL-6, and anti-TNF-α galactosidase, respectively. Then, the plates were washed and incubated with substrate solution. After incubation, the optical density was determined using an ELISA reader (575 nm of wavelength).
Western blot analysis
Similar to the ELISA, the dl-PAG tissues were removed. In order to determine the expression of PIC receptors on cell surface, PAG tissues were incubated with Sulfo-NHS-LC-Biotin (1 mg/ml, Pierce) for 30 min on ice as described previously.24 Because biotin is impermeable to the cell membrane, only proteins on the cell surface were biotinylated. The unbound biotin in the solution was removed by 5× wash of PAG tissues. PAG tissues were then homogenized and centrifuged at 13,500 × g (4°C) for 12 min. A sample (200 μg protein) was incubated with streptavidin beads (20 μl) for 3 h at 4°C. The beads were washed 3× with RIPA buffer and precipitated by centrifugation and collected. Sample buffer (50 μl) was added to the collected beads and boiled for 3 min. Beads were pelleted again by centrifugation, and the supernatant was collected. The supernatant was diluted to the same volume as the starting material (i.e., 200 μg total protein). Total and membrane samples in equal volume were applied to SDS-PAGE. Membranes were incubated with the rabbit anti-IL-1R, anti-IL-6R, and anti-TNFR1 primary antibodies (diluted at 1:500, obtained from Neuromics, Abcam Co, and/or Antibodies-Online Inc). After being fully washed, the membrane was incubated with horseradish peroxidase-linked antirabbit secondary antibody (1:250) and visualized for immunoreactivity. The membrane was also processed to detect β-actin for equal loading. The bands recognized by the primary antibody were visualized by exposure of the membrane onto an X-ray film. The film was then scanned, and the optical densities of protein bands were analyzed using the Scion image software. Then, values for densities of immunoreactive bands/β-actin band from the same lane were determined. Each of the values was then normalized to a control sample. Using the standard western blotting methods, expression of p-PI3K/p-Akt/p-mTOR/p-S6K1 and the total protein of PI3K/Akt/mTOR were also determined. All primary antibodies (diluted at 1:500) such as rabbit anti-p-PI3K/p-Akt/p-mTOR/p-S6K1 and rabbit anti-PI3K/Akt/mTOR were obtained from Neuromics, Abcam Co., and/or Antibodies-Online Inc.
Statistical analysis
All data were analyzed using a one-way analysis of variance. As appropriate, Tukey’s post hoc analyses were utilized to determine differences between groups. Values were presented as means ± standard error. For all analyses, differences were considered significant at P < 0.05. All statistical analyses were performed by using SPSS for Windows version 13.0 (SPSS Inc., Chicago, IL).
Results
Pain responses to mechanical and thermal stimuli
Bone cancer induced long-lasting pain behaviors in rats that were indicated by significantly increased mechanical and thermal sensitivity (Figure 1). Mechanical allodynia and thermal hyperalgesia were observed within a week after injection of Walker 256 cells and lasted for four weeks (P < 0.05 vs. control animals, n = 15 in each group). Note that no behavioral test was performed >4 weeks after the cell implantation in this experiment. Thus, in the current report, the rats with two weeks of inoculation of cancer cells were used in the experiments examining PIC and PI3K–mTOR signal pathways.
Pain responses after blocking PI3K–mTOR
We further examined the effects of blocking mTOR and PI3K using rapamycin (n = 12) and LY294002 (n = 16) in the dl-PAG on PWT and PWL in cancer rats. Figure 1 demonstrates that PWT and PWL were significantly increased after infusion of rapamycin and LY294002 (P < 0.05, indicated bone cancer rats vs. control rats). There were no significant differences in PWT and PWL between control rats and bone cancer rats with rapamycin and LY294002 (P > 0.05, cancer rats with rapamycin/LY294002 vs. control rats). Note that data presented in this figure were obtained from those rats in which microinjection site was localized within the dl-PAG.
In addition, data analysis was performed in eight rats in which that microinjection site was outside of the dl-PAG region as injection location controls. In five of those eight rats, PWT and PWL were 4.8 ± 0.9 g and 5.8 ± 1.2s with infusion of rapamycin (P > 0.05, cancer rats with rapamycin vs. cancer rats without PAG injection). Likewise, in three of those eight rats, PWT and PWL were 4.9 ± 1.1 g and 5.9 ± 1.3 s with infusion of LY294002 (P > 0.05, cancer rats with LY294002 vs. cancer rats without PAG injection).
Expression of PI3K/Akt/mTOR signal pathway
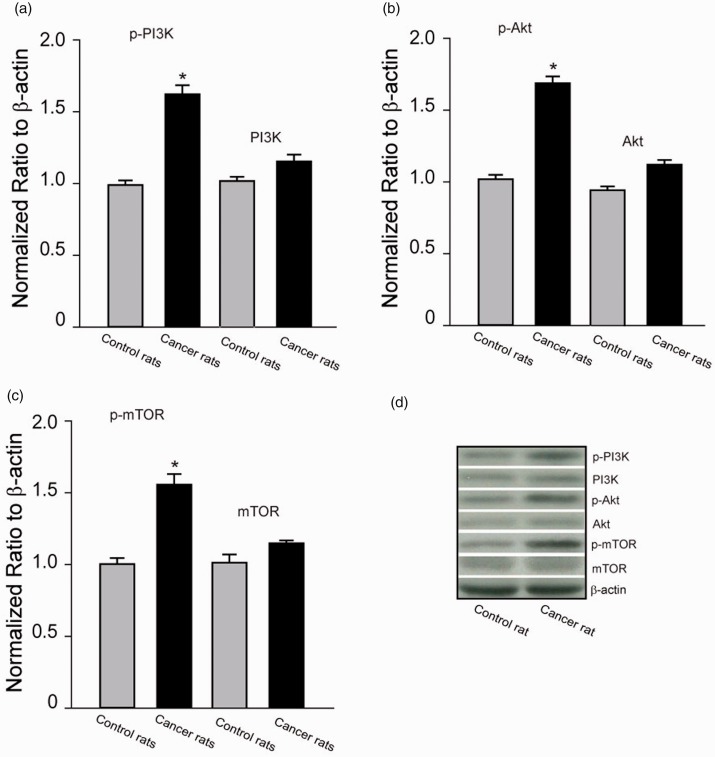
Figure 2(a) to (d) demonstrates expression of p-PI3K, p-Akt, and p-mTOR as well as the total protein of PI3K, Akt, and mTOR. P-PI3K, p-Akt, and p-mTOR were increased in the dl-PAG of bone cancer rats as compared with control rats (P < 0.05, cancer rats vs. control rats, n = 6–10 in each group). Note that total protein levels of PI3K, Akt, and mTOR were not significantly increased in bone cancer rats.
Figure 2.
Expression of PI3K/Akt/mTOR signal pathway. Averaged data (a to c) and typical bands (d) showing that p-PI3K, p-Akt, and p-mTOR were upregulated in the dl-PAG of bone cancer rats. The difference in total protein of PI3K, Akt, and mTOR was insignificant between two groups. *P < 0.05 versus control rats. n = 6–10 in each group. PI3K: phosphatidylinositide 3-kinase; mTOR: mammalian target of rapamycin; Akt: protein kinase B.
Levels of PICs and expression of PIC receptors
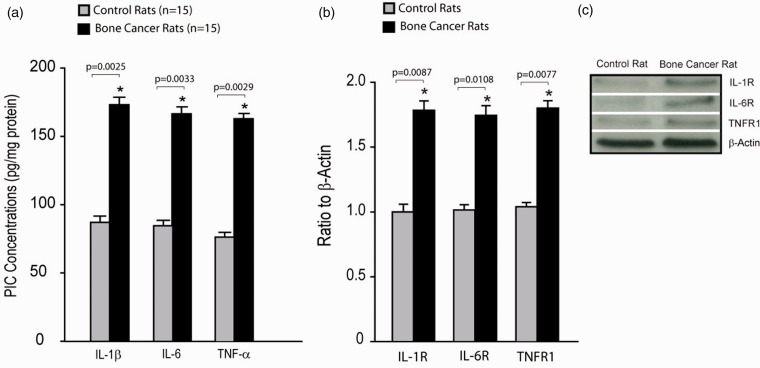
We also examined the levels of PICs as well as protein expression of membrane PIC receptors in the dl-PAG of bone cancer rats and control rats. Figure 3(a) shows that IL-1β, IL-6, and TNF-α were elevated in bone cancer rats as compared with control animals (n = 15 in each group). Figure 3(b) and (c) demonstrate that the expression of membrane PIC receptors (IL-1R, IL-6R, and TNFR1) in the dl-PAG was significantly increased in bone cancer rats as compared with control animals (P < 0.05 vs. their respective controls; n = 8–12).
Figure 3.
PIC levels and PIC receptor expression. (a) The levels of PICs were increased in the dl-PAG of bone cancer rats as compared with control rats (n = 15 in each group). *P < 0.01 versus control rats. P values are also shown. (b and c) Averaged data and typical bands showing the protein expression of PIC receptors (IL-1R, IL-6R, and TNFR1) in the dl-PAG. The expression levels of membrane PIC receptor were amplified in the PAG of bone cancer rats. *P < 0.01 versus control rats. The number of animals = 8–12 in each group. P values are also shown. PIC: pro-inflammatory cytokine; IL: interleukin; TNF: tumor necrosis factor; TNFR1: tumor necrosis factor receptor 1.
Pain responses after blocking PIC receptors
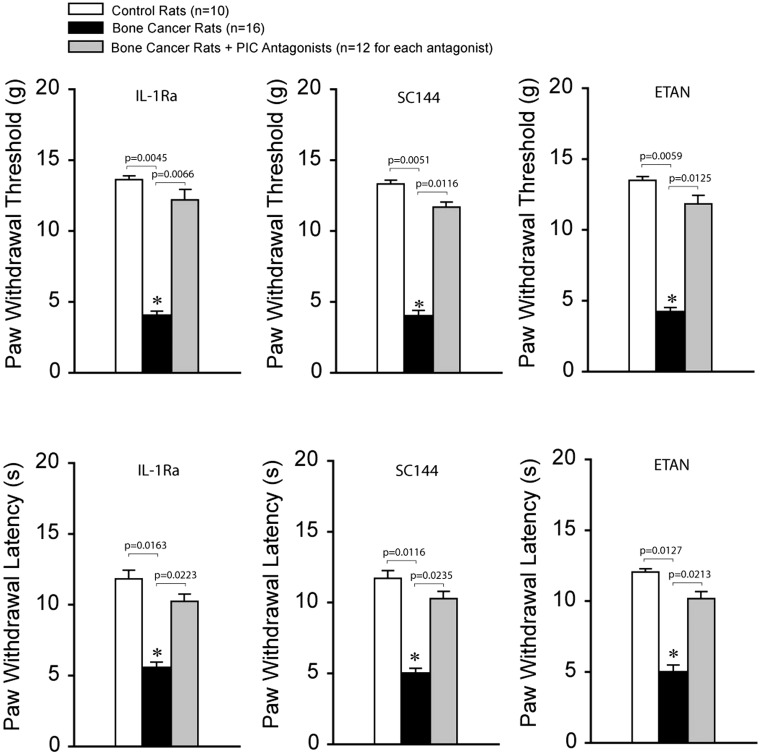
PWT and PWL were decreased in bone cancer rats (n = 16; P < 0.05 vs. control rats) as compared with control rats (n = 10). We further examined the effects of blocking PIC receptors (respective IL-1R, IL-6R, and TNFR1; n = 12 in each group) in the dl-PAG on PWT and PWL in bone cancer rats. Figure 4 demonstrates that PWT (top panels) and PWL (bottom panels) were significantly increased in bone cancer rats after infusion of each of PIC receptor antagonists (P < 0.05 vs. cancer rats without antagonists). Note that there were no differences in PWT and PWL between control rats and bone cancer rats with respective PIC receptor blocking (P > 0.05, cancer rats with each PIC inhibitor vs. control rats).
Figure 4.
Effects of blocking PIC receptors in the dl-PAG on pain responses to mechanical and thermal stimulation. Mechanical and thermal hyperalgesia appeared to be decreased in bone cancer rats (n = 16) as compared with control animals (n = 10). Infusion of respective PIC receptor inhibitors (IL-1Ra, SC144, and ETAN; n = 12 in each group) into the PAG attenuated hypersensitive pain responses in bone cancer rats. *P < 0.05 versus control rats and bone cancer rats that received infusion of inhibitors. P values are also shown. PIC: pro-inflammatory cytokine; IL: interleukin; ETAN: ■; SC144: ■.
Expression of PI3K–mTOR after blocking PIC receptors
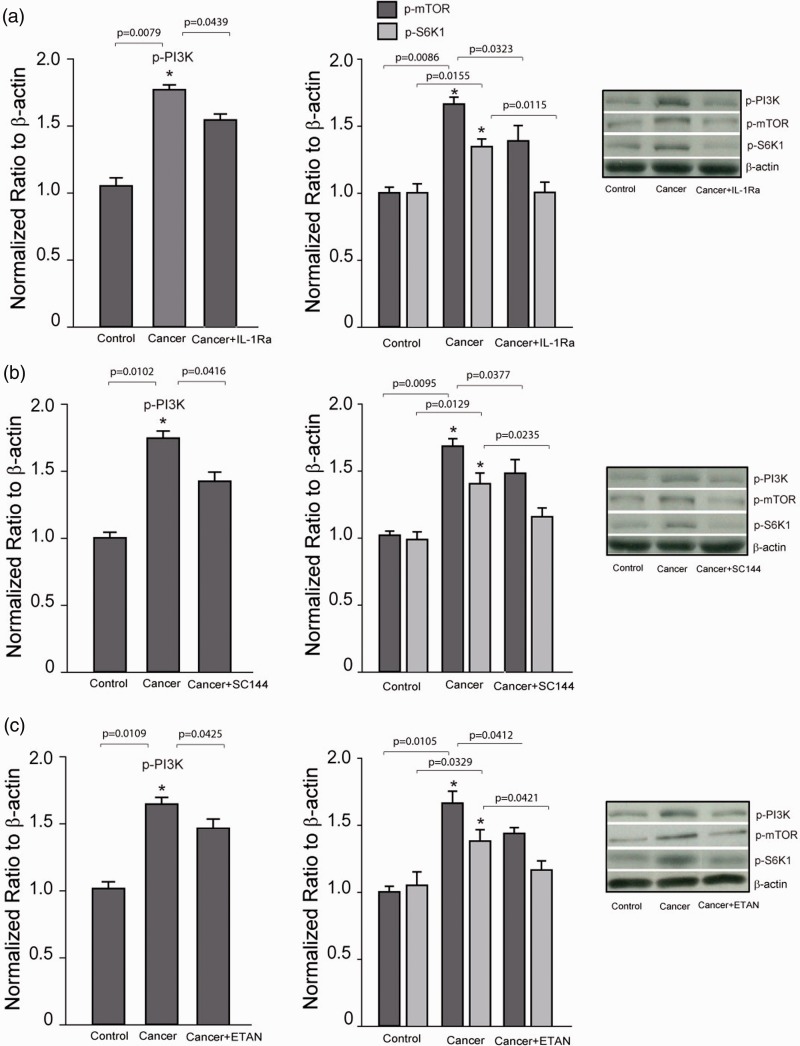
In order to determine the engagement of PI3K–mTOR signal in the effects of PICs, we further examined the expression of p-PI3K and p-mTOR/p-S6K1 after blocking each of PIC receptors. Figure 5(a) to (c) shows that infusion of respective PIC receptor inhibitors (IL-1Ra, SC144, and ETAN) into the PAG decreased the levels of p-PI3K and p-mTOR/p-S6K1 in bone cancer rats (P < 0.05, cancer rats without PIC receptor inhibitors vs. control rats and bone cancer rats with inhibitors; n = 6–12 in each group).
Figure 5.
Effects of blocking PIC receptors on expression of p-PI3K, p-mTOR, and p-S6K1. (a to c) Left and middle panels (averaged data) and right panel (typical band) showing that infusion of respective PIC receptor inhibitors (IL-1Ra, SC144, and ETAN) into the PAG decreased the levels of p-PI3K and p-mTOR/p-S6K1 in bone cancer rats. *P < 0.05 versus control rats and bone cancer rats with infusion of inhibitors. n = 6–12 in each group. P values are also shown. PI3K: phosphatidylinositide 3-kinase; mTOR: mammalian target of rapamycin; IL: interleukin; ETAN: ■; S6K1: ■; SC144: ■.
Discussion
Overall, the main findings of the present study are that (1) expression of p-PI3K/p-Akt/p-mTOR signal pathways is amplified in the dl-PAG of bone cancer rats; (2) IL-1β, IL-6, and TNF-α and their receptors including IL-1R, IL-6R, and TNFR1 in membrane expression are upregulated in the dl-PAG of bone cancer rats; (3) blocking PI3K–mTOR pathways in this brain region attenuates hypersensitive responses to mechanical and thermal stimuli in bone cancer rats; and (4) blocking individual PIC receptors (i.e., IL-1R, IL-6R, and TNFR1) in this brain region attenuates hypersensitive responses to mechanical and thermal stimuli in bone cancer rats likely via inhibition of PI3K–mTOR signal.
Evidence has suggested that antinociception is mediated partly by descending pathways arising from the midbrain PAG.25,26 Early studies showed that electrical stimulation or opioids microinjected into the PAG produced profound long-lasting antinociception.25,26 In particular, neural substrates are identified in the PAG of rats with neuropathic pain.27 Furthermore, previous studies showed that PIC mediators appear in the PAG, and activation of PICs in the PAG plays a role in modulating pain response or is involved in morphine withdrawal response.28,29 Nonetheless, to the best of our knowledge, data of our present study have shown for the first time that PIC–PI3K–mTOR signal pathways in the PAG plays a role in regulating mechanical and thermal pain responses in a rat model of bone cancer.
It is well known that IL-1β is involved in the immune response and signal transduction both in the periphery and the central nervous system.30 IL-1β produced in the nervous system regulates the function of neuron and glia cells.31 Prior studies specifically demonstrated that IL-1β contributes to inflammatory and neuropathic pain.32 Increased level of IL-1β has been observed in the cerebrospinal fluid of chronic pain patients33 and in the brainstem, contralateral thalamus/striatum and prefrontal cortex of rats with spared nerve injury.34 A prior study showed that inhibition of melanocortin 4 receptor in the PAG blunts mechanical allodynia and thermal hyperalgesia but also delays the development of pain facilitation induced by peripheral nerve injury.35 This further decreases the expression of levels of IL-1β, IL-6, and TNF-α.35 Treatments with anti-IL-1β neutralizing antibodies or with IL-1β receptor antagonist (IL-1Ra) have also been reported to attenuate or block the hyperalgesia induced by a various nociceptive injuries.32,36 Consistent with these prior findings, in the current report, we found that membrane expression of IL-1R was increased in the dl-PAG of bone cancer rats, and blocking IL-1R in this brain region attenuated hypersensitive responses to mechanical and thermal stimuli in bone cancer rats. The inhibitory effects of blocking IL-1R on pain responses were accompanied with attenuation of PI3K–mTOR signal pathways in the dl-PAG of bone cancer rats.
IL-6 complexes with membrane-bound or soluble IL-6R to stimulate cells expressing the signal transducer glycoprotein (gp130).37,38 Most cells are lacking of membrane-bound IL-6R and are thus unresponsive to IL-6. Nevertheless, they still react to IL-6 complexed with a soluble form of the IL-6R (sIL-6R) to activate gp130, a pathway called “trans-signaling.”37 Thus, in the current study, we used SC144, a gp130 inhibitor, to block IL-6-mediated signal transduction in order to examine engagement of the IL-6R in PI3K–mTOR signals and pain response thresholds to mechanical and thermal stimuli in bone cancer rats. Our data showed that membrane IL-6R was upregulated in the PAG of bone cancer rats, and SC144 injected into the PAG attenuated amplification of PI3K–mTOR and attenuated mechanical and thermal hyperalgesia.
The effects of TNF-α are via stimulation of two TNF-α receptor subtypes, TNFR1 and TNFR2.39 TNFR1 is present entirely on neuronal cells and plays a functional role, whereas TNFR2 is located predominantly on macrophages and/or monocytes in response to inflammation.40 Thus, in our current study, application of ETAN lessened PI3K–mTOR expression in the PAG of bone cancer rats and attenuated pain response, which is likely via TNFR1.
In the present study, we demonstrated that cell membrane PIC receptors are upregulated in the dl-PAG of bone cancer rats, indicating that PIC receptors trafficking to the cell membrane of PAG is particularly amplified in bone cancer rats.24 The underlying mechanism for the increase in trafficking of PIC receptors following the development of bone cancer needs to be determined. The elevated PICs were also observed in bone cancer rats in the present study. Accordingly, we assume that PICs are likely released from the glial cells, and this signal is likely to lead to upregulation of membrane PIC receptors. Nevertheless, it is speculated that the increased activities in the PIC pathways are likely to result in neuronal damage within the PAG since PICs is engaged in the process of apoptosis, which has been observed in brains.41
The GABAergic synaptic response was altered in the PAG neurons of rats with neuropathic pain.42 PICs and/or activation of PIC receptors can possibly alter GABAergic pathway.43 Prior studies showed that stimulation of this region of PAG led to antinociceptive effects.15,16 Thus, activation of PIC receptors within the PAG is likely to play a deinhibitory role in regulating the descending pain pathways. When PIC signals are blocked in the dl-PAG, the amplified pain response is attenuated as observed in our results. Our results also found that mechanical and thermal hyperalgesia were attenuated by blocking PI3K–mTOR pathways in the dl-PAG of cancer rats. Overall, this suggests that PIC activation influences GABAergic transmission within this region of PAG via the PI3K–mTOR and thereby amplifies pain response.
A prior study showed that the systemic injection of mTOR inhibitors attenuates the mTOR pathway in sensory neurons and in the spinal dorsal horn, and this further reduces the mechanical hypersensitivity that develops after nerve injury.44 In this prior study, since mTOR inhibitors were given systemically, it cannot be ruled out if the effects of mTOR inhibitors were via pain-related regions in the central nervous system. Note that the purpose of our study was to determine whether PI3K–mTOR signals within the PAG are intrinsically involved in bone cancer-induced pain because the PAG is an important region in regulating pain. In our results, rapamycin injected into the PAG attenuated mechanical and thermal hypersensitivity in cancer rats. Thus, mTOR signal within the PAG is likely to play its role per se. Nonetheless, it is also likely that the effects of rapamycin were via altering the releases of neurotransmitters from the terminals of the PAG neurons into the spinal dorsal horn because of descending projections from the PAG to the spinal dorsal horn.
Conclusions
PIC signal pathways are activated in the dl-PAG of bone cancer rats, and thereby PI3K–mTOR signals are amplified. These abnormalities are likely to contribute to the development of mechanical and thermal hyperalgesia in animals with bone cancer. Blocking PIC receptors inhibits expression of PI3K–mTOR pathway and alleviates pain responses induced the development of bone cancer. Results of this study provided a base for the mechanisms responsible for bone cancer pain. This further offers promising clues to target central nerve system for the development of new therapeutic strategies for managing intractable pain in patients with cancer.
Author Contributions
JZ and LW equally contributed to experimental designs and performance, data analysis, and drafting the manuscript. XP and ZS designed and oversaw the experiments and data and reviewed the paper as corresponding authors for this work.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Hanna M, Zylicz Z. (ed). Cancer pain. London: Springer, 2013, pp. vii and, 17. [Google Scholar]
- 2.van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 2007; 18: 1437–1449. [DOI] [PubMed] [Google Scholar]
- 3.Urch CE, Suzuki R. Pathophysiology of somatic, visceral, and neuropathic cancer pain In: Sykes N, Bennett MI, Yuan C-S. (eds) Clinical pain management: Cancer pain. London: Hodder Arnold, 2008, pp. 3–12. [Google Scholar]
- 4.Mantyh P. Bone cancer pain: causes, consequences, and therapeutic opportunities. Pain 2013; 154: S54–S62. [DOI] [PubMed] [Google Scholar]
- 5.Shor B, Gibbons JJ, Abraham RT, Yu K. Targeting mTOR globally in cancer: thinking beyond rapamycin. Cell Cycle 2009; 8: 3831–3837. [DOI] [PubMed] [Google Scholar]
- 6.Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, Abbruzzese JL. Metastatic patterns in adenocarcinoma. Cancer 2006; 106: 1624–1633. [DOI] [PubMed] [Google Scholar]
- 7.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev 2004; 18: 1926–1945. [DOI] [PubMed] [Google Scholar]
- 8.Banko JL, Poulin F, Hou L, DeMaria CT, Sonenberg N, Klann E. The translation repressor 4E-BP2 is critical for eIF4F complex formation, synaptic plasticity, and memory in the hippocampus. J Neurosci 2005; 25: 9581–9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron 2009; 61: 10–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geranton SM, Jimenez-Diaz L, Torsney C, Tochiki KK, Stuart SA, Leith JL, Lumb BM, Hunt SP. A rapamycin-sensitive signaling pathway is essential for the full expression of persistent pain states. J Neurosci 2009; 29: 15017–15027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price TJ, Rashid MH, Millecamps M, Sanoja R, Entrena JM, Cervero F. Decreased nociceptive sensitization in mice lacking the fragile X mental retardation protein: role of mGluR1/5 and mTOR. J Neurosci 2007; 27: 13958–13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Q, Fitzsimmons B, Steinauer J, Neill AO, Newton AC, Hua X-Y, Yaksh TL. Spinal phosphinositide 3-kinase-Akt-mammalian target of rapamycin signaling cascades in inflammation-induced hyperalgesia. J Neurosci 2011; 31: 2113–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asante CO, Wallace VC, Dickenson AH. Formalin-induced behavioural hypersensitivity and neuronal hyperexcitability are mediated by rapid protein synthesis at the spinal level. Mol Pain 2009; 5: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fumarola C, Bonelli MA, Petronini PG, Alfieri RR. Targeting PI3K/AKT/mTOR pathway in non small cell lung cancer. Biochem Pharmacol 2014; 90: 197–207. [DOI] [PubMed] [Google Scholar]
- 15.Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol 1995; 46: 575–605. [DOI] [PubMed] [Google Scholar]
- 16.Carrive P, Morgan MM. Periaqueductal gray In: Mai JK, Paxinos G. (eds) The human nervous system. 3rd ed. San Diego: Academic Press, 2012, pp. 367–400. [Google Scholar]
- 17.Craig AD. Distribution of brainstem projections from spinal lamina I neurons in the cat and the monkey. J Comp Neurol 1995; 361: 225–248. [DOI] [PubMed] [Google Scholar]
- 18.Skaper SD, Facci L, Zusso M, Giusti P. Neuroinflammation, mast cells, and glia: dangerous liaisons. Neuroscientist 2017; 23: 478–498. [DOI] [PubMed] [Google Scholar]
- 19.Gwak YS, Hulsebosch CE, Leem JW. Neuronal-glial interactions maintain chronic neuropathic pain after spinal cord injury. Neural Plast 2017; 2017: 2480689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lees JG, Makker PGS, Tonkin RS, Abdulla M, Park SB, Goldstein D, Moalem-Taylor G. Immune-mediated processes implicated in chemotherapy-induced peripheral neuropathy. Eur J Cancer 2017; 73: 22–29. [DOI] [PubMed] [Google Scholar]
- 21.Makker PGS, Duffy SS, Lees JG, Perera CJ, Tonkin RS, Butovsky O, Park SB, Goldstein D, Moalem-Taylor G. Characterisation of immune and neuroinflammatory changes associated with chemotherapy-induced peripheral neuropathy. PLoS One 2017; 12: e0170814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bao Y, Hou W, Yang L. Increased expression of protease-activated receptor 2 and 4 within dorsal root ganglia in a rat model of bone cancer pain. J Mol Neurosci 2014; 55: 706–714. [DOI] [PubMed] [Google Scholar]
- 23.Swanson LW. Brain maps: Structure of the rat brain. 2nd ed New York: Elsevier, 1998. [Google Scholar]
- 24.Xu GY, Huang LY. Ca2+/calmodulin-dependent protein kinase II potentiates ATP responses by promoting trafficking of P2X receptors. Proc Natl Acad Sci USA 2004; 101: 11868–11873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klemm WR. Habenular and interpeduncularis nuclei: shared components in multiple-function networks. Med Sci Monit 2004; 10: RA261–RRA73. [PubMed] [Google Scholar]
- 26.Millan MJ. Descending control of pain. Prog Neurobiol 2002; 66: 355. [DOI] [PubMed] [Google Scholar]
- 27.Guo J, Fu X, Cui X, Fan M. Contributions of purinergic P2X3 receptors within the midbrain periaqueductal gray to diabetes-induced neuropathic pain. J Physiol Sci 2015; 65: 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benamar K, Geller EB, Adler MW. Elevated level of the proinflammatory chemokine, RANTES/CCL5, in the periaqueductal grey causes hyperalgesia in rats. Eur J Pharmacol 2008; 592: 93–95. [DOI] [PubMed] [Google Scholar]
- 29.Hao S, Liu S, Zheng X, Zheng W, Ouyang H, Mata M, Fink D J. The role of TNFalpha in the periaqueductal gray during naloxone-precipitated morphine withdrawal in rats. Neuropsychopharmacology 2011; 36: 664–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heitmeier MR, Arnush M, Scarim AL, Corbett JA. Pancreatic beta-cell damage mediated by beta-cell production of interleukin-1. A novel mechanism for virus-induced diabetes. J Biol Chem 2001; 276: 11151–11158. [DOI] [PubMed] [Google Scholar]
- 31.Breder CD, Dinarello CA, Saper CB. Interleukin-1 immunoreactive innervation of the human hypothalamus. Science 1988; 240: 321–324. [DOI] [PubMed] [Google Scholar]
- 32.Sommer C. Cytokines and neuropathic pain In: Hansson P, Fields H, Hill R, Marchettini P. (eds) Neuropathic pain: pathophysiology and treatment. Seattle: IASP, 2001, pp. 37–62. [Google Scholar]
- 33.Alexander GM, van Rijn MA, van Hilten JJ, Perreault MJ, Schwartzman RJ. Changes in cerebrospinal fluid levels of pro-inflammatory cytokines in CRPS. Pain 2005; 116: 213–219. [DOI] [PubMed] [Google Scholar]
- 34.Apkarian AV, Lavarello S, Randolf A, Berra HH, Chialvo DR, Besedovsky HO, del Rey A. Expression of IL-1beta in supraspinal brain regions in rats with neuropathic pain. Neurosci Lett 2006; 407: 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chu H, Sun J, Xu H, Niu Z, Xu M. Effect of periaqueductal gray melanocortin 4 receptor in pain facilitation and glial activation in rat model of chronic constriction injury. Neurol Res 2012; 34: 871–888. [DOI] [PubMed] [Google Scholar]
- 36.Cunha JM, Cunha FQ, Poole S, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-1 receptor antagonist. Br J Pharmacol 2000; 130: 1418–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rose-John S, Heinrich PC. Soluble receptors for cytokines and growth factors: generation and biological function. Biochem J 1994; 300: 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taga T, Hibi M, Hirata Y, Yamasaki K, Yasukawa K, Matsuda T, Hirano T, Kishimoto T. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell 1989; 58: 573–581. [DOI] [PubMed] [Google Scholar]
- 39.MacEwan DJ. TNF receptor subtype signalling: differences and cellular consequences. Cellular Signalling. 2002; 14: 477–492. [DOI] [PubMed] [Google Scholar]
- 40.Probert L. TNF and its receptors in the CNS: the essential, the desirable and the deleterious effects. Neuroscience 2015; 302: 2–22. [DOI] [PubMed] [Google Scholar]
- 41.Gorman AM. Neuronal cell death in neurodegenerative diseases: recurring themes around protein handling. J Cell Mol Med 2008; 12: 2263–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hahm ET, Kim Y, Lee JJ, Cho YW. GABAergic synaptic response and its opioidergic modulation in periaqueductal gray neurons of rats with neuropathic pain. BMC Neurosci 2011; 12: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vezzani A, Viviani B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacol 2015; 96: 70–82. [DOI] [PubMed] [Google Scholar]
- 44.Obara I, Tochiki KK, Géranton SM, Carr FB, Lumb BM, Liu Q, Hunt SP. Systemic inhibition of the mammalian target of rapamycin (mTOR) pathway reduces neuropathic pain in mice. Pain 2011; 152: 2582–2595. [DOI] [PubMed] [Google Scholar]