Abstract
Oxidative stress and inflammation play a critical role in the initiation and progression of age-related ocular abnormalities as cataract, glaucoma, diabetic retinopathy, and macular degeneration. Therefore, phytochemicals with proven antioxidant and anti-inflammatory activities, such as carotenoids and polyphenols, could be of benefit in these diseases. We searched PubMed and Web of Science databases for original studies investigating the benefits of different carotenoids and polyphenols in age-related ophthalmic diseases. Our results showed that several polyphenols (such as anthocyanins, Ginkgo biloba, quercetin, and resveratrol) and carotenoids (such as lutein, zeaxanthin, and mezoxanthin) have shown significant preventive and therapeutic benefits against the aforementioned conditions. The involved mechanisms in these findings include mitigating the production of reactive oxygen species, inhibiting the tumor necrosis factor-α and vascular endothelial growth factor pathways, suppressing p53-dependent apoptosis, and suppressing the production of inflammatory markers, such as interleukin- (IL-) 8, IL-6, IL-1a, and endothelial leucocyte adhesion molecule-1. Consumption of products containing these phytochemicals may be protective against these diseases; however, adequate human data are lacking. This review discusses the role and mechanisms of polyphenols and carotenoids and their possible synergistic effects on the prevention and treatment of age-related eye diseases that are induced or augmented by oxidative stress and inflammation.
1. Introduction
Age-related ophthalmic diseases as cataract, age-related macular degeneration (AMD), glaucoma, and diabetic retinopathy are the main causes of progressive and irreversible vision loss worldwide [1–3]. These pathologies are joined by dry eye disease (DED), a prevalent eye disorder that affects the elderly population [4]. The pathogenic processes of these diseases are complex and unclear and sometimes depend on numerous factors. Unfortunately, most of these conditions are diagnosed in advanced stages at which effective treatments are not available [5]. As a result, both the improvement of diagnostic and therapeutic approaches and the prevention of age-related eye diseases have become global health priorities.
For this purpose, researchers have investigated the health benefits of several strategies to maintain visual acuity and prevent degenerative eye conditions [6, 7]. Experimental studies have found that fruit and vegetable consumption contributes to preserving vision and even reversing visual impairment [8, 9]. These beneficial effects have been attributed to the presence of some phytochemical compounds with bioactive properties, such as polyphenols and carotenoids [6, 10].
The pathophysiology of several eye diseases involves oxidative stress, and therefore, the antioxidant activity of phytochemicals is of particular importance. Carotenoids and polyphenols are plant-based molecules that have shown potent antioxidant and anti-inflammatory activities in several animal models of disease [11–15]. This review deals in depth with the role of antioxidant polyphenol and carotenoid properties in the prevention and treatment of age-related ophthalmic diseases, whose pathogeneses involve oxidative stress and inflammation.
1.1. Oxidative Stress: Implications for Age-Related Eye Diseases
Oxidative stress occurs due to imbalance in the redox hemostasis between prooxidant and antioxidant systems [16] and is involved in the pathogenesis of several inflammatory, degenerative, neoplastic, and cardiovascular disorders [17, 18]. Antioxidant enzymes (such as SOD, CAT, and GPx) can neutralize the production of toxic oxygen species, thus normalizing the body homeostasis [19]. Oxidative stress results from the inability of antioxidant enzymes to remove free radicals [20]. This imbalance leads to oxidative alteration of cellular targets, such as proteins, lipids, and DNA structures [18], ending in apoptosis or cellular necrosis [21]. A frequent risk element in numerous degenerative diseases in which oxidative stress plays a major role is aging. As humans advance in age, the rate of oxidative stress-induced cellular malfunction and death exceeds the rate of cellular regeneration [22, 23].
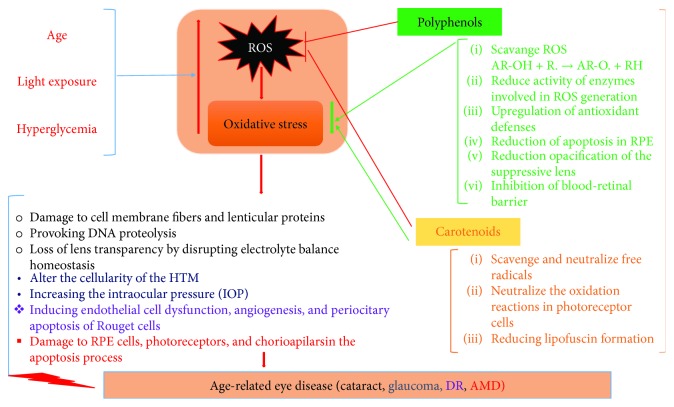
The eye is particularly vulnerable to oxidative stress due to its exposure to light, rich content of mitochondria, and high metabolic rate of photoreceptors. Due to the disequilibrium between the production and neutralization of reactive oxygen species (ROS), it results in oxidation of cellular constituents and ultimately malfunctions and degeneration of retinal tissues [24]. Figure 1 summarizes the implications of oxidative stress in age-related ocular diseases.
Figure 1.
Summarization of the implications of oxidative stress in age-related ocular diseases and effects of phytochemicals.
1.2. Cataract
In developing countries, cataract is the most prevalent cause of blindness and visual impairment in the elderly people. Currently, the management of a visually significant cataract is primarily surgical, which is the only available option to ophthalmologists. The pathogenesis of cataract involves Na+/K+ adenosine triphosphatase activity changes, oxidative stress, lens protein aggregation, polyol pathway activation, advanced glycation end-products, and genetic anomalies [25]. The contribution of ROS towards cataract pathogenesis occurs through (1) damage to cell membrane fibers and lenticular proteins, (2) provoking DNA proteolysis, and (3) loss of lens transparency by disrupting electrolyte balance homeostasis [26, 27].
1.3. Glaucoma
Glaucoma is an optical neuropathy, characterized by progressive degeneration of the retinal ganglion cells. The degeneration of these cells, which are actually neurons of the central nervous system (CNS) with axons in the optic nerve, causes progressive optical atrophy and irreversible visual loss [28]. In addition to ganglion cell loss, most glaucoma types are characterized by high intraocular pressure [29]. The diagnosis of open-angle glaucoma is often delayed due to the fact that its progression may be asymptomatic until a relatively late stage [30]. Several factors contribute to glaucoma pathophysiology, including aging, genetic predisposition, and exogenous environmental and endogenous factors.
Reactive oxygen species may damage the cells within the human trabecular meshwork (HTM), especially its endothelial cells. This consequently slows the drainage of the aqueous humor, increasing the intraocular pressure (IOP) [31]. Several studies reported oxidative stress and low levels of antioxidants as early events in patients with glaucoma [12, 32]. This is further exacerbated by the upregulated expression of the endothelial leukocyte adhesion molecule due to oxidative stress and activation of the interleukin-1 inflammatory cytokine [33–35]. Glaucoma cannot be cured; however, its progression may be delayed or prevented by reducing the IOP [36, 37]. The interest in investigating phytochemicals due to their antioxidant and anti-inflammatory properties has opened new treatment options with reduced side effects [34, 38].
1.4. Diabetic Retinopathy
Diabetic retinopathy (DR) is a major complication of diabetes mellitus. In the industrialized world, it is regarded as the leading cause of blindness in individuals who have not reached the retirement age [39]. Many interconnected mechanisms are implicated in the complex pathogenesis of DR, including oxidative stress [39]. Hyperglycemia induces many metabolic abnormalities in the retina, producing ROS, which are involved in the subsequent induction of oxidative stress [40]. Usually, this oxidative stress causes retinal lesions (inducing endothelial cell dysfunction, angiogenesis, and peri-ocitary apoptosis of Rouget cells/or mural cells) [40].
Oxidative stress plays a central role in DR development and in its critical phases: proliferative diabetic retinopathy (PDR) and diabetic macular edema (DME) [39]. The most glaring characteristics of DR are the vascular disturbances and retinal blood barrier (RBB) disturbance; both can be caused by oxidative stress [27, 41]. Consequently, their inhibitors may have protective effect on the retina. Antioxidants may be considered beneficial to DR because they reduce ROS production, neutralize free peroxide, and enhance the antioxidant defense system [40].
1.5. Age-Related Macular Degeneration (AMD)
This is a progressive, age-related degeneration of the underlying retinal pigment epithelium (RPE) and the retinal macula [42]. Although it is presumed that the cumulative repercussions of oxidative stress over years may be the first stimulus for AMD, the pathogenesis of AMD is yet uncertain [43]. However, RPE cells are considered to be the critical lesion site in AMD [44]. Excessive production and accumulation of ROS play a major role in the pathogenesis of AMD because their levels increase in the aging retina, even if retinal cells and RPE contain numerous antioxidants (enzymatic and nonenzymatic). The high level of ROS and attenuated antioxidant cell defense systems results in oxidative stress, leading to damage and apoptosis of RPE cells, photoreceptors, and chorioapilars [45, 46].
1.6. Dry Eye Disease
It is considered to be a multifactorial disease of the ocular surface and the tears that it manifests through visual disturbance, symptoms of discomfort, and tear film instability [47].
Recent laboratory and clinical studies have confirmed that the dry eye may be considered a chronic inflammatory disease and may be initiated by numerous intrinsic and extrinsic factors favoring an unstable and hyperosmolar lacrimal film [48].
Many times, some environmental factors are involved in dry eye disease; of these, we mention the following: exposure to chemical and physical pollutants, ultraviolet radiation (UV) and ozone, and chronic use of preserved eye drops (such as glaucoma treatment). These factors mentioned above increase the potential for oxidative stress and intensify the inflammation of the ocular surface. Phytochemicals with antioxidant and/or anti-inflammatory properties may be considered to be an alternative in the prevention and treatment of this disease [49]. Therefore, in the treatment of ocular disorders linked to oxidative stress, ameliorating the negative effects of ROS has been suggested to be a rational therapeutic strategy.
2. Materials and Methods
2.1. Identification of Reviewed Studies
We searched Medline (via PubMed) and Web of Science (WoS) databases for original studies that investigated the benefits of phytochemicals in age-related ocular diseases. We used the following keywords with different combinations: Phytochemicals OR Phytonutrient OR Plant-based OR Plant-derived OR Carotenoids OR Xanthophylls OR Lutein OR Polyphenols OR Anthocyanins AND Age-related OR Senile OR Elderly AND Eye disease OR Ophthalmic disease OR Cataract OR Glaucoma OR Macular degeneration OR Diabetic retinopathy OR Dry eye disease.
2.2. Study Selection
No restrictions by publication language or timing were applied. The retrieved studies were screened for eligibility. Overall, 2330 unique records were retrieved and screened for eligibility. Relevant findings were extracted and organized in a narrative approach.
3. Results and Discussion
3.1. Role of Polyphenols in the Prevention and Treatment of Age-Related Ophthalmic Diseases
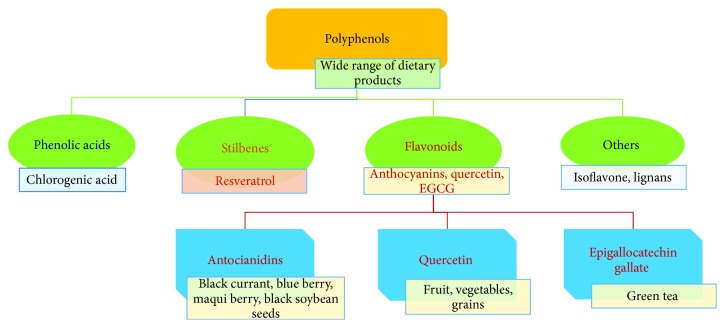
Polyphenols represent a broad group of phytochemicals with over 10000 different compounds [50], many of which have proven health benefits: antioxidant, anti-inflammatory, antiallergic, antimicrobial, and antiviral effects [51, 52]. They are classified into several groups based on their carbon backbone [53] as it is illustrated in Figure 2.
Figure 2.
Classification of main polyphenols investigated for age-related eye diseases and their natural sources.
The beneficial effects of polyphenols include: scavenging free radicals, ameliorating inflammation, and improving ocular blood flow and signal transduction [54]. There are recent studies describing new findings regarding the beneficial effect of polyphenol consumption on ocular health (reduction of apoptosis in the RPE, opacification of the suppressive lens, and inhibition of the blood-retinal barrier) [55].
The retina is highly susceptible to oxidative stress due to its rich content of polyunsaturated fatty acids and oxygen and its heavy exposure to light [56, 57]. In addition, oxidative stress can be involved in the production of severe inflammation by increasing the proinflammatory cytokines in the retinal tissue. These cytokines degrade the RBB and produce vascular cell death and apoptosis through tumor necrosis factor-α, chemotactic proteins, intercellular adhesion molecule 1, and interleukin- (IL-) 1β [54, 58].
Polyphenols can activate the process of transcription factor Nrf2, Nrf2 being considered to have the main role in protecting the cell from inflammation and oxidative stress [59]. The senso-redox transcription factor Nrf2 has an essential role in regulating the induction of detoxification and/or antioxidant phase II enzymes (HO-1, SOD, etc.); these enzymes have the effect of cellular defense against oxidative stress and exercise of the cytoprotective mechanism [60]. Numerous researches have studied and elucidated the molecular mechanisms considered to be responsible for activating Nrf2. A Kelch-associated Kelch homolog (ECH) associated with Kelch (Keap1)—cytoskeleton-binding protein—binds to Nrf2; it regulates the translocation of the protein to the nucleus, or its activation [61].
Nrf2 binds, after nuclear translocation, to the cis element (consensus called antireceptive element (ARE) or electrophilic response element (EpRE) present in the promoter region of genes encoding numerous antioxidant enzymes); it also binds to other transaction factors as follows: the small Maf-F/G/K, ARE coactivators which include the cAMP response-binding protein (CREB or CBP binding protein), p300, which may coordinate the transcription of the antioxidant gene driven by ARE [62, 63].
The mechanisms implied in the antioxidant activity of polyphenols include suppression of ROS formation (scavenging of ROS, inhibition of enzymes involved in their production, or protection or upregulation of antioxidant defenses) [64, 65]. Polyphenols can reduce the catalytic activity of enzymes involved in ROS generation, therefore reducing oxidative damage [66]. The explanation of the molecular mechanism of induction of the antioxidant enzyme by polyphone is still largely unrealized. There is, however, a universally accepted model for inducing ARE-mediated antioxidative gene expression. This mechanism involves the phosphorylation of serine and/or threonine residues of Nrf2 by protein kinases, resulting in ARE binding and subsequent increased nuclear accumulation of Nrf2 [67].
Some studies asserted that ROS formation increases free metal ions by reducing hydrogen peroxide and generating very reactive hydroxyl radicals. The reduced redox potentials of polyphenols can thermodynamically diminish free radical production by bonding them in chelates with metal ions such as iron and copper [68]. In addition, some polyphenols can inhibit the characteristic uncontrolled ocular angiogenesis in AMD by restoring the retinal structure and increasing the RPE function and choroidal blood flow [69]. By inhibiting oxidative stress and blocking the production of proinflammatory cytokines, polyphenols can attenuate vascular leakage and neovascularization in the retina of diabetic patients [70–72]. The effects of some polyphenols on age-related eye diseases are presented in Tables 1 and 2 and are discussed below.
Table 1.
Effects of some polyphenols on age-related eye diseases in human studies.
| Polyphenols | Natural source/dose | Cell type/type of study | Effects on chronic eye diseases | Ref | p |
|---|---|---|---|---|---|
| Anthocyanins | Bilberry | Retinal pigment epithelial cells (AREP 19) | Mediate a detergent-like perturbation of cell membranes and light-induced damage to the cell Inhibit AMD |
[80] | |
|
| |||||
| EGCG | 10, 25, 50, 75, 100, and 150 μM for 24 h | Human lens epithelial HLEB-3 cells | EGCG protects HLE cells from the mitochondria-mediated apoptosis (induced by H2O2 through the modulation of caspases, Bcl-2 family, and MAPK & Akt pathways) Cataract prevention |
[92] | |
| 3 months | Human patients/placebo-controlled, double-blind, crossover design 34 patients: 18 ocular hypertension; 16 open-angle glaucoma |
Neuroprotective function pattern-evoked electroretinograms increased in amplitude Favorable influence in inner retinal function in the eyes with early to moderately advanced glaucomatous damage | [94] | <0.01 | |
| 20 and 40 mM | Human RPE cell line ARPE-19 | AMD and DR prevention Inhibitor of ocular angiogenesis and its vascular permeability Treatment and prevention of ocular angiogenic diseases: age-related macular degeneration, diabetic retinopathy, and corneal neovascularization |
[95] | ||
| 20 and 40 mM | Human retinal vascular endothelial cell (HREC) (case of diabetes) | Inhibits the expression of vascular endothelial growth factor (VEGF) It ameliorated the negative effect of high glucose concentrations on the cell viability and apoptotic rate The protective effects of EGCG under high-glucose conditions may be attributed to the regulation of inflammatory cytokines and inhibition of the MAPK/ERK-VEGF pathway |
[96] | <0.01 | |
|
| |||||
| Ginkgo biloba | 40 mg GBE 2 times daily for 6 months | Human patients/experimental study, prospective, double blind | No significant changes were found in intraocular pressure and optic nerve head | [103] | >0.01 |
| Ginkgo biloba extract (40 mg, 3 times per day 4-week phases/8 weeks washout period) | Human patients/prospective, randomized, placebo-controlled crossover study | Changes in visual field and contrast sensitivity did not differ by treatment received or sequence | [104] | >0.2 | |
| 80 mg GBE 2 times daily | Human patients/retrospective study | Slowed the progression of visual field damage in patients with normal tension glaucoma | [105] | ||
| 80 mg GBE orally, twice a day for four weeks | Human patients/prospective, randomized, placebo study | Desirable effect on ocular blood flow in normal tension glaucoma patients | [106] | ||
| 40 mg, 3 times per day 4-week phases/8 weeks washout period/4 weeks of placebo treatment | Human patients/prospective, randomized, placebo-controlled, double-masked crossover trial | Improves preexisting visual field damage in some patients with normal tension glaucoma | [107] | ||
| Human patients/retrospective analysis | May be helpful in improving visual function in some individuals with NTG | [102] | |||
| Ginkgo biloba extract (EGB761) and ginkgolide B | Retina explants Three-dimensional tissue culture system |
Protecting RGCs against apoptosis It decreased cellular apoptosis and inhibited caspase-3 activation, suggesting that ginkgolide B can promote RGC axon growth by protecting RGCs against apoptosis |
[109] | ||
|
| |||||
| Quercetin | 50 μM | Cultured human RPE cells | AMD prevention It protects RPE cells from oxidative damage and cellular senescence in vitro in a dose-dependent manner Prevention of early AMD |
[113] | <0.001 |
| 50 μM | Cultured human RPE cells (ARPE-19) | AMD prevention It protects human RPE cells from oxidative stress in vitro, via inhibition of proinflammatory molecules & direct inhibition of the intrinsic apoptosis pathway |
[114] | ||
|
| |||||
| Resveratrol | Human lens epithelial cells | Cataract prevention It protects LECs from oxidative stress via the inhibition of the p53 pathway |
[118] | ||
| 5 and 10 mg/kg/day, 1 to 7 months | High-glucose culture Müller-treated cells | It is a therapeutic option to prevent diabetic retinopathy RSV (10, 20, and 30 mmol/L) significantly inhibited the HG-induced decreases in glutamate uptake, GS activity, GLAST, and GS expression |
[124] | <0.05 | |
| Longevinex | Human patients 3 clinical measures of visual function (Snellen visual acuity, contrast sensitivity, and glare recovery to a cone photostress stimulus) |
Efficacy against AMD | [125] | ||
Table 2.
Effects of some polyphenols on age-related eye diseases in animals.
| Polyphenols | Natural source/dose/period | Cell type/animal model | Effects in chronic eye diseases | Ref | p | |
|---|---|---|---|---|---|---|
| Anthocyanins | Grape skin Asamurasaki-2 (AS2) (Japanese black rice) and chikushi-akamochi-2 (CH2) (Japanese red rice) |
Sprague Dawley (SD) rat lens organ culture system | Inhibits selenite-induced cataractogenesis Inhibitory activities for lens opacity |
[79] | ||
| Bilberry | Retinal pigment epithelium (RPE) cells | Inhibits AMD Exhibited antioxidant activity of variable efficiency at 9 anthocyanins tested Cells exhibited a resistance to the membrane permeabilization that occurs because of the detergent-like action of A2E |
[80] | |||
| Blueberry; STZ, 60 mg/kg; and blueberry anthocyanins at 20, 40, and 80 mg/kg were given orally, 12 weeks | Rat retinas, male rats, 5 groups Streptozotocin-induced diabetic SD rats |
It can protect retinal cells from diabetes-induced oxidative stress and inflammation, and this may be regulated through Nrf2/HO-1 signaling | [83] | |||
| Bilberry (Vaccinium myrtillus) extract 100 mg/kg, orally, 6 weeks | Streptozotocin-induced diabetic SD rats | Prevention of diabetic retinopathy using a dietary bilberry supplement | [84] | |||
| Seed coat of black soybean 50 mg/kg daily, orally, for 1, 2, and 4 weeks after intraperitoneal injection of N-methyl-N-nitrosourea (MNU) | Animal model of retinal degeneration N-methyl-N-nitrosourea-induced RD rats |
Reduce retinal degeneration Anthocyanins (black soybean seeds) can protect retinal neurons from MNU-induced structural and functional damages, suggesting that it may be used as supplement to modulate RD |
[85] | |||
| Maqui berry (Aristotelia chilensis) | Murine photoreceptor cells (661 W) | Suppress the light-induced photoreceptor cell death by inhibiting ROS production; the inhibition of phosphorylated-p38 may be involved in the underlying mechanism | [86] | |||
| Dried cornelian cherry (Cornus mas L.) polar, iridoid-polyphenol-rich fraction 1 kg of fresh fruit: 4.0 g anthocyanin fraction + 1.5 g of pure loganic acid Each animal was given 0.7% loganic acid or polyphenolic fraction, intraconjunctival administration of 1 drop (50 μL) |
New Zealand white rabbits, aged between 6-12 months, were used: 7 males and 7 females | Intraocular pressure (IOP)—hypotensive effect for loganic acid (0.7%)—could be compared with the widely ophthalmologically used timolol 25% decrease in IOP was observed within the first 3 hours of use |
[87] | |||
|
| ||||||
| EGCG | Green tea leaf extract (Camellia sinensis) In vivo, cataract was induced in 9-day-old rat pups by a single subcutaneous injection of sodium selenite The treated pups were injected GTL extract intraperitoneally prior to selenite challenge and continued for 2 consecutive days thereafter Cataract incidence was evaluated on the 16th postnatal day by slit lamp examination |
Enucleated rat lenses | Inhibits selenite-induced cataractogenesis Green tea possesses significant anticataract potential and acts primarily by preserving the antioxidant defense system |
[91] | ||
| Intraperitoneal (25 mg/kg) intraocular (5 μL of 200 μM) | Rat retinal neurons | Glaucoma prevention It provides protection to retinal neurons from oxidative stress and ischemia/reperfusion |
[93] | |||
| 20 and 40 mM | Animal models | Significantly reduced vascular leakage and permeability by blood-retinal barrier breakdown in VEGF-induced animal models Treatment and prevention of ocular angiogenic diseases: age-related macular degeneration, diabetic retinopathy, and corneal neovascularization |
[95] | |||
| Nontoxic optimal concentration of EGCG used for the treatment of HCECs in vitro was 10 μg/mL IL-1β, IL-6, IL-8, and TNF-α and was significantly inhibited in inflamed HCECs treated with 10 μg/mL EGCG and 0.1% (w/v) HA (E10/HA) vs that in inflamed HCECs treated with either EGCG or HA alone | Rabbit DES model | Topical treatment with AT plus E10/HA increased tear secretion, reduced corneal epithelial damage, and maintained the epithelial layers and stromal structure The corneas of the E10/HA-treated rabbits showed fewer apoptotic cells, lower inflammation, and decreased IL-6, IL-8, and TNF-α levels In conclusion, we showed that AT plus E10/HA had anti-inflammatory and mucoadhesive properties when used as topical eye drops and were effective for treating DES in rabbits |
[98] | |||
|
| ||||||
| Ginkgo biloba | 5 μg of GBE, 4 times daily, for 14 days | Rabbits aged 7 weeks | Suppressed steroid-induced IOP elevation; it seems to prevent the adverse effects of DEX on TM cells | [100] | ||
|
| ||||||
| Quercetin | Quercetin 10 μM | Rat lens | Cataract prevention | [110] | ||
| The expression levels of BDNF, NGF, TrkB, synaptophysin, Akt, Bcl-2, cytochrome c, and caspase-3 using Western blotting techniques with and without QCT treatments were quantitated and compared with those of nondiabetic rats ELISA technique was used to determine the level of BDNF Caspase-3 activity and the level of glutathione were analyzed by biochemical methods |
Diabetic rat retina | Significant increase in the level of neurotrophic factors and inhibited the level of cytochrome c and caspase-3 activity The level of an antiapoptotic protein Bcl-2 was augmented It may protect the neuronal damage in diabetic retina by ameliorating the levels of neurotrophic factors and by inhibiting the apoptosis of neurons Quercetin suitable therapeutic agent to prevent neurodegeneration in diabetic retinopathy |
[112] | |||
|
| ||||||
| Quercetin and resveratrol | 0.01% QCT, 0.1% RES, 0.01% QCT + 0.1% RES (QCT + RES), or vehicle was topically applied | Desiccating stress (DS) mouse model | Reduced corneal staining in DS-exposed mice | [116] | QCT QCT + RES |
<10−3 <0.05 |
| IL-1α tear concentration was reduced by QCT, RES, and QCT + RES compared to DS + vehicle mice | <0.05, 0.01, and 0.01 | |||||
| CD4+ T cells increased in recipients of DS-exposed mice and were lower in recipients of QCT- and RES-treated mice | <0.05, <0.05 | |||||
| The anti-inflammatory effect of QCT, RES, and QCT + RES on DED experimental model suggests that their topical application could be used for DED treatment | ||||||
| 25 mg/kg/day quercetin by intraperitoneal injection daily, 2 months | Ccl2/Cx3cr1 double knockout (DKO) mice | Does not improve the retinal AMD-like lesions in the Ccl2(−/−)/Cx3cr1(−/−) (due to its insufficient suppression of the inflammatory and apoptosis pathways in the eye) | [113] | |||
| Quercetin 33.63 mg/kg/day and chlorogenic acid | Pigmented rabbits | AMD prevention Its alleviating retinal degeneration |
[115] | <0.05 | ||
|
| ||||||
| Resveratrol | 40 mg/kg 3 groups: (1) normal saline—% 5 ethanol injected i.p. on postpartum day 10; (2) Na selenite—30 nmol/g body wt. injected s.c. on day 10; and (3) Na selenite—s.c. on day 10 + resveratrol (40 mg/kg) i.p. on days 10-13 |
48 SD rat lens | Inhibits selenite-induced cataractogenesis It suppressed selenite-induced oxidative stress and cataract formation in rats |
[117] | ||
| 25 μM in DMSO, from Sigma, Saint Louis, MO | Porcine TM cells | Preventing or delaying of the abnormalities of the TM | [119] | |||
| 4 months oral resveratrol administration (5 mg/kg/day) | Streptozotocin-induced diabetic Wistar rats | Therapeutic supplement to prevent from diabetic retinopathy It improves diabetic retinopathy possibly through the oxidative stress—nuclear factor kappa B—apoptosis pathway |
[120] | |||
| 1 month after the 5th injection of streptozotocin or buffer—20 mg/kg, daily, for 4 weeks, and all mice were killed 2 months after the injections | Streptozotocin-induced diabetic C57BL/6 mice | Prevent diabetic retinopathy It decreases vascular lesions and VEGF induction in mouse retinas of early diabetes |
[121] | |||
| 10 mg/kg 30 days | 24 streptozotocin-induced diabetic Wistar albino male rats | Prevent diabetic retinopathy RSV suppressed the expression of eNOS, which is actively involved in the inflammation and healing process in chronic diabetes |
[122] | |||
| 5 mg/kg per day for 4 months | Streptozotocin-induced diabetic Wistar albino male rats | Prevent diabetic retinopathy Long-term resveratrol administration has beneficial anti-inflammatory properties in a rat model of diabetes |
[123] | |||
| 5 and 10 mg/kg/day, 1 to 7 months | Diabetic rat retina | Prevent diabetic retinopathy Significantly alleviated hyperglycemia and weight loss in diabetic rats Downregulated mRNA and protein expression of GLAST and GS in diabetic rat retina was prevented |
[124] | |||
3.1.1. Anthocyanins
Anthocyanins are flavonoid compounds with multiple biomedical functions [72, 73], including vision improvement [74]. Some recent scientific research clearly suggests that anthocyanins use several different mechanisms of action for biological beneficial effects on health. The free radical scavenging activity and antioxidant capacity are still the most common [75]. It has been shown that anthocyanidin isolates and high bioflavonoid mixtures in anthocyanidin provide several major actions: inhibition of enzymes, protection against DNA cleavage, anti-inflammatory activity, estrogen activity (modulation of the development of symptoms of hormone-dependent disease), stimulation of cytokine production thereby regulating immune responses, peroxidation, decrease of capillary permeability and fragility, and membrane hardening [67].
Several studies were performed to investigate their effects on asthenopia (eye fatigue). While some studies reported significant improvements in visual acuity [75], others [74, 76–78] reported contradictory effects for anthocyanins in this regard. Moreover, several experimental studies have reported antioxidant and protective effects at the eye level for anthocyanins, extracted from various fruits [79–81]. For example, anthocyanins extracted from grape skin were associated with inhibited opacity of the lens in sodium selenite-induced cataract in rats [78]. Anthocyanins from blackcurrant and blueberry extracts have demonstrated antioxidant effects at the level of RPE cells [80] by inhibiting the photooxidation of pyridinium bisretinoid (A2E) molecule and neutralizing oxygen free radicals [80, 81].
In an experimental study by Jang et al. [82], the accumulation of anthocyanins in the eye tissues of animals (after 4 weeks of dietary supplementation with blueberries) exhibited ocular protective effects and reversal of oxidative effects. Other studies performed on rats have shown that anthocyanins from blueberry extract can inhibit diabetes-induced retinal abnormalities, thus preventing DR induction [83, 84]. Moreover, anthocyanins extracted from black soybean seeds have shown their protective effects on the retinal neurons (from structural and functional damage produced by N-methyl-N-nitrosourea in rats) [85]. In vitro studies on Maqui berry extract have shown that it can protect the retinal cells against light-induced photoreceptor degeneration due to its content of anthocyanins [86]. Further in vivo and in vitro studies will be needed to establish the pleiotropic mechanisms of anthocyanins and to show how they can practically interfere in different visual processes [87, 88].
3.1.2. Epigallocatechin Gallate
Epigallocatechin gallate (EGCG) is the main flavonoid present in green tea, representing more than 50% of the whole amount of polyphenols in this type of tea [89]. Numerous studies indicate that the remarkable antioxidant properties of polyphenols (tea) may be due to the inhibition of ROS-generating enzymes. These enzymes contribute to the production of NO-mediated free radicals. In the study of Wu et al., in addition, the induction of the HO-1 enzyme in endothelial cells was also found due to the activation process of Akt and Nrf2 by EGCG, which gives the protective measure of endothelial cell protection against H2O2-mediated oxidative stress [90]. Other rodent studies have demonstrated the pleiotropic effect of EGCG. This effect wields a neuroprotective measure by modulation of antioxidative enzyme activity (SOD, GST), associated with suppression of ROS generation and with the protection of neuronal cells (from glycation-induced neurotoxicity) [67].
Some plausible mechanisms of explication of EGCG-induced Nrf2 activation are the oxidation or the modification of cysteine thiols present in Keap1 by ROS and/or via the active form of EGCG (during its redox cycling). However, it may be plausible that EGCH is mediated by ARE-mediated regulation of the antioxidant gene (by activation of MAPK, which ultimately activates Nrf2). However, there are numerous studies that have shown MAPK inhibition by phytochemicals, which eventually caused Nrf2 activity [60, 61].
Green tea leaf extract has been associated with an anticataract effect [91, 92], and short-term supplementation with EGCG has shown beneficial effects on the treatment of glaucoma through the protection of retinal neurons subjected to injuries due to high IOP [93, 94]. Further, it has been shown to suppress vascular endothelial growth factor (VEGF) and extracellular signal-regulated kinase (ERK) 1/2 signaling pathways [95, 96]. Supplements containing green tea, administered to diabetic rats, increase GSH levels and catalyze SOD activities, while reducing VEGF and TNF-α values and protecting retinal vasculature and retinal endothelial cells from/against apoptosis [97]. These data also describe possible mechanisms of beneficial effects of green tea on DR treatment [97]. Topical treatment with artificial tears (containing epigallocatechin gallate (EGCG) and hyaluronic acid (HA)) of DED rabbits had anti-inflammatory and mucoadhesive properties [98].
3.1.3. Ginkgo biloba
Ginkgo biloba (GB) is a plant that possesses several biological actions that encouraged researchers to investigate its benefits in the prevention or treatment of ocular diseases, especially glaucoma [99]. The GB extract significantly inhibited the steroid-induced increase of intraocular pressure in rabbits and prevented the adverse effects of dexamethasone on the trabecular meshwork cells [100]. Moreover, the GB extract was associated with positive effects on the improvement of deteriorated visual field in patients with normotensive (open-angle) glaucoma [101–107]. Further, its ability to prevent cell membrane damage by free radicals that may aid stop the evolution of AMD was demonstrated [108]. Through the ginkgolide B terpenoid contained in the GB extract, it protects retinal ganglion cells and promotes their growth in vitro by antiapoptosis [109].
3.1.4. Quercetin
Quercetin is a flavonoid present in many fruits and vegetables. Studies have reported that quercetin effectively protects against hydrogen peroxide-induced cataracts and diabetes-induced retinal lesions [110, 111]. It is well known that oxidative stress is the major cause of neurodegenerative accountability, by its action in steering neurotrophic factors and activating apoptosis (in retinal diabetes). Some studies have shown that treatment with quercetin in diabetic rats causes a substantial increase in neurotrophic factor levels and an inhibition of cytochrome activity and a caspase-3 activity in retinal diabetes. Furthermore, the level of an antiapoptotic Bcl-2 protein increases in the diabetic retina treated with quercetin. It can be argued that quercetin can protect neuronal damage in retinal diabetes, ameliorates neurotrophic factor levels, and inhibits neuronal apoptosis [112].
In in vitro RPE cells, quercetin inhibits oxidative stress that causes apoptosis as highlighted in experimental studies [113, 114]. Wang et al. demonstrated that quercetin protects the retina subjected to oxidative stress and inflammation, induced by light [115], which could provide new strategies to impede AMD occurrence and progression. The use in artificial tears of quercetin in the experimental model, alone or in combination with resveratrol, suggests that their topical application could be used for DED treatment [116].
3.1.5. Resveratrol
Resveratrol (trans-3,40,5-trihydroxystilbene) is a polyphenolic phytoalexin that belongs to the stilbene family (a group of compounds that consist of 2 aromatic rings joined by a methylene bridge) that is mainly found in grape seed and skin, as well as fruit berries [69]. In a study by Doganay et al. in experimental rats, resveratrol effectively suppressed cataract development after sodium selenite exposure and increased the lenticular levels of reduced glutathione [117]. Moreover, it ameliorated the damages caused by hydrogen peroxide in the lens epithelial cell morphology by inhibiting p53-dependent apoptosis (by activating sirtuin-1) [118].
In another study by Luna et al., resveratrol treatment was associated with a significant downregulation of glaucoma marker expression, caused by chronic oxidative stress in the trabecular meshwork cells through its antiapoptotic effect [69, 119]. Moreover, it suppressed the production of proinflammatory markers, comprising IL-8, IL-6, IL-1a, and endothelial leucocyte adhesion molecule-1 (ELAM-1) [119].
At the retinal level, diabetes-induced oxidative stress, apoptosis, proinflammatory cytokine production, and NF-κB activation have been shown to be inhibited by resveratrol. Destruction of diabetic-induced neuronal cell and vascular hyper permeability were stopped by resveratrol treatment [120–124]. The ocular structure and activity in three patients with AMD were enhanced over a two- to three-year survey, implying the efficiency of resveratrol in AMD treatment [125].
3.2. Role of Carotenoids in the Prevention and Treatment of Age-Related Ophthalmic Diseases
Carotenoids are a group of lipo-soluble pigments comprising over 600 natural compounds, divided into three classes of xanthophylls, carotenes, and lycopene [126, 127]. Although they are widespread in nature (plants, fruits, vegetables, fungi, bacteria, algae, and fish) [128, 129], only about 40 carotenoids are present in the human diet, of which 20 were found in tissues and biological fluids [126]. At the level of the human macula, lutein, zeaxanthin, mezoxanthin, and xanthophyll carotenoids are responsible for the yellow pigmentation of the fovea and have been chemically identified [130] A diet high in these carotenoids is associated with a low incidence of age-related eye diseases, such as AMD and cataracts [131, 132].
Lutein and zeaxanthin are obtained exclusively from food and are found especially in vegetables with green leaves and in yellow and orange fruits and vegetables. Meso-zeaxanthin is rarely found in diet and is therefore considered a metabolite of the other ingested carotenoids [133–135]. Due to the conformational similarity between lutein and meso-zeaxanthin [136], it was considered that lutein is the direct precursor of meso-zeaxanthin; a hypothesis which was confirmed by some experimental studies [137, 138]. The presence of meso-zeaxanthin in ocular tissues only (macula, retina, and RPE/choroid) [139] indicates that lutein conversion in meso-zeaxanthin is produced at the eye level.
Carotenoids are very powerful antioxidants that can scavenge and neutralize free radicals, such as hydroxyl radical and superoxide anion [140]. The association of carotenoid supplementation with a lower incidence of ocular diseases is primarily due to the ability of macular carotenoids like zeaxanthin and lutein to neutralize the oxidation reactions in photoreceptor cells [136, 141, 142]. All these eye-protective nutrients undergo the oxidation process and a series of transformations which protect the macula. 3-Hydroxy-β,ε-caroten-3′-one is a substance that was identified as the direct oxidation product of lutein present in the human eye and in monkey retinas [136].
Another protective function that was reported for carotenoids is reducing lipofuscin formation. With age, the RPE cumulates lipofuscin, which is a fluorescent protein-lipid mixture containing by-products of vitamin A metabolism, along with lipid peroxidation products [143, 144]. This compound is noxious to the mitochondria and was found to induce apoptosis in cultured RPE cells upon exposure to blue light [145–147]. Interestingly, the amount of lipofuscin formed in vivo and in cultured RPE cells was found to be diminished in the presence of lutein and zeaxanthin [143, 148]. The effects of macular carotenoids on age-related eye diseases are presented in Table 3.
Table 3.
Effects of some carotenoids on age-related eye diseases.
| Carotenoid | Characteristics of the study | Cell type/animal model | Effects in chronic eye diseases | Ref. |
|---|---|---|---|---|
| Lutein and zeaxanthin | Multicenter, randomized, double-masked, placebo-controlled phase 3 study with a 2 × 2 factorial design (2006–2012) Participants were randomized to receive lutein 10 mg + zeaxanthin 2 mg, DHA 350 mg + EPA 650 mg, lutein + zeaxanthin and DHA + EPA, or placebo All participants were also asked to take the original AREDS formulation or accept a secondary randomization to 4 variations of the AREDS formulation, including elimination of beta-carotene, lowering of zinc dose, or both |
4203 human patients, age 50-85 years | Median follow-up: 5 years, with 1940 study eyes, 1608 participants, progressing to advanced AMD Kaplan-Meier probabilities of progression to advanced AMD by 5 years were 31% (493 eyes, 406 participants), for placebo, 29% (468 eyes, 399 participants), for lutein + zeaxanthin, 31% (507 eyes, 416 participants), for DHA + EPA, and for lutein + zeaxanthin and DHA + EPA, 30% (472 eyes, 387 participants) Comparison with placebo demonstrated no statistically significant reduction in progression to advanced AMD (hazard ratio 0.90 (98.7% CI, 0.76-1.07); p = 0.12 for lutein + zeaxanthin; 0.97 (98.7% CI, 0.82-1.16); p = 0.70 for DHA + EPA; 0.89 (98.7% CI, 0.75-1.06); p = 0.10 for lutein + zeaxanthin and DHA + EPA) No apparent effect of beta-carotene elimination or lower-dose zinc on progression to advanced AMD More lung cancers were noted in the beta-carotene vs no beta-carotene groups (23 (2.0%) vs 11 (0.9%), nominal p = 0.04), mostly in former smokers Addition of lutein + zeaxanthin, DHA + EPA, or both to the AREDS formulation did not further reduce risk of progression to advanced AMD Because of potential increased incidence of lung cancer in former smokers, lutein + zeaxanthin could be an appropriate carotenoid substitute in the AREDS formulation |
[152] |
| Prospective study, in 1980 Repeated administration of a food frequency questionnaire during 12 years of follow-up |
Human patients (female), age 45-71 years | Decrease the risk of cataracts, p = 0.04, foods rich in these carotenoids may decrease the risk of cataracts severe enough to require extraction | [155] | |
| Prospective study, in 1986 Repeated administration of carotenoids and other nutrients—frequency questionnaire during 8 years of follow-up |
36644 human patients (male), age 45-75 years | May decrease the risk of cataracts severe enough to require extraction; this relation appears modest in magnitude As recommendations—to consume vegetables/fruit high in carotenoids daily; p = 0.03 |
[156] | |
| 1354 people eligible, 246 developed a nuclear cataract (level 4 or 5 opacity) Nuclear opacity was assessed on a 5-point ordinal scale using lens photographs taken at baseline (1988-1990) and at follow-up (1993-1995) Food frequency questionnaire |
Human patients, adults aged 43-84 years | Decrease the risk of nuclear cataracts Possible protective influence of lutein and vitamins E and C on the development of nuclear cataracts; the evidence provides weak support for these associations |
[157] | |
| Usual nutrient intake = average intake from 5 food frequency questionnaires (were collected during a 13- to 15-year period before the evaluation of lens opacities). The duration of vitamin supplement use was determined from 7 questionnaires collected during this same period | 478 human patients (nondiabetic female), aged 53-73 years | Prevention of age-related cataract The prevalence of nuclear opacities was significantly lower (p = 0.04) for women who used a vitamin C supplement for ≥10 years relative to women who never used vitamin C supplements (odds ratio, 0.36; 95% confidence interval, 0.18-0.72) Plasma measures of vitamins C and E taken at the eye examination were inversely associated with the prevalence of nuclear opacities |
[158] | |
| Study for individual carotenoids and tocopherols in serum, quality-controlled HPLC method One-way ANOVA analysis and logistic regression analysis were applied |
138 human patients with senile cataracts | No association The relation carotenoids-cataracts is biologically plausible; serum carotenoid levels are highly dependent on dietary intake and may not be clinically relevant biomarkers for cataract risk |
[160] | |
|
| ||||
| Macular pigments | The optical density of MP was measured psychophysically | 46 subjects, age 21-81 years with healthy maculae and in 9 healthy eyes known to be at high risk of AMD | Decrease the risk of AMD age-related decline (optical density of MP) at volunteers with no ocular disease (right eye: r(2) = 0.29, p = 0.0006; left eye: r(2) = 0.29, p < 0.0001) Healthy eyes (predisposed to AMD) had significantly less MP than healthy eyes at no such risk (Wilcoxon's signed rank test: p = 0.015) Supplemental lutein and zeaxanthin may delay, avert, or modify the course of this disease |
[162] |
| Lutein (L) and zeaxanthin (Z) extracted from each tissue sample were determined by HPLC | 56 donors with AMD and 56 controls were cut into 3 concentric regions centered on the fovea | The results are inconsistent with a model that attributes a loss of L and Z in the retina to the destructive effects of AMD | [163] | |
| The relative risk for AMD—estimated according to dietary indicators of antioxidant status, controlling risk factors (smoking), by using multiple logistic regression analyses | 356 case subjects, diagnosed with the advanced stage of AMD within 1 year prior to their enrollment, aged 55-80 years 520 control subjects |
Increasing the consumption of foods rich in certain carotenoids (dark-green, leafy vegetables) may decrease the risk of developing advanced or exudative AMD | [165] | |
|
| ||||
| Lutein | Measuring macular pigment optical density (MPOD) and retinal sensitivity Vitamin supplementation (lutein 10 mg/day, zeaxanthin 2 mg/day), 3 months later—repeated ophthalmological examination |
20 patients with medium/large drusen, 19 with RPD, and 15 control subjects | The mean MPOD significantly increased in RPD (p = 0.002), showing no more difference compared with controls (p = 0.3); no significant changes were found in mean retinal sensitivity and BCVA (p = 0.3 and p = 0.7) Medium/large drusen did not show significant changes on MPOD, retinal sensitivity, and BCVA (p = 0.5, p = 0.7, and p = 0.7, respectively) Different pathophysiology for RPD as compared with medium/large drusen |
[168] |
|
| ||||
| Lutein and zeaxanthin | Dietary intake | People over age 40 years (n = 8222) | No association with AMD Higher levels of lutein and zeaxanthin in the diet were related to lower odds for pigmentary abnormalities, one sign of early ARM (odds ratio among persons in high vs low quintiles = 0.1, 95% confidence interval: 0.1, 0.3) and of late ARM (odds ratio = 0.1, 95% confidence interval: 0.0, 0.9) |
[170] |
| Dietary intake + supplement intake of antioxidant vitamins and zinc at baseline and the 5-year incidence of early age-related maculopathy A 145-item food frequency questionnaire (FFQ) was used to assess nutrient intakes |
During 1992-1994, 3654 people, aged > 49 years (82% of those are eligible) were examined for the Blue Mountains Eye Study baseline 5 years later, 2335 persons (75% of known survivors) were reexamined |
No evidence of protection associated with dietary antioxidant or zinc intakes | [171] | |
|
| ||||
| Lutein + lycopene | Dietary intake Cross-sectional study Photodocumented retinal status and HPLC to measure plasma carotenoid concentrations |
111 individuals with type 2 diabetes in a community-based study | Modulation of retinopathy risk A higher concentration of PVA carotenoids was associated with greater odds of diabetic retinopathy, after adjustment for risk factors (p = 0.049) |
[172] |
|
| ||||
| Zeaxanthin | Diet supplemented with 0.02% or 0.1% Zx | Age-matched normal rats | Inhibits diabetic retinopathy Zx supplementation has the potential to inhibit the development of retinopathy in diabetic patients |
[173] |
|
| ||||
| Lutein | Lutein, lutein + insulin 12 weeks | Streptozotocin-induced diabetic rats | Preventing the development of cataracts Treatment is useful in preventing the development of cataracts in streptozotocin-induced diabetic rats |
[174] |
|
| ||||
| Carotenoids and vitamins A, C, and E | Multicenter (5 ophthalmology centers in the USA) eye disease case control study | 356 case subjects, diagnosed with an advanced stage of AMD within 1 year prior to their enrollment, aged 55 to 80 years, and residing near a participating clinical center The 520 control subjects were from the same geographic areas as case subjects, had other ocular diseases, and were frequency matched to cases according to age and sex |
Dietary intake of carotenoids associated with a lower risk for AMD Adjusting for other risk factors for AMD—those in the highest quintile of carotenoid intake had a 43% lower risk for AMD compared with those in the lowest quintile (odds ratio, 0.57; 95% confidence interval, 0.35 to 0.92; p for trend = 0.02) Among the specific carotenoids, lutein and zeaxanthin, which are primarily obtained from dark-green, leafy vegetables, were most strongly associated with a reduced risk for AMD (p for trend = 0.001) Several food items rich in carotenoids were inversely associated with AMD A higher frequency of intake of spinach or collard greens was associated with a substantially lower risk for AMD (p for trend < 0.001) The intake of preformed vitamin A (retinol) was not appreciably related to AMD Neither vitamin E nor total vitamin C consumption was associated with a statistically significant reduced risk for AMD; a possibly lower risk for AMD was suggested among those with higher intake of vitamin C from foods Increasing the consumption of foods rich in certain carotenoids (dark-green, leafy vegetables) may decrease the risk of developing advanced or exudative AMD |
[153] |
3.2.1. Lutein, Zeaxanthin, and Meso-zeaxanthin
Through their antioxidant and anti-inflammatory properties and ability to filter blue light, macular pigments (MP) play a major role in reducing the incidence of eye disorders, especially AMD [149, 150]. Several studies have examined the role of MP on visual function, and most have reported a positive influence [151]. It has also been found that the supplementation with all three MP is superior to formulations containing only two nutrients [151].
Epidemiological research and a number of large-scale clinical trials (such as Age-Related Eye Disease Study 2 (AREDS2)) have directed the researchers' attention to the functional benefits and potential ocular health of these three xanthophyll carotenoids, which are consumed through the diet or using supplements [136]. AREDS2 is a randomized, placebo-controlled study designed to demonstrate whether supplementation with lutein (10 mg/day) and zeaxanthin (2 mg/day) can slow the rate of progression of AMD. Addition in primary analyses of lutein + zeaxanthin to the AREDS formulation did not further reduce risk of progression to advanced AMD [152]. A five USA ophthalmology center eye disease case control study demonstrated that a higher dietary intake of some carotenoids (lutein and zeaxanthin) is associated with reduced AMD risk [153].
At the lens level, there are only lutein and zeaxanthin, which protect the eye against photooxidative damage and filter the blue light (which by generating ROS can produce photodamage) [154]. Data on their role in cataract is not unitary; some epidemiological studies have reported an inverse association between cataract risk and macular pigment density [155–158], while others reported no association [159] or positive association [160]. These differences can be explained by the fact that the etiology and evolution of the disease can be dependent on the type of cataract (nuclear sclerotic, cortical, and posterior subcapsular) [161].
Several observational and interventional studies implied that subjects with increased dietary ingestion and/or high plasma concentrations of lutein and zeaxanthin have a lower risk of AMD [162–167]. Moreover, MP supplementation has been shown to enhance macular pigment optic consistency in the reticular pseudodrusen [168, 169], which correlates with a low risk for AMD.
However, other studies failed to record such association [170, 171]. Moreover, various studies [172, 173] showed the function of carotenoids in DR improvement. Zeaxanthin has been demonstrated to suppress diabetes-induced retinal oxidative lesions and increased retinal levels of VEGF and ICAM-1 [173]. Similarly, lutein could diminish oxidative stress and lipid peroxidation in diabetic retinal tissues [174, 175]. Other carotenoids (e.g., lycopene and astaxanthin) have been shown to diminish oxidative stress and apoptosis, triggered by hyperglycemia in retinal tissues [176]. Future studies should determine the optimal dosage and routes of administration for these phytochemicals in the prevention or treatment of age-related eye diseases.
4. Conclusions and Implications for Future Research
In recent years, more attention has been paid to plant extracts used in the treatment of age-related eye diseases. Several polyphenols (such as anthocyanins, Ginkgo biloba, quercetin, and resveratrol) and carotenoids (such as lutein, zeaxanthin, and mezoxanthin) have shown significant preventive and therapeutic benefits against the aforementioned conditions. The involved mechanisms in these findings include mitigating the production of reactive oxygen species, inhibiting the tumor necrosis factor-α and vascular endothelial growth factor pathways, suppressing p53-dependent apoptosis, and suppressing the production of inflammatory markers, such as interleukin- (IL-) 8, IL-6, IL-1a, and endothelial leucocyte adhesion molecule-1.
Moreover, research into the mechanisms by which these compounds act and their role on molecular signaling indicates possible synergic interactions between compounds with complementary antioxidant and anti-inflammatory mechanisms of the two classes. The combination of bacterial carotenoids and some phenolic compounds has led to an increase in antioxidant activity of carotenoids [177]. On the other hand, the combination of lutein with certain polyphenols led to a decrease in absorption of lutein [178]. The combination of antioxidant compounds with complementary action mechanisms could be the key to optimizing the action of phytochemicals with antioxidant effect.
Research on synergistic combinations of carotenoids and polyphenols has shown that in certain combinations, polyphenols and carotenoids can interact synergically in the inhibition of several proinflammatory pathways. It has recently been discovered that these polyphenols of curcumin and carnosic acid produce (synergistically) the inhibitory effect of some carotenoids (lycopene, lutein, and beta-carotene) on the secretion of inflammatory mediators such as NO, TNF-alpha, and PGE2. In addition, synergism is significantly increased when polyphenol (curcumin or carnosic acid) combines with two of the aforementioned carotenoids; this synergistic effect occurs on the binary combinations of carnosic acid or curcumin and lycopene, beta-carotene, or lutein. At the same time, the synergistic anti-inflammatory effect that we are talking about is noticeable when carotenoids are present in combination with other polyphenols (quercetin, resveratrol, and gallic acid) [179, 180].
Consequently, future research should further establish and clarify the precise mechanism of action by which these compounds include extra and intracellular sites under healthy and pathological conditions. Knowing the mechanisms of action would enhance the acceptance and implementation of these compounds for use in clinical practice. Although beneficial effects of plant-based diets or phytochemicals on reducing the risk of chronic diseases have been shown through various epidemiological and in vitro/in vivo studies, much more mechanistic and clinical evidence is required to define a particular phytochemical as an inhibitor of specific cellular pathways or identify the plant-derived active compound with specific therapeutic properties. Moreover, researchers should further investigate the toxicological aspects of these phytochemicals and determine the optimal doses, routes of administration, and exact mechanism of action.
Abbreviations
- AMD:
Age-related macular degeneration
- RBB:
Retinal blood barrier
- DME:
Diabetic macular edema
- DR:
Diabetic retinopathy
- EGCG:
Epigallocatechin gallate
- ERK:
Extracellular signal-regulated kinase
- GB:
Ginkgo biloba
- GSH:
Glutathione
- IL:
Interleukin
- IOP:
Intraocular pressure
- MP:
Macular pigment
- PDR:
Proliferative diabetic retinopathy
- ROS:
Reactive oxygen species
- RPE:
Retinal pigment epithelium
- VEGF:
Vascular endothelial growth factor.
Contributor Information
Mohamed M. Abdel-Daim, Email: abdeldaim.m@vet.suez.edu.eg.
Esraa Ghanem, Email: esraaghanem@azhar.edu.eg.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Simona Bungau, Mohamed M. Abdel-Daim, and Delia Mirela Tit contributed equally to this work.
References
- 1.Rauf A., Imran M., Suleria H. A. R., Ahmad B., Peters D. G., Mubarak M. S. A comprehensive review of the health perspectives of resveratrol. Food & Function. 2017;8(12):4284–4305. doi: 10.1039/c7fo01300k. [DOI] [PubMed] [Google Scholar]
- 2.London D. S., Beezhold B. A phytochemical-rich diet may explain the absence of age- related decline in visual acuity of Amazonian hunter-gatherers in Ecuador. Nutrition Research. 2015;35(2):107–117. doi: 10.1016/j.nutres.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Abu-Amero K. K., Kondkar A. A., Chalam K. V. Resveratrol and ophthalmic diseases. Nutrients. 2016;8(4):p. 200. doi: 10.3390/nu8040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding J., Sullivan D. A. Aging and dry eye disease. Experimental Gerontology. 2012;47(7):483–490. doi: 10.1016/j.exger.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhone M., Basu A. Phytochemicals and age-related eye diseases. Nutrition Reviews. 2008;66(8):465–472. doi: 10.1111/j.1753-4887.2008.00078.x. [DOI] [PubMed] [Google Scholar]
- 6.West A. L., Oren G. A., Moroi S. E. Evidence for the use of nutritional supplements and herbal medicines in common eye diseases. American Journal of Ophthalmology. 2006;141(1):157–166. doi: 10.1016/j.ajo.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 7.Nelson J. L., Bernstein P. S., Schmidt M. C., von Tress M. S., Askew E. W. Dietary modification and moderate antioxidant supplementation differentially affect serum carotenoids, antioxidant levels and markers of oxidative stress in older humans. The Journal of Nutrition. 2003;133(10):3117–3123. doi: 10.1093/jn/133.10.3117. [DOI] [PubMed] [Google Scholar]
- 8.Cho E., Seddon J. M., Rosner B., Willett W. C., Hankinson S. E. Prospective study of intake of fruits, vegetables, vitamins, and carotenoidsand risk of age-related maculopathy. Archives of Ophthalmology. 2004;122(6):883–892. doi: 10.1001/archopht.122.6.883. [DOI] [PubMed] [Google Scholar]
- 9.Tang L., Zhang Y., Jiang Y., et al. Dietary wolfberry ameliorates retinal structure abnormalities in db/db mice at the early stage of diabetes. Experimental Biology and Medicine. 2011;236(9):1051–1063. doi: 10.1258/ebm.2011.010400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milani A., Basirnejad M., Shahbazi S., Bolhassani A. Carotenoids: biochemistry, pharmacology and Treatment. British Journal of Pharmacology. 2017;174(11):1290–1324. doi: 10.1111/bph.13625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Čejková J., Štípek S., Crkovská J., et al. UV rays, the prooxidant/antioxidant imbalance in the cornea and oxidative eye damage. Physiological Research. 2004;53(1):1–10. [PubMed] [Google Scholar]
- 12.Chen J., Patil S., Seal S., McGinnis J. F. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nature Nanotechnology. 2006;1(2):142–150. doi: 10.1038/nnano.2006.91. [DOI] [PubMed] [Google Scholar]
- 13.Kowluru R. A., Kanwar M. Oxidative stress and the development of diabetic retinopathy: contributory role of matrix metalloproteinase-2. Free Radical Biology and Medicine. 2009;46(12):1677–1685. doi: 10.1016/j.freeradbiomed.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de la Camara C. M. F., Salom D., Sequedo M. D., et al. Altered antioxidant-oxidant status in the aqueous humor and peripheral blood of patients with retinitis pigmentosa. PLoS One. 2013;8(9, article e74223) doi: 10.1371/journal.pone.0074223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kowluru R. A., Chan P. S. Oxidative stress and diabetic retinopathy. Experimental Diabetes Research. 2007;2007:12. doi: 10.1155/2007/43603.43603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alderton W. K., Cooper C. E., Knowles R. G. Nitric oxide synthases: structure, function and inhibition. The Biochemical Journal. 2001;357(3):593–615. doi: 10.1042/bj3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abushouk A. I., Ismail A., Salem A. M. A., Afifi A. M., Abdel-Daim M. M. Cardioprotective mechanisms of phytochemicals against doxorubicin-induced cardiotoxicity. Biomedicine and Pharmacotherapy. 2017;90:935–946. doi: 10.1016/j.biopha.2017.04.033. [DOI] [PubMed] [Google Scholar]
- 18.Halliwell B., Gutteridge J. M. C. Free Radicals in Biology and Medicine. 5th. Oxford University Press; 2015. [DOI] [PubMed] [Google Scholar]
- 19.Lightfoot T., Skibola C., Smith A., et al. Polymorphisms in the oxidative stress genes, superoxide dismutase, glutathione peroxidase and catalase and risk of non-Hodgkin’s lymphoma. Haematologica. 2006;91(9):1222–1227. [PubMed] [Google Scholar]
- 20.Halliwell B. Oxidative stress and cancer: have we moved forward? Biochemical Journal. 2007;401(1):1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- 21.Remacle J., Raes M., Toussaint O., Renard P., Rao G. Low levels of reactive oxygen species as modulators of cell function. Mutation Research. 1995;316(3):103–122. doi: 10.1016/0921-8734(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 22.Cabrera M. P., Chihuailaf R. H. Antioxidants and the integrity of ocular tissues. Veterinary Medicine International. 2011;2011:8. doi: 10.4061/2011/905153.905153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carneiro Â., Andrade J. P. Nutritional and lifestyle interventions for age-related macular degeneration: a review. Oxidative Medicine and Cellular Longevity. 2017;2017:13. doi: 10.1155/2017/6469138.6469138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitra R., Conleyand S., Naash M. Therapeutic approach of nanotechnology for oxidative stress induced ocular neurodegenerative diseases. In: Bowes Rickman C., LaVail M., Anderson R., Grimm C., Hollyfield J., Ash J., editors. Retinal Degenerative Diseases. Vol. 854. Cham: Springer; 2016. pp. 463–469. (Advances in Experimental Medicine and Biology). [DOI] [PubMed] [Google Scholar]
- 25.Erdurmus M., Simavli H., Aydın B. Chapter 3 - cataracts: an overview. In: Preedy V. R., editor. Handbook of Nutrition, Diet and the Eye. San Diego, CA, USA: Academic Press; 2014. pp. 21–28. [DOI] [Google Scholar]
- 26.Bhuyan K. C., Bhuyan D. K. Molecular mechanism of cataractogenesis: III. Toxic metabolites of oxygen as initiators of lipid peroxidation and cataract. Current Eye Research. 1984;3(1):67–82. doi: 10.3109/02713688408997188. [DOI] [PubMed] [Google Scholar]
- 27.Spector A. Oxidative stress-induced cataract: mechanism of action. FASEB Journal. 1995;9(12):1173–1182. doi: 10.1096/fasebj.9.12.7672510. [DOI] [PubMed] [Google Scholar]
- 28.Weinreb R. N., Aung T., Medeiros F. A. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sacca S. C., Cartiglia C., Izzotti A. Chapter 4 - glaucoma: an overview. In: Preedy V. R., editor. Handbook of Nutrition, Diet and the Eye. San Diego, CA, USA: Academic Press; 2014. pp. 29–40. [DOI] [Google Scholar]
- 30.Liu Q., Ju W. K., Crowston J. G., et al. Oxidative stress is an early event in hydrostatic pressure–induced retinal ganglion cell damage. Investigative Ophthalmology and Visual Science. 2007;48(10):4580–4589. doi: 10.1167/iovs.07-0170. [DOI] [PubMed] [Google Scholar]
- 31.Sacca S. C., Izzotti A. Oxidative stress and glaucoma: injury in the anterior segment of the eye. Progress in Brain Research. 2008;173:385–407. doi: 10.1016/S0079-6123(08)01127-8. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Riquelme N., Villalba C., Tormo C., et al. Endothelin-1 levels and biomarkers of oxidative stress in glaucoma patients. International Ophthalmology. 2014;35(4):527–532. doi: 10.1007/s10792-014-9979-8. [DOI] [PubMed] [Google Scholar]
- 33.Izzotti A., Bagnis A., Sacca S. C. The role of oxidative stress in glaucoma. Mutation Research/Reviews in Mutation Research. 2006;612(2):105–114. doi: 10.1016/j.mrrev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Head K. A. Natural therapies for ocular disorders, part two: cataracts and glaucoma. Alternative Medicine Review. 2001;6(2):141–166. [PubMed] [Google Scholar]
- 35.Mozaffarieh M., Flammer J. Is there more to glaucoma treatment than lowering IOP? Survey of Ophthalmology. 2007;52(6):S174–S179. doi: 10.1016/j.survophthal.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Calkins D. J. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Progress in Retinal and Eye Research. 2012;31(6):702–719. doi: 10.1016/j.preteyeres.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tham Y. C., Li X., Wong T. Y., Quigley H. A., Aung T., Cheng C. Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 38.Ritch R. Complementary therapy for the treatment of glaucoma: a perspective. Ophthalmology Clinics of North America. 2005;18(4):597–609. doi: 10.1016/j.ohc.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Lopez-Galvez M. I., Lavado F. M., Pastor J. C. Chapter 5 - Diabetic retinopathy: an overview. In: Preedy V. R., editor. Handbook of Nutrition, Diet and the Eye. San Diego, CA, USA: Academic Press; 2014. pp. 41–51. [DOI] [Google Scholar]
- 40.Li C., Miao X., Li F., et al. Oxidative stress-related mechanisms and antioxidant therapy in diabetic retinopathy. Oxidative Medicine and Cellular Longevity. 2017;2017:15. doi: 10.1155/2017/9702820.9702820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frey T., Antonetti D. A. Alterations to the blood-retinal barrier in diabetes: cytokines and reactive oxygen species. Antioxidants and Redox Signaling. 2011;15(5):1271–1284. doi: 10.1089/ars.2011.3906. [DOI] [PubMed] [Google Scholar]
- 42.Datta S., Cano M., Ebrahimi K., Wang L., Handa J. T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Progress in Retinal and Eye Research. 2017;60:201–218. doi: 10.1016/j.preteyeres.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hollyfield J. G., Bonilha V. L., Rayborn M. E., et al. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nature Medicine. 2008;14(2):194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaarniranta K., Salminen A., Eskelinen E.-L., Kopitz J. Heat shock proteins as gatekeepers of proteolytic pathways—implications for age-related macular degeneration (AMD) Ageing Research Reviews. 2009;8(2):128–139. doi: 10.1016/j.arr.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Lu L., Hackett S. F., Mincey A., Lai H., Campochiaro P. A. Effects of different types of oxidative stress in RPE cells. Journal of Cellular Physiology. 2006;206(1):119–125. doi: 10.1002/jcp.20439. [DOI] [PubMed] [Google Scholar]
- 46.Zarbin M. A. Current concepts in the pathogenesis of age-related macular degeneration. Archives of Ophthalmology. 2004;122(4):598–614. doi: 10.1001/archopht.122.4.598. [DOI] [PubMed] [Google Scholar]
- 47.Seen S., Tong L. Dry eye disease and oxidative stress. Acta Ophthalmologica. 2018;96(4):e412–e420. doi: 10.1111/aos.13526. [DOI] [PubMed] [Google Scholar]
- 48.Pflugfelder S. C., de Paiva C. S. Pathophysiology of dry eye disease: what we know and future directions for research. Ophthalmology. 2017;124(11):S4–S13. doi: 10.1016/j.ophtha.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dogru M., Wajamatsu T., Kojima T., et al. The role of oxidative stress and inflammation in dry eye disease. Cornea. 2009;28(Supplement 1):S70–S74. doi: 10.1097/ico.0b013e3181ae8689. [DOI] [Google Scholar]
- 50.Li A.-N., Li S., Zhang Y.-J., Xu X. R., Chen Y. M., Li H. B. Resources and biological activities of natural polyphenols. Nutrients. 2014;6(12):6020–6047. doi: 10.3390/nu6126020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pietta P., Minoggio M., Bramati L. Plant polyphenols: structure, occurrence and bioactivity. Studies in Natural Products Chemistry. 2003;28:257–312. doi: 10.1016/S1572-5995(03)80143-6. [DOI] [Google Scholar]
- 52.Mohammadian A., Moradkhani S., Ataei S., et al. Antioxidative and hepatoprotective effects of hydroalcoholic extract of Artemisia absinthium L. in rat. Journal of Herbmed Pharmacology. 2016;5(1):29–32. [Google Scholar]
- 53.Milenkovic D., Jude B., Morand C. miRNA as molecular target of polyphenols underlying their biological effects. Free Radical Biology and Medicine. 2013;64:40–51. doi: 10.1016/j.freeradbiomed.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 54.Srinivasan K. Chapter 42 - polyphenols in vision and eye health. In: Preedy V. R., editor. Handbook of Nutrition, Diet and the Eye. San Diego, CA, USA: Academic Press; 2014. pp. 413–421. [DOI] [Google Scholar]
- 55.Xu Z., Sun T., Li W., Sun X. Inhibiting effects of dietary polyphenols on chronic eye diseases. Journal of Functional Foods. 2017;39:186–197. doi: 10.1016/j.jff.2017.10.031. [DOI] [Google Scholar]
- 56.Anderson R. E., Rapp L. M., Wiegand R. D. Lipid peroxidation and retinal degeneration. Current Eye Research. 1984;3(1):223–227. doi: 10.3109/02713688408997203. [DOI] [PubMed] [Google Scholar]
- 57.Vallabh N. A., Romano V., Willoughby C. E. Mitochondrial dysfunction and oxidative stress in corneal disease. Mitochondrion. 2017;36:103–113. doi: 10.1016/j.mito.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 58.Zou Y., Chiou G. C. Apigenin inhibits laser-induced choroidal neovascularization and regulates endothelial cell function. Journal of Ocular Pharmacology and Therapeutics. 2006;22(6):425–430. doi: 10.1089/jop.2006.22.425. [DOI] [PubMed] [Google Scholar]
- 59.Gopinath B., Liew G., Kifley A., et al. Dietary flavonoids and the prevalence and 15-y incidence of age-related macular degeneration. The American Journal of Clinical Nutrition. 2018;108(2):381–387. doi: 10.1093/ajcn/nqy114. [DOI] [PubMed] [Google Scholar]
- 60.Lee J. H., Khor T. O., Shu L., Su Z.-Y., Fuentes F., Kong A.-N. T. Dietary phytochemicals and cancer prevention: Nrf 2 signaling, epigenetics, and cell death mechanisms in blocking cancer initiation and progression. Pharmacology and Therapeutics. 2013;137(2):153–171. doi: 10.1016/j.pharmthera.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Na H.-K., Surh Y.-J. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food and Chemical Toxicology. 2008;46(4):1271–1278. doi: 10.1016/j.fct.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 62.Dhakshinamoorthy S., Jaiswal A. K. Small maf (MafG and MafK) proteins negatively regulate antioxidant response element-mediated expression and antioxidant induction of the NAD(P)H:Quinone oxidoreductase1 gene. Journal of Biological Chemistry. 2000;275(51):40134–40141. doi: 10.1074/jbc.M003531200. [DOI] [PubMed] [Google Scholar]
- 63.Shen G., Hebbar V., Nair S., et al. Regulation of Nrf 2 transactivation domain activity: the differential effects of mitogen-activated protein kinase cascades and synergistic stimulatory effect of Raf and CREB-binding protein. Journal of Biological Chemistry. 2004;279(22):23052–23060. doi: 10.1074/jbc.M401368200. [DOI] [PubMed] [Google Scholar]
- 64.Cardozo L. F. M. F., Pedruzzi L. M., Stenvinkel P., et al. Nutritional strategies to modulate inflammation and oxidative stress pathways via activation of the master antioxidant switch Nrf 2. Biochimie. 2013;95(8):1525–1533. doi: 10.1016/j.biochi.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 65.Mishra A., Sharma A. K., Kumar S., Saxena A. K., Pandey A. K. Bauhinia variegata leaf extracts exhibit considerable antibacterial, antioxidant, and anticancer activities. BioMed Research International. 2013;2013:10. doi: 10.1155/2013/915436.915436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumar S., Sharma U. K., Sharma A. K., Pandey A. K. Protective efficacy of Solanum xanthocarpum root extracts against free radical damage: phytochemical analysis and antioxidant effect. Cellular and Molecular Biology. 2012;58(1):174–181. [PubMed] [Google Scholar]
- 67.Upadhyay S., Dixit M. Role of polyphenols and other phytochemicals on molecular signaling. Oxidative Medicine and Cellular Longevity. 2015;2015:15. doi: 10.1155/2015/504253.504253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mishra A., Kumar S., Pandey A. K. Scientific validation of the medicinal efficacy of Tinospora cordifolia. The Scientific World Journal. 2013;2013:8. doi: 10.1155/2013/292934.292934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bola C., Bartlett H., Eperjesi F. Resveratrol and the eye: activity and molecular mechanisms. Graefe's Archive for Clinical and Experimental Ophthalmology. 2014;252(5):699–713. doi: 10.1007/s00417-014-2604-8. [DOI] [PubMed] [Google Scholar]
- 70.Sulaiman R. S., Basavarajappa H. D., Corson T. W. Natural product inhibitors of ocular angiogenesis. Experimental Eye Research. 2014;129:161–171. doi: 10.1016/j.exer.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kumar B., Gupta S. K., Nag T. C., et al. Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats. Experimental Eye Research. 2014;125:193–202. doi: 10.1016/j.exer.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 72.Wallace T. C. Anthocyanins in cardiovascular disease. Advances in Nutrition. 2011;2(1):1–7. doi: 10.3945/an.110.000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wallace T. C., Slavin M., Frankenfeld C. L. Systematic review of anthocyanins and markers of cardiovascular disease. Nutrients. 2016;8(1):p. 32. doi: 10.3390/nu8010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee J., Lee H. K., Kim C. Y., et al. Purified high-dose anthocyanoside oligomer administration improves nocturnal vision and clinical symptoms in myopia subjects. British Journal of Nutrition. 2005;93(06):895–899. doi: 10.1079/BJN20051438. [DOI] [PubMed] [Google Scholar]
- 75.Sole P., Rigal D., Peyresblanques J. Effects of cyaninoside chloride and Heleniene on mesopic and scotopic vision in myopia and night blindness. Journal Francais d’ Ophtalmologie. 1984;7:35–39. [PubMed] [Google Scholar]
- 76.Levy Y., Glovinsky Y. The effect of anthocyanosides on night vision. Eye. 1998;12(6):967–969. doi: 10.1038/eye.1998.250. [DOI] [PubMed] [Google Scholar]
- 77.Zadok D., Levy Y., Glovinsky Y. The effect of anthocyanosides in a multiple oral dose on night vision. Eye. 1999;13(6):734–736. doi: 10.1038/eye.1999.218. [DOI] [PubMed] [Google Scholar]
- 78.Muth E. R., Laurent H. M., Jasper P. The effect of bilberry nutritional supplementation on high visual acuity and contrast sensitivity. Alternative Medicine Review. 2000;5(2):164–173. [PubMed] [Google Scholar]
- 79.Morimitsu Y., Kubota K., Tashiro T., Hashizume E., Kamiya T., Osawa T. Inhibitory effect of anthocyanins and colored rice on diabetic cataract formation in the rat lenses. International Congress Series. 2002;1245:503–508. doi: 10.1016/S0531-5131(02)00919-6. [DOI] [Google Scholar]
- 80.Jang Y., Zhou J., Nakanishi K., Sparrow J. R. Anthocyanins protect against A2E photooxidation and membrane permeabilization in retinal pigment epithelial cells. Photochemistry and Photobiology. 2005;81(3):529–536. doi: 10.1562/2004-12-14-RA-402.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Milbury P. E., Graf B., Curran-Celentano J. M., Blumberg J. B. Bilberry (Vaccinium myrtillus) anthocyanins modulate heme oxygenase-1 and glutathione S transferase expression in ARPE-19 cells. Investigative Ophthalmology and Visual Science. 2007;48(5):2343–2349. doi: 10.1167/iovs.06-0452. [DOI] [PubMed] [Google Scholar]
- 82.Kalt W., Blumberg J. B., McDonald J. E., et al. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. Journal of Agricultural and Food Chemistry. 2008;56(3):705–712. doi: 10.1021/jf071998l. [DOI] [PubMed] [Google Scholar]
- 83.Song Y., Huang L., Yu J. Effects of blueberry anthocyanins on retinal oxidative stress and inflammation in diabetes through Nrf 2/HO-1 signaling. Journal of Neuroimmunology. 2016;301:1–6. doi: 10.1016/j.jneuroim.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 84.Kim J., Kim C. S., Lee Y. M., Sohn E., Jo K., Kim J. S. Vaccinium myrtillus extract prevents or delays the onset of diabetes-induced blood-retinal barrier breakdown. International Journal of Food Science and Nutrition. 2015;66(2):236–242. doi: 10.3109/09637486.2014.979319. [DOI] [PubMed] [Google Scholar]
- 85.Paik S. S., Jeong E., Jung S. W., et al. Anthocyanins from the seed coat of black soybean reduce retinal degeneration induced by N-methyl-N-nitrosourea. Experimental Eye Research. 2012;97(1):55–62. doi: 10.1016/j.exer.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 86.Tanaka J., Kadekaru T., Ogawa K., Hitoe S., Shimoda H., Hara H. Maqui berry (Aristotelia chilensis) and the constituent delphinidin glycoside inhibit photoreceptor cell death induced by visible light. Food Chemistry. 2013;139(1–4):129–137. doi: 10.1016/j.foodchem.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 87.Szumny A. Application of cornelian cherry iridoid-polyphenolic fraction and loganic acid to reduce intraocular pressure. Evidence-Based Complementary and Alternative Medicine. 2015;2015:8. doi: 10.1155/2015/939402.939402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tremblay F., Waterhouse J., Nason J., Kalt W. Prophylactic neuroprotection by blueberry- enriched diet in a rat model of light-induced retinopathy. The Journal of Nutritional Biochemistry. 2013;24(4):647–655. doi: 10.1016/j.jnutbio.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 89.Oliveira M. R., Nabavi S. F., Daglia M., Rastrelli L., Nabavi S. M. Epigallocatechin gallate and mitochondria—a story of life and death. Pharmacological Research. 2016;104:70–85. doi: 10.1016/j.phrs.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 90.Wu C. C., Hsu M. C., Hsieh C. W., Lin J. B., Lai P. H., Wung B. S. Upregulation of heme oxygenase-1 by epigallocatechin-3-gallate via the phosphatidylinositol 3-kinase/Akt and ERK pathways. Life Sciences. 2006;78(25):2889–2897. doi: 10.1016/j.lfs.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 91.Gupta S. K., Halder N., Srivastava S., Trivedi D., Joshi S., Varma S. D. Green tea (Camellia sinensis) protects against selenite induced oxidative stress in experimental cataractogenesis. Ophthalmic Research. 2002;34(4):258–263. doi: 10.1159/000063881. [DOI] [PubMed] [Google Scholar]
- 92.Yao K., Ye P., Zhang L., Tan J., Tang X., Zhang Y. Epigallocatechin gallate protects against oxidative stress-induced mitochondria-dependent apoptosis in human lens epithelial cells. Molecular Vision. 2008;14:217–223. [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang B., Safa R., Rusciano D., Osborne N. N. Epigallocatechin gallate, an active ingredient from green tea, attenuates damaging influences to the retina caused by ischemia/reperfusion. Brain Research. 2007;1159:40–53. doi: 10.1016/j.brainres.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 94.Falsini B., Marangoni D., Salgarello T., et al. Effect of epigallocatechin-gallate on inner retinal function in ocular hypertension and glaucoma: a short-term study by pattern electroretinogram. Graefe's Archive for Clinical and Experimental Ophthalmology. 2009;247(9):1223–1233. doi: 10.1007/s00417-009-1064-z. [DOI] [PubMed] [Google Scholar]
- 95.Lee H. S., Jun J. H., Jung E. H., Koo B., Kim Y. Epigalloccatechin-3-gallate inhibits ocular neovascularization and vascular permeability in human retinal pigment epithelial and human retinal microvascular endothelial cells via suppression of MMP-9 and VEGF activation. Molecules. 2014;19(8):12150–12172. doi: 10.3390/molecules190812150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang L., Zhang Z. K., Liang S. Epigallocatechin-3-gallate protects retinal vascular endothelial cells from high glucose stress in vitro via the MAPK/ERK-VEGF pathway. Genetics and Molecular Research. 2016;15(2) doi: 10.4238/gmr.15027874. [DOI] [PubMed] [Google Scholar]
- 97.Kumar B., Gupta S. K., Nag T. C., Srivastava S., Saxena R. Green tea prevents hyperglycemia-induced retinal oxidative stress and inflammation in streptozotocin-induced diabetic rats. Ophthalmic Research. 2012;47(2):103–107. doi: 10.1159/000330051. [DOI] [PubMed] [Google Scholar]
- 98.Tseng C. L., Hung Y. J., Chen Z. Y., Fang H. W., Chen K. H. Synergistic effect of artificial tears containing epigallocatechin gallate and hyaluronic acid for the treatment of rabbits with dry eye syndrome. PLoS One. 2016;11(6, article e0157982) doi: 10.1371/journal.pone.0157982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ritch R. Potential role for Ginkgo biloba extract in the treatment of glaucoma. Medicine Hypotheses. 2000;54(2):221–235. doi: 10.1054/mehy.1999.0025. [DOI] [PubMed] [Google Scholar]
- 100.Jia L., Sun L., Fan D. S. P., Lam D. S. C., Pang C. P., Yam G. H. F. Effect of topical Ginkgo biloba extract on steroid-induced changes in the trabecular meshwork and intraocular pressure. Archives of Ophthalmology. 2008;126(12):1700–1706. doi: 10.1001/archophthalmol.2008.512. [DOI] [PubMed] [Google Scholar]
- 101.Quaranta L., Riva I., Floriani I. Ginkgo biloba extract improves visual fi eld damage in some patients affected by normal-tension glaucoma. Investigative Ophthalmology and Visual Science. 2014;55(4, article 2417) doi: 10.1167/iovs.14-13942. [DOI] [PubMed] [Google Scholar]
- 102.Shim S. H., Kim J. M., Choi C. Y., Kim C. Y., Park K. H. Ginkgo biloba extract and bilberry anthocyanins improve visual function in patients with normal tension glaucoma. Journal of Medicinal Food. 2012;15(9):818–823. doi: 10.1089/jmf.2012.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dewi Sari M., Sihotang D. A., Lelo A. Ginkgo biloba extract effect on oxidative stress marker malonildialdehyde, redox enzyme glutathione peroxidase, visual field damage, and retinal nerve fiber layer thickness in primary open angle glaucoma. International Journal of PharmTech Research. 2016;9(3):158–166. [Google Scholar]
- 104.Guo X., Kong X., Huang R. Effect of Ginkgo biloba on visual field and contrast sensitivity in Chinese patients with normal tension glaucoma: a randomized, crossover clinical trial. Investigative Ophthalmology and Visual Science. 2014;55(1):p. 110. doi: 10.1167/iovs.13-13168. [DOI] [PubMed] [Google Scholar]
- 105.Lee J., Sohn S. W., Kee C. Effect of ginkgo biloba extract on visual field progression in normal tension glaucoma. Journal of Glaucoma. 2013;22(9):780–784. doi: 10.1097/IJG.0b013e3182595075. [DOI] [PubMed] [Google Scholar]
- 106.Park J. W., Kwon H. J., Chung W. S., Kim C. Y., Seong G. J. Short-term effects of ginkgo biloba extract on peripapillary retinal blood flow in normal tension glaucoma. Korean Journal of Ophthalmology. 2011;25(5):323–328. doi: 10.3341/kjo.2011.25.5.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Quaranta L., Bettelli S., Uva M. G., Semeraro F., Turano R., Gandolfo E. Effect of Ginkgo biloba extract on preexisting visual field damage in normal tension glaucoma. Ophthalmology. 2003;110(2):359–362. doi: 10.1016/S0161-6420(02)01745-1. [DOI] [PubMed] [Google Scholar]
- 108.Evans J. R. Ginkgo biloba extract for age-related macular degeneration. Cochrane Database of Systematic Review. 2000;1, article 23440785 doi: 10.1002/14651858.CD001775. [DOI] [PubMed] [Google Scholar]
- 109.Wang Z. Y., Mo X. F., Jiang X. H., Rong X. F., Miao H. M. Ginkgolide B promotes axonal growth of retina ganglion cells by anti-apoptosis in vitro. Sheng Li Xue Bao. 2012;64(4):417–424. [PubMed] [Google Scholar]
- 110.Sanderson J., McLauchlan W. R., Williamson G. Quercetin inhibits hydrogen peroxide- induced oxidation of the rat lens. Free Radical Biology and Medicine. 1999;26(5-6):639–645. doi: 10.1016/S0891-5849(98)00262-7. [DOI] [PubMed] [Google Scholar]
- 111.Kyselova Z. The nutraceutical potential of natural products in diabetic cataract prevention. Journal of Food and Nutrition Research. 2012;51(4):185–200. [Google Scholar]
- 112.Ola M. S., Ahmed M. M., Shams S., Al-Rejaie S. S. Neuroprotective effects of quercetin in diabetic rat retina. Saudi Journal of Biological Sciences. 2017;24(6):1186–1194. doi: 10.1016/j.sjbs.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kook D., Wolf A. H., Yu A. L., et al. The protective effect of quercetin against oxidative stress in the human RPE in vitro. Investigative Ophthalmology and Visual Science. 2008;49(4):1712–1720. doi: 10.1167/iovs.07-0477. [DOI] [PubMed] [Google Scholar]
- 114.Cao X., Liu M., Tuo J., Shen D., Chan C. C. The effects of quercetin in cultured human RPE cells under oxidative stress and in Ccl 2/Cx3cr1 double deficient mice. Experimental Eye Research. 2010;91(1):15–25. doi: 10.1016/j.exer.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Y., Zhao L., Wang C., et al. Protective effect of quercetin and chlorogenic acid, two polyphenols widely present in edible plant varieties, on visible light-induced retinal degeneration in vivo. Journal of Functional Foods. 2017;33:103–111. doi: 10.1016/j.jff.2017.02.034. [DOI] [Google Scholar]
- 116.Abengózar-Vela A., Schaumburg C. S., Stern M. E., Calonge M., Enríquez-de-Salamanca A., González-García M. J. Topical quercetin and resveratrol protect the ocular surface in experimental dry eye disease. Ocular Immunology and Inflammation. 2018;2018:1–10. doi: 10.1080/09273948.2018.1497664. [DOI] [PubMed] [Google Scholar]
- 117.Doganay S., Borazan M., Iraz M., Cigremis Y. The effect of resveratrol in experimental cataract model formed by sodium selenite. Current Eye Research. 2006;31(2):147–153. doi: 10.1080/02713680500514685. [DOI] [PubMed] [Google Scholar]
- 118.Zheng T., Lu Y. SIRT1 protects human lens epithelial cells against oxidative stress by inhibiting p53-dependent apoptosis. Current Eye Research. 2016;41(8):1068–1075. doi: 10.3109/02713683.2015.1093641. [DOI] [PubMed] [Google Scholar]
- 119.Luna C., Li G., Liton P. B., et al. Resveratrol prevents the expression of glaucoma markers induced by chronic oxidative stress in trabecular meshwork cells. Food and Chemical Toxicology. 2009;47(1):198–204. doi: 10.1016/j.fct.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Soufi F. G., Ad-Nejad D. M., Ahmadieh H. Resveratrol improves diabetic retinopathy possibly through oxidative stress – nuclear factor κB – apoptosis pathway. Pharmacological Reports. 2012;64(6):1505–1514. doi: 10.1016/S1734-1140(12)70948-9. [DOI] [PubMed] [Google Scholar]
- 121.Kim Y. H., Kim Y. S., Roh G. S., Choi W. S., Cho G. J. Resveratrol blocks diabetes-induced early vascular lesions and vascular endothelial growth factor induction in mouse retinas. Acta Ophthalmologica. 2012;90(1):e31–e37. doi: 10.1111/j.1755-3768.2011.02243.x. [DOI] [PubMed] [Google Scholar]
- 122.Yar A. S., Menevse S., Dogan I., et al. Investigation of ocular neovascularization–related genes and oxidative stress in diabetic rat eye tissues after resveratrol treatment. Journal of Medicinal Food. 2012;15(4):391–398. doi: 10.1089/jmf.2011.0135. [DOI] [PubMed] [Google Scholar]
- 123.Ghadiri Soufi F., Arbabi-Aval E., Kanavi M. R., Ahmadieh H. Anti-inflammatory properties of resveratrol in the retinas of type 2 diabetic rats. Clinical and Experimental Pharmacology and Physiology. 2015;42(1):63–68. doi: 10.1111/1440-1681.12326. [DOI] [PubMed] [Google Scholar]
- 124.Zeng K., Yang N., Wang D., et al. Resveratrol prevents retinal dysfunction by regulating glutamate transporters, glutamine synthetase expression and activity in diabetic retina. Neurochemical Research. 2016;41(5):1050–1064. doi: 10.1007/s11064-015-1793-9. [DOI] [PubMed] [Google Scholar]
- 125.Richer S., Patel S., Sockanathan S., Ulanski L., II, Miller L., Podella C. Resveratrol based oral nutritional supplement produces long-term beneficial effects on structure and visual function in human patients. Nutrients. 2014;6(10):4404–4420. doi: 10.3390/nu6104404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jomova K., Valko M. Health protective effects of carotenoids and their interactions with other biological antioxidants. European Journal Medicinal Chemistry. 2013;70:102–110. doi: 10.1016/j.ejmech.2013.09.054. [DOI] [PubMed] [Google Scholar]
- 127.Rutz J. K., Borges C. D., Zambiazi R. C., da Rosa C. G., da Silva M. M. Elaboration of microparticles of carotenoids from natural and synthetic sources for applications in food. Food Chemistry. 2016;202:324–333. doi: 10.1016/j.foodchem.2016.01.140. [DOI] [PubMed] [Google Scholar]
- 128.Tapiero H., Townsend D. M., Tew K. D. The role of carotenoids in the prevention of human pathologies. Biomedicine and Pharmacotherapy. 2004;58(2):100–110. doi: 10.1016/j.biopha.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.El-Agamey G., Lowe M., McGarvey D. J., et al. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Archives of Biochemistry and Biophysics. 2004;430(1):37–48. doi: 10.1016/j.abb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 130.Nussbaum J. J., Pruett R. C., Delori F. C. Historic perspectives. Macular yellow pigment. The first 200 years. Retina. 1981;1(4):296–310. doi: 10.1097/00006982-198101040-00007. [DOI] [PubMed] [Google Scholar]
- 131.Bone R. A., Landrum J. T., Cao Y., Howard A. N., Alvarez-Calderon F. Macular pigment response to a supplement containing meso-zeaxanthin, lutein and zeaxanthin. Nutrition & Metabolism. 2007;4(1):p. 12. doi: 10.1186/1743-7075-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bone R. A., Landrum J. T., Friedes L. M., et al. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Experimental Eye Research. 1997;64(2):211–218. doi: 10.1006/exer.1996.0210. [DOI] [PubMed] [Google Scholar]
- 133.Krinsky N. I., Landrum J. T., Bone R. A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annual Review of Nutrition. 2003;23(1):171–201. doi: 10.1146/annurev.nutr.23.011702.073307. [DOI] [PubMed] [Google Scholar]
- 134.Landrum J. T., Bone R. A. Lutein, zeaxanthin, and the macular pigment. Archives of Biochemistry and Biophysics. 2001;385(1):28–40. doi: 10.1006/abbi.2000.2171. [DOI] [PubMed] [Google Scholar]
- 135.Thurnham D. I., Nolan J. M., Howard A. N., Beatty S. Macular response to supplementation with differing xanthophyll formulations in subjects with and without age-related macular degeneration. Graefe's Archive for Clinical and Experimental Ophthalmology. 2015;253(8):1231–1243. doi: 10.1007/s00417-014-2811-3. [DOI] [PubMed] [Google Scholar]
- 136.Bernstein P. S., Li B., Vachali P. P., et al. Lutein, zeaxanthin, and meso-zeaxanthin: the basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Progress in Retinal and Eye Research. 2016;50:34–66. doi: 10.1016/j.preteyeres.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Johnson E. J., Neuringer M., Russell R. M., Schalch W., Snodderly D. M. Nutritional manipulation of primate retinas, III: effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Investigative Ophthalmology and Visual Science. 2005;46(2):692–702. doi: 10.1167/iovs.02-1192. [DOI] [PubMed] [Google Scholar]
- 138.Bhosale P., Serban B., Zhao D. Y., Bernstein P. S. Identification and metabolic transformations of carotenoids in ocular tissues of the Japanese quail Coturnix japonica. Biochemistry. 2007;46(31):9050–9057. doi: 10.1021/bi700558f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Khachik F., de Moura F. F., Zhao D. Y., Aebischer C. P., Bernstein P. S. Transformations of selected carotenoids in plasma, liver, and ocular tissues of humans and in nonprimate animal models. Investigative Ophthalmology and Visual Science. 2002;43(11):3383–3392. [PubMed] [Google Scholar]
- 140.Stahl W., Nicolai S., Briviba K., et al. Biological activities of natural and synthetic carotenoids: induction of gap junctional communication and singlet oxygen quenching. Carcinogenesis. 1997;18(1):89–92. doi: 10.1093/carcin/18.1.89. [DOI] [PubMed] [Google Scholar]
- 141.Krinsky N. I. Antioxidant functions of carotenoids. Free Radical Biology and Medicine. 1989;7(6):617–635. doi: 10.1016/0891-5849(89)90143-3. [DOI] [PubMed] [Google Scholar]
- 142.Krinsky N. I. Possible biologic mechanisms for a protective role of xanthophylls. Journal of Nutrition. 2002;132(3):540S–542S. doi: 10.1093/jn/132.3.540S. [DOI] [PubMed] [Google Scholar]
- 143.Bhosale P., Serban B., Bernstein P. S. Retinal carotenoids can attenuate formation of A2E in the retinal pigment epithelium. Archives of Biochemistry and Biophysics. 2009;483(2):175–181. doi: 10.1016/j.abb.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bernstein P. S., Khachik F., Carvalho L. S., Muir G. J., Zhao D. Y., Katz N. B. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Experimental Eye Research. 2001;72(3):215–223. doi: 10.1006/exer.2000.0954. [DOI] [PubMed] [Google Scholar]
- 145.Sparrow J. R., Nakanishi K., Parish C. A. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Investigative Ophthalmology and Visual Science. 2000;41(7):1981–1989. [PubMed] [Google Scholar]
- 146.Sparrow J. R., Cai B. Blue light-induced apoptosis of A2E-containing RPE: involvement of caspase-3 and protection by Bcl-2. Investigative ophthalmology & visual science. 2001;42(6):1356–1362. [PubMed] [Google Scholar]
- 147.Suter M., Reme C., Grimm C., et al. Age-related macular degeneration. The lipofusion component N-retinyl-N-retinylidene ethanolamine detaches proapoptotic proteins from mitochondria and induces apoptosis in mammalian retinal pigment epithelial cells. Journal of Biology Chemistry. 2000;275(50):39625–39630. doi: 10.1074/jbc.M007049200. [DOI] [PubMed] [Google Scholar]
- 148.Sundelin S. P., Nilsson S. E. Lipofuscin-formation in retinal pigment epithelial cells is reduced by antioxidants. Free Radical Biology and Medicine. 2001;31(2):217–225. doi: 10.1016/S0891-5849(01)00573-1. [DOI] [PubMed] [Google Scholar]
- 149.Madaan T., Choudhary A. N., Gyenwalee S., et al. Lutein, a versatile phyto-nutraceutical: an insight on pharmacology, therapeutic indications, challenges and recent advances in drug delivery. PharmaNutrition. 2017;5(2):64–75. doi: 10.1016/j.phanu.2017.02.005. [DOI] [Google Scholar]
- 150.Aleman T. S., Duncan J. L., Bieber M. L., et al. Macular pigment and lutein supplementation in retinitis pigmentosa and usher syndrome. Investigative Ophthalmology and Visual Science. 2001;42(8):1873–1881. [PubMed] [Google Scholar]
- 151.Akuffo K. O., Nolan J. M., Peto T., et al. Relationship between macular pigment and visual function in subjects with early age-related macular degeneration. British Journal of Ophthalmology. 2016;101(2):190–197. doi: 10.1136/bjophthalmol-2016-308418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.The Age-Related Eye Disease Study 2 (AREDS2) Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309(19):2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 153.Seddon J. M., Ajani U. A., Sperduto R. D., et al. Dietary carotenoids, vitamin A, C and E, and advanced age-related macular degeneration. Eye disease case-control study group. JAMA. 1994;272(18):1413–1420. doi: 10.1001/jama.1994.03520180037032. [DOI] [PubMed] [Google Scholar]
- 154.Alves-Rodrigues A., Shao A. The science behind lutein. Toxicology Letters. 2004;150(1):57–83. doi: 10.1016/j.toxlet.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 155.Chasan-Taber L., Willett W. C., Seddon J. M., et al. A prospective study of carotenoid and vitamin A intakes and risk of cataract extraction in US women. The American Journal of Clinical Nutrition. 1999;70(4):509–516. doi: 10.1093/ajcn/70.4.509. [DOI] [PubMed] [Google Scholar]
- 156.Brown L., Rimm E. B., Seddon J. M., et al. A prospective study of carotenoid intake and risk of cataract extraction in US men. The American Journal of Clinical Nutrition. 1999;70(4):517–524. doi: 10.1093/ajcn/70.4.517. [DOI] [PubMed] [Google Scholar]
- 157.Lyle B. J., Mares-Perlman J. A., Klein B. E., Klein R., Greger J. L. Antioxidant intake and risk of incident age-related nuclear cataracts in the Beaver Dam Eye Study. American Journal of Epidemiology. 1999;149(9):801–809. doi: 10.1093/oxfordjournals.aje.a009895. [DOI] [PubMed] [Google Scholar]
- 158.Jacques P. F., Chylack L. T., Jr., Hankinson S. E., et al. Long-term nutrient intake and early age-related nuclear lens opacities. Archives of Ophthalmology. 2001;119(7):1009–1019. doi: 10.1001/archopht.119.7.1009. [DOI] [PubMed] [Google Scholar]
- 159.Valero M. P., Fletcher A. E., De Stavola B. L., Vioque J., Alepuz V. C. Vitamin C is associated with reduced risk of cataract in a Mediterranean population. The Journal of Nutrition. 2002;132(6):1299–1306. doi: 10.1093/jn/132.6.1299. [DOI] [PubMed] [Google Scholar]
- 160.Olmedilla B., Granado F., Blanco L., et al. Serum status of carotenoids and tocopherols in patients with age-related cataracts: a case-control study. The Journal of Nutrition Health and Aging. 2002;6:66–68. [PubMed] [Google Scholar]
- 161.Ma L., Dou H. L., Wu Y. Q., et al. Lutein and zeaxanthin intake and the risk of age-related macular degeneration: a systematic review and meta-analysis. British Journal of Nutrition. 2012;107(3):350–359. doi: 10.1017/S0007114511004260. [DOI] [PubMed] [Google Scholar]
- 162.Beatty S., Murray I. J., Henson D. B., Carden D., Koh H., Boulton M. E. Macular pigment and risk for age-related macular degeneration in subjects from a northern European population. Investigative Ophthalmology and Visual Science. 2001;42(2):439–446. [PubMed] [Google Scholar]
- 163.Bone R. A., Landrum J. T., Mayne S. T., Gomez C. M., Tibor S. E., Twaroska E. E. Macular pigment in donor eyes with and without AMD: a case-control study. Investigative Ophthalmology and Visual Science. 2001;42(1):235–240. [PubMed] [Google Scholar]
- 164.Bernstein P. S., Zhao D.-Y., Wintch S. W., Ermakov I. V., McClane R. W., Gellermann W. Resonance Raman measurement of macular carotenoids in normal subjects and in age-related macular degeneration patients. Ophthalmology. 2002;109(10):1780–1787. doi: 10.1016/S0161-6420(02)01173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Seddon J. M., Ajani U. A., Sperduto R. D., et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration: eye disease case-control study group. JAMA. 1994;272(18):1413–1420. doi: 10.1001/jama.1994.03520180037032. [DOI] [PubMed] [Google Scholar]
- 166.Snellen E. L., Verbeek A. L., Van Den Hoogen G. W., Cruysberg J. R., Hoyng C. B. Neovascular age-related macular degeneration and its relationship to antioxidant intake. Acta Ophthalmology Scandinavica. 2002;80(4):368–371. doi: 10.1034/j.1600-0420.2002.800404.x. [DOI] [PubMed] [Google Scholar]
- 167.Gale C. R., Hall N. F., Phillips D. I., Martyn C. N. Lutein and zeaxanthin status and risk of age-related macular degeneration. Investigative Ophthalmology & Visual Science. 2003;44(6):2461–2465. doi: 10.1167/iovs.02-0929. [DOI] [PubMed] [Google Scholar]
- 168.Corvi F., Souied E. H., Falfoul Y., et al. Pilot evaluation of short-term changes in macular pigment and retinal sensitivity in different phenotypes of early age-related macular degeneration after carotenoid supplementation. British Journal of Ophthalmology. 2016;101(6):770–773. doi: 10.1136/bjophthalmol-2016-309115. [DOI] [PubMed] [Google Scholar]
- 169.Ma L., Liu R., Du J. H., Liu T., Wu S., Liu X. Lutein, zeaxanthin and meso-zeaxanthin supplementation associated with macular pigment optical density. Nutrients. 2016;8(7):p. 426. doi: 10.3390/nu8070426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mares Perlman J. A., Fisher A. I., Klein R., et al. Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the third national health and nutrition examination survey. American Journal of Epidemiology. 2001;153(5):424–432. doi: 10.1093/aje/153.5.424. [DOI] [PubMed] [Google Scholar]
- 171.Flood V., Smith W., Wang J. J., Manzi F., Webb K., Mitchell P. Dietary antioxidant intake and incidence of early age-related maculopathy: the Blue Mountains Eye Study. Ophthalmology. 2002;109(12):2272–2278. doi: 10.1016/S0161-6420(02)01263-0. [DOI] [PubMed] [Google Scholar]
- 172.Brazionis L., Rowley K., Itsiopoulos C., O’Dea K. Plasma carotenoids and diabetic retinopathy. British Journal of Nutrition. 2009;101(2):270–277. doi: 10.1017/S0007114508006545. [DOI] [PubMed] [Google Scholar]
- 173.Kowluru R. A., Menon B., Gierhart D. L. Beneficial effect of zeaxanthin on retinal metabolic abnormalities in diabetic rats. Investigative Ophthalmology and Visual Science. 2008;49(4):1645–1651. doi: 10.1167/iovs.07-0764. [DOI] [PubMed] [Google Scholar]
- 174.Arnal E., Miranda M., Almansa I., et al. Lutein prevents cataract development and progression in diabetic rats. Graefe's Archive for Clinical and Experimental Ophthalmology. 2009;247(1):115–120. doi: 10.1007/s00417-008-0935-z. [DOI] [PubMed] [Google Scholar]
- 175.Arnal E., Miranda M., Johnsen-Soriano S., et al. Beneficial effect of docosahexanoic acid and lutein on retinal structural, metabolic, and functional abnormalities in diabetic rats. Current Eye Research. 2009;34(11):928–938. doi: 10.3109/02713680903205238. [DOI] [PubMed] [Google Scholar]
- 176.Dong L.-Y., Jin J., Lu G., Kang X.-L. Astaxanthin attenuates the apoptosis of retinal ganglion cells in db/db mice by inhibition of oxidative stress. Marine Drugs. 2013;11(12):960–974. doi: 10.3390/md11030960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sy C., Dangles O., Borel P., Caris-Veyrat C. Interactions between carotenoids from marine bacteria and other micronutrients: impact on stability and antioxidant activity. Marine Drugs. 2015;13(11):7020–7039. doi: 10.3390/md13117020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Reboul E., Thap S., Tourniaire F., et al. Differential effect of dietary antioxidant classes (carotenoids, polyphenols, vitamins C and E) on lutein absorption. British Journal of Nutrition. 2007;97(03):440–446. doi: 10.1017/S0007114507352604. [DOI] [PubMed] [Google Scholar]
- 179.Zelkha M., Levy R., Paran E., Sharoni Y., Levy J. Synergistic combinations of carotenoids and polyphenols. US Patent 2017/0035713 A1, 2017.
- 180.Mirela D. Research Notes Regarding Analytical and Pharmacological Approach of Some Drug and Phytochemicals, [Ph. D. Thesis] University of Oradea; 2018. https://www.uoradea.ro/display17733. [Google Scholar]