Abstract
OBJECTIVES
Previous work suggests that aortic root and valve prostheses alter blood flow patterns in the ascending aorta, creating aberrant haemodynamics compared with those of healthy volunteers. Various valve designs have been proposed to better restore physiological haemodynamics. In this study, magnetic resonance imaging (MRI) was used to non-invasively assess three-dimensional (3D) ascending aortic haemodynamics after aortic root replacement (ARR) with a mechanical valved conduit postulated to create less turbulent blood flow.
METHODS
Ten patients (40 ± 9 years) underwent transthoracic echocardiography and contrast-enhanced multidimensional four-dimensional (4D) flow MRI at 1.5 T after ARR with an On-X mechanical valved conduit. Preoperative 4D flow MRI was available in 7 patients. Ten age- and gender-matched healthy volunteers (42 ± 13 years) were also analysed to characterize physiological flow. The presence of vortex/helix formation was graded by two blinded observers. Peak transvalvular pressure gradients were computed using the simplified Bernoulli equation. Patients' postoperative pressure gradients and helicity/vorticity grades were compared with preoperative gradients and those from healthy volunteers.
RESULTS
Intra- and interobserver ratings showed good agreement (κ = 0.93, P < 0.01 and κ = 0.84, P < 0.01, respectively). Highly helical and/or vortical flow was observed in all patients preoperatively, which was significantly reduced postoperatively (P < 0.01 and <0.01, respectively), restoring similar flow patterns similar to those seen in volunteers (P = 0.56 and 0.56). Peak transvalvular pressure gradients (ΔP) were also significantly reduced [43 ± 21 vs 12 ± 7 mmHg, P < 0.05 (Echo); 48 ± 22 vs 16 ± 9 mmHg, P < 0.05 (MRI)], but remained significantly higher than those of volunteers (6 ± 1 mmHg, P < 0.01).
CONCLUSIONS
Preliminary evidence suggests that ARR with an On-X mechanical valve significantly reduces aberrant aortic haemodynamics, producing flow patterns that resemble those in healthy volunteers.
Keywords: Aortic valve replacement, Mechanical heart valve prosthesis, Magnetic resonance imaging
INTRODUCTION
Previous work has suggested that both aortic root geometry and valve mechanics can affect flow patterns in the ascending aorta. Less compliant synthetic root material may lead to increased systolic peak velocities by eliminating the normal physiological Windkessel effect [1], and mechanical and bioprosthetic valves can create highly erratic flow patterns and asymmetric areas of wall shear stress (WSS) in the ascending aorta [2, 3]. Although the clinical implications of such flow patterns are unknown, research continues to suggest that abnormal mechanical stress on vessel walls can increase atherogenesis, wall degeneration and remodelling underlying common vascular pathologies [4]. In cases of aortic root replacement (ARR), the graft material is not susceptible to these effects; however, these potential risks do apply to the downstream native tissue and anastomosis regions.
Since the development of the first valve prostheses, a wide array of valve designs have been engineered to improve patients' outcomes. On-X mechanical valves have recently been shown to provide lower anticoagulation targets [5] and transvalvular pressure gradients postoperatively [6]. The producer of these mechanical valves has also claimed that their unique design features, such as a flared inlet, may restore more physiological aortic haemodynamics. However, three-dimensional (3D) visualization of pre- and postoperative flow patterns have been performed for various mechanical prostheses, stented/stentless bioprostheses and autografts, showing highly aberrant postoperative aortic haemodynamics in all cases [3, 7]. On-X valves have been shown to create a strong central flow and low turbulence [8–10], but no analysis of pre- and postoperative 3D flow patterns for these valves has been performed. In light of this, we conducted a pilot investigation of how ARR with this mechanical valved conduit may affect transvalvular pressure gradients and ascending aortic haemodynamics in terms of flow helicity and vorticity. We hypothesized that the geometry of this valve was unique enough that it could result in flow patterns distinct to those observed previously with other valves.
PATIENTS AND METHODS
This retrospective chart review was approved by a Northwestern University IRB with a waiver of informed consent. In 2 cases, patients were recruited for postoperative examinations with informed consent.
Patient population
Patients and healthy volunteers were retrospectively identified from previous groups who had undergone contrast-enhanced four-dimensional (4D) flow magnetic resonance imaging (MRI) at 1.5 or 3.0 T (MAGNETOM Aera, Skyra Scanner, Siemens Medical Systems AG, Erlangen, Germany) at our institution. Subjects were previously excluded from participating if they had a contraindication to MRI—such as pacemarkers, cochlear implants or aneurysm clips—were unable/unwilling to give informed consent, <18 or >89 years old, had a GFR <30 ml/min, had or planned to undergo another contrast-enhanced MRI within 24 h or had undergone kidney and/or liver transplantation. Five identified patients were excluded for undergoing aortic valve replacement rather than ARR and/or having a history of aortic dissection. Ten patients were identified with aortic valve and/or root pathology who had undergone 4D flow MRI after ARR with an On-X Ascending Aortic Prosthesis (CryoLife, Inc., Kennesaw, GA, USA) between April 2013 and March 2015. Preoperative 4D flow MRI was available in 7 patients. Ten age- and gender-matched, healthy volunteers were also identified who had undergone the same imaging studies between June 2012 and June 2013 in order to characterize physiological flow patterns.
Surgical technique
All 10 patients underwent modified Bentall procedures with reattachment of the left and right coronary arteries to the Gelweave Dacron Valsalva Graft (Vascutek Ltd, Inchinnan, UK) portion of the valved conduit [11]. Average valve size: 26.0 ± 1.4 mm, valved conduit graft size: 25.8 ± 0.6 mm, maximum skirt diameter: 33.8 ± 0.6 mm, graft length 11 cm and hemiarch replacement graft size: 25.2 ± 1.0 mm. All valves were oriented with leaflet openings facing the right and left coronary ostia to maximize coronary blood flow [12].
Magnetic resonance imaging technique
All study subjects received electrocardiographic (ECG)-gated, time-resolved (CINE) cardiac MR imaging to evaluate cardiac function and valve morphology. After administration of an FDA-approved gadolinium-based agent [Multihance (0.1–0.2 mmol/kg), Magnevist (0.1–0.2 mmol/kg) or Ablavar (0.03 mol/kg)] for contrast-enhanced MRI, subjects also underwent time-resolved 3D phase-contrast MR imaging with three-directional velocity encoding (4D flow MRI) to assess aortic haemodynamics. 4D flow MRI was obtained during free breathing, using respiratory and prospective ECG gating in an oblique sagittal orientation for optimal measurement of blood flow velocities throughout the thoracic aorta [13]. Pulse sequence parameters were as follows: flip angle = 15°, echo time = 2.2–2.5 ms, repetition time = 36.8–39.2 ms, tri-directional velocity-encoding gradient = 150–300 cm/s, slice thickness 2.4–3.6 mm, field of view = 240–330 × 240–400, receiver bandwidth 453–460 Hz, spatial resolution = 2.1–3.4 mm × 2.1–2.5 mm × 2.2–3.0 mm.
Magnetic resonance data processing
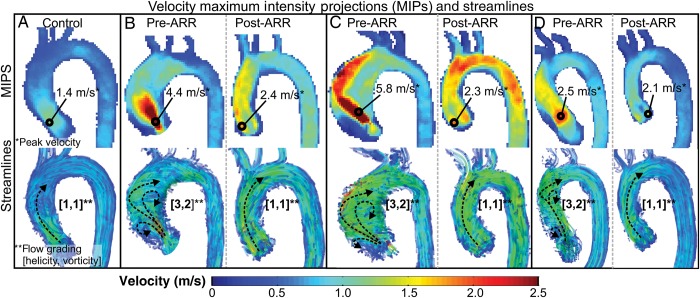
The 4D flow MRI data were corrected for noise, eddy currents, Maxwell terms and velocity aliasing in MatLab (The MathWorks, Natick, MA, USA). A phase contrast magnetic resonance angiography was generated to permit the 3D segmentation of the thoracic aorta using the commercially available software (Mimics, Materialise, Leuven, Belgium) [14]. The 3D segmentation was used to mask the velocity field for the generation of a maximum intensity projection (MIP) image of the velocity magnitude at peak systole (as defined by the peak transvalvular flow rate (Fig. 1). Further analysis was completed in EnSight (CEI, Apex, NC, USA), allowing for time-resolved streamline visualization.
Figure 1:
Example maximum intensity projections (MIPs) and flow patterns in a healthy volunteer (A) and 3 patients pre- and post-aortic root replacement (AAR) (B–D). Three-dimensional (3D) velocity MIPs were used to find peak velocity and pressure gradient (top row). Systolic streamlines were used to visualize and grade 3D flow patterns (bottom row, bulk flow patterns are illustrated with black dotted arrows).
Peak transvalvular pressure gradients were computed from peak velocities identified in the MIP images, using the simplified Bernoulli equation (ΔP = 4v2, where ΔP is the pressure gradient and v is the peak velocity in the region of the vena contracta). The presence of vortex or helix formation was graded separately by two blinded observers (Alex J. Barker and Eric J. Keller), using semi-quantitative scales of flow patterns. Grading was repeated by one observer (Eric J. Keller) 2 months after the initial analysis. Helical flow was defined as rotational motion around the longitudinal axis of the vessel and graded from 1 to 3: flow rotation <180°, flow rotation >180° and flow rotation >360°, respectively. Vorticity was defined by the number of vertical rotational flow patterns with cohesive pathline structures greater than 1 cm in diameter graded from 1 to 3: no large vortices, 1–2 large vortices, >2 large vortices (see Fig. 1 for examples).
Echocardiography imaging technique
Transthoracic echocardiography (TTE) was performed before and after ARR, using one of the following systems: Sequoia 256 (Siemens Healthcare, Malvern, PA, USA); Philips Sonos 7500 or IE33 (Philips Medical Systems, Andover, MA, USA) or GE Vivid 7 (GE Healthcare, Waukesha, WI, USA). The aortic valve was assessed in the parasternal long- and short-axis views, and Doppler measurements were taken with standard Doppler beam alignment, averaged over three cardiac cycles [15]. Peak and mean transvalvular pressure gradients were calculated from peak and mean velocities, respectively, using the simplified Bernoulli equation. Effective orifice areas (EOAs) were calculated using velocity time integrals via Doppler TTE. The EOA was not assessable on postoperative MRI due to artefact from the mechanical valve. EOA indices (EOAi) were calculated by dividing patients' EOAs by their body surface areas to assess patient–prosthesis mismatch, using <0.85 cm2/m2 as the defining threshold [16].
Statistical analysis
All analyses were performed using SPSS software (IBM, Chicago, IL, USA). Intra- and interobserver agreement for helicity and vorticity grading was assessed separately via kappa analyses. Patients' postoperative pressure gradients and helicity grades were compared with preoperative and control values via one-tailed t-tests and Wilcoxon signed-rank tests, respectively. Peak transvalvular pressure gradients obtained from TTE and 4D flow MRI were compared via two-tailed, paired t-tests and Bland–Altman analyses. A P-valve of ≤0.05 was considered statistically significant.
RESULTS
Demographics and surgical outcomes
Subject demographics are summarized in Table 1. Of the 10 patients who met the inclusion criteria, there were 9 males (90%) with a mean age of 40 ± 9 years. At the time of surgery, this cohort had significant cardiovascular disease including aortic regurgitation (80%), aortic stenosis (50%), hypertension (40%) and dyslipidaemia (10%). All had ascending aortic aneurysms of the aortic root and/or mid-ascending aorta with an average maximum diameter of 4.8 ± 0.4 cm. The majority (8/10) had congenital bicuspid aortic valves and 1 of the 2 patients with a tricuspid valve had a history of Marfan syndrome, likely accounting for the extent of valvular and aortic disease in this relatively young cohort. The 10 volunteers were matched for gender (90% male, P = 1) and age (42 ± 13 years, P = 0.61) and had no history of significant cardiovascular disease per our healthy volunteer inclusion criteria.
Table 1:
Patient and healthy volunteers’ demographics
| Parameter | Patients | Healthy volunteers | P-value |
|---|---|---|---|
| Age | 40 ± 9 | 42 ± 13 | 0.61 |
| Gender (M/F) | 9/1 | 9/1 | 1.00 |
| BAV | 8/10 (80%) | 0/10 (0%) | – |
| TAV | 2/10 (20%) | 10/10 (100%) | – |
| BSA (m2) | 2.0 ± 0.2 | – | – |
| TAA maximum diameter (cm) | 4.8 ± 0.4 | – | – |
| Mild aortic stenosis | 1/10 (10%) | – | – |
| Moderate aortic stenosis | 1/10 (10%) | – | – |
| Severe aortic stenosis | 2/10 (20%) | – | – |
| Mild aortic insufficiency | 3/10 (30%) | – | – |
| Moderate aortic insufficiency | 3/10 (30%) | – | – |
| Severe aortic insufficiency | 2/10 (20%) | – | – |
| Hypertension | 4/10 (40%) | – | – |
| Dyslipidaemia | 1/10 (10%) | – | – |
| Marfan syndrome | 1/10 (10%) | – | – |
Results reported as mean ± SD unless labelled otherwise. Aortic stenosis was defined by aortic jet velocity [2.6–2.9 m/s (mild), 3.0–4.0 m/s (moderate) and >4.0 m/s (severe)]. Aortic insufficiency was defined by the regurgitant fraction [30–39% (mild), 40–49% (moderate) and ≥50% (severe)].
BAV: bicuspid aortic valve; BSA: body surface area; TAA: thoracic aortic aneurysm; TAV: tricuspid aortic valve.
All ARRs were technically successful with no intraoperative complications and mean cardiopulmonary bypass and cross-clamp times of 167 ± 33 and 140 ± 29 min, respectively. One 23/24 mm, three 25/26 mm and six 27/29/26 mm On-X valved Gelweave conduits were used. Patients had lengths of stay of 7 ± 3 days. Postoperative complications occurred in 3 patients including 2 cases of symptomatic atrial fibrillation and 1 case of bilateral subdural haemorrhage. All were efficiently and effectively managed, causing no further issues. On average, TTE and 4D flow MRI were performed 50 ± 17 and 39 ± 12 days prior to surgery and 5 ± 2 and 36 ± 32 days after surgery. The average postoperative EOA and EOA indices on Doppler echocardiography were 2.3 ± 0.3 and 1.1 ± 0.2 cm2, respectively. Patient–prosthesis mismatch, as defined by an EOA index <0.85, was identified in 2 patients (see Table 2 for the summary of surgical outcomes).
Table 2:
Surgical summary
| Parameter | Prevalence |
|---|---|
| 23 mm On-X valve; 24 mm Gelweave Conduit | 1/10 (10%) |
| 25 mm On-X valve; 26 mm Gelweave Conduit | 3/10 (30%) |
| 27/29 mm On-X valve; 26 mm Gelweave Conduit | 6/10 (60%) |
| Cardiopulmonary bypass time (min) | 167 ± 33 |
| Cross-clamp time (min) | 140 ± 29 |
| Length of stay (days) | 7 ± 3 |
| Postoperative complications | 3/10 (30%) |
| Effective orifice area via TTE (mm) | 2.3 ± 0.3 |
| Effective orifice area index via TTE | 1.1 ± 0.2 |
Results reported as mean ± SD unless labelled otherwise.
TTE: transthoracic echocardiography.
Intra- and interobserver reliability
Interobserver agreement for helicity and vorticity grading was high (κ = 0.88, P < 0.001 and κ = 0.79, P < 0.001, respectively). Intraobserver agreement was similarly high (κ = 0.93, P < 0.001 for helicity and vorticity).
Aortic haemodynamics
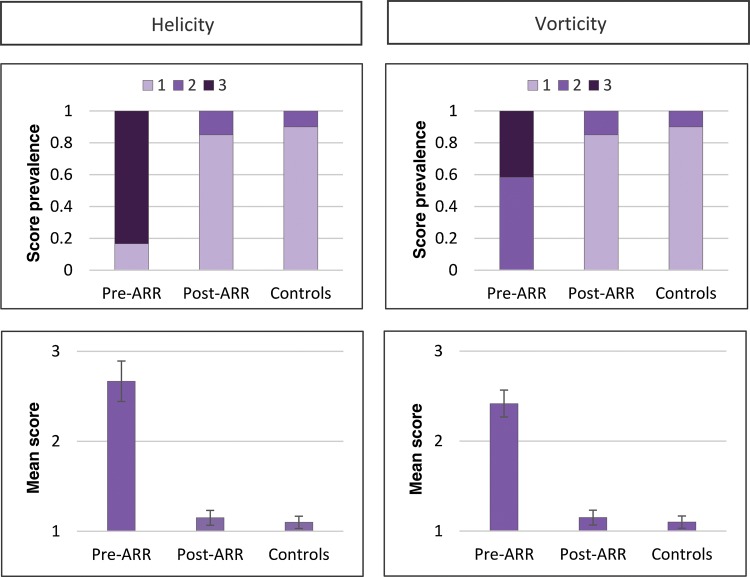
The mean times between pre- and postoperative MRI and between MRI and TTE were 86 ± 109 days [7–326 days] and 33 ± 72 days [0–288 days], respectively. Blood flow visualization was feasible in all but one 4D flow MRI study due to incomplete coverage of the aorta. The healthy volunteers were found to have mild helical flow or vortices, leading to mean helicity and vorticity grades of 1.1 ± 0.1 and 1.1 ± 0.1, respectively. Prior to ARR, the ascending aortic blood flow appeared markedly aberrant in nearly all patients with significant helical flow and/or large vortices (Table 3). As such, the mean helicity and vorticity grades for patients pre-ARR were 2.7 ± 0.3 and 2.5 ± 0.2, respectively. Following ARR, ascending aortic haemodynamics were dramatically less aberrant with mean helicity and vorticity grades of 1.2 ± 0.1 and 1.2 ± 0.1, respectively. Helicity and vorticity grades were significantly lower post-ARR (P < 0.001 and <0.001) with no significant differences compared with healthy volunteers (P = 0.56 and 0.56). See Table 3 for a summary of haemodynamic measurements and Fig. 2 for comparison of semi-qualitative grades. See Videos 1 and 2 for example pre- and post-ARR flow visualizations, respectively.
Table 3:
Haemodynamic measurements in patients and healthy volunteers
| Parameter | Patients pre-ARR | Patients post-ARR | Healthy volunteers | P-value (pre- versus post-ARR) | P-value (post-ARR versus volunteers) |
|---|---|---|---|---|---|
| Mean transvalvular ΔP (TTE, mmHg) | 25.9 ± 13.6 | 7.0 ± 4.0 | – | 0.03 | – |
| Peak transvalvular ΔP (TTE, mmHg) | 43.1 ± 21.0 | 12.4 ± 7.0 | – | 0.01 | – |
| Peak transvalvular ΔP (4D flow MRI, mmHg) | 47.8 ± 22.1 | 15.9 ± 8.7 | 6.4 ± 0.4 | 0.04 | <0.01 |
| Helicity grade (1–3) | 2.7 ± 0.3 | 1.2 ± 0.1 | 1.1 ± 0.1 | <0.01 | 0.56 |
| Vorticity grade (1–3) | 2.5 ± 0.2 | 1.2 ± 0.1 | 1.1 ± 0.1 | <0.01 | 0.56 |
Results reported as mean ± SEM.
ARR: aortic root replacement; ΔP: pressure gradient.
Figure 2:
Comparison of helicity and vorticity grading in patients and healthy volunteers. Upper row illustrates the frequency of each helicity or vorticity grade in patients' pre- and post-aortic root replacement (ARR) as well as healthy volunteers. Lower row illustrates mean grade ± SEM.
Video 1: Pre-aortic root replacement (ARR) ascending aortic haemodynamics in a 48-year old man with severe aortic insufficiency and an ascending aortic aneurysm.
Video 2: Post-aortic root replacement (ARR) ascending aortic haemodynamics in the same 48-year old patient in Video 1 with a 27-mm On-X valved conduit.
No significant difference was found between peak transvalvular pressure gradients assessed via 4D flow MRI compared with echocardiography in patients pre- and post-ARR (P > 0.05) with an average difference of 4.00 mmHg (−0.73 to 8.74 mmHg). See Fig. 3 for comparison and Bland–Altman analysis. Both modalities showed increased preoperative peak velocities, and thus, peak pressure gradients across patients' aortic valves [43.1 ± 21.0 mmHg (Echo); 47.8 ± 22.1 mmHg (4D flow MRI)]. These pressure gradients were significantly reduced following ARR [12.4 ± 7.0 mmHg (P = 0.01, Echo); 15.9 ± 8.7 mmHg (P = 0.04, 4D flow MRI)]. The mean transvalvular pressure gradients assessed via TTE were also significantly reduced (25.9 ± 13.6 vs 7.0 ± 4.0 mmHg, P = 0.03). However, unlike the helicity and vorticity grades, post-ARR peak transvalvular pressure gradients (via 4D flow MRI) remained significantly higher than those in healthy volunteers (P = 0.001).
Figure 3:
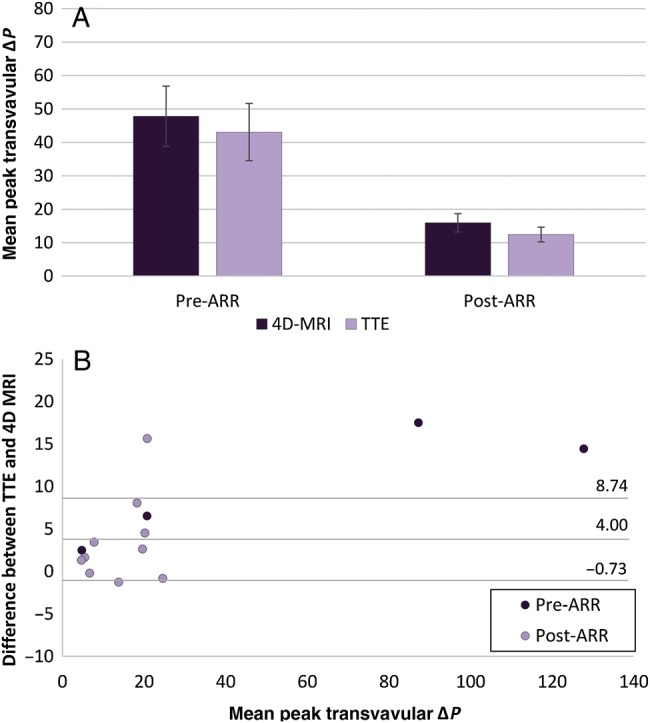
Comparison of four-dimensional (4D) flow magnetic resonance imaging (MRI) and transthoracic echocardiography (TTE) assessment of peak transvalvular pressure gradients. (A) Mean peak transvalvular pressure gradients ± SEM for patients pre- and post-aortic root replacement (ARR) via 4D flow MRI (dark purple) and TTE (light purple). Pre-ARR (P = 0.62). Post-ARR (P = 0.24). (B) Bland–Altman plot comparing difference between TTE and 4D flow MRI measurements and means for patients pre-ARR (dark purple) and post-ARR (light purple).
DISCUSSION
In light of previous studies illustrating aberrant ascending aortic haemodynamics following aortic valve replacement with various prostheses, we sought to investigate whether ARR with a mechanical valved conduit designed to reduce turbulent flow would produce less erratic blood flow patterns. As found previously [6], the On-X mechanical valve significantly reduced transvalvular pressure gradients. However, we also performed a similar qualitative analysis of 3D aortic haemodynamics to those done for other valves [3, 7] and observed a significant reduction in helical and vortical flow postoperatively. Patients' postoperative ascending aortic flow patterns were found to be comparable (P > 0.05) with those of healthy volunteers despite differences in the distensibility of the ascending aortas in these two groups. There remained a non-significant trend towards greater helicity and vorticity in patients following ARR, but our results suggest that postoperative haemodynamics in this pilot cohort approached those of the healthy volunteers.
The flow patterns we observed were likely the result of both aortic root and valve geometries. Previous 4D flow MRI studies of valve-sparing ARR with a similar Dacron graft material and shape as this investigation yielded increased ascending aortic velocities but similar helical flow gradings to controls. Interestingly, a reduction in helical flow was independent of native valve geometry (tricuspid aortic valve versus bicuspid aortic valve), but was not achieved in patients who underwent ARR with a bioprosthesis [1, 7]. Even the configuration of the sinuses of Valsalva may affect haemodynamics. Recreation of sinus-like dilatations near the aortic valve has been shown to produce vortical flow in these dilatations similar to physiological sinus flow patterns that are lost when the root prosthesis is a straight tube [17]. These findings underscore the importance of aortic root geometry in producing physiological flow patterns; however, other 4D flow MRI studies have demonstrated distinct, aberrant flow patterns with various valve prostheses irrespective of root geometry. For example, St Jude mechanical valves yielded the highest postoperative vorticity grades, whereas the Medtronic stented bioprosthesis resulted in prominent helical flow, underscoring the importance of aortic valve geometry [3]. Aberrant preoperative flow patterns observed in our cohort were a consequence of both valvular and aortic pathology. Similarly, both the On-X valve and root reconstruction using the Dacron graft likely contributed to the less aberrant postoperative flow patterns. Future work assessing flow patterns following aortic valve replacement with On-X valves alone is necessary to independently assess the haemodynamic effects of the On-X valve and reconstructed root geometry.
Design features of the On-X valve, such as the flared inlet and larger length-to-diameter ratio, may form a more cohesive central flow jet by mimicking the natural left ventricular outflow tract [9, 10]. It is also possible that the ability for valve leaflets to fully open reduces erratic flow observed with other bileaflet mechanical valves [3, 8]. Single leaflet mechanical valves and bileaflet valves that do not open to a full 90° create more diffuse flow jets, leading to more aberrant 3D flow patterns [8]. However, this study was not designed to have the sensitivity to illustrate a cause-and-effect relationship between individual design features and ascending aortic haemodynamics. Our results from this pilot cohort only suggest that this mechanical valve may be unique in its ability to reduce helical and vortical flow patterns in the ascending aorta and that these postoperative flow patterns seem to better match those of healthy volunteers with central flow jets and minimal (<180°), right-handed, helical flow during systole [18].
The clinical implications of these findings are currently unknown, but reducing aberrant postoperative haemodynamics in the setting of ARR may reduce the risk of rare but serious late complications such as aneurysm formation, thrombosis or leakage at the distal graft–aorta anastomosis. Previous authors [19, 20] have observed that commonly used aortic grafts, such as woven Dacron, are less compliant, reducing ventricular–arterial coupling. Their work suggests that this compliance mismatch between grafts and native tissue can cause abnormal WSS and flow disturbances at distal anastomoses, leading to intimal hyperplasia and platelet aggregation, respectively. Furthermore, less-compliant grafts can increase total systolic pressure and WSS downstream since the proximal aorta is a major determinant of total arterial impedance. Thus, reduction of aberrant postoperative flow patterns may offset some deleterious effects of compliance mismatch and ultimately patients' risk of late postoperative complications [7].
Recent work has also suggested that abnormal haemodynamics may have a significant role in the development of vascular disease. Vascular endothelial cells experience three mechanical forces: pressure, stretch/tension and WSS; of these, WSS seems particularly important in altering endothelial cell permeability, lipid uptake and proliferation [21]. Physiological WSS is necessary for promoting endothelial homeostasis, but WSS above or below certain thresholds appears to promote atherogenesis, cellular misalignment and remodelling via apoptosis and proliferation [4]. In light of this work, authors have hypothesized that asymmetric areas of WSS created by eccentric blood flow in the setting of valvular disease or post-ARR with certain prostheses may increase one's risk of wall degeneration and aneurysm formation in the native ascending aorta [3, 7]. However, further work is needed to better support this theory with longitudinal studies with more compatible methods. We chose to not investigate WSS since our cohort underwent ARR and thus have limited susceptibility to WSS changes in the ascending aorta.
In light of this early work, our observation of a mechanical valve significantly reducing erratic aortic blood flow to resemble healthy volunteers is encouraging. Similar to previous reports [6, 22], post-ARR transvalvular pressure gradients remained elevated compared with healthy volunteers. This may be due to our use of ‘peak’ as opposed to ‘mean’ velocity gradients. Mean measurements are lower than peak gradients, and this matches the pattern of our postoperative peak gradients [12.4 ± 7.0 mmHg (Echo); 15.9 ± 8.7 mmHg (4D flow MRI)] being higher than the mean gradients reported in other studies for this prosthesis (∼10 mmHg) [6, 22]. The assessment of mean pressure gradients from 4D flow MRI has yet to be developed. However, higher postoperative peak pressure gradients may reflect valve prosthesis–patient mismatch that was observed in 20% of our small cohort. Prostheses with smaller EOAs than patients' native valves are known to increase postoperative, transvalvular pressure gradients [16]. Prosthesis–patient mismatch is also more common in patients with higher BSAs [16]. Our patients' mean BSA of 2.0 ± 0.2 m2 suggests that this cohort tended to have larger body habituses, and thus, more risk of higher postoperative transvalvular pressure gradients.
Although this study was not designed to compare 4D flow MRI and echocardiographic measurements, the similarity of peak transvalvular pressure gradient measurements by these two modalities is an important finding. Echocardiographic evaluation of valve morphology and blood flow is standard due to its wide availability, ease at the bedside and high temporal resolution. However, cardiovascular magnetic resonance (CMR) imaging avoids limitations caused by the acoustic window, beamline orientation and image quality in some patients and regions. Previous work has shown echocardiographic and two-dimensional velocity-encoding CMR to provide comparable velocity and pressure gradient measurements [23, 24]. 4D flow MRI has the advantage of assessing velocity in three dimensions rather than one, but studies comparing 4D flow MRI and 2D MRI sequences have suggested that 4D flow MRI underestimates stenotic pressure gradients, perhaps due to its relatively low temporal resolution [25]. However, these studies identified peak velocity in the 4D flow data by manually placing a 2D plane and then measuring peak velocity, potentially missing the peak velocity in the aortic volume (i.e. in the region of the vena contracta). Conversely, our use of 3D volumetric segmentation combined with a velocity MIP takes into account all three principal velocity directions in the entire volume of interest, allows for easy identification of the vena contracta and appears to provide comparable transvalvular pressure data to echocardiography. We suspect that these advantages are the reason for the better results than those of previous studies comparing 4D flow MRI with echocardiography. This finding is encouraging for the future of CMR and requires further investigation.
Our study had important limitations. A small pilot cohort of patients and healthy volunteers was analysed. Although this afforded statistically significant results, larger studies should be performed comparing post-ARR haemodynamics with various prosthetic aortic valves assessing the impact of different postoperative haemodynamics on long-term patient outcomes, similar to a recent study that did not investigate On-X valves [3]. The retrospective nature of our analysis also introduces a higher risk of sampling bias, but we included all patients found who met our inclusion/exclusion criteria. The qualitative nature of our blood flow grading system introduced reader variability, but kappa statistics suggested excellent agreement between observers, and similar flow grading schemes have been used previously with good results [3, 7]. Preoperative aortic pathology, such as ascending aortic aneurysms, likely accounted for some of the aberrant flow that would have been corrected by ARR independent of valve design. However, no non-native aortic valve has been able to produce 3D, in vivo haemodynamics that so closely approach flow patterns observed in healthy volunteers. Finally, the stiffness of patients' ascending aortas following ARR may have influenced the changes in flow observed, but aberrant flow patterns have been found to remain following ARR with bioprostheses [7] in contrast to what we found with an On-X valved conduit.
In summary, we assessed the effects of ARR with an On-X mechanical valved conduit and its impact on haemodynamics in the ascending aorta of 10 patients, comparing postoperative flow patterns with those of 10 gender-/age-matched healthy volunteers. Our results suggest that this particular mechanical aortic valve significantly reduces aberrant ascending aortic haemodynamics in contrast to flow patterns described in previous reports for other commercially available valve designs [3]. The clinical implications of these findings are unknown but may reduce the risk of long-term complications such as aneurysm formation at the distal anastomotic line or distal aorta.
Future work is necessary to elucidate the effects of specific valve design features on ascending aortic haemodynamics. Furthermore, there are little data on the effects of abnormal aortic haemodynamics on clinical outcomes. As such, prospective, long-term studies will be necessary to better understand the links between prosthetic aortic valve design, aortic haemodynamics and clinically meaningful outcomes.
Funding
This work was supported by NIH K25 HL119608, NIH R01 HL115828 and the Northwestern's Melman Bicuspid Aortic Valve Program at the Bluhm Cardiovascular Institute.
Conflict of interest: none declared.
ACKNOWLEDGEMENTS
Research reported in this publication was supported, in part, by the Northwestern University Bluhm Cardiovascular Institute (BCVI) Clinical Trials Unit. The authors thank Edouard Seeman for his help with data collection.
REFERENCES
- 1. Semaan E, Markl M, Malaisrie SC, Barker A, Allen B, McCarthy P et al. Haemodynamic outcome at four-dimensional flow magnetic resonance imaging following valve-sparing aortic root replacement with tricuspid and bicuspid valve morphology. Eur J Cardiothorac Surg 2014;45:818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kvitting JP, Dyverfeldt P, Sigfridsson A, Franzen S, Wigstrom L, Bolger AF et al. In vitro assessment of flow patterns and turbulence intensity in prosthetic heart valves using generalized phase-contrast MRI. J Magn Resonan Imaging 2010;31:1075–80. [DOI] [PubMed] [Google Scholar]
- 3. von Knobelsdorff-Brenkenhoff F, Trauzeddel RF, Barker AJ, Gruettner H, Markl M, Schulz-Menger J. Blood flow characteristics in the ascending aorta after aortic valve replacement--a pilot study using 4D-flow MRI. Int J Cardiol 2014;170:426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cecchi E, Giglioli C, Valente S, Lazzeri C, Gensini GF, Abbate R et al. Role of hemodynamic shear stress in cardiovascular disease. Atherosclerosis 2011;214:249–56. [DOI] [PubMed] [Google Scholar]
- 5. Puskas J, Gerdisch M, Nichols D, Quinn R, Anderson C, Rhenman B et al. Reduced anticoagulation after mechanical aortic valve replacement: interim results from the prospective randomized on-X valve anticoagulation clinical trial randomized Food and Drug Administration investigational device exemption trial. J Thorac Cardiovasc Surg 2014;147:1202–10; discussion 10–1. [DOI] [PubMed] [Google Scholar]
- 6. Dohmen G, Schmitz C, Steinseifer U, Hatam N, Hilgers RD, Autschbach R et al. Influence of aortic dimensions on the hemodynamic performance of small aortic valve prostheses: impact on patient/prosthesis mismatch. Thorac Cardiovasc Surg 2011;59:449–53. [DOI] [PubMed] [Google Scholar]
- 7. Collins JD, Semaan E, Barker A, McCarthy PM, Carr JC, Markl M et al. Comparison of hemodynamics after aortic root replacement using valve-sparing or bioprosthetic valved conduit. Ann Thorac Surg 2015;100:1556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akutsu T, Matsumoto A. Influence of three mechanical bileaflet prosthetic valve designs on the three-dimensional flow field inside a simulated aorta. J Artif Org 2010;13:207–17. [DOI] [PubMed] [Google Scholar]
- 9. Dasi LP, Simon HA, Sucosky P, Yoganathan AP. Fluid mechanics of artificial heart valves. Clin Exp Pharmacol Physiol 2009;36:225–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kheradvar A, Groves EM, Falahatpisheh A, Mofrad MK, Hamed Alavi S, Tranquillo R et al. Emerging trends in heart valve engineering: part IV. Computational modeling and experimental studies. Ann Biomed Eng 2015;43:2314–33. [DOI] [PubMed] [Google Scholar]
- 11. Malaisrie SC, Duncan BF, Mehta CK, Badiwala MV, Rinewalt D, Kruse J et al. The addition of hemiarch replacement to aortic root surgery does not affect safety. J Thorac Cardiovasc Surg 2015;150:118–24e2. [DOI] [PubMed] [Google Scholar]
- 12. Kleine P, Scherer M, Abdel-Rahman U, Klesius AA, Ackermann H, Moritz A. Effect of mechanical aortic valve orientation on coronary artery flow: comparison of tilting disc versus bileaflet prostheses in pigs. J Thorac Cardiovasc Surg 2002;124:925–32. [DOI] [PubMed] [Google Scholar]
- 13. Markl M, Harloff A, Bley TA, Zaitsev M, Jung B, Weigang E et al. Time-resolved 3D MR velocity mapping at 3T: improved navigator-gated assessment of vascular anatomy and blood flow. J Magn Reson Imaging 2007;25:824–31. [DOI] [PubMed] [Google Scholar]
- 14. Bock J, Kreher B, Hennig J, Markl M. Optimized pre-processing of time-resolved 2D and 3D phase contrast MRI data. In: Conference Optimized Pre-processing of Time-resolved 2D and 3D Phase Contrast MRI Data, Berlin, Germany, 2007, p. 3138. [Google Scholar]
- 15. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39e14. [DOI] [PubMed] [Google Scholar]
- 16. Pibarot P, Dumesnil JG. Prosthesis-patient mismatch: definition, clinical impact, and prevention. Heart 2006;92:1022–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Markl M, Draney MT, Miller DC, Levin JM, Williamson EE, Pelc NJ et al. Time-resolved three-dimensional magnetic resonance velocity mapping of aortic flow in healthy volunteers and patients after valve-sparing aortic root replacement. J Thorac Cardiovasc Surg 2005;130:456–63. [DOI] [PubMed] [Google Scholar]
- 18. Kilner PJ, Yang GZ, Mohiaddin RH, Firmin DN, Longmore DB. Helical and retrograde secondary flow patterns in the aortic arch studied by three-directional magnetic resonance velocity mapping. Circulation 1993;88:2235–47. [DOI] [PubMed] [Google Scholar]
- 19. Matsumoto T, Naiki T, Hayashi K. Flow visualization analysis in a model of artery-graft anastomosis. Biomed Mater Eng 1992;2:171–83. [PubMed] [Google Scholar]
- 20. Simon-Kupilik N, Schima H, Huber L, Moidl R, Wipplinger G, Losert U et al. Prosthetic replacement of the aorta is a risk factor for aortic root aneurysm development. Ann Thorac Surg 2002;73:455–9. [DOI] [PubMed] [Google Scholar]
- 21. Pan S. Molecular mechanisms responsible for the atheroprotective effects of laminar shear stress. Antioxid Red Signal 2009;11:1669–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smadi O, Garcia J, Pibarot P, Gaillard E, Hassan I, Kadem L. Accuracy of Doppler-echocardiographic parameters for the detection of aortic bileaflet mechanical prosthetic valve dysfunction. Eur Heart J Cardiovasc Imaging 2014;15:142–51. [DOI] [PubMed] [Google Scholar]
- 23. Caruthers SD, Lin SJ, Brown P, Watkins MP, Williams TA, Lehr KA et al. Practical value of cardiac magnetic resonance imaging for clinical quantification of aortic valve stenosis: comparison with echocardiography. Circulation 2003;108:2236–43. [DOI] [PubMed] [Google Scholar]
- 24. Dyverfeldt P, Bissell M, Barker AJ, Bolger AF, Carlhall CJ, Ebbers T et al. 4D flow cardiovascular magnetic resonance consensus statement. J Cardiovasc Magn Resonan 2015;17:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bock J, Frydrychowicz A, Lorenz R, Hirtler D, Barker AJ, Johnson KM et al. In vivo noninvasive 4D pressure difference mapping in the human aorta: phantom comparison and application in healthy volunteers and patients. Magn Resonan Med 2011;66:1079–88. [DOI] [PubMed] [Google Scholar]