Abstract
The spatio-temporal scalp distribution of multisensory auditory–somatosensory integration was investigated in typically developing children ages 6–13. Event-related potentials were recorded from 32 scalp electrodes while participants watched a silent cartoon. Three types of sensory stimulation were presented pseudo-randomly: auditory clicks, somatosensory median nerve electrical pulses, or simultaneous auditory and somatosensory stimuli. No behavioral responses were required of the participant. To examine integration, responses to simultaneous auditory and somatosensory stimulation were compared to the sum of unisensory auditory plus unisensory somatosensory responses for four time-windows: (60–80 ms, 80–110 ms, 110–150 ms and 180–220 ms). Results indicated significant multisensory integration occurred in central/post-central scalp regions between 60–80 ms in the hemisphere contralateral to the side of somatosensory stimulation and between 110–150 ms in the hemisphere ipsilateral to the side of somatosensory stimulation. Between 180–220 ms, significant multisensory integration was evident in central/post-central regions in both hemispheres as well as midline scalp regions. This study suggests that children exhibit differential processing of multisensory compared to unisensory stimuli, as has previously been reported in adults.
Keywords: Tactile, Interaction, Evoked potential, Modulation, Multimodal, Cross-modal
1. Introduction
The ability of the brain to integrate information from all sensory systems is a fundamental neural process. Sensory input converges in specific brain regions which integrate information about elements of the external world creating a foundation for determining the significance of events and producing meaningful responses.
Multisensory integration (MSI) is frequently evaluated using a method that compares a model of the neural activity representing the sum of the responses to two unisensory stimuli with the neural response to the simultaneous presentation of the same two stimuli (Foxe et al., 2000; Giard and Peronnet, 1999; Molholmet al., 2002; Talsma and Woldorff, 2005; Teder-Sälejärvi et al., 2002). This approach assumes that if populations of neurons responding to auditory and somatosensory stimulation are intermixed but do not interact, responses to multisensory stimulation will be equivalent to the linear sum of the unisensory auditory and somatosensory responses (Di et al., 1994). In addition, if differences are found between multisensory and summed unisensory responses cortical regions that are uniquely activated by multisensory stimulation are identified where MSI is likely occurring. This approach has identified numerous brain regions involved in MSI in animals (Stein and Meredith, 1993) and humans (Calvert and Thesen, 2004). However, it should be noted that this approach may not be sensitive to all areas of multisensory convergence where MSI occurs (Foxe et al., 2000).
MSI in humans is often examined using electrophysiological recordings, specifically, event-related potentials (ERPs). Most ERP studies of MSI examine auditory–visual integration (Fort et al., 2002; Giard and Peronnet, 1999; Molholm et al., 2002; Teder-Sälejärvi et al., 2002). However two recent ERP studies examined auditory–somatosensory MSI in adults, finding evidence of auditory–somatosensory integration in central/post-central scalp regions contralateral to the side of somatosensory stimulation in auditory association cortex at <50 ms (Foxe et al., 2000; Murray et al., 2005). These studies suggest that MSI occurs earlier in the hierarchical sensory processing streamin regions previously thought to be unisensory (Foxe and Schroeder, 2005).
Auditory–somatosensory MSI has been reported both contralateral and ipsilateral to the side of somatosensory stimulation using magnetoencephalography (MEG). For example, Gobbelé et al. (2003) reported MSI contralateral to the side of somatosensory stimulation between 75–85 ms and between 105–130 ms localized in the posterior parietal cortex and between secondary somatosensory cortex and auditory cortex, respectively (Gobbelé et al., 2003). Reported by Lütkenhöner et al. (2002) was MSI contralateral to the side of somatosensory stimulation in the majority of subjects at 140 ms and 220 ms localized to the secondary somatosensory and auditory cortex (Lütkenhöner et al., 2002). However, Lütkenhöner et al., 2002 also reported MSI ipsilateral to the side of somatosensory stimulation in some subjects (Lütkenhöner et al., 2002). One additional study also reported ipsilateral-only MSI between 70–100 ms that was localized in secondary somatosensory cortex (Lam et al., 1999). Unresolved are the exact brain regions where auditory–somatosensory MSI occurs.
No study of auditory–somatosensory MSI in children has been previously reported. Therefore, the primary purpose of this study was to extend MSI research to typically developing children. Objectives were determining whether typically developing children exhibit MSI, whether spatio-temporal patterns of MSI are similar to those reported in adults, and whether developmental effects on MSI are present over the range of ages tested (6–13) (Bruneau et al., 1997; Ponton et al., 2000; Zumsteg and Wieser, 2002). This research is intended to serve as a precursor to studying MSI in children with various clinical diagnoses. A further goal of this study was investigating the timing of MSI in the hemispheres contralateral and ipsilateral to the side of somatosensory stimulation over four time-windows: (60–80 ms, 80–100 ms, 110–150 ms and 180–220 ms). Hypothesized was that integration would occur at an early time-frame contralateral to the side of somatosensory stimulation and at a later time-frame ipsilateral to the side of somatosensory stimulation based on the subsequent inter-hemispheric transfer of auditory and contralateral somatosensory input to the hemisphere ipsilateral to the side of somatosensory stimulation. Exploration of the spatio-temporal patterns of MSI over the entire cortical sensory processing network was completed to aid future hypothesis generation.
2. Results
2.1. Event-related potentials: visual inspection
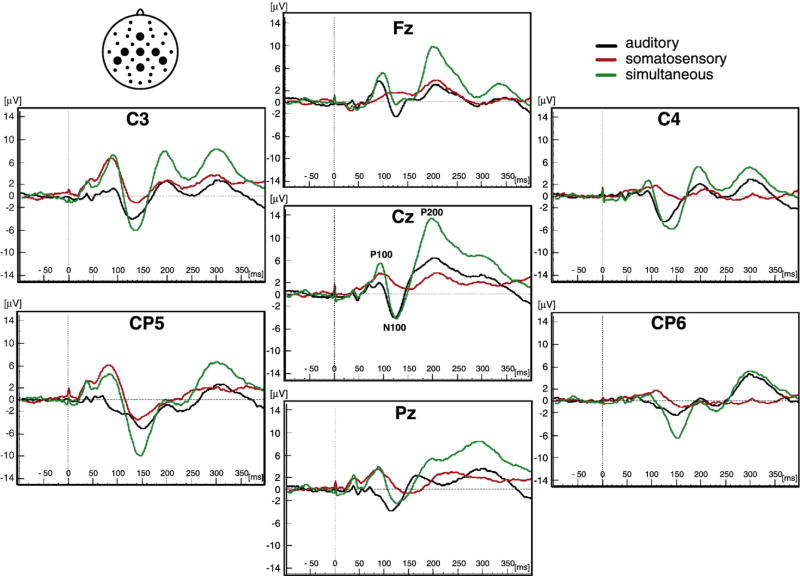
Grand average unisensory auditory, unisensory somatosensory and simultaneous auditory-somatosensory ERPs are displayed for select electrode sites (Fig. 1). The auditory ERP exhibited a series of ERP components identified at electrode site Cz with grand average peak latencies as follows: the P100 at 95 ms, the N100 at 125 ms, and the P200 at 200 ms. The somatosensory ERP exhibited similar ERP components identified at electrode site C3, over the scalp region contralateral to the side of median nerve stimulation. The grand average peak latencies were: the P100 at 85 ms, the N100 at 140 ms, and the P200 at 200 ms. At electrode site Cz, multisensory stimulation produced ERPs that had similar peak latencies but higher peak amplitudes compared to the unisensory auditory and unisensory somatosensory ERPs. However, this pattern varied depending on the time-windows and electrode sites that were examined.
Fig. 1.
Event Related Potentials (ERPs). The grand averages of event related potentials (ERPs) for auditory (black traces), somatosensory (red traces) and simultaneous auditory and somatosensory stimuli (green traces) are shown for a sample of 21 children. The ERPs are shown at midline electrode sites (Fz, Cz and Pz) and central/post-central electrode sites (C3, CP5, C4 and CP6). The P100, N100 and P200 ERP amplitude peaks are labeled at electrode site Cz. The placement of the electrode sites are indicated in a top view schematic in the upper left corner.
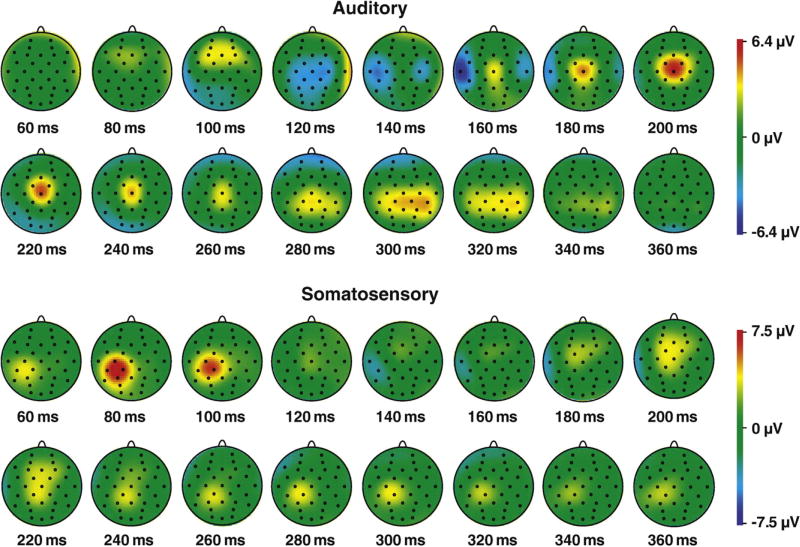
Topographical maps of the spatio-temporal distribution of unisensory ERPs are displayed (Fig. 2). For the auditory ERP, the maximal response was observed: for P100 over frontal electrode sites, for the N100 over central/post-central and temporal electrode sites, and for the P200 over central electrode sites. For the somatosensory ERP, the maximal response was observed for P100 and N100 over left central/post-central electrode sites and for the P200 over midline electrode sites Fz, Cz and Pz.
Fig. 2.
ERP voltage maps. The spatio-temporal distribution of grand average auditory and somatosensory ERPs are displayed in 20 ms intervals. The key to the right shows the range of voltages (in µV) for both types of ERPs.
2.2. Statistical analyses of MSI
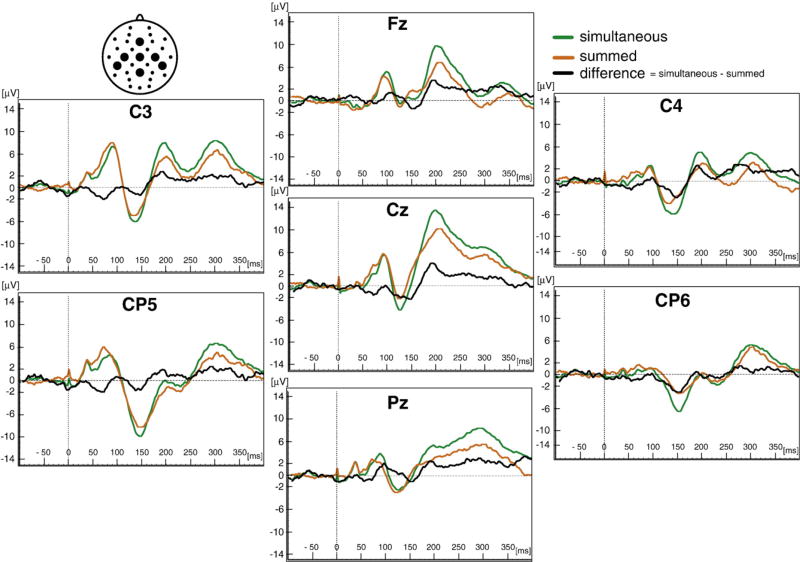
ERPs elicited by simultaneous stimulation were visually compared to ERPs derived by summing the unisensory ERPs (Fig. 3). The morphology of the simultaneous and summed waveforms was similar; however, they were not equivalent, suggesting that MSI might be occurring. Difference waves calculated by subtracting the simultaneous and summed ERPs further suggested MSI.
Fig. 3.
Auditory–somatosensory multisensory integration (MSI). The grand averages of ERPs for 21 children are shown for the seven electrode sites (Fz, Cz Pz, C3, CP5, C4 and CP6) designated in the schematic in the upper left-hand corner. Simultaneous auditory and somatosensory ERPs (green traces) are compared to the sum of unisensory auditory added to unisensory somatosensory ERPs (orange traces). Difference waves (black traces = simultaneous – summed) identify possible scalp locations for MSI.
To examine MSI, ERPs elicited by simultaneous stimulation were statistically compared to the summed ERPs at three midline electrode sites (Fz, Cz and Pz). Comparisons of averaged amplitudes calculated for four time-windows: (60–80 ms, 80–110 ms, 110–150 ms, 180–220 ms) found a significant main effect of stimulus type for the time-window between 180–220 ms (F(1,20) = 5.84, p = 0.03). The effect size estimated by the partial eta squared was moderate (0.25).
To test the hypothesis that early MSI occurs contralateral to the side of median nerve stimulation, stimulus type (simultaneous vs. summed) was compared for electrode sites C3 and CP5 for the four time-windows. A significant main effect of stimulus type was found for the earliest time-window, 60–80 ms, (F(1, 20) = 4.90, p = 0.04); and for the latest time-window, 180–220 ms, (F(1, 20) = 4.60, p = 0.04).
Estimated effect sizes were moderate (0.20 and 0.19, respectively). No significant main effect of stimulus type was found for the other two time-windows, 80–110 ms and 110–150 ms.
To test the hypothesis that later MSI occurs ipsilateral to the side of median nerve stimulation, stimulus type (simultaneous vs. summed) was compared for electrode sites C4 and CP6 at the four time-windows. A significant main effect of stimulus type was found at one time-window, 110–150 ms, (F(1, 20) = 4.776, p = 0.04). The effect size at this time-window was also moderate (0.19). No significant main effect of stimulus type was found for the other three time-windows.
2.3. Exploratory analysis of the spatio-temporal distribution of MSI
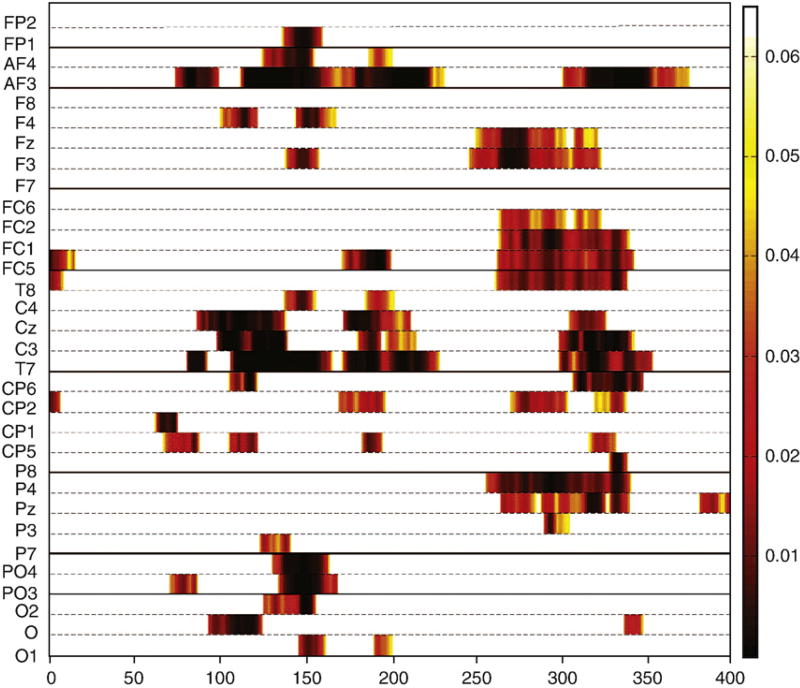
The statistical significance (p values) of comparisons of simultaneous vs. summed responses across the entire electrode array for the 400 ms recording epoch are displayed (Fig. 4). Demonstrated was that MSI occurred at central/post-central scalp regions beginning around 65 ms and continuing until approximately 230 ms. MSI was also evident at anterior frontal regions and posterior parietal and parietal/occipital regions over a similar time-frame. In addition, integration effects were apparent at frontal, central and parietal scalp regions between 250–350 ms.
Fig. 4.
P-values for MSI. Significant p values are displayed over time for 32 electrodes from running t-tests comparing the simultaneous (AS) and summed (A+S) ERPs. Statistically significant p values ranging from 0.01 to 0.05 are represented by the color code (key is to the right of graph). Time is plotted on the x-axis from 0 to 400 ms. Electrode sites are plotted on the y-axis.
2.4. Exploratory analysis of age effects on multisensory ERPs
Age (in months) and the averaged amplitude of the simultaneous ERP were correlated for the four time-frames at seven electrode sites (Fz, Cz, Pz, C3, CP5, C4, CP6). Correlation values ranged from −0.01 for electrode site CP6 during the 110–150 ms time-window, to 0.32 for electrode site Cz during the 180–220 ms time-window; all were non-significant.
3. Discussion
This is the first study to provide evidence for cortical MSI between audition and somatosensation in typically developing children. The key findings were: 1) significant differences between responses to simultaneous auditory and somatosensory stimulation and summed responses to unisensory auditory and somatosensory stimulation, between 180–220 ms for midline electrode sites (Fz, Cz, Pz), 2) significant MSI contralateral to the side of somatosensory stimulation for electrode sites C3 and CP5 at two time-windows: 60–80 ms and 180–220 ms and, 3) significant MSI ipsilateral to the side of somatosensory stimulation for electrode sites C4, CP6 at one time-window: 110–150 ms. Thus cross-validation with another sample is suggested to confirm conclusions of the present study.
3.1. Unisensory and multisensory ERP components
This study identified ERP components with unisensory auditory and somatosensory stimulation that are typically reported in ERP studies: the P100, the N100 and the P200 (for review, see (Luck, 2005; Regan, 1989). Similar ERP components were identified for multisensory stimulation that were consistent with adult literature (Foxe et al., 2000; Murray et al., 2005). Voltage maps demonstrated that the typical spatiotemporal distribution for unisensory auditory and somatosensory ERPs was consistent with previously reported results (Näätänen and Picton, 1987).
3.2. MSI in children
Previous ERP studies examining cortical auditory-somatosensory MSI are only in adults. Findings for children in this sample were consistent with findings in adults e.g. significant differences were found in adults between simultaneous auditory-somatosensory responses and summed unisensory responses during specific time-windows for midline and central/post-central scalp regions (Foxe et al., 2000; Murray et al., 2005).
3.3. MSI at electrode sites Fz, Cz and Pz
Preliminary evidence that MSI can be reliably measured in children was suggested by significant MSI found at midline scalp regions (Fz, Cz and Pz) during the latest time-window tested between 180–220 ms. This finding is consistent with a previous adult study on auditory–somatosensory integration that reported MSI in a time-window between 200–300 ms (Murray et al., 2005). Further exploration of MSI at additional electrode sites as a next step in this study was logical.
3.4. Contralateral MSI at electrode sites C3 and CP5
Understanding the timing of the onset of MSI is crucial to explaining the relationship between sensory processing and behavior. Previous ERP literature suggests that MSI begins early, around 50 ms in adults, contralateral to the side of somatosensory stimulation [Foxe and Schroeder, 2005; Murray et al., 2005]. MEG studies have identified MSI occurring between 75–85 ms (Gobbelé et al., 2003). The results of this study of children suggest that significant MSI occurs relatively early, between 60–80 ms, contralateral to the side of somatosensory stimulation at central/post-central electrode sites C3 and CP5; however, the onset of MSI in children cannot be definitively determined because earlier time-windows were not tested.
Earlier integration effects were not examined because the number of trials presented in this study was limited to 300 (100 auditory, 100 somatosensory, 100 auditory–somatosensory) and very early integration effects are small in amplitude requiring a large number of trials to enhance the signal-to-noise ratio and detect MSI.
The number of trials presented in this study was minimized due to pilot testing indicating that young children (6 years) could only reliably participate in the protocol for 30 min and were intolerant of stimulation rates faster than those used here (ISI = 4 s). Further rationale for limiting the number of trials was that the previous literature suggests a long, pseudorandom inter-stimulus interval is needed to allow for the recovery of the ERP, and to prevent contamination from anticipatory slow wave potentials (Teder-Sälejärvi et al., 2002). Thus the total number of trials presented was limited to 300. Therefore, the earliest time-frame when differences could be examined in this study was between 60–80 ms.
Yet, the relatively early MSI reported here could be consistent with the early MSI found in adults occurring during the falling phase of the P50 (Foxe et al., 2000). The earliest time-window tested here coincided with a trough that occurred between 60–80 ms at electrode sites contralateral to the side of somatosensory stimulation and prior to the time-window tested for the P100 (80–110). Reported longer latency responses to unisensory stimulation in children compared to adults could account for a delay in MSI (Ponton et al., 2000; Zumsteg and Wieser, 2002).
In this study, MSI was also found at contralateral electrode sites between 180–220 ms, consistent with previous adult literature (Lütkenhöner et al., 2002; Murray et al., 2005). However, MSI is extremely sensitive to variation in the experimental methodology, including the relative salience of the stimuli and the sensory experience of the participant, therefore direct comparisons of results must be considered cautiously (Fort et al., 2002; Giard and Peronnet, 1999; Schürmann et al., 2006).
3.5. Ipsilateral MSI at electrode sites C4 and CP6
Significant MSI was found at electrode sites C4 and CP6, ipsilateral to the side of somatosensory stimulation between 110–150 ms. Neither of the recent ERP studies examining auditory–somatosensory integration in adults specifically addresses the timing of contralateral vs. ipsilateral MSI. However, previous literature using MEG supports MSI during a similar time-frame (Gobbelé et al., 2003; Lütkenhöner et al., 2002). In one study using a paradigm very similar to the one used in this study (e.g. clicks were delivered binaurally via earphones and somatosensory stimuli were via air puffs delivered to the right thumb), clear auditory–somatosensory integration was found at 140 ms and at 220 ms (Lütkenhöner et al., 2002). It was reported by the authors that at 140 ms, MSI was contralateral to the side of somatosensory stimulation for most participants, although some participants showed MSI ipsilateral to the side of somatosensory stimulation. It was suggested that for participants with strong interactions in the hemisphere contralateral to the side of somatosensory stimulation, contralateral secondary somatosensory cortex plays an important role in MSI whereas for those subjects for whom interactions in contralateral secondary somatosensory cortex are weak, a contribution from the auditory cortex may be unmasked that contributes to MSI that is found ipsilateral to the side of somatosensory stimulation (Lütkenhöner et al., 2002). Because MEG is sensitive only to tangential current flow while ERPs are sensitive to both tangential and radial current flow, comparisons between studies using these methodologies are speculative. However findings obtained with both methodologies suggest the inter-hemispheric transfer of somatosensory information facilitates subsequent integration of auditory and somatosensory input in the hemisphere ipsilateral to the side of somatosensory stimulation during the time-frame of 110 to 150 ms.
3.6. Exploratory analysis of the spatio-temporal distribution of MSI
Examination of significant MSI using a t-map analysis confirmed MSI identified in ANOVA analyses at midline and central-post/central electrode sites between 65–230 ms, before and during the P100, N100 and P200 multisensory ERP components. T-maps also showed significant MSI during a similar time-frame mainly for anterior–frontal, temporal and posterior parietal scalp regions not formally examined in this study.
Finally, the t-map showed significant MSI between 250–350 ms widely distributed across the scalp, which was indicative of possible MSI during the time-frame of the P300 ERP component.
3.7. Unisensory and multisensory ERPs in adults vs. children: effects of age
The effect of development on unisensory auditory and somatosensory ERP components has been investigated in previous literature (Ponton et al., 2000; Zumsteg and Wieser, 2002); however, the effect of development on multisensory ERP components has not been reported. In general, the long latency auditory ERP components (P100, N100 and P200) show a gradual decrease in latency from age 5 to adolescence, whereas amplitude tends to show a gradual increase (Ponton et al., 2000). However, the observed pattern of auditory ERP maturation depends on the scalp location at which the responses were recorded and on the generators of the response (Bruneau et al., 1997; Ponton et al., 2000). Moreover, mid-latency somatosensory ERPs show a substantial increase in the latency and amplitude of ERP components with age (Zumsteg and Wieser, 2002).
No previous studies have documented the effects of development in children on multisensory auditory–somatosensory integration measured with ERPs. Although this study found no significant relation between age (in months) and the averaged multisensory ERP amplitude across the four time-windows tested, findings must be considered preliminary as several factors may account for the non-significant association. First, sensory maturation effects occurring between 6–13 years of age may be related differentially to each of the two unisensory components of the multisensory ERP, in essence cancelling out developmental effects on the multisensory response. Second, important sensory maturation occurs before and after the time period between 6–13 years, therefore limiting the sample to this age range may have eliminated maturational effects that might be observed if younger and/or older children were included. Third, averaged amplitude within a particular time-window may not be the best measure of development effects on sensory ERP components, compared to peak amplitudes, for example. Fourth, the sample size is small and thus may not be representative of the population. Clearly, subsequent investigation with a larger, age stratified sample of children is required to further inform the effects of development on MSI.
3.8. Neural generators: auditory–somatosensory MSI
Likely, numerous cortical regions are involved in generating auditory–somatosensory MSI, as even unisensory ERPs are known to have multiple cortical generators (Näätänen and Picton, 1987). Although no specific analysis of neural generators was conducted in this study, findings of MSI at central/post-central electrode sites are consistent with a neural generator in the region between secondary auditory and secondary somatosensory cortex, as has been reported previously (Foxe et al., 2002). Suggestive of an additional generator in the mesencephalic reticular formation is finding significant MSI at the vertex during the time-frame of the P200 ERP component as a mesencephalic contribution to the auditory P200 has been previously proposed (Ponton et al., 2000).
4. Conclusion
This study is the first to report auditory–somatosensory MSI in a sample of typically developing school-aged children. The findings suggest that auditory–somatosensory MSI does occur in typically developing children, seen in significant differences between responses to multisensory stimulation and summed unisensory responses. Significant MSI occurred at central/post-central and midline scalp locations between 180–220 ms. Significant early MSI (60–80 ms) was found in the hemisphere contralateral to the side of somatosensory stimulation, and later MSI (110–150 ms) was found in the ipsilateral hemisphere. Replication with a larger and developmentally stratified sample will provide a basis for future work comparing the MSI of children who are typically developing to the MSI of children with developmental and behavioral disorders in whom MSI has received limited study.
5. Experimental procedures
5.1. Participants
Twenty-one (19 males), neurologically normal, paid volunteers, ages 6 to 13 years (M = 9.84, SD = ±2.15) participated. Participants and their parents provided written consent, using procedures that were approved by the local University.
5.2. Sensory stimulation
Three types of stimulation were presented: 1) click sounds (80 dB, 3 ms duration) delivered binaurally via earphones (Etymotic Research, Inc. (ER-1), Elk Grove Village, IL, USA), 2) constant current pulses delivered to the median nerve (0.5–2.5 mA, 400 µs) via a bar electrode placed approximately 2 cm proximal to the right wrist, and 3) simultaneous onset auditory and somatosensory stimulation as described in 1 and 2 above. Each type of stimulus was delivered one-hundred times in a pseudo-random order with an average inter-stimulus interval of 4 s (range 3–5 s).
To determine the appropriate intensity of median nerve stimulation for each participant, a threshold for detection was found by presenting current pulses that were stepped-up from zero in 0.10 mA increments until the participant reported a tingling feeling near their wrist. The current was set to 200% of the threshold (mean intensity = 1.36 mA).
5.3. Data acquisition and reduction
A 32 channel BioSemi ActiveTwo system (Cortech Solutions, Willmington, NC, US) with electrodes positioned according to the American Electroencephalographic Society Guidelines (1994) (Jasper, 1958) was used for continuous EEG recording. The Common Mode Sense (CMS) active electrode and Driven Right Leg (DRL) passive electrode were used as the reference and ground respectively (http://www.biosemi.com/faq/cms&drl.htm). Recordings were digitally sampled at 1024. Hz. Off-line data reduction using Brain Vision Analyzer software (Brain Products GmbH, Munich, DE) included re-referencing to an average of the two earlobes, filtering (0.1–100 Hz; roll-off = 12 dB/octave), and segmenting ERPs. Segments were 100 ms pre-stimulus (baseline) to 400 ms post-stimulus. Trials with blinks/large eye movements >150 mV based on horizontal and vertical electro-oculograms and trials with other artifact >150 mV were rejected. Accepted number of segments per stimulus type ranged from 41 to 94 trials (M = 60). The following variables were generated for each participant: 1) averaged auditory evoked potential, 2) averaged somatosensory evoked potential, 3) averaged simultaneous auditory and somatosensory evoked potential, 4) averaged summed auditory plus somatosensory evoked potential, and 5) averaged difference potential (the average of the summed unisensory responses subtracted from the average of the simultaneous responses). Spline-interpolated voltage maps of the unisensory ERPs provided voltage distributions across all 32 channels.
ERPScore (Segalowitz, 1999) was used to create and score averaged amplitudes for the simultaneous and summed responses at seven sites (Fz, Cz, Pz, C3, CP5, C4, CP6). Averaged amplitudes were calculated for four time-windows: (60–80 ms, 80–110 ms, 110–150 ms and 180–220 ms) by deriving an area measure between each ERP waveform and the 0 µV baseline. Time-windows were selected based on the centers of the major ERP components visually identified in the grand average multisensory responses, and refined based on visual inspection of the individual data.
5.4. Statistical analyses
MSI was first examined through a comparison of averaged amplitudes using a within subjects, repeated measures, two-way (stimulus type by electrode site) analysis of variance (ANOVA) for each time-window. The stimulus types were simultaneous and summed and the electrode sites were Fz, Cz and Pz. Two additional within subjects, repeated measures, two-way ANOVAs were used to examine MSI by hemisphere.
Factors were stimulus type (simultaneous and summed) by electrode site (C3, CP5 and C4, CP6). Alpha level was set at p <0.05 for all statistical tests. Based on the model provided by Molholm et al. (2002) (Molholm et al., 2002), an exploratory analysis was conducted to examine the spatio-temporal properties of MSI using multiple point-wise running paired t-tests (two tailed), with MSI defined as 10 consecutive data points (10.24 ms at 1024 Hz digitization rate) meeting an alpha level criterion of 0.05. This exploratory method met strict standards for conducting multiple t-tests (Guthrie and Buckwald, 1991). A spatio-temporal map was produced showing significant p values for all 32 electrode sites over the entire recording epoch.
The effect of age on MSI was examined by Pearson product moment correlations between age and averaged amplitude of the simultaneous auditory–somatosensory ERP at each electrode site for all four time-windows.
Acknowledgments
The authors wish to thank the children and their parents who participated in this study as well as The Wallace Research Foundation, The Children's Hospital Research Institute, and The General Clinical Research Centers Program at The Children's Hospital of Denver for support. Special thanks to Sarah A. Schoen, PhD, OTR for statistical support and to Wen-Pin Chang, Marianne Reale and Tracy Goldenberg for research assistance.
References
- Bruneau N, Roux S, Guerin P, Barthelemy C, Lelord G. Temporal prominence of auditory evoked potentials (N1 wave) in 4–8-year-old children. Psychophysiology. 1997;34(1):32–38. doi: 10.1111/j.1469-8986.1997.tb02413.x. [DOI] [PubMed] [Google Scholar]
- Calvert GA, Thesen T. Multisensory integration: methodological approaches and emerging principles in the human brain. J. Physiol. Paris. 2004;98(1–3):191–205. doi: 10.1016/j.jphysparis.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Di S, Brett B, Barth DS. Polysensory evoked potentials in rat parietotemporal cortex: Combined auditory and somatosensory responses. Brain Res. 1994;642(1–2):267–280. doi: 10.1016/0006-8993(94)90931-8. [DOI] [PubMed] [Google Scholar]
- Fort A, Delpuech C, Pernier J, Giard MH. Dynamics of cortico-subcortical cross-modal operations involved in audio-visual object detection in humans. Cereb. Cortex. 2002;12:1031–1039. doi: 10.1093/cercor/12.10.1031. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Morocz IA, Murray MM, Higgins BA, Javitt DC, Schroeder CE. Multisensory auditory-somatosensory interactions in early cortical processing revealed by high-density electrical mapping. Cogn. Brain Res. 2000;10(1–2):77–83. doi: 10.1016/s0926-6410(00)00024-0. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Schroeder CE. The case for feedforward multisensory convergence during early cortical processing. Neuroreport. 2005;16(5):419–423. doi: 10.1097/00001756-200504040-00001. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Wylie GR, Martinez A, Schroeder CE, Javitt DC, Guilfoyle D, Ritter W, Murray MM. Auditory-somatosensory multisensory processing in auditory association cortex: An fMRI study. J. Neurophysiol. 2002;88:540–543. doi: 10.1152/jn.2002.88.1.540. [DOI] [PubMed] [Google Scholar]
- Giard MH, Peronnet F. Auditory-visual integration during multimodal object recognition in humans: A behavioral and electrophysiological study. J. Cogn. Neurosci. 1999;11(5):473–490. doi: 10.1162/089892999563544. [DOI] [PubMed] [Google Scholar]
- Gobbelé R, Schürmann M, Forss N, Juottonen K, Buchner H, Hari R. Activation of the human posterior parietal and temporoparietal cortices during audiotactile interaction. Neuroimage. 2003;20(1):503–511. doi: 10.1016/s1053-8119(03)00312-4. [DOI] [PubMed] [Google Scholar]
- Guthrie D, Buckwald JS. Significance testing of difference potentials. Psychophysiology. 1991;28:240–244. doi: 10.1111/j.1469-8986.1991.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The 10–20 electrode system of the International Federation. Electroencephalogr. Clin. Neurophysiol. 1958;10(2):370–375. [PubMed] [Google Scholar]
- Lam K, Kakigi R, Kaneoke Y, Naka D, Maeda K, Suzuki H. Effects of visual and auditory stimulation on somatosensory evoked magnetic fields. Clin. Neurophysiol. 1999;110:295–304. doi: 10.1016/s0168-5597(98)00059-8. [DOI] [PubMed] [Google Scholar]
- Luck SJ. An introduction to the event-related potential technique. MIT Press; Cambridge: 2005. [Google Scholar]
- Lütkenhöner B, Lammertmann C, Simões C, Hari R. Magnetoencephalographic correlates of audiotactile interaction. Neuroimage. 2002;15(3):509–522. doi: 10.1006/nimg.2001.0991. [DOI] [PubMed] [Google Scholar]
- Molholm S, Ritter W, Muttay MM, Javitt DC, Schroeder CE, Foxe JJ. Multisensory auditory–visual interactions during early sensory processing in humans: a high density electrical mapping study. Cogn. Brain Res. 2002;14:115–128. doi: 10.1016/s0926-6410(02)00066-6. [DOI] [PubMed] [Google Scholar]
- Murray MM, Molholm S, Michel CM, Heslenfeld DJ, Ritter W, Javitt DC, Schroeder CE, Foxe JJ. Grabbing your ear: rapid auditory–somatosensory multisensory interactions in low-level sensory cortices are not constrained by spatial alignment. Cereb. Cortex. 2005;15:963–974. doi: 10.1093/cercor/bhh197. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Picton TW. The N1 wave of the human electric and magnetic response to sound: a review and analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Eggermont JJ, Kwong B, Don M. Maturation of human central auditory system activity: evidence from multi-channel evoked potentials. Clin Neurophysiol. 2000;111(2):220–236. doi: 10.1016/s1388-2457(99)00236-9. [DOI] [PubMed] [Google Scholar]
- Regan D. Human brain electrophysiology: evoked potentials and evoked magnetic fields in science and medicine. Brain Res. 1989;101:345–354. [Google Scholar]
- Schürmann M, Caetano G, Hlushchuck Y, Jousmäki V, Hari R. Touch activates human auditory cortex. Neuroimage. 2006;30:1325–1331. doi: 10.1016/j.neuroimage.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ. ERPScore program: peak and area analysis of event-related potentials. Brock University; St. Catherines: 1999. [Google Scholar]
- Stein BE, Meredith MA. The Merging of the Senses. MIT Press; Cambridge: 1993. [Google Scholar]
- Talsma D, Woldorff MG. Selective attention and multisensory integration: multiple phases of effects on the evoked brain activity. J. Cogn. Neurosci. 2005;17(7):1098–1114. doi: 10.1162/0898929054475172. [DOI] [PubMed] [Google Scholar]
- Teder-Sälejärvi WA, McDonald JJ, Di Russo F, Hillyard SA. An analysis of audio-visual crossmodal integration by means of event-related potential (ERP) recordings. Cogn. Brain Res. 2002;14:106–114. doi: 10.1016/s0926-6410(02)00065-4. [DOI] [PubMed] [Google Scholar]
- Zumsteg D, Wieser HG. Effects of aging and sex on middle-latency somatosensory evoked potentials: normative data. Clin. Neurophysiol. 2002;113:681–685. doi: 10.1016/s1388-2457(02)00054-8. [DOI] [PubMed] [Google Scholar]