Abstract
Vocal fold tissue engineering requires biomimetic scaffolds with an appropriate matrix stiffness closely matching that of the natural vocal folds to maintain function. Traditionally, poly(ε-caprolactone) (PCL) and thermoplastic polyurethane (TPU) have been employed as the primary matrix materials for vocal fold electrospun scaffolds. However, not all of the scaffolds fabricated thus far matched the human vocal fold tissues. Poly(glycerol sebacate) (PGS) is a non-cytotoxic and biodegradable soft elastomer that has shown promising results for soft tissue engineering applications. However, no work has been done to employ this biomaterial to construct vocal fold scaffolds. In this study, PGS has been synthesized and blended with thermoplastic polyurethane (TPU) to produce vocal fold scaffolds with improved hydrophilicity and compliance by electrospinning. The resulting scaffolds were found to have mechanical properties mimicking those of the vocal fold lamina propria extracellular matrix (ECM). An unusual leaf-like structure was obtained when using 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) as the solvent. Other suitable fibrous scaffolds were also obtained when using acetic acid and 2,2,2-trifluoroethanol (TFE) as binary solvents. A biological evaluation of these TPU/PGS scaffolds showed better cell spreading and significantly improved cell proliferation as compared to TPU-only scaffolds (p < 0.01), thereby suggesting potential applications for vocal fold tissue engineering.
1. Introduction
The lamina propria of the human vocal fold is made of soft connective tissue which is easily damaged by various environmental factors, diseases and injuries [1]. Injury to the vocal folds results in scar tissue, thereby altering vocal biomechanics and subsequently causing hoarseness [2, 3]. According to recent estimates, up to 9% of adults in the general population will experience a voice disorder and detrimentally affect their daily lives [3]. To date, there has been little success in the treatment of vocal fold scarring [4–6]. Although great progress has been made toward the fabrication of scaffolds, there is little advancement in electrospun materials that reproducing the mechanical properties of vocal fold, especially an appropriate matrix stiffness [3].
Electrospinning, which applies electrical forces to produce nano- to microfiber mats, is an extremely promising method for the preparation of tissue engineering scaffolds [7–8], especially for thin vocal folds [9]. Until now, there have been only two reports using electrospinning to prepare tissue engineering scaffolds for the vocal folds. Jia et al. [10] used fibrous poly(ε-caprolactone) (PCL) scaffolds, prepared by electrospinning, to emulate the lamina propria structure of the vocal folds. Combining the synthetic fibrous scaffolds with high-frequency vibratory stimulation created by a bioreactor, these scaffolds served as a potent modulator of mesenchymal stem cells (MSCs) functions and for the engineering of functional vocal fold tissues. Thibeault et al. [3] also used nanofibrous TPU scaffolds to mimic the structure of the vocal fold lamina propria. The results suggested that both the aligned structure and elastin-like polypeptide coating were beneficial for human vocal fold fibroblast (HVFF) growth. Unfortunately, not all of the scaffolds matched the mechanical properties of human vocal fold tissue. Since matrix stiffness is a vital tissue engineering parameter for directing tissue development and subsequently encouraging regeneration [11–13], preparing an appropriate biomaterial to better match the stiffness of human vocal folds is of the utmost importance.
Traditional biomaterials such as poly(lactic acid) and poly(ε-caprolactone) are biocompatible and biodegradable in vivo [14–15]; however, their elastic moduli are too high compared to human vocal folds [16]. Elastomeric biomaterials such as poly(ether urethane urea) and poly(caprolactone-co-lactic acid) are an important class of polymers used in the medical, pharmaceutical, and biomedical fields [17]. Although their mechanical properties can be tuned by the ratio of hard segments to soft segments [18], these elastomers also have moduli in the MPa–GPa range, which is also stiffer than human vocal fold lamina propria that has moduli in the range of 50 KPa to 730 KPa depending on the gender, age and spatial location of the tissue[19]. Therefore, in order to overcome this limitation, it is essential to develop an elastomeric biomaterial with suitable mechanical properties.
Poly(glycerol sebacate) (PGS), a non-cytotoxic and biodegradable soft elastomer, has already shown promising results for soft tissue engineering applications [20] such as cardiac muscle [21], cartilage [22], vascular structures [23], and nerve conduits [24]. This soft elastomer has a modulus in the range of 0.04–1.2 MPa depending on the reaction condition [20, 25–26] that mimicked the mechanical stiffness of human vocal fold [19]. However, no work has been done to develop this remarkable material to construct scaffold for vocal fold tissue engineering applications. On the other hand, PGS has a relatively fast degradation rate and it is hard to electrospin by itself [27–33]. Biodegradable thermoplastic polyurethane (TPU) is a kind of elastomeric, easy to electrospin biomaterial having slower degradation rate and approaching matrix stiffness to native vocal fold [3, 17]. Therefore, it is envisioned that through blending TPU with PGS, it would be possible to develop a suitable biological substrate for vocal fold scaffold tissue engineering application.
In this work, we focused on material formulation and synthesis, process optimization, and mechanical property regulation. We synthesized fibrous scaffolds using elastomeric TPU and PGS with mechanical properties similar to native human vocal fold. No work has been done to spin these two biomaterials together, so the electrospinning parameters were optimized based on the morphologies of the scaffolds. The chemical composition, thermal properties, surface wettability, and mechanical properties were characterized for the TPU/PGS hybrid scaffolds at different blend ratios. Furthermore, the biocompatibility, including the cell morphology and cell proliferation of the fabricated composites, was also assessed.
2. Materials and methods
2.1. Materials
Medical grade TPU (Tecoflex SG-93A) was generously donated by Lubrizol, Countryside, USA. Glycerol and sebacic acid for synthesizing PGS were purchased from Sigma–Aldrich (Milwaukee, WI, U.S.A.). Chloroform (CF), N,N-dimethylformamide (DMF), acetic acid, and 2,2,2-trifluoroethanol (TFE) were also purchased from Sigma–Aldrich (Milwaukee, WI, U.S.A.). 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) was purchased from Oakwood Products, Inc. (West Columbia, SC). The materials were all used as received without further purification.
2.2. Synthesis of poly(glycerol sebacate) (PGS)
PGS was synthesized based on previously published polycondensation methods [25, 32–33]. Briefly, equimolar glycerol and sebacic acid were heated to 130°C in a 250 ml two-neck round-bottom flask under a nitrogen atmosphere for 24 hours. The pressure was then gradually decreased to 50 mTorr and the reaction was continued for 36 hours. The resulting yellowish colored, highly viscous PGS was cooled and stored in a closed glass container at room temperature until further use.
2.3. Fabrication of TPU–PGS fibrous scaffolds
For preparation, an appropriate amount of TPU pellets were dissolved in a mixture of chloroform (CF) and N,N-dimethylformamide (DMF) (v/v=6:4) to achieve a 6% (w/v) solution. The TPU/ PGS blends were also dissolved in chloroform (CF) and N,N-dimethylformamide (DMF) (v/v=6:4) at various polymer ratios (6:6, 6:4, and 6:2) and the concentration of TPU was kept at 6% (w/v). A single solvent 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) and a binary solvents 2,2,2-trifluoroethanol (TFE) and acetic acid at 5:5 v/v were used to spin another set of TPU/PGS blends. The concentrations and ratios between TPU and PGS were the same as the CF/DMF solution. Next, the prepared solution was injected into a 10 ml syringe that was connected by Teflon tubing as well as an 18-gauge blunted needle. The electrospinning parameters were kept the same for all of the solvent systems. The applied voltage was 18 kV, the flow rate was kept at 0.5 ml/h, and the distance from the needle to the collector was 20 cm. The temperature and humidity at ambient condition were around 25 °C and 30%, respectively. Flat aluminum foil was used as a collector to gather all of the electrospun fibers.
2.4. Characterization methods
2.4.1. Morphological characterization (SEM)
The microstructure and morphologies of obtained fibrous scaffolds were observed by scanning electron microscopy (SEM) (LEO GEMINI 1530) with an accelerating voltage of 10 kV. Samples were coated with gold for 40 s before imaging. The fiber diameters and diameter distributions were analyzed from SEM images using Image-Pro Plus software. At least fifty fibers were measured from three SEM images to calculate the average geometric information.
2.4.2. Pore size distributions and porosity
The mean pore size and pore size distributions of electrospun scaffolds were determined by a capillary flow porometer (CFP-1500A). A low surface tension liquid (Galwick, 16 mN/m) was used as the wetting fluid in the test.
The porosities of the scaffolds were investigated using the gravimetric analysis method [34–35]. Based on the water contact angle test, isopropyl alcohol was used as the wetting liquid for the TPU scaffolds and water was used for the composite scaffolds. The weight of the samples was measured before and after liquid penetration. The porosity was calculated using the following equation,
| (1) |
where Wwet and Wdry are the weight of the wet and dry membranes respectively, ρl is the density of the wetting liquid, and S and T are the effective area and thickness of the membrane, respectively.
2.4.3. Fourier transform infrared spectroscopy (FTIR)
Fourier transform infrared (FTIR) spectra of the fabricated electrospun scaffolds were obtained using a Bruker Tensor 27 FTIR instrument. All of the samples were measured in transmittance mode in the range of 4000 to 600 cm−1 with a resolution of 4 cm−1.
2.4.4. Thermogravimetric analysis (TGA)
The thermal properties of the nanocomposites were determined by thermogravimetric analysis (TGA) (TGA Q50, TA Instruments). Samples of approximately 10 mg were heated from room temperature to 600 °C at a heating rate of 10 °C/min. The scaffold weight was measured against the temperature.
2.4.5. Differential scanning calorimetry (DSC)
The thermal properties and crystalline behavior of the TPU–PGS scaffolds were determined using a differential scanning calorimeter (DSC) (Q20, TA instruments, USA). Pre-weighted scaffolds (around 5–6 mg) were sealed in aluminum pans and heated at 10 °C/min from –70 °C to 160 °C. All tests were carried out under the protection of a nitrogen atmosphere and an empty aluminum pan was used as a reference.
2.4.6. Water contact angle (WCA) tests
All of the samples were placed in a vacuum oven at 30 °C for 3 days to ensure that all of the solvent had evaporated before performing the water contact angle tests. The surface water contact angles (WCAs) of the nanofibrous scaffolds were tested using the sessile drop method in air at ambient temperature using a video contact angle instrument (Dataphysics OCA 15, Germany). The droplet size was fixed at 4 μL, and the surface contact angle was determined 15 s after the water droplet was deposited on the surface of the mats. Five specimens for each group were tested at different surface locations on the scaffolds to calculate the average value.
2.4.7. Mechanical properties under wet conditions
The mechanical properties of the blended TPU–PGS scaffolds were characterized using an Instron 5967 universal testing machine with a 50 N load cell and a crosshead speed of 5 mm/min. The electrospun scaffolds were cut into rectangular shapes with dimension of 20 × 10 mm2 (length × width), and the thickness of the samples were around 100 μm as measured by a caliper before testing. Each specimen was soaked in Dulbecco’s Phosphate-Buffered Saline (DPBS) for 1 h before being measured. In order to avoid slippage during the tensile test, the samples were griped form two ends using double-sided foam tape. A preload of 0.01 N was applied on the scaffolds before the start of the experiment. The slope of the linear portion of the stress–strain curve was calculated as the elastic modulus. Five specimens were tested for each ratio and the average values and standard deviations were reported.
2.5. Biocompatibility evaluations
2.5.1. Cell culture and seeding
Swiss mouse NIH 3T3 fibroblasts were used for the biocompatibility evaluations on the prepared scaffolds. The cells were cultured in high-glucose DMEM (Dulbecco’s Modified Eagle Medium) (Invitrogen), supplemented with 10% fetal bovine serum (FBS) (WiCell), and 1% penicillin-streptomycin (Invitrogen). Fibroblasts were cultured in 6-well tissue culture-treated polystyrene (TCPS) plates (BD Falcon) and incubated at 5% CO2 and 37 °C. Cells were routinely passaged at a 1:20 ratio before reaching ~80–90% confluence, and the culture medium was replaced every two days. Before cell seeding, all of the samples were sterilized by ultraviolet (UV) light irradiation for 30 minutes on each side and placed into 24-well TCPS plates. NIH 3T3 fibroblasts were dissociated with trypsin-EDTA for 5 min and then seeded onto fibrous substrates at a density of 20,000 cells/well for cell morphology observation and proliferation studies. All tests were done with at least three replicates.
2.5.2. Cytoskeleton fluorescent staining
The shape and cytoskeleton organization of the fibroblast cells were analyzed after 3, 6, and 9 days of culture. For these assays, cellular substrates were fixed on ice with 4% paraformaldehyde solution in DPBS for 15 minutes. Cells were then gently rinsed with DPBS three times and permeabilized with 0.5% Triton X-100 in DPBS at room temperature for 10 minutes. The cells were washed three additional times with DPBS and stained with biotium phalloidin CF™568 for the actin filament and 4’, 6-diamidino-2-phenylindole (DAPI) for the cell nuclei for 1 h at room temperature. Samples were then washed with DPBS and imaged under a fluorescence microscope (Olympus, Shinjuku, Tokyo, Japan).
2.5.3. Cell proliferation assay
Cell proliferation assays were conducted after 3, 6, and 9 days culture using MTT assays (Promega). This assay utilized formazan that was bioreduced upon entering a live cell. The resulting products predictably altered the absorbance of the media in which the cells resided. Standard curves were established and confirmed by comparison with hemocytometer readings prior to the experiments. Upon testing, 500 µL of 500 µg/mL MTT dissolved in media was put onto five samples of each type. After incubating for 4 hours at room temperature, the media containing MTT was aspirated off and 200 µL of Dimethyl sulfoxide (DMSO) were added. The number of cells was calculated by measuring the absorbance at 560 nm for each sample, with a reference wavelength of 750 nm.
2.6. Statistical analysis
All of the quantitative results were expressed as mean ± standard deviation: (SD). Statistical analyses were performed using a one-way analysis of variance (ANOVA). A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Morphology of TPU fibrous scaffolds
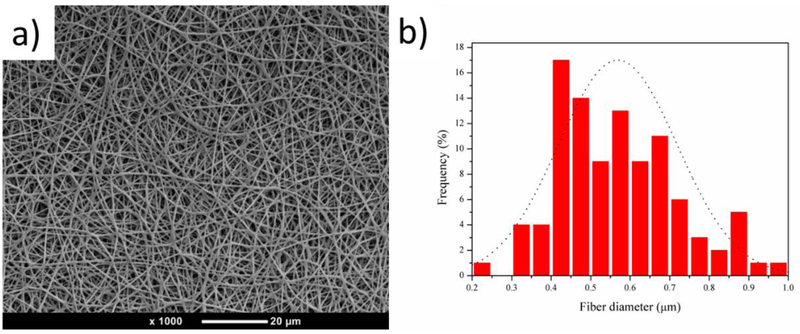
TPU nanofibrous scaffolds were obtained by electrospinning 6% (W/V) TPU/CF/DMF solution as discussed in the Materials and Methods section above. Figure 1 (a) shows an SEM image of a random electrospun TPU nanofibrous scaffold that was smooth and bead-free. Scaffolds were further characterized by measuring the fiber diameters using the Image-Pro Plus software. The average diameter of TPU nanofibers was 568 nm, and the diameter of the nanofibers ranged from 244 to 957 nm, as is illustrated in Figure 1 (b).
Figure 1.
(a) SEM image of an electrospun TPU nanofibrous scaffold, and (b) the fiber diameter distribution of a TPU nanofibrous scaffold.
3.2. Morphology of TPU–PGS hybrid fibrous scaffolds
The selection of a suitable solvent system is indispensable for successful electrospinning. Thus this part was focused on the optimization of the TPU/PGS electrospinning process with regard to the type of solvent used in electrospun solution.
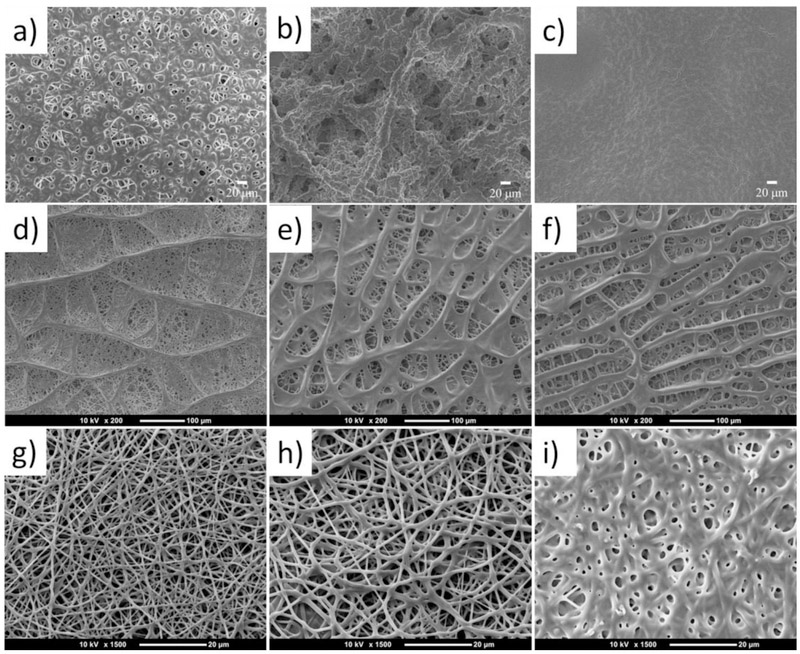
First, TPU/PGS blend fibers were prepared by using CF/DMF as the solvent (first row of Figure 2). Single fibers can be seen in some area when the blend ratio between TPU and PGS was 6:2, but meanwhile the fibers began to fuse to each other in some area (Figure 2(a)). For spinning solution with a 6:4 TPU/PGS blend, the fibers were all fused to each other without perfect shape. Further increasing the blend ratio to 6:6 TPU/PGS, all the materials fused together and the microstructure of the membranes looked like a sheet rather than a fibrous matrix. This phenomenon maybe caused by the difference between the evaporation pressure and boiling temperature of CF and DMF. TPU/PGS is easier to phase separate during electrospinning process when using CF and DMF binary solvent system, and the phase separation intensified with the increasing ratio of PGS.
Figure 2.
SEM images of the TPU/PGS blended nanofibrous scaffolds from an electrospun solution in CF/DMF at various ratios between TPU and PGS, including (a) 6:2, (b) 6:4, (c) 6:6, in HFIP at various ratios between TPU and PGS, including (d) 6:2, (e) 6:4, (f) 6:6, and in TFE/acetic acid at various ratios between TPU and PGS, including (g) 6:2, (h) 6:4, and (i) 6:6.
1,1,1,3,3,3 - hexafluoroisopropanol (HFIP) was also tried to electrospin TPU/PGS blend (second row of Figure 2). A fascinating structure was obtained when this kind of solvent was used. When the ratio between TPU and PGS was 6:2, the microstructure of the fibers looked like a leaf, as can be seen in Figure 2 (d). The main stem of this interesting structure became smaller by increasing the blend ratio of PGS (Figure 2 (e) and (f)). The boiling point for HFIP was 59°C and the vapor pressure was 120 mmHg at 20 °C; thus, it evaporated easily at room temperature. The generation of this structure may have been due to the phase separation phenomenon between TPU and PGS when they were dissolved in HFIP during the electrospinning process. By increasing the ratio of PGS, the phase separation phenomenon intensified. Thus, when the ratio of PGS increased to 4 or 6, the main stem of this interesting structure became compact, as illustrated in the first row of Figure 2. As far as we know, this unique structure has not been reported in other studies, and it may have unique properties when used in applications such as waste water filtration [36]. Further research on this structure is planned for the future.
To obtain a uniform fibrous scaffold in which fibers has a perfect shape, 2,2,2-trifluoroethanol (TFE) and acetic acid 5:5 v/v binary solvents were used to spin the blends. Uniform fibers with perfect shape were obtained when the ratio between TPU and PGS was 6:2 and 6:4. The diameter of the fibers was related to the ratio of PGS. An increase in PGS content led to an increase in the diameters of the fibers, as shown in Figures 2 (g) and (h). A further increase in the content of PGS resulted in a fusion of the fibers, as illustrated in Figure 2 (i), which was similar to prior work [28]. This was because PGS is less viscous and has a low spinnablity because of low-molecular-weight, and when the ratio of PGS is increased, the phase separation intensified between TPU and PGS, subsequently some fibers began to fuse to each other [37–39]. Based on the morphological investigation, electrospun TPU:PGS fibrous scaffolds at various ratios, including pure TPU, 6:4, and 6:2, were fabricated and are characterized in the next sections.
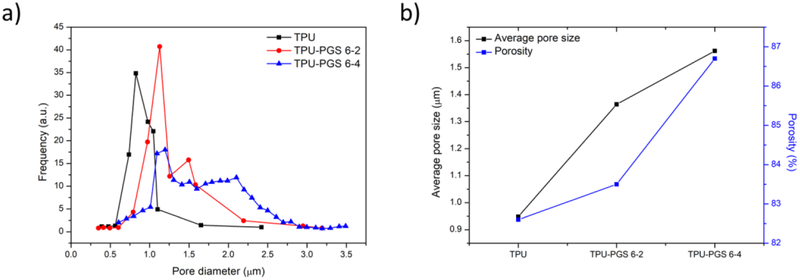
The average diameter of the composite electrospun fibers were further characterized by measuring the fiber diameters using the Image-Pro Plus software. The average diameter of TPU-PGS 6–2 fibers was 725 nm, and ranged from 258 to 1062 nm. The average diameter of TPU-PGS 6–4 fibers was 956 nm—higher than the other two kinds of scaffolds—and ranged from 273 nm to 1686 nm. This increasing fiber diameter trend could be attributed to the decreased conductivity of the composite solutions. Figure 3 (a) shows the pore size distribution obtained from the fibrous membranes. It can be seen that as the PGS content of the scaffold increased, the pore size distribution shifted to higher and wider value ranges. As shown in Figure 3 (b), the mean pore size and porosity of the fibrous membranes both increased with increasing PGS content. This was because the composite fibrous membranes had larger average fiber diameters and wider diameter value ranges as illustrated above. The porosities of all of the scaffolds were higher than 80%, which is an attractive asset for tissue engineering applications as higher porosities indicate more surfaces for cell culture media to pass through [40].
Figure 3.
(a) Pore size distribution, and (b) average pore size and porosity of the TPU/PGS blended nanofibrous scaffolds.
3.3. Analysis of fiber composition
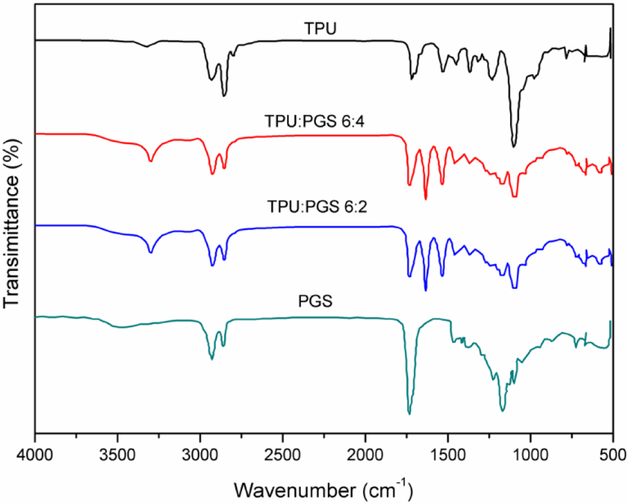
The purpose of using FTIR was to confirm the existence of both TPU and PGS in the electrospun matrices. FTIR spectra of pure TPU, PGS, and TPU/PGS at ratios of 6:4 and 6:2 are shown in Figure 4. Generally, three primary bands were detected at 1692 cm−1 (amide I C=O stretching), 1531 cm−1 (amide II N–H in-plane bending), and 1232 cm−1 (amide III C–N stretching) in the pure TPU spectrum. Other bands, such as the asymmetric CH2 stretching vibration at 2929 cm−1 and the symmetric CH2 stretching vibration at 2858 cm−1, were also observed in the spectrum. Furthermore, TPU exhibited the stretching of ether group C–O–C at 1100 cm–1, as well as the stretching vibrations of N–H bonds located around 3320 cm-1. The PGS spectrum also exhibited stretch vibration peaks for the alkyl group (–CH2) at 2927 cm−1, 2859 cm−1, and 1384 cm-1. The ester bands presented stretching vibration C=O bonds at 1732 cm−1, and the carbonyl stretching bonds (C–O) at 1170 cm-1. There was also a broad band around 3500 cm−1 that indicated the hydroxyl bond (–OH) stretch vibration. The blended fibrous scaffolds showed not only overlapping peaks at 2927 cm−1, 2859 cm−1, and 1384 cm−1 for the alkyl group, but also unique TPU and PGS peaks, thus confirming the material composition of the composite fibers.
Figure 4.
FTIR spectra of the electrospun fibrous scaffolds for pure TPU and TPU/PGS at 6:4 and 6:2 ratios compared with a pure PGS sheet.
3.4. Thermal properties
Thermal behavior determines the physical properties of the tissue engineering scaffold, such as mechanical stability and degradation. Therefore, thermal behavior is an important characteristic property to be extensively studied while fabricating any new polymer composite [41]. To gain deeper insight into the thermal properties of the electrospun TPU/PGS scaffolds, we performed TGA and DSC tests on the samples.
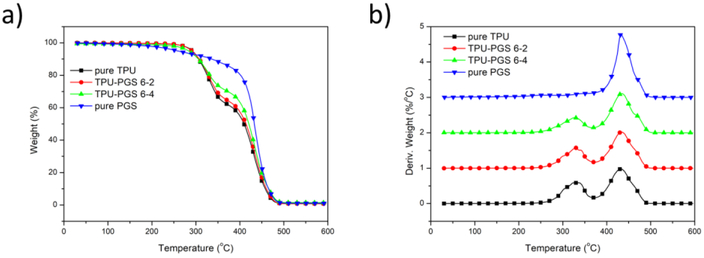
As shown in Figure 5, pure PGS material started to decompose at approximately 433 °C. For pure TPU nanofibrous scaffolds, two obvious weight loss steps could be seen, belonging to the decomposition of the hard and soft segments. Because the amide bonds in the hard segments were easier to cleave than the ether bonds in the soft segments [42], the first decomposition temperature of 333 °C belonged to the hard segments, while the second one at 432 °C belonged to the soft segments. In addition, as the content of PGS increased, the scaffolds had a lower proportion of hard segments and better thermal stability.
Figure 5.
(a) TGA and (b) DTG results of pure TPU, TPU:PGS 6:2, TPU–PGS 6:4 electrospun fibrous scaffolds, and pure PGS.
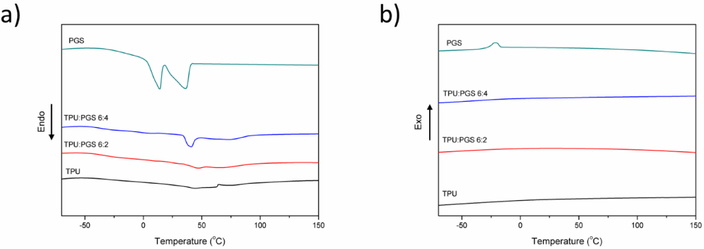
DSC was further used to determine the effect of the addition of PGS on the thermal properties of the electrospun TPU–PGS scaffolds. Figure 6 shows the DSC thermograms (heating cycle and cooling cycle) of PGS, TPU, and TPU–PGS scaffolds. Pure PGS polymer showed two melting endotherms (first sharp and second broad bands) with a Tm1 of 12.5 °C and a Tm2 of 42.67 °C, similar to that reported in the literature [43–44]. In addition, a crystallization peak appeared during the cooling cycle with a crystallization temperature of –15 °C, which was similar to a previous report [43]. For pure TPU scaffolds, all of the samples were amorphous and lacked obvious melting peaks and crystallization peaks [45]. When PGS was added to the scaffolds, a melting endotherm appeared at a blend ratio of 6:4. Furthermore, the melting endotherm disappeared when there was a lower amount of PGS. The melting temperature shown for the blended polymers was the highest (approximately 43 °C), similar to previous findings [46]. No crystallization peak appeared when the TPU and PGS were blended together. Overall, the results indicated that TPU and PGS were partly miscible after the electrospinning process.
Figure 6.
Differential scanning calorimetry results of TPU, TPU:PGS 6:4, and TPU:PGS 6:2 electrospun scaffolds and PGS: (a) heating cycle and (b) cooling period.
3.5. Surface wettability
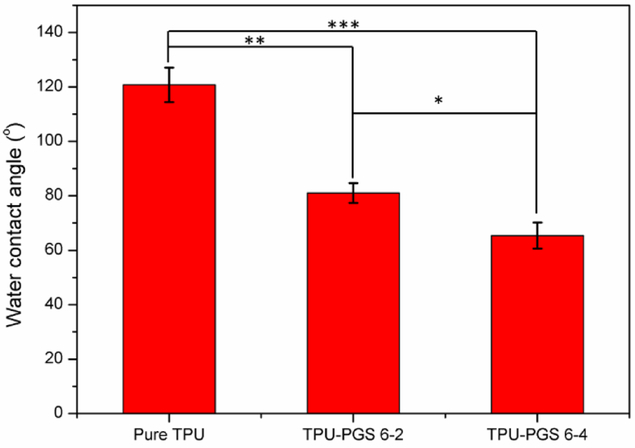
It has been reported that improved hydrophilicity is better for cell adhesion, proliferation, and infiltration in materials intended for tissue engineering [47]. Thus, the water contact angle—which can directly reflect the surface wettability of scaffolds—was measured. As shown in Figure 7, the water contact angle for pure TPU scaffolds was around 120.8±6.4°, which showed a greater hydrophobic behavior. The water contact angle changed to 81.0±3.7° for TPU/PGS 6:2, showing that the incorporation of PGS greatly improved the hydrophilicity of the scaffolds. The contact angle of a water droplet on TPU/PGS blend nanofibrous scaffolds decreased with increasing PGS content (65.4±4.8° for TPU/PGS 6:4). The enhanced hydrophilicity was due to the addition of PGS, which has been reported previously [48–49]. Thus, these properties affect the cellular responses in the blended scaffolds as water molecules at the interfaces influence cell adhesion which will be evaluated in a later section.
Figure 7.
Water contact angles of the TPU and TPU/PGS blend nanofibrous scaffolds (statistical significance was shown as *p < 0.05, **p < 0.01, ***p < 0.001).
3.6. Mechanical properties
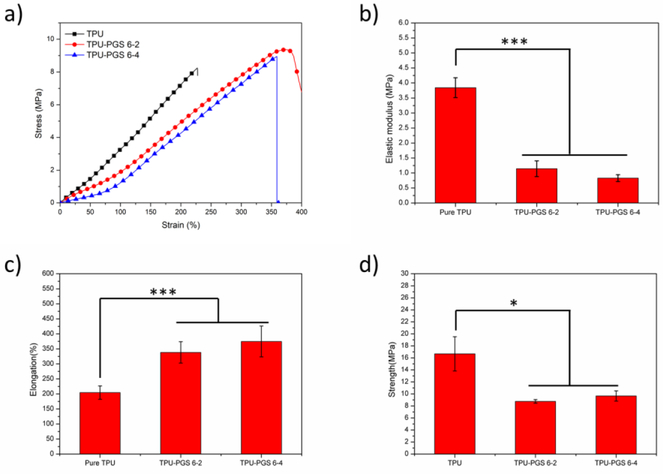
Tissues within the body exhibit different mechanical properties depending on their functionalities. The mechanical properties of tissue scaffolds are among a crucial set of parameters that significantly influence cell functions, including cell adhesion, motility, organization, and differentiation [50–51]. Therefore, uniaxial tensile testing under wet condition was done to investigate the mechanical properties of the resulting fibrous scaffolds. Representative stress–strain curves for the scaffolds can be seen in Figure 8(a). All scaffolds exhibited similar strain–stress trends—a linear region followed by a non-linear region without the necking phenomenon. The elastic modulus, maximum elongation, and ultimate tensile strength were obtained from the uniaxial tensile tests (Figures 8(b)–(d)). The elastic modulus of the TPU/PGS blended scaffolds changed from 3.16±0.28 MPa for pure TPU to 1.14 ± 0.26 MPa for TPU/PGS 6:2 and 0.83±0.12MPa for TPU/PGS 6:4 scaffolds. The elongation-at-break and Young’s modulus showed a similar trend. With the increasing content of PGS in the fiber, the elongation-at-break increased from 204.7 ± 22.2% for pure TPU scaffolds to 338.6 ± 35.6% for TPU/PGS 6:2 and 374.9 ± 51.4% for TPU/PGS 6:4. The ultimate tensile strength of the scaffolds decreased after adding PGS; pure TPU had an ultimate tensile strength of 16.69 ± 2.85 MPa, TPU/PGS 6:2 had an ultimate tensile strength of 8.76 ± 0.30 MPa, and TPU/PGS 6:4 had an ultimate tensile strength of 9.67 ± 0.85 MPa. This tendency was similar to a previous report [52]. Based on surface wettability observations, adding PGS to the scaffolds also enhanced the hydrophilicity of the matrix, thus capturing more water molecules in the scaffold. Either the water molecules or the addition of PGS had a plasticizing effect on the mechanical properties of the blended scaffolds, thereby decreasing the stiffness and improving the elongation of the blended scaffolds [32].
Figure 8.
Mechanical properties of TPU:PGS fibrous scaffolds. (a) The stress–strain curve, (b) Young’s modulus, (c) elongation-at-break, and (d) ultimate tensile strength (statistical significance was shown as *p < 0.05, **p < 0.01, ***p < 0.001).
3.7. In vitro cytotoxicity assays
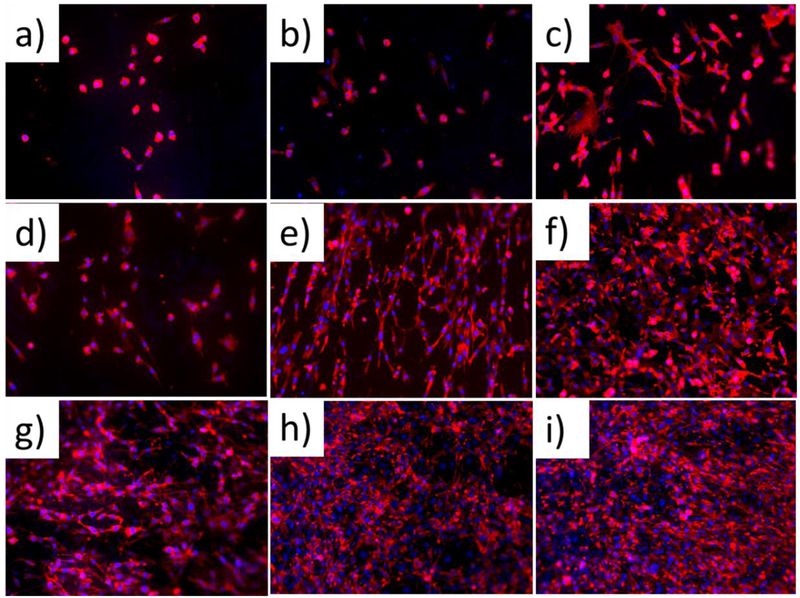
The cell morphology and cytoskeleton organization of TPU and PGS blended scaffolds were characterized by fluorescent microscopy, with F-actin labeled with red and nuclei labeled with blue (Figure 9). After 3 days of culture, cells displayed a spherical shape on the pure TPU scaffolds (Figure 9 (a)), which is a non-spreading, non-biocompatible morphology for fibroblasts. However, a healthy, spindle-like morphology began to show on hybrid scaffolds, with almost all of the fibroblasts displaying a spreading, biocompatible morphology with an increased ratio of PGS (Figures 9(b) and (c)). Continuing to culture the cells for 6 days, cells on the pure TPU began to spread (Figure 9(d)) and all of the fibroblasts became highly stretched on the hybrid scaffolds (Figures 9(e) and (f)), suggesting that they spread easily on the TPU and PGS composites. In addition, fibroblasts on the blended scaffolds joined together and/or overlapped one another, whereas cells remained separated on the pure TPU scaffolds. This signified that cells on the hybrid scaffolds interacted very well with each other. A significant increase in cell number and cell spreading could be seen on all three kinds of matrixes after 9 days of cell culture, thus indicating that TPU and PGS were both biocompatible (Figures 9 (g), (h), and (i)). Similar to 6 days of cell culture, by day 9, more cells spread across the area of the composites (Figures 9 (h) and (i)). Overall, adding PGS to the nanofibrous scaffolds resulted in the largest cell spreading area and strongest cell interactions.
Figure 9.
Cytoskeleton of cells grown on (a, d, g) pure TPU scaffolds, (b, e, h) TPU/PGS 6:2 scaffolds, and (c, f, i) TPU/PGS 6:4 scaffolds after culturing for 3 days (the first row), 6 days (the second row), and 9 days (the third row), respectively.
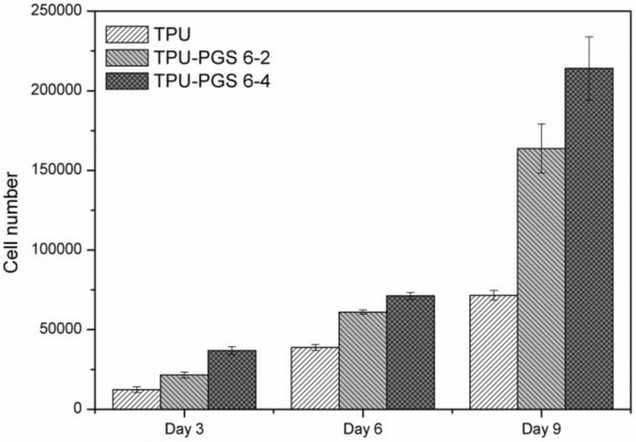
As is well known that the quantitative cell proliferation results demonstrated the cytocompatibility of a scaffold, which play a vital role in indicating the potential tissue engineering applications of the substrate. In this study, the quantitative cell proliferation results after 3, 6, and 9 days of in vitro cell culture were investigated and shown in Figure 10. Obviously, more cells could be observed on the scaffolds after 6 and 9 days of in vitro cell culture than 3 days, indicating better proliferation within 9 days. On the other side, it can be clearly seen that more cells survived on the blended scaffolds, with higher cell proliferation rates linked to higher ratios of PGS for day 3 results. The increasing trend between pure TPU and composite scaffolds continued throughout the cell culture process. These results were consistent with the immunofluorescent staining results (Figure 9), suggesting that the growth and proliferation of 3T3 fibroblasts on the TPU/PGS surfaces were better than that on the TPU-only scaffold.
Figure 10.
Cell proliferation on the scaffolds after culturing for 3 days, 6 days, and 9 days determined by MTT assay.
4. Discussion
Most tissue cells are not viable when suspended in a fluid, thus normal tissue cells must not only adhere to a solid but also pull on their microenvironment and subsequently respond to the matrix stiffness in ways that relate to tissue elasticity [53]. For instance, some cells have important phenotypes when they are cultured on soft materials that is essential to their function. Hence, constructing a fibrous matrix with elasticity that approximates the lamina propria of the human vocal fold is of the utmost importance. According to Kellehera et al.[19], the longitudinal elastic modulus varies greatly with spatial location along lamina propria of the vocal fold, from 50 KPa to 730 KPa. Traditional biomaterials such as poly(lactic acid) and poly(ε-caprolactone), even elastomeric biomaterials such as poly(ether urethane urea) and poly(caprolactone-co-lactic acid), have moduli in the MPa–GPa range, which are too high compared to vocal fold lamina propria[16–19]. The present investigation focused on developing a soft elastomer PGS and constructing fibrous scaffolds by elastomeric TPU and PGS to mimic the mechanical stiffness of human vocal fold.
To date, there has been no reports of electrospinning TPU and PGS together, so electrospinning parameters need to be first optimized. The solvent system used in preparing electrospun solutions plays a vital role in its spinnability, because dissolving polymer in an appropriate solvent is the first and foremost step in the electrospinning process [54]. The volatility, vapor pressure and boiling point of the solvents have a significant influence on the evaporation rate and the drying time during the jet thinning process. Hence, the selection of a suitable solvent system is indispensable for successful electrospinning. TPU/PGS fibers fused to each other even when small proportions of PGS was added, when using a CF and DMF binary solvent system. However, fibers with perfect shape can be obtained when using a TFE and acetic acid binary solvent system. A unique leaf-like structure was acquired by using single solvent HFIP to prepare the electrospun solution. These results further verified the importance of solvent system selection for electrospinning. However, further investigations need to be completed to disclose the formation mechanism of leaf-like structure and its potential applicational value. Besides solvent system selection for electrospinning TPU with PGS, another challenge for fabricating TPU–PGS scaffolds is to crosslink the hydroxyl groups on the molecular chains of PGS without ruining the fibrous structure. Fibrous scaffolds will melt under the traditional harsh PGS crosslinking conditions, especially at the high crosslinking temperature (around 130°) [28]. Hence, like some previous works [44], the PGS in the composite scaffolds were not crosslinked in this work. We will try to develop new a crosslinking method by chemical modification of PGS or by adding crosslinking agents in our future work.
To characterize the matrix stiffness, uniaxial tensile tests were performed on the hybrid scaffold. The results showed that TPU/PGS 6:4 hybrid scaffold (830 kPa) approximate the stiffness of human vocal fold (less than 730 kPa)[19]. Compared to previous studies [3, 10], TPU/PGS hybrid electrospun matrix is a better choice as a vocal fold tissue scaffold from mechanical aspects. However, it should be noted that the uniaxial tensile elastic moduli of the scaffolds were still a little higher than the vocal fold lamina propria [19, 55]. On the other hand, due to the aligned structured ECM networks of the vocal fold lamina propria, constructing scaffold comprised of aligned fibers is also necessary for vocal fold tissue engineering. Normally, aligned fibers lead to a higher matrix stiffness, hence PGS-only fibrous scaffolds should be constructed to better match human vocal fold tissue. One method could be spinning PGS and water soluble polymer together by core-shell electrospinning with leaching out the water soluble polymer [28]. Meanwhile, combining electrospun fibrous networks with soft hydrogels maybe also exhibit mechanical anisotropy and morphology analogous to the human vocal fold lamina propria [56].
3T3 fibroblast cells were cultured on the scaffolds over a 3, 6 and 9 days period. A more flourishing living state on the hybrid scaffold was attributed to the modulated surface wettability when adding PGS into the scaffolds, because cells may not adhere and proliferate well on scaffolds with a wettability higher than 90° [57–58]. In particular, the water contact angle of TPU/PGS 6:4 scaffolds was 65.4°, which is most favorable for protein adsorption and initial attachment of cells based on previous studies [59–60]. It has been widely acknowledged that the mechanical properties of extracellular matrixes—especially stiffness—have a profound impact on cell adhesion [61], spreading [62], migration [63], proliferation [64], and differentiation [65–66]. Hence, the softness of the scaffolds, especially after adding PGS, could also influence cell proliferation. It should be noted that 3T3 fibroblasts are a frequently used cell line to check the biocompatibility of scaffolds and were used for our initial biocompatibility tests. Human vocal fold fibroblast lines established from human tissue samples will be used in our future works. Overall, PGS is a good candidate biomaterial for fabricating soft tissues such as vocal fold tissue.
5. Conclusion
In this study, we successfully fabricated fibrous scaffolds by blending a PGS elastomer with TPU. Different structures could be obtained by electrospinning the same materials using different solvent systems. The presence of PGS significantly improved the hydrophilicity of the scaffolds. Meanwhile, the elastic modulus decreased as expected, while the maximum elongation increased after adding PGS to the scaffolds, approaching to those of the human vocal fold lamina propria. In vitro cell culture results indicated improved biocompatibility for hybrid matrixes compared to TPU-only scaffolds. Overall, the electrospun TPU/PGS composite membrane possessed some of the desirable attributes as a candidate for fabricating vocal fold tissue engineering scaffolds.
Acknowledgements
The authors would like to acknowledge the support of the Wisconsin Institute for Discovery (WID), National Center for International Joint Research of Micro-Nano Molding Technology and the China Scholarship Council (CSC), the financial support of NIH NIDCD grant R0104336, the National Science Fund (Grant No. 11372286, 11372287), the International Technological Cooperation Project (Grant No. 2015DFA30550).
References
- [1].Ramig LO, Verdolini K, Treatment efficacy: Voice disorders, Journal of Speech, Language, and Hearing Research, 41 (1): S101–S116, 1998. [DOI] [PubMed] [Google Scholar]
- [2].Branski RC, Verdolini K, Sandulache V, Rosen CA, Hebda PA, Vocal fold wound healing: A review for clinicians, Journal of Voice: Official Journal of the Voice Foundation, 20 (3): 432–442, 2006. [DOI] [PubMed] [Google Scholar]
- [3].Hughes LA, Gaston J, McAlindon K, Woodhouse KA, Thibeault SL, Electrospun fiber constructs for vocal fold tissue engineering: Effects of alignment and elastomeric polypeptide coating, Acta Biomaterialia, 13: 111–120, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gugatschka M, Graupp M, Friedrich G. Applications of tissue engineering in modern laryngology[J]. European Surgery, 2013, 45(3): 136–141. [Google Scholar]
- [5].Wrona EA, Peng R, Amin MR, et al. Extracellular Matrix for Vocal Fold Lamina Propria Replacement: A Review[J]. Tissue Engineering Part B: Reviews, 2016, 22(6): 421–429. [DOI] [PubMed] [Google Scholar]
- [6].Li L, Stiadle JM, Lau HK, et al. Tissue engineering-based therapeutic strategies for vocal fold repair and regeneration[J]. Biomaterials, 2016, 108: 91–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jaganathan SK, Mani MP, Ayyar M, et al. Engineered electrospun polyurethane and castor oil nanocomposite scaffolds for cardiovascular applications[J]. Journal of Materials Science, 2017, 52(18): 10673–10685. [Google Scholar]
- [8].Li T, Ding X, Tian L, et al. Engineering BSA-dextran particles encapsulated bead-on-string nanofiber scaffold for tissue engineering applications[J]. Journal of Materials Science, 2017, 52(18): 10661–10672. [Google Scholar]
- [9].Agarwal S, Wendorff JH, Greiner A, Progress in the field of electrospinning for tissue engineering applications, Adv Mater, 21 (32–33): 3343–3351, 2009. [DOI] [PubMed] [Google Scholar]
- [10].Tong ZX, Duncan RL, Jia XQ, Modulating the behaviors of mesenchymal stem cells via the combination of high-frequency vibratory stimulations and fibrous scaffolds, Tissue Eng Pt A, 19 (15–16): 1862–1878, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gaston J, Bartlett RS, Klemuk SA, Thibeault SL, Formulation and characterization of a porous, elastomeric biomaterial for vocal fold tissue engineering research, The Annals of Otology, Rhinology, and Laryngology, 123 (12): 866–874, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Miri AK, Mechanical characterization of vocal fold tissue: A review study, Journal of Voice: Official Journal of the Voice Foundation, 28 (6): 657–667, 2014. [DOI] [PubMed] [Google Scholar]
- [13].Sánchez-Arévalo FM, Muñoz-Ramírez LD, Álvarez-Camacho M, et al. Macro-and micromechanical behaviors of poly (lactic acid)–hydroxyapatite electrospun composite scaffolds[J]. Journal of Materials Science, 2017, 52(6): 3353–3367. [Google Scholar]
- [14].Wolf MT, Dearth CL, Sonnenberg SB, Loboa EG, Badylak SF, Naturally derived and synthetic scaffolds for skeletal muscle reconstruction, Advanced Drug Delivery Reviews, 84: 208–221, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jing X, Jin E, Mi HY, et al. Hierarchically decorated electrospun poly (ε-caprolactone)/nanohydroxyapatite composite nanofibers for bone tissue engineering[J]. Journal of Materials Science, 2015, 50(12): 4174–4186. [Google Scholar]
- [16].Cipitria A, Skelton A, Dargaville TR, Dalton PD, Hutmacher DW, Design, fabrication and characterization of PCL electrospun scaffolds-a review, J Mater Chem, 21 (26): 9419–9453, 2011. [Google Scholar]
- [17].Sobczak M, Biodegradable polyurethane elastomers for biomedical applications - synthesis methods and properties, Polym-Plast Technol, 54 (2): 155–172, 2015. [Google Scholar]
- [18].Yilgor I, Yilgor E, Wilkes GL, Critical parameters in designing segmented polyurethanes and their effect on morphology and properties: A comprehensive review, Polymer, 58: A1–A36, 2015. [Google Scholar]
- [19].Kelleher J E, Zhang K, Siegmund T, et al. Spatially varying properties of the vocal ligament contribute to its eigenfrequency response[J]. Journal of the mechanical behavior of biomedical materials, 2010, 3(8): 600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rai R, Tallawi M, Grigore A, Boccaccini AR, Synthesis, properties and biomedical applications of poly(glycerol sebacate) (PGS): A review, Prog Polym Sci, 37 (8): 1051–1078, 2012. [Google Scholar]
- [21].Bhana B, Iyer RK, Chen WL, Zhao R, Sider KL, Likhitpanichkul M, Simmons CA, Radisic M, Influence of substrate stiffness on the phenotype of heart cells, Biotechnology and Bioengineering, 105 (6): 1148–1160, 2010. [DOI] [PubMed] [Google Scholar]
- [22].Jeong CG, Hollister SJ, A comparison of the influence of material on in vitro cartilage tissue engineering with PCL, PGS, and POC 3D scaffold architecture seeded with chondrocytes, Biomaterials, 31 (15): 4304–4312, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee KW, Stolz DB, Wang Y, Substantial expression of mature elastin in arterial constructs, Proceedings of the National Academy of Sciences of the United States of America, 108 (7): 2705–2710, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sundback CA, Shyu JY, Wang YD, Faquin WC, Langer RS, Vacanti JP, Hadlock TA, Biocompatibility analysis of poly(glycerol sebacate) as a nerve guide material, Biomaterials, 26 (27): 5454–5464, 2005. [DOI] [PubMed] [Google Scholar]
- [25].Wang Y, Ameer GA, Sheppard BJ, Langer R, A tough biodegradable elastomer, Nature Biotechnology, 20 (6): 602–606, 2002. [DOI] [PubMed] [Google Scholar]
- [26].Chen QZ, Bismarck A, Hansen U, Junaid S, Tran MQ, Harding SE, Ali NN, Boccaccini AR, Characterisation of a soft elastomer poly(glycerol sebacate) designed to match the mechanical properties of myocardial tissue, Biomaterials, 29 (1): 47–57, 2008. [DOI] [PubMed] [Google Scholar]
- [27].Bettinger CJ, Biodegradable Elastomers for tissue engineering and cell–biomaterial interactions, Macromol Biosci, 11 (4): 467–482, 2011. [DOI] [PubMed] [Google Scholar]
- [28].Jeffries EM, Allen RA, Gao J, Pesce M, Wang Y, Highly elastic and suturable electrospun poly(glycerol sebacate) fibrous scaffolds, Acta Biomaterialia, 18: 30–39, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Masoumi N, Larson BL, Annabi N, Kharaziha M, Zamanian B, Shapero KS, Cubberley AT, Camci-Unal G, Manning KB, Mayer JE Jr., Khademhosseini A, Electrospun PGS:PCL microfibers align human valvular interstitial cells and provide tunable scaffold anisotropy, Advanced Healthcare Materials, 3 (6): 929–939, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kharaziha M, Nikkhah M, Shin SR, Annabi N, Masoumi N, Gaharwar AK, Camci-Unal G, Khademhosseini A, PGS: Gelatin nanofibrous scaffolds with tunable mechanical and structural properties for engineering cardiac tissues, Biomaterials, 34 (27): 6355–6366, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hu J, Kai D, Ye H, Tian L, Ding X, Ramakrishna S, Loh XJ, Electrospinning of poly (glycerol sebacate)-based nanofibers for nerve tissue engineering, Materials Science & Engineering. C, Materials for Biological Applications, 70 (Pt 2): 1089–1094, 2017. [DOI] [PubMed] [Google Scholar]
- [32].Wang Y, Kim YM, Langer R, In vivo degradation characteristics of poly(glycerol sebacate), Journal of Biomedical Materials Research. Part A, 66 (1): 192–197, 2003. [DOI] [PubMed] [Google Scholar]
- [33].Engelmayr GC Jr., Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE, Accordion- like honeycombs for tissue engineering of cardiac anisotropy, Nature Materials, 7 (12): 1003–1010, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ke H, Feldman E, Guzman P, et al. Electrospun polystyrene nanofibrous membranes for direct contact membrane distillation[J]. J Membrane Sci, 515: 86–97, 2016. [Google Scholar]
- [35].Islam MS, McCutcheon JR, S Rahaman M. A high flux polyvinyl acetate-coated electrospun nylon 6/SiO2 composite microfiltration membrane for the separation of oil-in-water emulsion with improved antifouling performance[J]. J Membrane Sci, 537: 297–309, 2017. [Google Scholar]
- [36].Ramakrishna S, Fujihara K, Teo WE, Yong T, Ma ZW, Ramaseshan R, Electrospun nanofibers: Solving global issues, Mater Today, 9 (3): 40–50, 2006. [Google Scholar]
- [37].Sant S, Hwang CM, Lee SH, Khademhosseini A, Hybrid PGS-PCL microfibrous scaffolds with improved mechanical and biological properties, Journal of Tissue Engineering and Regenerative Medicine, 5 (4): 283–291, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yi F, LaVan DA, Poly(glycerol sebacate) nanofiber scaffolds by core/shell electrospinning, Macromol Biosci, 8 (9): 803–806, 2008. [DOI] [PubMed] [Google Scholar]
- [39].Salehi S, Fathi M, Javanmard SH, Bahners T, Gutmann JS, Ergun S, Steuhl KP, Fuchsluger TA, Generation of PGS/PCL blend nanofibrous scaffolds mimicking corneal stroma structure, Macromol Mater Eng, 299 (4): 455–469, 2014. [Google Scholar]
- [40].Shrestha S, Shrestha BK, Kim JI, et al. Electrodeless coating polypyrrole on chitosan grafted polyurethane with functionalized multiwall carbon nanotubes electrospun scaffold for nerve tissue engineering, Carbon, 136: 430–443, 2018. [Google Scholar]
- [41].Augustine R, Nethi S K, Kalarikkal N, et al. Electrospun polycaprolactone (PCL) scaffolds embedded with europium hydroxide nanorods (EHNs) with enhanced vascularization and cell proliferation for tissue engineering applications[J]. Journal of Materials Chemistry B, 2017, 5(24): 4660–4672. [DOI] [PubMed] [Google Scholar]
- [42].Buczek O, Krowarsch D, Otlewski J, Thermodynamics of single peptide bond cleavage in bovine pancreatic trypsin inhibitor (BPTI), Protein Science: A Publication of the Protein Society, 11 (4): 924–932, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sant S, Iyer D, Gaharwar AK, Patel A, Khademhosseini A, Effect of biodegradation and de novo matrix synthesis on the mechanical properties of valvular interstitial cell-seeded polyglycerol sebacate-polycaprolactone scaffolds, Acta Biomaterialia, 9 (4): 5963–5973, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gaharwar AK, Nikkhah M, Sant S, Khademhosseini A, Anisotropic poly (glycerol sebacate)- poly (ε-caprolactone) electrospun fibers promote endothelial cell guidance, Biofabrication, 7 (1): 015001, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mi HY, Jing X, Salick MR, Cordie TM, Peng XF, Turng LS, Properties and fibroblast cellular response of soft and hard thermoplastic polyurethane electrospun nanofibrous scaffolds, Journal of Biomedical Materials Research. Part B, Applied Biomaterials, 103 (5): 960–970, 2015. [DOI] [PubMed] [Google Scholar]
- [46].Salehi S, Bahners T, Gutmann JS, Gao SL, Mader E, Fuchsluger TA, Characterization of structural, mechanical and nano-mechanical properties of electrospun PGS/PCL fibers, Rsc Adv, 4 (33): 16951–16957, 2014. [Google Scholar]
- [47].D’Sa RA, Raj J, Dickinson PJ, McCabe F, Meenan BJ, Human fetal osteoblast response on poly(methyl methacrylate)/polystyrene demixed thin film blends: Surface chemistry vs topography effects, Acs Appl Mater Inter, 8 (24): 14920–14931, 2016. [DOI] [PubMed] [Google Scholar]
- [48].Kerativitayanan P, Gaharwar AK, Elastomeric and mechanically stiff nanocomposites from poly(glycerol sebacate) and bioactive nanosilicates, Acta Biomaterialia, 26: 34–44, 2015. [DOI] [PubMed] [Google Scholar]
- [49].Gaharwar AK, Patel A, Dolatshahi-Pirouz A, Zhang HB, Rangarajan K, Iviglia G, Shin SR, Hussain MA, Khademhosseini A, Elastomeric nanocomposite scaffolds made from poly (glycerol sebacate) chemically crosslinked with carbon nanotubes, Biomater Science, 3 (1): 46–58, 2015. [DOI] [PubMed] [Google Scholar]
- [50].Engler AJ, Sen S, Sweeney HL, Discher DE, Matrix elasticity directs stem cell lineage specification, Cell, 126 (4): 677–689, 2006. [DOI] [PubMed] [Google Scholar]
- [51].Discher DE, Janmey P, Wang YL, Tissue cells feel and respond to the stiffness of their substrate, Science, 310 (5751): 1139–1143, 2005. [DOI] [PubMed] [Google Scholar]
- [52].Masoumi N, Larson BL, Annabi N, Kharaziha M, Zamanian B, Shapero KS, Cubberley AT, Camci-Unal G, Manning KB, Mayer JE, Khademhosseini A, Electrospun PGS:PCL microfibers align human valvular interstitial cells and provide tunable scaffold anisotropy, Advanced Healthcare Materials, 3 (6): 929–939, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Discher DE, Janmey P, Wang Y. Tissue cells feel and respond to the stiffness of their substrate[J]. Science, 2005, 310(5751): 1139–1143. [DOI] [PubMed] [Google Scholar]
- [54].Bhardwaj N, Kundu SC. Electrospinning: a fascinating fiber fabrication technique[J]. Biotechnology advances, 2010, 28(3): 325–347. [DOI] [PubMed] [Google Scholar]
- [55].Caton T, Thibeault SL, Klemuk S, et al. Viscoelasticity of hyaluronan and nonhyaluronan based vocal fold injectables: implications for mucosal versus muscle use[J]. The Laryngoscope, 2007, 117(3): 516–521. [DOI] [PubMed] [Google Scholar]
- [56].Bas O, De-Juan-Pardo EM, Meinert C, et al. Biofabricated soft network composites for cartilage tissue engineering[J]. Biofabrication, 2017, 9(2): 025014. [DOI] [PubMed] [Google Scholar]
- [57].Tuteja A, Choi W, Ma ML, Mabry JM, Mazzella SA, Rutledge GC, McKinley GH, Cohen RE, Designing superoleophobic surfaces, Science, 318 (5856): 1618–1622, 2007. [DOI] [PubMed] [Google Scholar]
- [58].Ma ML, Gupta M, Li Z, Zhai L, Gleason KK, Cohen RE, Rubner MF, Rutledge GC, Decorated electrospun fibers exhibiting superhydrophobicity, Adv Mater, 19 (2): 255, 2007. [Google Scholar]
- [59].Wei J, Igarashi T, Okumori N, et al. Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts[J]. Biomedical Materials, 2009, 4(4): 045002. [DOI] [PubMed] [Google Scholar]
- [60].Tamada Y, Ikada Y. Effect of preadsorbed proteins on cell adhesion to polymer surfaces[J]. Journal of colloid and interface science, 1993, 155(2): 334–339. [Google Scholar]
- [61].Liao YC, Ma YT, Huang CH, Yu J, Hsiao HM, Rigidity guided cell attachment on inkjet-printed patterns, ACS Appl Mater Interfaces, 4 (7): 3335–3339, 2012. [DOI] [PubMed] [Google Scholar]
- [62].Chaudhuri O, Gu L, Darnell M, Klumpers D, Bencherif SA, Weaver JC, Huebsch N, Mooney DJ, Substrate stress relaxation regulates cell spreading, Nature Communications, 6: 6364, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lo CM, Wang HB, Dembo M, Wang YL, Cell movement is guided by the rigidity of the substrate, Biophysical Journal, 79 (1): 144–152, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM, Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture, Science, 329 (5995): 1078–1081, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Fu J, Wang YK, Yang MT, Desai RA, Yu X, Liu Z, Chen CS, Mechanical regulation of cell function with geometrically modulated elastomeric substrates, Nature Methods, 7 (9): 733–736, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ye K, Cao L, Li S, Yu L, Ding J, Interplay of matrix stiffness and cell-cell contact in regulating differentiation of stem cells, ACS Appl Mater Interfaces, 8 (34): 21903–21913, 2016. [DOI] [PubMed] [Google Scholar]