Abstract
Purpose
To summarize the evidence comparing the effectiveness of short and long courses of oral antibiotics for infections treated in outpatient settings.
Methods
We identified systematic reviews of randomized controlled trials for children and adults with bacterial infections treated in outpatient settings from Medline, Embase, CINAHL, Cochrane Database of Systematic Reviews and The Database of Review of Effects. Data were extracted on the primary outcome of clinical resolution and secondary outcomes.
Results
We identified 30 potential reviews, and included 9. There was no difference in the clinical cure for children treated with short or long course antibiotics for Group A streptococcal tonsillopharyngitis (OR 1.03, 95% CI:0.97, 1.11); community acquired pneumonia (RR 0.99, 95% CI:0.97, 1.01); acute otitis media [<2 years old OR: 1.09 (95% CI:0.76, 1.57); ≥2 years old OR: 0.85 (95% CI:0.60, 1.21)]; or urinary tract infection (RR 1.06, 95% CI:0.64, 1.76). There was no difference in the clinical cure for adults treated with short or long course antibiotics for acute bacterial sinusitis (RR 0.95, 95% CI:0.81, 1.21); uncomplicated cystitis in non-pregnant women (RR 1.10, 95% CI:0.96, 1.25), or elderly women (RR: 0.98, 95% CI:0.62, 1.54); acute pyelonephritis (RR 1.03, 95% CI:0.80, 1.32); or community acquired pneumonia (RR: 0.96, 95% CI:0.74, 1.26). We found inadequate evidence about the effect on antibiotic resistance.
Conclusions
This overview of systematic reviews has identified good quality evidence that short course antibiotics are as effective as longer courses for most common infections managed in ambulatory care. The impact on antibiotic resistance and associated treatment failure requires further study.
Keywords: Adult, antibacterial agents, bacterial infections, general practice, pediatrics, review
Introduction
Antibiotics are one of the most frequently prescribed medication classes worldwide. Outpatient prescriptions for antibiotics are the most common prescriptions to children in the USA and Europe and are one of the most common prescriptions amongst adults (1–4). Worldwide antibiotic prescriptions are increasing with notable differences in antibiotic prescribing both across nations and between geographic regions within national boundaries (5).
Though many factors contribute to the development of antibiotic resistance, the frequency and duration of antibiotic prescriptions clearly play a role in its development (6). Antibiotic dosing duration with few exceptions (tuberculosis) is not based on rigorous evaluation, rather on historical precedent (7). Despite this, patients are frequently recommended to complete a full antibiotic course (even if they have recovered), and there is a perception that resistance develops if they do not complete a full antibiotic course. Since the risk of antibiotic-related adverse events is cumulative with increasing exposure, long courses of antibiotics may be more likely to induce adverse events than short-courses (8).
Shifting primary care practice away from current usual practice of longer courses of antibiotics could reduce overall antibiotic consumption in primary care, and be an important strategy in combatting global rates of antibiotic resistance. An increasing number of primary research and systematic reviews suggest short-course antibiotics may be sufficient to treat bacterial infections in outpatient settings, yet, with some notable exceptions, the use of short (or shorter) courses of antibiotics is not standard practice in most outpatient settings to the knowledge of the authors. The presentation of the evidence for such shorter courses together may help shift the paradigm on antibiotic prescribing. Therefore, the objective of this systematic overview is to critically appraise and summarize the evidence from systematic reviews comparing the effectiveness of short-courses to long-courses (as defined by the disease process standard of care) of oral antibiotics for the clinical resolution of bacterial infections commonly encountered by adults and children in primary care settings.
Methods
Search
We developed a detailed search strategy in collaboration with an information specialist (author: NR) to identify systematic reviews that compared the effects of short versus long courses of the same antibiotic in children and adults (full search strategy in Supplementary Table 1). The search strategy was applied to five bibliographic databases: Medline (OvidSP) [1946-present], Embase (OvidSP) [1974-present] and CINAHL (OvidSP) [1980-present], Cochrane Database of Systematic Reviews (Issue 11 of 12, 2013) and The Database of Review of Effects (Issue 4 of 4, 2013) (Supplementary Table 2). Searches were not restricted by language or country of origin of primary studies; however, only systematic reviews published in English were eligible. Search date was April 6, 2016.
Inclusion and exclusion criteria
We included systematic reviews of randomized controlled trials (RCTs) for children and adults with bacterial infections that are commonly treated in outpatient or primary care settings. We excluded reviews of infections routinely or exclusively treated in hospitalized patients; of parasitic, fungal or viral etiology; involving patients with immune compromise (e.g. HIV, cancer); that included combination, topical, or intravenous antibiotic administration; with mixed etiology; and of strategies to reduce uptake of antibiotics such as use of delayed prescriptions. Eligible reviews included trials that compared antibiotic prescriptions described as short course (i.e. antibiotic duration 2 or more days shorter than longer course treatments) with longer duration courses of antibiotics based on disease specific standards of care. We did not include individual articles, or abstracts not included in systematic reviews.
Our primary outcome was comparison of resolution of clinical symptoms between longer and shorter courses of therapy. Secondary outcomes included microbiological cure (8–12), adverse events (8–13), relapse (9,11,12,14), recurrence of illness (9,11), and other (including: treatment failure (13,14), compliance with therapy (10), worsening disease (8,13), resistance (10), and mortality (14)) (Supplementary Table 3).
Article selection
Two pairs of authors (pediatrics: EDH and MT; adults: SM and IO) screened citations for inclusion initially using title and abstract, and then reviewed full text of potentially eligible articles for inclusion. Where eligibility remained uncertain, authors used consensus to determine final eligibility. Where two or more systematic reviews were identified on the same infection and/or same participants, we selected one review for inclusion using the following criteria, which were evaluated by two authors independently: (i) higher quality and (ii) published most recently. Quality of the included systematic reviews was independently assessed by the same pairs of authors using the Assessment of Multiple Systematic Reviews (AMSTAR) scale (15). On the 11-point AMSTAR scale, quality is defined by score as low if 0–3, medium is 4–7 and high if 8–11 (15).
Data extraction
Data from included reviews were extracted on a pre-specified form by one author and checked for accuracy by a second with disagreements resolved by consensus. We extracted data on year of publication, patient population, clinical setting, antibiotic regimens compared (including antibiotic type, dosing schedules, definitions of short and long/standard courses), which of our predefined primary and secondary outcomes were measured and how they were defined. Countries where the studies took place were grouped into income levels based on World Bank criteria (16). The overall quality of the studies included within each systematic review were described in the text and Table 1 based on one of two risk of bias tools either their Jadad score—a score to define the quality of a randomized control trial (RCT) (17), or their assessment for risk of bias (18). Summary data from included reviews were extracted for our primary and secondary outcomes.
Table 1.
Characteristics of included systematic reviews
| Study | Population | Studies (patients) | Countries (world bank criteria) | Diagnostic criteria | Antibiotic regimen | Definition of clinical resolutionb | Overall quality of included studies within reviews |
|---|---|---|---|---|---|---|---|
| Falagas, 2008(a) | Children and young adults ≤25 years old with group A strep tonsillopharyngitis | 7 (1410)a | Netherlands, Italy, Switzerland, Sweden, Canada, USA (high income) | Clinical criteria, or Clinical criteria + culture or serology or rapid antigen | Same antibiotic, same daily dose | Complete or substantial resolution of symptoms and signs at end-of- therapy evaluation | Moderate |
| Haider, 2011 | Children 2–59 months old with non-severe community acquired pneumonia | 4 (6177) | India, Pakistan, Philippines, Indonesia, Bangladesh (lower-middle income) | Clinical criteria | Same antibiotic, same daily dose | Respiratory rate returned to normal | Moderate/good |
| Kozyrskyj, 2010 | Children 3 months to 14 years old with acute otitis media (AOM) | 49 (12045) | Bulgaria, Canada, Costa Rica, Chile, China, Denmark, the Dominican Republic, Egypt, Finland, France, Greece, Guatemala, Israel, Italy, Latvia, Mexico, Peru, Romania, Saudi Arabia, Spain, Sweden, Switzerland, Turkey, UK, USA (lower-middle, upper-middle, and high income) | Clinical criteria | Same antibiotics (12); different (37) | Treatment failure, including lack of clinical resolution, relapse or recurrence of AOM over 1 month follow up. | Low |
| Michael, 2010 | Children 3 months to 18 years old with urinary tract infection | 10 (652) | Canada, Costa Rica, Denmark, Hungary, Kuwait, Sweden, USA (upper-middle and high income) | Urine culture | Same antibiotic, same daily dosage | Persistent bacteriuria at the end of treatment | Low |
| Falagas, 2008(b) | Adults with acute bacterial sinusitis | 12 (4430) | Argentina, Belgium, Canada, Chile, Estonia, Finland, France, Germany, Ireland, Italy, Lithuania, Poland, South Africa (upper- middle and high income) | Clinical criteria | Same antibiotic, same daily dosage | Complete resolution, or improvement of symptoms and signs at the test of cure visit | Moderate/good |
| Katchman, 2005 | Adult 16–65 years old non-pregnant women with uncomplicated urinary tract infection | 32 (9605) | Denmark, France, Israel, Japan, Mexico, the Netherlands, Norway, Spain, Sweden, UK, USA (upper-middle and high income) | Clinical criteria + culture | Same antibiotic, same daily dosage (15); same antibiotic, different daily dosage (3); different antibiotics (14); | Persistence or recurrence of symptoms within 2 weeks follow-up | Moderate |
| Kyriakidou, 2008 | Adults ≥15 years old with acute pyelonephritis | 2 (185)c | The Netherlands, Sweden, USA (high income) | Clinical criteria + culture | Same antibiotic, same daily dosage | Resolution of symptoms and signs at test of cure visit | Moderate |
| Li, 2007 | Adults ≥12 years old with mild to moderate community acquired pneumonia | 8 (1540)d | Canada, Croatia, France, Israel, Italy, Japan, the Netherlands, Poland, South Africa, Spain, USA (upper-middle and high income) | Clinical criteria + radiology | Different antibiotics | Failure to achieve clinical improvement or cure; based on clinical symptoms and need for additional antibiotics | Moderate/good |
| Lutters, 2008 | Older women (>60 years) with acute uncomplicated lower tract urinary tract infection | 6 (431) | Canada, Denmark, France, Germany, Ireland, Israel, Italy, the Netherlands (high income) | Clinical criteria + culture | Same antibiotic (2), same daily dosage (2) | Persistence of symptoms | Moderate |
aOnly studies of individuals up to 25 years of age were included in the review, total review included 11 studies and 2750 patients.
bSecondary outcome definitions can be found in Supplementary Table 3.
cTwo additional studies were included in systematic review that compared IV antibiotics, therefore, they were excluded in our analyses.
dSeven studies were included in the systematic review that compared IV antibiotics, therefore, they were excluded in our analyses.
Analysis
We reported results descriptively, identifying and explaining differences between studies and any resulting heterogeneity. Data were presented comparing short and long duration antibiotics and summarized separately for adults and children, and for all primary and secondary outcomes of interest. We report the quality of the included studies within each systematic review, as described by the systematic review authors. We determined the AMSTAR score for each systematic review.
Results
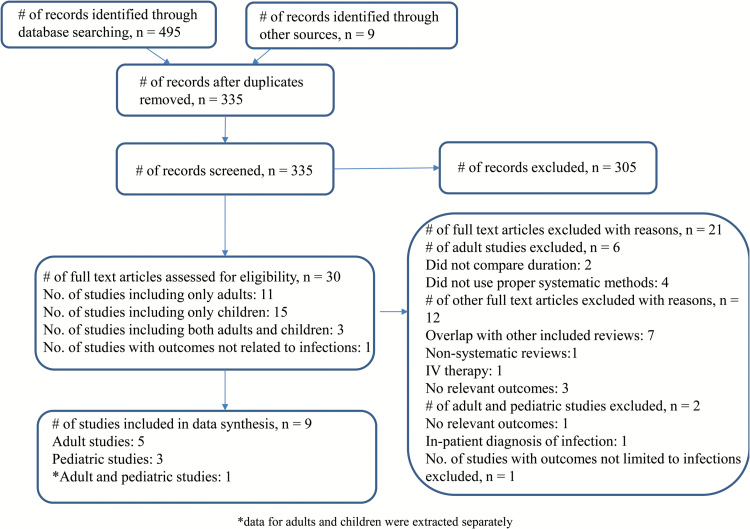
After removal of duplicates, we screened 335 articles and identified 30 eligible reviews (Figure 1). We excluded 21 reviews because they did not include duration (n = 2) or use formal systematic review methods (n = 4), overlapped with other included reviews (n = 7), were non-systematic or clinical reviews (n = 1), included intravenous therapy exclusively (n = 1), contained no relevant outcomes (n = 4), focused on inpatient infections (n = 1) or the outcome was not limited to infection (n = 1). Therefore, a total of nine reviews were included. The infections included in the four pediatric systematic reviews were streptococcal tonsillopharyngitis (9), community acquired pneumonia (14), acute otitis media (13) and urinary tract infection (UTI) (10); those in the five adult systematic reviews were acute bacterial sinusitis (11), uncomplicated UTI in non-pregnant women (8), acute pyelonephritis (12), community acquired pneumonia (19) and acute uncomplicated lower UTI in elderly women (20). The primary studies were conducted in low, lower-middle, upper-middle and high income countries. The AMSTAR scores ranged from 9–10 for the pediatric studies and 7–10 for the adult studies (Supplementary Table 4).
Figure 1.
Flow chart showing the process for inclusion of systematic reviews.
Comparison of short- versus long-duration antibiotics in children
Tonsillopharyngitis
We included one systematic review of 7 studies of 1410 children and young people (age range 1–25 years) with Group A streptococcal (GAS) tonsillopharyngitis (9). The included studies were published between 1972 and 2003, all conducted in high income countries and the overall quality was moderate, as assessed by Jadad scores that ranged from 1 to 4 (Table 1). There was no difference in rates of clinical cure between 5–7 days and 10 days of treatment using the same antibiotic, at the same daily dose (OR 1.03, 95% CI: 0.97, 1.11) (Table 2) (9). There was no difference in microbiobiological eradication (OR: 1.06, 95% CI: 0.99, 1.13) or adverse events (OR: 1.23, 95% CI: 0.68, 2.22) between those treated with a short antibiotic course or longer antibiotic course (Supplementary Table 5).
Table 2.
Comparison of effectiveness of short and long courses of antibiotics in children
| Condition (Source systematic review) | Primary Outcome | Definition of short versus long courses | Studies (#) | Patients (#) | Relative effect of short compared to long duration (95%CI) | Interpretation of summary risk statistic | Duration of antibiotic course supported by results |
|---|---|---|---|---|---|---|---|
| Group A Streptococcal tonsillopharyngitis (Falagas, 2008) | Clinical success | 5–7 days versus 10 days | 4 | 823 | 1.03 (0.97–1.11) | OR > 1 supports short course | No difference for short versus long |
| Community acquired pneumonia (Haider, 2011) | Clinical cure | 3 versus 5 days | 3 | 5763 | 0.99 (0.97, 1.01) | RR > 1 supports long course | No difference for short versus long |
| Acute otitis media (Kozyrskyj, 2010) | Treatment failure <2 years old | >48 hours versus ≥ 7 daysa | 5 | 570 | 1.09 (0.76, 1.57) | OR > 1 supports long course | No difference for short versus long |
| Treatment failure ≥2 years old | >48 hours versus ≥7 daysa | 6 | 1064 | 0.85 (0.60, 1.21) | No difference for short versus long | ||
| Urinary tract infection (Michael, 2010) | Bacteriuria after completing treatment | 2–4 days versus 7–14 days | 8 | 423 | 1.06 (0.64, 1.76) | RR >1 supports long course | No difference for short versus long |
aShort course in this study is defined as >48 up to 7 days, and long is ≥7 days.
Community acquired pneumonia
We included one systematic review of 4 studies of 6177 children aged 2–59 months old with non-severe community acquired pneumonia (14). The included studies were published between 1999 and 2004, all conducted in lower-middle income countries, and the overall quality was moderate/good, with three studies reporting adequate sequence generation, allocation concealment, blinding and addressing incomplete outcome data (Table 1). There was no significant difference in clinical cure between children who received 3 days compared to 5 days of antibiotics (RR 0.99, 95% CI: 0.97–1.01), (Table 2) (14). Subgroup analyses by type of antibiotic showed similar results: there were no significant differences in clinical cure in children who received 3 versus 5 days of amoxicillin (RR 0.99, 95% CI: 0.97, 1.02) or 3 day versus 5 days of cotrimoxazole (1.00, 95% CI: 0.97–1.03). The rates of treatment failure and relapse for children receiving short versus long courses of antibiotics were also not significantly different (Supplementary Table 5).
Acute otitis media
We included one systematic review of 49 studies of 12045 children aged 3 months to 14 years old with acute otitis media (13). Its included studies were published from 1982–2006, conducted in lower-middle, upper-middle and high income countries, and the overall quality was low, with the majority unclear regarding adequate sequence generation, allocation concealment, incomplete outcome data and adequate blinding (Table 1). This review measured risk of treatment failure rather than clinical cure. Neither children younger than 2 years nor those 2 years and older were more likely to experience treatment failure with short course treatment (<2 years: RR: 1.09, 95% CI: 0.76, 1.57; ≥2 years: RR 0.85, 95% CI: 0.60, 1.21), (Table 2). However, adverse gastrointestinal side effects were significantly less likely with shorter than longer courses of antibiotics at all ages (RR: 0.72, 95% CI: 0.60, 0.87) (Supplementary Table 5).
Urinary tract infection
We included one systematic review of 10 studies of 652 children aged 3 months to 18 years old with urinary tract infection (10). Its included studies were published between 1979 and 2008, conducted in upper-middle and high income countries, and the overall quality was low; the majority of the studies had unclear allocation concealment, and no or unstated blinding for the subject/investigator (Table 1). None of the studies stated if the outcome assessor was blinded or if they performed an intention-to-treat (ITT) analysis. The rates of loss to follow-up ranged from zero to 23%. There was no significant difference in clinical cure, as measured by UTI at the end of treatment, in children who received antibiotics for 2–4 days compared to 7–14 days (RR 1.06, 95% CI: 0.64, 1.76), (Table 2) (10). Similarly, there was no significant difference in persistence of bacteriuria, recurrence of UTI, persistent bacteriuria with abnormal urinary tract imaging or resistance to antibiotics between children given short versus long courses of antibiotics (Supplementary Table 5) (10).
Comparison of short versus long duration antibiotics in adults
Acute bacterial sinusitis
We included one systematic review of 12 studies of 4430 adults with acute bacterial sinusitis (11). Its included studies were published from 1995–2006, conducted in upper middle or high income countries, and the overall quality was moderate/good and reported Jadad scores ranged from 3–5. (Table 1) There was no significant difference in clinical cure rates between adults given 3–7 days versus 6–10 days of the same therapy (RR 0.95, 95% CI: 0.81, 1.12), (Table 3) (11). The effects on secondary outcomes of microbiological efficacy, relapses, and adverse events were all similar for short versus long courses of antibiotics (11). Individuals in the subgroup who took a shorter antibiotic course had a lower risk of adverse events than those who took a longer antibiotic course (OR: 0.79, 95% CI: 0.63, 0.89) (Supplementary Table 6).
Table 3.
Comparison of effectiveness of short and long courses of antibiotics in adults
| Condition (source systematic review) | Primary Outcome | Definition of short versus long courses (days) | Studies (#) | Patients (#) | Relative effect of short compared to long duration (95%CI) | Interpretation of summary risk statistic | Duration of antibiotic course supported by results |
|---|---|---|---|---|---|---|---|
| Acute bacterial sinusitis (Falagas 2008(b)) | Clinical cure/ success | 3–7 versus 6–10 | 12 | 4430 | 0.95 (0.81, 1.12) | OR > 1 supports short course | No difference for short versus long |
| Non-pregnant women with uncomplicated UTI (Katchman, 2005) | Symptomatic failure | 3 versus ≥5 | 17 | 5029 | 0.99 (0.89, 1.10) | RR > 1 supports long course | No difference for short versus long |
| Acute pyelonephritis (Kyriakidou 2008) | Clinical success | 7–14 versus 14–42 | 2 | 185 | 1.03 (0.80, 1.32) | OR > 1 supports short course | No difference for short versus long |
| Community-acquired pneumonia (Li et al. 2007)a | Clinical failure | ≤7 versus >7 | 8 | 1540 | 0.96 (0.74, 1.26) | RR > 1 supports long course | No difference for short versus long |
| Acute uncomplicated lower UTI in older women (Lutters, 2008) | Clinical failure | 3–6 versus 7–14 | 4 | 395 | 0.98 (0.62, 1.54) | RR > 1 supports long course | No difference for short versus long |
aFour additional studies were included in systematic review that compared IV antibiotics; therefore, they were excluded in our analyses.
Non-pregnant women with uncomplicated UTI
We included one systematic review of 32 studies of 9605 non-pregnant adult women with uncomplicated UTI (8). Its included studies were published from 1980 to 1999, were conducted in upper middle or high income countries, and the overall quality was moderate, only half of included trials were considered adequately randomized and about a third were double-blinded (Table 1). However, few of the studies presented ITT analyses or reported adequate allocation concealment. There was no significant difference in the proportion of participants who achieved clinical resolution within two weeks of follow-up for those given 3 days versus 5 days or longer of antibiotics (RR 1.10, 95% CI: 0.96, 1.25), (Table 3) (8). Symptomatic failure at 8 weeks was also similar between short and long course groups. However, the risk of bacteriological failure at 8 weeks was lower in women treated with long course antibiotic therapy, while risk of discontinuation of therapy and adverse events were lower for those on short course antibiotics (Supplementary Table 6).
Acute pyelonephritis
We included one systematic review of 2 studies of 185 adults with acute pyelonephritis (12). Its included studies were published between 1987–1988 all conducted in high-income countries, and the overall quality of the studies was moderate with Jadad scores ranging from 2–5 (Table 1). There was no difference in clinical cure rates between short (7–14 days) versus long (14–42 days) courses of antibiotics (RR: 1.03, 95% CI: 0.80–1.32), (Table 3) (12). There were also no differences in bacteriological efficacy, relapse, adverse events, withdrawal or recurrence between those given long versus short courses (Supplementary Table 6).
Community-acquired pneumonia
We included one systematic review of 8 studies of 1540 adults with community acquired pneumonia (19). Its included studies were published between 1981 and 2005, conducted in upper middle and high income countries, and the overall quality of the studies was moderate/good, with Jadad scores ≥3 (Table 1). There were no significant differences in the rates of clinical improvement when given different antibiotics for 3–7 days compared to 7 days or longer (RR: 0.96, 95% CI: 0.74, 1.26), (Table 3) (19). The review conducted a sensitivity analysis including only the high quality studies, which showed no significant difference in clinical improvement rates between short and long courses (Supplementary Table 6). There was also no significant difference in mortality rates between short and longer course groups (Supplementary Table 6).
Acute uncomplicated UTI in older women
We included one systematic review of 6 studies of 431 adult women older than 60 years with acute uncomplicated cystitis (20). Its included studies were published between 1981 and 2005, all conducted in high income countries, and the overall quality of included studies was moderate; the majority of the included trials were unclear in their reporting of allocation concealment, trial participants and the outcome assessors were not blinded to the treatment, and ITT analyses were not included in the results (Table 1). There was no significant difference in the rates of clinical cure in participants given short (3–6 days) versus longer courses (7–14 days) when comparing different antibiotics (RR 0.98, 95% CI: 0.62, 1.54), (Table 3) (20). In addition, rates of bacteriological persistence of UTI at ≥2 weeks and adverse drug reactions were equivalent among women treated with short and long courses (Supplementary Table 6).
Discussion
Main findings
Adults and children treated in outpatient settings with uncomplicated UTI or mild / moderate community acquired pneumonia, adults treated for acute sinusitis or acute pyelonephritis, and children treated for tonsillopharyngitis or acute otitis media all had similar clinical cure rates when given shorter courses of antibiotics compared to those receiving longer courses. Moreover, the similar clinical cure rates were not offset by differential risks of relapse or remission, where these data were reported. For six of these conditions (i.e. tonsillopharyngitis, acute otitis media, acute bacterial sinusitis, non-pregnant women with uncomplicated UTI, acute pyelonephritis and acute uncomplicated UTI in elderly women) shorter courses of antibiotics were also associated with lower rates of adverse effects. There was an overall absence of evidence about the impact on treatment duration on antibiotic resistance and associated treatment failure. Because clinical cure is similar between short and long course therapy for the included conditions, and because the harms associated with longer course therapy (adverse drug reactions, development of antimicrobial resistance, etc.) can only be equivalent or increased, we recommend the adoption of shorter courses of antibiotics for the conditions outlined in this review.
The quality of the systematic reviews was moderate to high with AMSTAR scores of 7–10; however, the quality of the included studies was variable. Overall the studies in the pediatric reviews were of low-moderate quality while those in the adult reviews were of moderate-good quality. The majority of the pediatric and adult studies were conducted in middle and high income countries. We found few data on comparative effectiveness of short and long courses of antibiotics in low income countries, where baseline risks, immunization rates, complication rates and treatment access may differ substantially from middle or high income countries.
Comparison to existing international guidelines
The current practice guidelines in the US (21–27), the UK (28–32), Australia (33) and Canada (34–36) vary in their recommendations for the type and duration of antibiotics for the infections included in our study.
Guidelines for children
Our review supports decreasing the duration of treatment for GAS tonsillopharyngitis from 10 days to 5–7 days, with the caveat that there is a lack of robust evidence on comparative effectiveness on rare complications such as acute rheumatic fever. Among guidelines for AOM there is considerable variability in the inclusion of a duration recommendation for the treatment particularly among children <2 years old, however, our study supports shorter course therapy (<7 days) for children of all ages as is recommended in the Canadian guidelines for children >2years old (36,37). For AOM treatment the Australian guidelines are the only ones that avoid routine use of antibiotics for AOM. All guidelines recommend longer duration therapy for children with pneumonia rather than the 3 days found to be acceptable in our study. The UK guidelines for UTI already meet those recommended by our review of 2–4 days, however, other guidelines may benefits from decreasing to this range as well.
Guidelines for adults
There is considerable variation across country guidelines for sinusitis, the Australian and UK guidelines avoid antibiotic use during the first 7–18 days of symptoms, and the duration of treatment recommended is longer in all other countries than the 3–7 days recommended by our study. The guidelines in most countries are in line with our findings that treatment of uncomplicated UTI for 3–7 days, and acute pyelonephritis for 7 days is not associated with worse outcomes. However, 10–14 days is still recommended in Australia for acute pyelonephritis. The guidelines for treatment of pneumonia in the US, UK and Australia align with the shorter duration therapy, however, the Canadian Infectious Disease and Thoracic Society guidelines recommend treatment for 7–14 days for community acquired pneumonia (34).
Strengths and limitations
To our knowledge, this is the first overview of systematic reviews that has critically appraised and summarized comparative effectiveness data for common bacterial infections treated in primary care settings. We believe that many primary care clinicians have not adopted evidence that we have summarized here due to the diversity of reviews of individual clinical conditions, and their complexity of reporting—we suspect few have the time and resources to unravel such variable reporting.
Our review has several limitations. We were unable to collate evidence about the effect of antibiotic resistance, due to poor reporting quality of this information across included studies. Additionally, none of the included reviews provided sufficient data to comment on the comparative effectiveness of short and long courses for rare outcomes associated with several common infections, such as hospitalization for community acquired pneumonia, or development of post infectious complications (e.g. acute rheumatic fever following GAS tonsillopharyngitis). However, data from other sources indicate that these complications are now extremely rare in outpatient settings in high income countries and should not be used to justify treatment in most patients (38). If shorter courses of therapy are adopted for GAS tonsillopharyngitis it will be important to monitor for rare outcomes such as acute rheumatic fever.
Unfortunately, not all systematic reviews included all age ranges, therefore, some age ranges (for example children >59 months with pneumonia) were not included for particular diagnoses. While we believe that a systematic review of systematic reviews provides a readily digestible format for clinicians, research and policy makers, this method is limited by the dates of the included studies within each systematic review rather than the most up to date primary literature. The literature would benefit from additional systematic reviews on cellulitis and sexually transmitted infections, which are commonly seen in outpatient settings, however, there were no fitting reviews for inclusion in this study. Additionally, a new systematic review of children with pneumonia over a wider age range and in high-income countries would be helpful to clinicians in the provision of care.
Implications for clinicians
Our review provides a comprehensive resource that primary care clinicians can use to make evidence-based decisions for patients with several common infections to guide antibiotic duration decisions. While the authors are aware that many clinicians now use short courses of antibiotics to treat adult women with UTI, overall use of short courses does not appear to be common practice. For patients and clinicians, this may change the commonly held view (for most infections) that 7 day courses of antibiotics are required. Further, a study of antibiotic prescribing for children in ambulatory settings found that antibiotic prescribing occurs twice as often as is indicated for acute respiratory tract infections, supporting the important ongoing efforts of antibiotic stewardship (39). While reductions in antibiotic prescribing rates have occurred particularly for pediatric practices in the US, rates among adults and the elderly have remained static or increased, and overall rates appear at least double what is indicated (39). In combination with Centers for Disease Control and Prevention, and other antibiotic stewardship efforts, our research provides an alternative to typical long course therapies.
Implications for research
While some antibiotic stewardship strategies have been effective in reducing antibiotic use rates and improving antibiotic selection, research is needed to help implement our wider findings that short courses appear as effective as longer ones for most common infections in ambulatory care. Interventions to integrate short courses into prescribing tools, educational materials and other resources need to be evaluated, and their effectiveness compared to or in combination with other strategies. Further research as to whether or not decreasing dosing duration impacts antimicrobial resistance and associated treatment failure both locally, regionally and globally would contribute to ongoing efforts to expand this approach (40).
Implications for policy makers
For policy makers involved in antibiotic stewardship, our findings should be used to encourage public and provider education on appropriate prescribing, so that when antibiotics are used the options of short course prescribing are encouraged. Prior research has shown that frequently patients are unaware of antibiotic resistance (41), further policy work around the indications for antibiotics and the option for shorter courses may be helpful. We encourage guidelines committees from relevant bodies (e.g. Infectious Disease Society of America etc.) to consider decreasing dosing duration for common bacterial infections treated in outpatient settings.
Conclusions
This overview of systematic reviews has identified quality evidence that short course antibiotics are as effective as longer courses for adults and children treated in outpatient settings with tonsillopharyngitis, acute otitis media, uncomplicated UTI or mild / moderate community acquired pneumonia, and for adults treated for acute sinusitis or acute pyelonephritis managed in ambulatory care. Antibiotics are a valuable resource, but one that is at risk from the combination of growing resistance and few new antibiotics being developed. In order to preserve antibiotics, our review suggests that a short course of antibiotics may be suitable for several common infections in adults and children.
Supplementary Material
Supplementary data are available at Family Practice online.
Declaration
Funding: CCB is supported by the National Institute for Health Research Health Protection Research Unit, Healthcare Associated Infections and Antimicrobial Resistance, at the University of Oxford. EED-H’s time was supported by the Ruth L. Kirschstein National Research Service Award (#T32HP10002). All other funding was provided by departmental support.
Ethical approval: this systematic review did not require human subjects review.
Conflict of interest: none.
Supplementary Material
References
- 1. Hersh AL, Shapiro DJ, Pavia AT, Shah SS. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics 2011; 128: 1053–61. [DOI] [PubMed] [Google Scholar]
- 2. Holstiege J, Schink T, Molokhia M, et al. Systemic antibiotic prescribing to paediatric outpatients in 5 European countries: a population-based cohort study. BMC Pediatr 2014; 14: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee GC, Reveles KR, Attridge RT, et al. Outpatient antibiotic prescribing in the United States: 2000 to 2010. BMC Med 2014; 12: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Little P, Stuart B, Francis N, et al. ; GRACE consortium. Effects of internet-based training on antibiotic prescribing rates for acute respiratory-tract infections: a multinational, cluster, randomised, factorial, controlled trial. Lancet 2013; 382: 1175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Laxminarayan R, Duse A, Wattal C, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis 2013; 13: 1057–98. [DOI] [PubMed] [Google Scholar]
- 6. Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010; 340: c2096. [DOI] [PubMed] [Google Scholar]
- 7. Horsburgh CR, Shea KM, Phillips P, Lavalley M. Randomized clinical trials to identify optimal antibiotic treatment duration. Trials 2013; 14: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Katchman EA, Milo G, Paul M, et al. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am J Med 2005; 118: 1196–207. [DOI] [PubMed] [Google Scholar]
- 9. Falagas ME, Vouloumanou EK, Matthaiou DK, Kapaskelis AM, Karageorgopoulos DE. Effectiveness and safety of short-course vs long-course antibiotic therapy for group a beta hemolytic streptococcal tonsillopharyngitis: a meta-analysis of randomized trials. Mayo Clin Proc 2008; 83: 880–9. [PubMed] [Google Scholar]
- 10. Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database Syst Rev 2003; 1:CD003966. [DOI] [PubMed] [Google Scholar]
- 11. Falagas ME, Karageorgopoulos DE, Grammatikos AP, Matthaiou DK. Effectiveness and safety of short vs. long duration of antibiotic therapy for acute bacterial sinusitis: a meta-analysis of randomized trials. Br J Clin Pharmacol 2009; 67: 161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kyriakidou KG, Rafailidis P, Matthaiou DK, Athanasiou S, Falagas ME. Short- versus long-course antibiotic therapy for acute pyelonephritis in adolescents and adults: a meta-analysis of randomized controlled trials. Clin Ther 2008; 30: 1859–68. [DOI] [PubMed] [Google Scholar]
- 13. Kozyrskyj A, Klassen TP, Moffatt M, Harvey K. Short-course antibiotics for acute otitis media. Cochrane Database Syst Rev 2010; 9: Cd001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haider BA, Saeed MA, Bhutta ZA. Short-course versus long-course antibiotic therapy for non-severe community-acquired pneumonia in children aged 2 months to 59 months. Cochrane Database Syst Rev 2008; 2: CD005976. [DOI] [PubMed] [Google Scholar]
- 15. Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007; 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. The World Bank Data: Country and Lending Programs. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed on 17 April 2017). [Google Scholar]
- 17. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 18. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2009. [Google Scholar]
- 19. Li JZ, Winston LG, Moore DH, Bent S. Efficacy of short-course antibiotic regimens for community-acquired pneumonia: a meta-analysis. Am J Med 2007; 120: 783–90. [DOI] [PubMed] [Google Scholar]
- 20. Lutters M, Vogt-Ferrier N. Antibiotic duration for treating uncomplicated, symptomatic lower urinary tract infections in elderly women. status and date: Edited (conclusions changed), published in. 2008; 3: CD001535. [DOI] [PubMed] [Google Scholar]
- 21. Mandell LA, Wunderink RG, Anzueto A, et al. ; Infectious Diseases Society of America; American Thoracic Society. Infectious diseases society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44 (suppl 2): S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roberts KB; Subcommittee on Urinary Tract Infection SCoQI, Management Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 2011; 128 (3): 595–610. [DOI] [PubMed] [Google Scholar]
- 23. Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics 2013; 131: e964–99. [DOI] [PubMed] [Google Scholar]
- 24. Bradley JS, Byington CL, Shah SS, et al. ; Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis 2011; 53: e25–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis 2012; 55: 1279–82. [DOI] [PubMed] [Google Scholar]
- 26. Chow AW, Benninger MS, Brook I, et al. ; Infectious Diseases Society of America. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis 2012; 54: e72–e112. [DOI] [PubMed] [Google Scholar]
- 27. Gupta K, Hooton TM, Naber KG, et al. ; Infectious Diseases Society of America; European Society for Microbiology and Infectious Diseases. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52: e103–20. [DOI] [PubMed] [Google Scholar]
- 28. National Collaborating Centre for Women and Children’s Health. Urinary tract infection in under 16s: diagnosis and management. 2007. https://www.nice.org.uk/guidance/cg54 (accessed on 17 April 2017). [Google Scholar]
- 29. National Clinical Guideline Centre. Pneumonia in adults: diagnosis and management National Institute for Health and Care Excellence; 2014. https://www.nice.org.uk/guidance/cg191/ (accessed on 17 April 2017). [Google Scholar]
- 30. National Institute for Health and Care Excellence. Urinary tract infections in adults. National Institute for Health and Care Excellence; 2015. https://www.nice.org.uk/guidance/qs90 (accessed on 17 April 2017). [Google Scholar]
- 31. National Institute for Health and Care Excellence. Otitis Media—Acute. http://cks.nice.org.uk/otitis-media-acute#!topicsummary (accessed on 17 April 2017). [Google Scholar]
- 32. National Institute for Health and Care Excellence.. Prescribing of antibiotics for self-limiting respiratory tract infections in adults and children in primary care: National Institute for Health and Clinical Excellence; 2008. http://guidance.nice.org.uk/CG69 (accessed on 17 April 2017). [PubMed] [Google Scholar]
- 33. Antibiotic Expert Groups. Therapeutic guidelines: Antibiotic. Melbourne: Therapeutic Guidelines Limited, 2016. [Google Scholar]
- 34. Mandell LA, Marrie TJ, Grossman RF, Chow AW, Hyland RH; Canadian Infectious Disease Society; Canadian Thoracic Society Summary of Canadian guidelines for the initial management of community-acquired pneumonia: an evidence-based update by the Canadian Infectious Disease Society and the Canadian Thoracic Society. Can Respir J 2000; 7: 371–82. [DOI] [PubMed] [Google Scholar]
- 35. Robinson JL, Finlay JC, Lang ME, Bortolussi R; Canadian Paediatric Society, Infectious Diseases and Immunization Committee, Community Paediatrics Committee Urinary tract infections in infants and children: Diagnosis and management. Paediatr Child Health 2014; 19: 315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Le Saux N, Robinson JL; Canadian Paediatric Society, Infectious Diseases and Immunization Committee Management of acute otitis media in children six months of age and older. Paediatr Child Health 2016; 21: 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Le Saux N, Robinson JL; Canadian Paediatric Society, Infectious Diseases and Immunization Committee Uncomplicated pneumonia in healthy Canadian children and youth: Practice points for management. Paediatr Child Health 2015; 20: 441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shulman ST, Stollerman G, Beall B, Dale JB, Tanz RR. Temporal changes in streptococcal M protein types and the near-disappearance of acute rheumatic fever in the United States. Clin Infect Dis 2006; 42: 441–7. [DOI] [PubMed] [Google Scholar]
- 39. Kronman MP, Zhou C, Mangione-Smith R. Bacterial prevalence and antimicrobial prescribing trends for acute respiratory tract infections. Pediatrics 2014; 134: e956–65. [DOI] [PubMed] [Google Scholar]
- 40. Leibovici L, Paul M, Garner P, et al. Addressing resistance to antibiotics in systematic reviews of antibiotic interventions. J Antimicrob Chemother. 2016. [DOI] [PubMed] [Google Scholar]
- 41. Brooks L, Shaw A, Sharp D, Hay AD. Towards a better understanding of patients’ perspectives of antibiotic resistance and MRSA: a qualitative study. Fam Pract 2008; 25: 341–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.