Abstract
Background:
Vitamin D plays an important role in nervous health and depression. Vitamin D deficiency and anxiety affect diabetic status. The purpose of this study was to determine the effect of vitamin D supplementation on anxiety, depression, and inflammation in diabetic women with anxiety.
Methods:
In this randomized controlled trial, totally 51 women with type 2 diabetes (T2DM) and vitamin D deficiency were randomly allocated to receive one oral pearl of 50,000 IU vitamin D3 (26 women) or a placebo (25 women) fortnightly for 16 weeks. Anthropometric indices, sun exposure, dietary intake, depression, anxiety, and stress scores and biochemical biomarkers including high sensitivity C-reactive protein (hs-CRP) and interleukin-10 (IL-10) were measured at the baseline and after 16-week supplementation.
Results:
Mean ± SD age of participant was 47.43 ± 9.57 years old. Baseline values were not different between the groups. Anxiety score changes were significantly lower in vitamin D group than the controls (P = 0.001). Within group comparison indicated that depression in supplement group with lower vitamin D levels was significantly reduced. Serum hs-CRP reduced (P = 0.01), while IL-10 concentrations increased (P = 0.04) in the intervention group.
Conclusions:
Vitamin D supplementation can improve mood status and anti-inflammatory biomarkers in female diabetics with anxiety and vitamin D deficiency.
Keywords: Anxiety, diabetes, inflammation, Vitamin D, women
Introduction
Recently, in addition to the function of vitamin D on calcium metabolism, bone, proliferation, differentiation, and immune modulation, few evidence indicates that vitamin D plays an important role in the brain, nervous system health, and depression.[1] Both vitamin D deficiency and insufficiency show global burden[2] that is more prevalent in women.[3]
The anxiety disorders include some of the most common and disabling psychiatric illnesses. Anxiety is characterized by excessive and persistent worry that can reduce the ability to perform normal activities in life.[4]
A systematic review and meta-analysis has indicated that diabetes is related to increased risk of anxiety disorders.[5] Uncontrolled blood sugar is associated with anxiety and the rates of generalized anxiety disorder (GAD) is higher in diabetes.[6]
Tumor necrosis factor-alpha (TNF-α) and C-reactive protein (CRP) are increased in anxiety due to inflammation and psychoneuroimmunologic pathways.[7] Anxiety can weaken the immune system[8] while some studies show that diabetic patients have higher levels of inflammatory markers such as CRP, interleukin-1 (IL-1), IL-6, TNF-α, interstitial cellular adhesion molecule-1, and vascular cellular adhesion molecule-1.[9] On the other hand, evidence suggests that both depression and anxiety stimulate inflammation in diabetic patient.[10]
Previous interventions have shown the impact of vitamin D deficiency on depression,[11] however, limited studies have been carried out to illustrate the association of anxiety disorders with serum levels of vitamin D.[12] Penckofer et al. reported 6-month intervention with 50,000 IU vitamin D significantly decreased depression and anxiety in type 2 diabetes mellitus (T2DM) who had depression.[13] Supplementation with vitamin D reduced anxiety levels in premenstrual syndrome in adolescents who had severe vitamin D deficiency.[14] At present, there have been no studies regarding the effect of vitamin D supplementation in women with T2DM affected by anxiety. Therefore, the main aim of this study was to examine the potential impact of vitamin D on anxiety in diabetic women with vitamin D deficiency.
Methods
Participants
In the present randomized double-blind placebo-controlled clinical trial, 64 women with T2DM were recruited from Shahr-e-Kord Diabetes Clinic, Iran. The participants were T2DM women between 20 to 60 years old diagnosed with mild, moderate, or severe anxiety. All participants had vitamin D deficiency or insufficiency [10 (ng/mL) ≤ serum vitamin D ≤30 (ng/mL)]. Diagnosis of T2DM was on the bases of the World Health Organization guidelines.[15]
Anxiety was determined by the Depression, Anxiety and Stress Scales (DASS- 21) questionnaire apply for clinical and non-clinical populations that had previously been validated in Iranian population.[16] Each question of DASS-21 has four scales (0 = did not apply to me at all, 1 = applied to me to some degree or some of the time, 2 = applied to me to a considerable degree or a good part of the time, and 3 = applied to me very much or most of the time) to determine the symptoms of anxiety and depression. The sum of scores for each subscale should be multiplied to 2 to evaluate original depression anxiety stress scale of 42 items. Patient's scores on DASS-21 scale can run between 0 to 42. Cut-off points for anxiety score are 0–7, 8–9, 10–14, 15–19, and more than 20 to classify patients into normal, mild, moderate, severe, and extremely severe state of anxiety, respectively.[17,18]
General characteristics of the participants including age, educational levels, suffering from other diseases, duration of T2DM, and medication history were collected through face-to-face interview pre-intervention.
Patients that had neurological or psychiatric disorders, took any medications for depression or vitamin D/multivitamin supplements during the last 4 month, consumed alcohol, were pregnant, or lactating were excluded. The purpose of the study was explained for eligible participants. Women who had the aforementioned criteria and agreed to participate in the study completed informed consent form before commencing the intervention.
Study design, randomization, and blindness
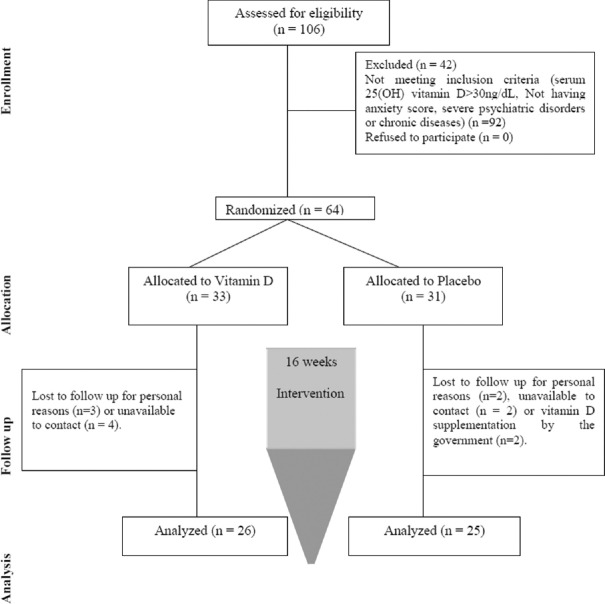
Current study was a randomized double-blind placebo-controlled clinical trial. Block randomization (with block sizes of two) was performed using the Random Allocation Software: (RAS). Participants were randomly allocated into either intervention or placebo group [Figure 1]. The intervention and placebo groups received 50,000 IU cholecalciferol and paraffin soft gel capsules, respectively, fortnightly for 16 weeks. Since normal upper limit of vitamin D intake is 10,000 IU/day, the recommended dosage was regarded safe.[19]
Figure 1.
Algorithm of study participants in the two groups
All participants and investigators including the laboratory staff were blind to randomization. Placebo was similar to vitamin D soft gels in color and size and they were presented in dark bottles (A, B) coded by a subject who was not involved in any procedures of the study. Both supplements were produced by Zahravi Pharm Co; Tabriz, Iran.
Sun exposure time
At the beginning and end of the study, sun exposure rate of participants were assessed through a validated questionnaire. Duration of exposure to sunlight [the average minutes (min)/hours (h) of a usual day in the previous week] was asked using a questionnaire.[20] The duration of sun exposure was classified as 0–10 min; 10 min–1 h; 1–2 h; and >2 h. Furthermore, the time of exposure to sunlight during a day was also asked and classified as follows: 7 am–10 am; 10 am–3 pm; and 3 pm–5 pm. At the end of the questionnaire, the part of the body that was exposed to sunlight was asked from all participants. Because of the intense sunlight, the highest score was the exposure of sunlight at 10–3 o’clock.[21]
Dietary assessment
Dietary intakes were assessed by a single researcher using a valid 3-day food record (2 working days and 1 weekend) at pre- and post-intervention. Nutritionist IV software program (First Databank Inc., Hearst Corp., San Bruno, CA, USA) modified for Iranian food was used for dietary analysis.
Physical activity assessment
The physical activity level of participants was determined using the short version of International Physical Activity Questionnaire (IPAQ) validated for Iranians.[22] at pre- and post-intervention.
Anthropometric indices assessment
Anthropometric indices such as height (by a graded wall to the nearest of 0.1 cm), weight (fasting weight was measured with the accuracy of 0.1 kg using Seca scales), waist circumferences (WC) was measured at the midway between lower rib and iliac crest, and hip circumferences (HC) was recorded using standard methods[23] at the baseline and post-intervention by a trained nutritionist. Body mass index (BMI) was calculated by dividing weight (kg) to square height (m2).
Sample size determination
Considering 0.05 as type 1 error and 0.2 as type 2 error (power = 80%) based on previous study and using standard deviation equal to 1 and change in mean equal to 0.81 of high sensitivity CRP (hs-CRP), 24 subjects were needed in each group. We added 10% to sample size because of covering possible dropouts to reach 25 participants in each group.[24]
Laboratory analyses
A total of 10 ml of fasting blood samples were collected from each participant after 12 h fasting at the beginning and at the end of study. The blood samples were centrifuged to separate serums and they were then stored at −80°C until further analysis. Serum 25-hydroxy vitamin D levels were quantitatively measured using the enzyme-linked immunosorbent assay (ELISA) method (Monobind ELISA kit, USA). Serum hs-CRP concentrations were assayed using ELISA kit (Diagnostics Biochem Canada Inc, Ontario, Canada).
Statistical analysis
We analyzed data using the SPSS software, version 17 (SPSS Inc, Chicago, IL). The Shapiro–Wilk test was performed to evaluate the normality of the distributions. The between-group comparisons for baseline characteristics were done using independent t-test or Mann–Whitney U test for variables with normal and non-normal distribution, respectively. Wilcoxon test and paired t tests were applied for within-group analysis. We used analysis of covariance (ANCOVA) test adjusted for baseline values and confounders such as fat and vitamin C intake to compare the two groups pre- and post-intervention. P-values <0.05 were considered statistically significant. Between- and within-group analyses of subgroups were performed with the similar methods used previously.
Ethical consideration
The present study was carried out in accordance with the guidelines described in the Declaration of Helsinki.[25] It was approved by the Medical Ethics Committee of Isfahan University of Medical Sciences (Reg. No: IR. MUI. REC.1395.3736). This study has been registered in the Iranian registry of clinical trials (http://www.irct.ir) under the number IRCT20170927036451N1.
Results
Sixty-four TDM2 women with mean ± SD age of 47.43 ± 9.57 years were included to our study. Finally, 51 participants (26 patients in the vitamin D and 25 patients in the placebo groups) completed the trial [Figure 1]. Demographic information including education, BMI, physical activity, sun exposure, waist-to-hip ratio, and serum 25-hydroxy vitamin D at the beginning and post-intervention showed no significant differences [Table 1]. Compliance rate of taking soft pearls was >90% in the two groups.
Table 1.
Baseline characteristic of participants in the study groups
| Variables | Supplement group (n=26) | Placebo group (n=25) | P |
|---|---|---|---|
| Age (year) | 48.5±7.58a | 46.32±11.16a | 0.42† |
| FBS (mg/dL) | 166.63±63.63 | 158.32±54.14 | 0.38† |
| Education, n (%) | |||
| Primary/high school | 18 (69.40) | 13 (52.00) | 0.42* |
| Diploma | 4 (15.30) | 7 (28.00) | |
| University degree | 4 (15.3) | 5 (20.00) | |
| Exposure to sunlight score | 31.02±24.67a | 32.54±6.71a | 0.85† |
| BMI (kg/m2) | 30.21±4.42a | 29.19±6.41a | 0.16† |
| Waist-to-hip ratio | 0.94±0.05 | 0.91±0.10 | |
| Physical activity (MET/hours*day) | 31.48±18.12a | 30.21±33.05a | 0.09† |
| Use of sunscreen cream, n (%) | |||
| Never | 14 (53.84) | 14 (56.00) | 0.92† |
| Sometimes | 7 (26.92) | 5 (20.00) | |
| Very often | 3 (11.54) | 3 (12.00) | |
| Always | 2 (7.70) | 3 (12.00) | |
| 25-hydroxy vitamin D (ng/mL) | 21.32±5.85a | 20.73±6.47a | 0.37† |
†By independent t-test, *By Chi-square test, aMean±SD. SD=Standard deviation, FBS=Fasting blood sugar, BMI=Body mass index, MET=Metabolic equivalent of task
Analysis of 3-day food records showed no significant differences in terms of nutrients and energy intake, except for fat (P = 0.02) and vitamin C (P = 0.02) at the baseline [Table 2].
Table 2.
Comparison of dietary intake, anthropometric indices, and physical activity in the study groups pre- and post-intervention
| Variables | Supplement group (n=26) | Placebo group (n=25) | P† |
|---|---|---|---|
| Weight (kg) | |||
| Baseline | 74.55±12.20 | 74.40±16.10 | 0.97† |
| 16 weeks | 75.13±12.29 | 75.76±16.33 | 0.88† |
| P*,‡ | 0.64 | 0.06 | |
| Waist circumference (cm) | |||
| Baseline | 100.87±15.02 | 99.36±14.62 | 0.71 |
| 16 weeks | 100.47±9.61 | 99.52±13.54 | 0.87 |
| P‡ | 0.85 | 0.92 | |
| Waist-to-hip ratio | |||
| Baseline | 0.94±0.05 | 0.92±0.10 | 0.18 |
| 16 weeks | 0.95±0.56 | 0.92±0.11 | 0.18 |
| P‡ | 0.02 | 0.01 | |
| BMI (kg/m2) | |||
| Baseline | 30.21±4.42 | 29.20±6.41 | 0.16 |
| 16 weeks | 30.42±4.30 | 29.73±6.48 | 0.21 |
| P¥ | 0.55 | 0.08 | |
| 25(OH) D3 (ng/mL) | |||
| Baseline | 21.32±5.85 | 20.73±6.47 | 0.81 |
| 16 weeks | 29.11±11.70 | 22.4±11.36 | 0.04 |
| P¥ | <0.001** | 0.89 | |
| Energy intake (kCal/day) | |||
| Baseline | 2337.92±410.20 | 2203.60±442.12 | 0.26 |
| 16 weeks | 2276.82±448.80 | 2084.20±257.66 | 0.07 |
| P‡ | 0.07 | 0.24 | |
| Carbohydrate intake (g/day) | |||
| Baseline | 356.29±84.41 | 322.25±66.19 | 0.11 |
| 16 weeks | 343.28±73.36 | 311.16±56.25 | 0.86 |
| P‡ | 0.11 | 0.44 | |
| Protein intake (g/day) | |||
| Baseline | 91.80±15.57 | 86.99±15.07 | 0.33 |
| 16 weeks | 86.90±17.92 | 100.48±9.61 | 0.44 |
| P‡ | 0.57 | 0.98 | |
| Fat intake (g/day) | |||
| Baseline | 64.29±10.61 | 56.25±11.51 | 0.02** |
| 16 weeks | 62.24±15.20 | 53±13.93 | 0.43† |
| P‡ | 0.40 | 0.54 | |
| Vitamin C intake (mg/day) | |||
| Baseline | 125.66±58.79 | 84.72±54.17 | 0.02** |
| 16 weeks | 101.81±51.50 | 88.43±49.50 | 0.38 |
| P‡ | 0.07 | 0.68 | |
| Vitamin D intake (mcg/day) | |||
| Baseline | 0.84±1.28 | 0.56±0.72 | 0.33 |
| 16 weeks | 1.06±1.23 | 0.77±0.79 | 0.33 |
| P¥ | 0.48 | 0.27 | |
| Saturated fatty acids intake (g/day) | |||
| Baseline | 17.89±9.16 | 16.11±11.24 | 0.27 |
| 16 weeks | 16.73±8.72 | 12.67±6.23 | 0.07 |
| P¥ | 0.10 | 0.33 | |
| Monounsaturated fatty acids intake (g/day) | |||
| Baseline | 25.54±11.11 | 21.41±7.06 | 0.12 |
| 16 weeks | 24.40±14.80 | 19.84±6.91 | 0.18 |
| P‡ | 0.56 | 0.28 | |
| Polyunsaturated fatty acids intake (g/day) | |||
| Baseline | 19.93±5.51 | 20.36±9.23 | 0.84 |
| 16 weeks | 18.27±4.21 | 16.96±4.24 | 0.26 |
| P‡ | 0.04 | 0.84 | |
| Exposure to sunlight score | |||
| Baseline | 31.02±24.67 | 32.54±33.05 | 0.85 |
| 16 weeks | 40.29±35.23 | 32.80±25.19 | 0.54 |
| P‡ | 0.44 | 0.98 | |
| Physical activity (MET/hours*day) | |||
| Baseline | 31.48±18.12 | 25.81±15.81 | 0.09 |
| 16 weeks | 30.28±16.99 | 24.95±12.96 | 0.19 |
| P¥ | 0.50 | 0.99 |
Variables are represented as mean±SD. †By independent t-test, ‡By paired sample t-test, ¥By Wilcoxon signed rank test, *Indicates between groups comparisons, **P<0.05. SD=Standard deviation, BMI=Body mass index, MET=Metabolic equivalent of task
Anthropometric variables, sun exposure, and physical activity of both the groups did not change after intervention.
The mean ± SD of anxiety score at the beginning of the study in supplement group was 12.31 ± 4.26 which indicated moderate anxiety. At the end of intervention in vitamin D group, mean ± SD of anxiety severity was 9.15 ± 4.54 this average is classified as mild anxiety level.
Significant reduction in anxiety score was seen in vitamin D group vs. control group (P = 0.001), [Table 1].
Depression changes were not different significantly in vitamin D group vs. controls (P > 0.05), while the depression score significantly decreased post-intervention in vitamin D group, that determined by within-group analyses [Table 3]; (P = 0.03).
Table 3.
Effects of vitamin D on inflammatory biomarkers, anxiety, stress, and depression
| Variables | Intervention group (n=26) | Placebo group (n=25) | P | P* |
|---|---|---|---|---|
| Anxiety | ||||
| Baseline | 12.31±4.26 | 10.44±5.05 | 0.16† | <0.001** |
| 16 weeks | 9.15±4.54 | 13.28±4.61 | 0.02†,** | |
| Change | -3.15±4.65 | 1.64±4.75 | 0.001†,** | |
| P (within group)‡ | <0.001** | 0.10 | ||
| Stress | ||||
| Baseline | 13.27±5.38 | 1.84±4.29 | 0.68† | 0.42 |
| 16 weeks | 12.2±6.63 | 13.28±4.61 | 0.51† | |
| Change | -1.04±4.68 | -0.56±2.80 | 0.66† | |
| P (within group)‡ | 0.26 | 0.33 | ||
| Depression | ||||
| Baseline | 11.88±4.84 | 12.44±5.09 | 0.69† | 0.29 |
| 16 weeks | 9.88±3.88 | 11.08±5.28 | 0.36† | |
| Change | -2.00±4.38 | -1.36±4.07 | 0.59† | |
| P (within group)¥ | 0.03** | 0.11 | ||
| hs-CRP (mg/L) | ||||
| Baseline | 3.23 (1.37, 9.06) | 4.12 (2.32, 10.65) | 0.35₤ | <0.001** |
| 16 weeks | 2.39 (1.18, 7.93) | 4.29 (2.56, 10.23) | 0.05₤ | |
| Change | -0.27 (-0.89, -0.22) | 2.58 (-0.21, 1.21) | 0.01₤ | |
| P (within group)¥ | 0.01** | 0.16 | ||
| IL-10 (ng/mL) | ||||
| Baseline | 80.25 (61.75, 102.00) | 93.00 (70.75, 115.00) | 0.22₤ | 0.02** |
| 16 weeks | 93.00 (64.27, 157.27) | 83.00 (66.90, 106.00) | 0.44₤ | |
| Change | 8.80 (-2.86, 27.75) | 2.1 (-11.00, 10.70) | 0.04₤ | |
| P (within group)¥ | 0.02** | 0.93 | ||
Variables are represented mean±SD or median (25th, 75th percentiles). *By ANCOVA for between groups’ comparisons (adjustment was made for baseline values, fat, and vitamin C intake), †By Independent t-test, ‡By paired sample t-test, ¥Obtained from Wilcoxon signed rank test, ₤Obtained from Mann-Whitney test, **P<0.05. SD=Standard deviation, hs-CRP=high sensitivity C-reactive protein, IL-10=interleukin 10
Patients were classified into two subgroups as 10> serum vitamin D ≥20 and 20> serum vitamin D ≥30. Subgroup analysis indicated that anxiety score decreased in supplement group in both categories of serum vitamin D (P < 0.5). Depression score in supplement group with lower vitamin D levels was significantly reduced (P = 0.04). Stress did not change significantly in any of the groups and also in vitamin D subgroups [Table 4]. Reduction in hs-CRP levels was significant in patients who took vitamin D supplement in both categories (P = 0.01).
Table 4.
Comparison of the baseline and post-intervention values of variables based on serum vitamin D (ng/mL) status in the study groups
| Variables | 10 >Vitamin D ≥20 | 20 >Vitamin D >30 | ||||
|---|---|---|---|---|---|---|
| Intervention group (n=9) | Placebo group (n=9) | P | Intervention group (n=17) | Placebo group (n=16) | P | |
| Anxiety | ||||||
| Baseline | 12.89±3.14 | 11.11±5.16 | 0.06† | 12.00±4.82 | 11.12±5.51 | 0.63† |
| 16 weeks | 9.78±3.68 | 10.78±4.04 | 0.06† | 9.82±4.34 | 12.18±4.26 | 0.12† |
| Change | -5±4.92 | 2.67±2.31 | 0.01†,** | -2.18±3.33 | 1.06±2.73 | 0.03†,** |
| P (within group)‡ | 0.02 | 0.24 | 0.05 | 0.27 | ||
| Stress | ||||||
| Baseline | 13.22±4.23 | 12.77±2.63 | 0.79† | 13.29±6.02 | 14.44±4.98 | 0.33† |
| 16 weeks | 7.88±3.89 | 11.89±3.69 | 0.96† | 11.88±4.97 | 13.56±5.01 | 0.43† |
| Change | -0.33±3.33 | -00±1.73 | 0.84† | 0.25 | 0.24 | 0.70† |
| P (within group)‡ | 0.83 | 0.99 | -1.42±3.91 | -0.88±2.87 | ||
| Depression | ||||||
| Baseline | 12.77±4.14 | 9.22±4.15 | 0.46† | 11.40±5.22 | 13.18±5.06 | 0.33† |
| 16 weeks | 12.89±6.27 | 12.78±4.02 | 0.68† | 9.94±4.05 | 11.25±5.00 | 0.42† |
| Change | -3.00±2.71 | -0.33±2.57 | 0.14† | -1.47±2.71 | 1.94±3.32 | 0.76† |
| P (within group)‡ | 0.04 | 0.78 | 0.22 | 0.09 | ||
| hs-CRP (mg/L) | ||||||
| Baseline | 2.52 (1.19, 12.19) | 2.79 (1.54, 4.27) | 0.73₤ | 3.35 (1.80, 7.05) | 7.18 (2.71, 12.31) | 0.09₤ |
| 16 weeks | 2.21 (1.12, 9.09) | 2.56 (1.84, 5.51) | 0.07₤ | 2.56 (1.18, 7.88) | 6.01 (3.02, 11.89) | 0.04₤ |
| Change | -0.75 (-1.82, -0.14) | 0.35 (-0.39, 0.86) | 0.01₤,** | -0.21 (-0.86, 0.27) | 0.23 (-1.51, 1.41) | 0.08₤ |
| P (within group)¥ | 0.008** | 0.23 | 0.03 | 0.26 | ||
| IL-10 (ng/mL) | ||||||
| Baseline | 68.00 (48.70, 111.20) | 99.00 (59.30, 118.50) | 0.34₤ | 85.00 (62.50, 107.00) | 90.00 (74.12, 116.00) | 0.48₤ |
| 16 weeks | 92.00 (48.80, 143.25) | 83.00 (63.50, 114.50) | 0.76₤ | 94.00 (81.25, 164.50) | 83.50 (68.85, 109.25) | 0.26₤ |
| Change | 4.40 (-6.35, 19.00) | 00.00 (-10.40, 6.20) | 0.34₤ | 10.00 (-3.25, 49.10) | 2.55 (-20.75, 14.76) | 0.08₤ |
| P (within group)¥ | 0.31 | 0.67 | 0.67 | 0.83 | ||
Variables are represented mean±SD or median (25th, 75th percentiles). †By independent t-test, ‡By paired sample t-test, ¥Obtained from Wilcoxon signed rank test, ₤Obtained from Mann-Whitney test, **P<0.05. SD=Standard deviation, hs-CRP=high sensitivity C-reactive protein, IL-10=Interleukin 10
Discussion
Our study showed that 16 weeks supplementation with 50,000 IU vitamin D decreased anxiety, hs-CRP and increased IL-10 concentrations in T2DM women with anxiety for a period of 4 months. Recently Penckofer et al. reported that vitamin D supplementation for 6 month can decrease depression and anxiety in diabetic patient with symptoms of depression.[13] Several studies have shown that vitamin D deficiency is associated to poorer mental health.[26,27] However, overall effect of vitamin D in a meta-analysis reported no significant reduction in depression after supplementation. It is worthy to note that most studies examined the individuals with low depression and sufficient serum levels of vitamin D at the baseline.[28]
Vitamin D supplementation reduced anxiety in this trial. Bičíková et al. showed that lower serum level of calcidiol was related to anxiety in patients with anxiety disorders assessed by Clinical Global Impressions Scale (CGI).[29] Ataie-Jafari have indicated that anxiety, poor quality sleep, depression, and worry were related to vitamin D deficiency in Iranian adolescences.[30] A trial by Dean et al. indicated that vitamin D has no therapeutic effects on anxiety and depression in healthy adult.[31] A study carried out on the effect of daily supplementation with dosage of 5000 IU vitamin D in young adults did not show any changes in anxiety scores,[31] however, another study reported that anxiety improved in diabetic patients received 50,000 IU vitamin D on weekly basis for 6 month.[13]
Depression score did not change significantly, however, subgroup analysis in accordance to the baseline 25-hydroxy vitamin D indicated that depression scale decreased in group with lower levels of serum vitamin D. Although recent studies indicated serum 25(OH) vitamin D concentrations have inverse relation with symptoms of depression.[32] Other studies have reported non-significant effect of vitamin D on depression in individuals with lower scores of depression,[33,34] justifying the challenge between subgroups analysis and overall result. Furthermore, vitamin D treatment for longer periods may exert therapeutic effect to ameliorate depression in patients with clinically proven depression.[35] Future randomized controlled trials are needed to examine the effect of vitamin D on patients with vitamin D deficiency and depression.
In the present study, there was no improvement in patients’ stress scale. Black et al. in a cross-sectional study did not find any relationships between stress and serum level of vitamin D assessed through DASS-21.[12] Wang et al. reported that vitamin D could not reduce physiological distress in hospitalized patients with vitamin D and vitamin C deficiency.[36]
We found that vitamin D supplementation for 4-month period decreased hs-CRP while increased IL-10 levels. Previous observational studies have revealed that diabetic patients have significantly higher hs-CRP concentrations than healthy people.[37] Moreover, anxiety is associated with high serum levels of hs-CRP,[38] while IL-10 level, as an anti-inflammatory biomarker, is low in patients with anxiety.[7] Improving inflammatory markers in GAD could help reduce anxiety.[39] It seems that anxiety disorders are associated with defective activity of hypothalamus-pituitary-adrenal axis (HPA), higher pro-inflammatory, and low anti-inflammatory cytokines.[40,41]
Diabetic patients are at higher risk of oxidative stress, heart disease, and anxiety disorder.[42,43] Vitamin D supplementation is effective for reducing serum hs-CRP concentrations,[44] however, some authors did not report any changes in women with gestational diabetes.[45] The difference in the results of studies could be due to supplementation dosage, treatment period, and the population studied. The higher serum concentration of hs-CRP at baseline is effective in result of vitamin D interventions.[46] Supplementation with vitamin D for 35 days increased IL-10 in vitamin D insufficient subjects [serum 25(OH) D <29 ng/mL].[47] Furthermore, 3-month supplementation with 50,000 IU vitamin D increased IL-10 in multiple sclerosis patients.[48] Subgroup analysis showed that the increase in IL-10 in both subgroups with vitamin D supplementation was non-significant and could be attributed to smaller sample size in subgroups.
Vitamin D supplement had no significant effects on BMI and anthropometric indices as previous studies also confirmed these results.[49,50,51] A meta-analysis indicated that serum vitamin D has inverse relationship with fat mass, however, vitamin D supplementation was not effective on fat mass.[52]
Recently, it has been reported vitamin D responsive factors such as vitamin D receptors (VDRs) and the regions of serotonin receptors and tryptophan hydroxylase, have relationship with depression.[53] On the other hand, another study did not find any association between vitamin D levels, polymorphism of vitamin D receptor, and depression.[54] In the future, more studies are needed to show the mediatory role of genes in vitamin D impact.
As a limitation of our study, we were unable to measure the seasonal changes that could affect the outcome. However, the intervention was not conducted in different seasons. The strength of our study was the continuous follow-up during the intervention.
Conclusions
Vitamin D supplementation could improve anti-inflammatory biomarkers and reduce cardiovascular risk factor and anxiety in T2DM women with anxiety and vitamin D deficiency. More studies are warranted to clarify the beneficial effects of supplementation with vitamin D on male diabetes patients having anxiety.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.DeLuca GC, Kimball SM, Kolasinski J, Ramagopalan SV, Ebers GC. Review: The role of Vitamin D in nervous system health and disease. Neuropathol Appl Neurobiol. 2013;39:458–84. doi: 10.1111/nan.12020. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. The Vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017;18:153–65. doi: 10.1007/s11154-017-9424-1. [DOI] [PubMed] [Google Scholar]
- 3.Kazemi A, Sharifi F, Jafari N, Mousavinasab N. High prevalence of Vitamin D deficiency among pregnant women and their newborns in an Iranian population. J Womens Health (Larchmt) 2009;18:835–9. doi: 10.1089/jwh.2008.0954. [DOI] [PubMed] [Google Scholar]
- 4.Gale C, Oakley-Browne M. Generalised anxiety disorder. Evidence Based Ment Health. 2004;7:32–3. [Google Scholar]
- 5.Smith KJ, Béland M, Clyde M, Gariépy G, Pagé V, Badawi G, et al. Association of diabetes with anxiety: A systematic review and meta-analysis. J Psychosom Res. 2013;74:89–99. doi: 10.1016/j.jpsychores.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Grigsby AB, Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. Prevalence of anxiety in adults with diabetes: A systematic review. J Psychosom Res. 2002;53:1053–60. doi: 10.1016/s0022-3999(02)00417-8. [DOI] [PubMed] [Google Scholar]
- 7.Furtado M, Katzman MA. Neuroinflammatory pathways in anxiety, posttraumatic stress, and obsessive compulsive disorders. Psychiatry Res. 2015;229:37–48. doi: 10.1016/j.psychres.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira TB, Kasahara TM, Barros PO, Vieira MM, Bittencourt VC, Hygino J, et al. Dopamine up-regulates Th17 phenotype from individuals with generalized anxiety disorder. J Neuroimmunol. 2011;238:58–66. doi: 10.1016/j.jneuroim.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Luis-Rodríguez D, Martínez-Castelao A, Górriz JL, De-Álvaro F, Navarro-González JF. Pathophysiological role and therapeutic implications of inflammation in diabetic nephropathy. World J Diabetes. 2012;3:7–18. doi: 10.4239/wjd.v3.i1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajebrahimi B, Kiamanesh A, Asgharnejad Farid AA, Asadikaram G. Type 2 diabetes and mental disorders; a plausible link with inflammation. Cell Mol Biol (Noisy-le-grand) 2016;62:71–7. doi: 10.14715/cmb/2016.62.13.13. [DOI] [PubMed] [Google Scholar]
- 11.Jhee JH, Kim H, Park S, Yun HR, Jung SY, Kee YK, et al. Vitamin D deficiency is significantly associated with depression in patients with chronic kidney disease. PLoS One. 2017;12:e0171009. doi: 10.1371/journal.pone.0171009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Black LJ, Jacoby P, Allen KL, Trapp GS, Hart PH, Byrne SM, et al. Low Vitamin D levels are associated with symptoms of depression in young adult males. Aust N Z J Psychiatry. 2014;48:464–71. doi: 10.1177/0004867413512383. [DOI] [PubMed] [Google Scholar]
- 13.Penckofer S, Byrn M, Adams W, Emanuele MA, Mumby P, Kouba J, et al. Vitamin D supplementation improves mood in women with type 2 diabetes. J Diabetes Res. 2017;2017:8232863. doi: 10.1155/2017/8232863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tartagni M, Cicinelli MV, Tartagni MV, Alrasheed H, Matteo M, Baldini D, et al. Vitamin D supplementation for premenstrual syndrome-related mood disorders in adolescents with severe hypovitaminosis D. J Pediatr Adolesc Gynecol. 2016;29:357–61. doi: 10.1016/j.jpag.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 16.Samani S, Joukar B. A study on the reliability and validity of the short form of the depression anxiety stress scale (DASS21) Journal Of Social Science And Humanities Of Shiraz University. 2007;26:65–77. [Google Scholar]
- 17.Henry JD, Crawford JR. The short-form version of the depression anxiety stress scales (DASS-21): Construct validity and normative data in a large non-clinical sample. Br J Clin Psychol. 2005;44:227–39. doi: 10.1348/014466505X29657. [DOI] [PubMed] [Google Scholar]
- 18.Asghari A, Saed F, Dibajnia P. Psychometric properties of the depression anxiety stress scales-21 (DASS-21) in a non-clinical Iranian sample. Int J Psychol. 2008;2:82–102. [Google Scholar]
- 19.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of Vitamin D deficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 20.Hejazi E, Amani R, SharafodinZadeh N, Cheraghian B. Comparison of antioxidant status and Vitamin D levels between multiple sclerosis patients and healthy matched subjects. Mult Scler Int. 2014;2014:539854. doi: 10.1155/2014/539854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Webb AR. Who, what, where and when-influences on cutaneous Vitamin D synthesis. Prog Biophys Mol Biol. 2006;92:17–25. doi: 10.1016/j.pbiomolbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Fogelholm M, Malmberg J, Suni J, Santtila M, Kyröläinen H, Mäntysaari M, et al. International physical activity questionnaire: Validity against fitness. Med Sci Sports Exerc. 2006;38:753–60. doi: 10.1249/01.mss.0000194075.16960.20. [DOI] [PubMed] [Google Scholar]
- 23.Newby PK, Muller D, Hallfrisch J, Andres R, Tucker KL. Food patterns measured by factor analysis and anthropometric changes in adults. Am J Clin Nutr. 2004;80:504–13. doi: 10.1093/ajcn/80.2.504. [DOI] [PubMed] [Google Scholar]
- 24.Jamilian M, Foroozanfard F, Rahmani E, Talebi M, Bahmani F, Asemi Z, et al. Effect of two different doses of Vitamin D supplementation on metabolic profiles of insulin-resistant patients with polycystic ovary syndrome. Nutrients. 2017;9 doi: 10.3390/nu9121280. pii: E1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.General Assembly of the World Medical Association. World medical association declaration of helsinki: Ethical principles for medical research involving human subjects. J Am Coll Dent. 2014;81:14–8. [PubMed] [Google Scholar]
- 26.Kjærgaard M, Joakimsen R, Jorde R. Low serum 25-hydroxyvitamin D levels are associated with depression in an adult norwegian population. Psychiatry Res. 2011;190:221–5. doi: 10.1016/j.psychres.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 27.Belvederi Murri M, Respino M, Masotti M, Innamorati M, Mondelli V, Pariante C, et al. Vitamin D and psychosis: Mini meta-analysis. Schizophr Res. 2013;150:235–9. doi: 10.1016/j.schres.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 28.Gowda U, Mutowo MP, Smith BJ, Wluka AE, Renzaho AM. Vitamin D supplementation to reduce depression in adults: Meta-analysis of randomized controlled trials. Nutrition. 2015;31:421–9. doi: 10.1016/j.nut.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 29.Bičíková M, Dušková M, Vítků J, Kalvachová B, Řípová D, Mohr P, et al. Vitamin D in anxiety and affective disorders. Physiol Res. 2015;64(Suppl 2):S101–3. doi: 10.33549/physiolres.933082. [DOI] [PubMed] [Google Scholar]
- 30.Ataie-Jafari A, Qorbani M, Heshmat R, Ardalan G, Motlagh ME, Asayesh H, et al. The association of Vitamin D deficiency with psychiatric distress and violence behaviors in Iranian adolescents: The CASPIAN-III study. J Diabetes Metab Disord. 2015;14:62. doi: 10.1186/s40200-015-0191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dean AJ, Bellgrove MA, Hall T, Phan WM, Eyles DW, Kvaskoff D, et al. Effects of Vitamin D supplementation on cognitive and emotional functioning in young adults – a randomised controlled trial. PLoS One. 2011;6:e25966. doi: 10.1371/journal.pone.0025966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SH, Seok H, Kim DS. Relationship between serum Vitamin D levels and symptoms of depression in stroke patients. Ann Rehabil Med. 2016;40:120–5. doi: 10.5535/arm.2016.40.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Liu Y, Lian Y, Li N, Liu H, Li G, et al. Efficacy of high-dose supplementation with oral Vitamin D3 on depressive symptoms in dialysis patients with Vitamin D3 insufficiency: A Prospective, randomized, double-blind study. J Clin Psychopharmacol. 2016;36:229–35. doi: 10.1097/JCP.0000000000000486. [DOI] [PubMed] [Google Scholar]
- 34.Kjærgaard M, Waterloo K, Wang CE, Almås B, Figenschau Y, Hutchinson MS, et al. Effect of Vitamin D supplement on depression scores in people with low levels of serum 25-hydroxyvitamin D: Nested case-control study and randomised clinical trial. Br J Psychiatry. 2012;201:360–8. doi: 10.1192/bjp.bp.111.104349. [DOI] [PubMed] [Google Scholar]
- 35.Shaffer JA, Edmondson D, Wasson LT, Falzon L, Homma K, Ezeokoli N, et al. Vitamin D supplementation for depressive symptoms: A systematic review and meta-analysis of randomized controlled trials. Psychosom Med. 2014;76:190–6. doi: 10.1097/PSY.0000000000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sepehrmanesh Z, Kolahdooz F, Abedi F, Mazroii N, Assarian A, Asemi Z, et al. Vitamin D supplementation affects the beck depression inventory, insulin resistance, and biomarkers of oxidative stress in patients with major depressive disorder: A randomized, controlled clinical trial. J Nutr. 2016;146:243–8. doi: 10.3945/jn.115.218883. [DOI] [PubMed] [Google Scholar]
- 37.Bellia A, Garcovich C, D’Adamo M, Lombardo M, Tesauro M, Donadel G, et al. Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects. Intern Emerg Med. 2013;8:33–40. doi: 10.1007/s11739-011-0559-x. [DOI] [PubMed] [Google Scholar]
- 38.Tayefi M, Shafiee M, Kazemi-Bajestani SMR, Esmaeili H, Darroudi S, Khakpouri S, et al. Depression and anxiety both associate with serum level of hs-CRP: A gender-stratified analysis in a population-based study. Psychoneuroendocrinology. 2017;81:63–9. doi: 10.1016/j.psyneuen.2017.02.035. [DOI] [PubMed] [Google Scholar]
- 39.Vieira MM, Ferreira TB, Pacheco PA, Barros PO, Almeida CR, Araújo-Lima CF, et al. Enhanced Th17 phenotype in individuals with generalized anxiety disorder. J Neuroimmunol. 2010;229:212–8. doi: 10.1016/j.jneuroim.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 40.Koh KB, Lee Y. Reduced anxiety level by therapeutic interventions and cell-mediated immunity in panic disorder patients. Psychother Psychosom. 2004;73:286–92. doi: 10.1159/000078845. [DOI] [PubMed] [Google Scholar]
- 41.Lenze EJ, Mantella RC, Shi P, Goate AM, Nowotny P, Butters MA, et al. Elevated cortisol in older adults with generalized anxiety disorder is reduced by treatment: A placebo-controlled evaluation of escitalopram. Am J Geriatr Psychiatry. 2011;19:482–90. doi: 10.1097/JGP.0b013e3181ec806c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bertoni AG, Burke GL, Owusu JA, Carnethon MR, Vaidya D, Barr RG, et al. Inflammation and the incidence of type 2 diabetes: The multi-ethnic study of atherosclerosis (MESA) Diabetes Care. 2010;33:804–10. doi: 10.2337/dc09-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas J, Jones G, Scarinci I, Brantley P. A descriptive and comparative study of the prevalence of depressive and anxiety disorders in low-income adults with type 2 diabetes and other chronic illnesses. Diabetes Care. 2003;26:2311–7. doi: 10.2337/diacare.26.8.2311. [DOI] [PubMed] [Google Scholar]
- 44.Ngo DT, Sverdlov AL, McNeil JJ, Horowitz JD. Does Vitamin D modulate asymmetric dimethylarginine and C-reactive protein concentrations? Am J Med. 2010;123:335–41. doi: 10.1016/j.amjmed.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 45.Asemi Z, Karamali M, Esmaillzadeh A. Effects of calcium-Vitamin D co-supplementation on glycaemic control, inflammation and oxidative stress in gestational diabetes: A randomised placebo-controlled trial. Diabetologia. 2014;57:1798–806. doi: 10.1007/s00125-014-3293-x. [DOI] [PubMed] [Google Scholar]
- 46.Chen N, Wan Z, Han SF, Li BY, Zhang ZL, Qin LQ, et al. Effect of Vitamin D supplementation on the level of circulating high-sensitivity C-reactive protein: A meta-analysis of randomized controlled trials. Nutrients. 2014;6:2206–16. doi: 10.3390/nu6062206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barker T, Rogers VE, Levy M, Templeton J, Goldfine H, Schneider ED, et al. Supplemental Vitamin D increases serum cytokines in those with initially low 25-hydroxyvitamin D: A randomized, double blind, placebo-controlled study. Cytokine. 2015;71:132–8. doi: 10.1016/j.cyto.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 48.Ashtari F, Toghianifar N, Zarkesh-Esfahani SH, Mansourian M. Short-term effect of high-dose Vitamin D on the level of interleukin 10 in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled clinical trial. Neuroimmunomodulation. 2015;22:400–4. doi: 10.1159/000439278. [DOI] [PubMed] [Google Scholar]
- 49.Salekzamani S, Mehralizadeh H, Ghezel A, Salekzamani Y, Jafarabadi MA, Bavil AS, et al. Effect of high-dose Vitamin D supplementation on cardiometabolic risk factors in subjects with metabolic syndrome: A randomized controlled double-blind clinical trial. J Endocrinol Invest. 2016;39:1303–13. doi: 10.1007/s40618-016-0507-8. [DOI] [PubMed] [Google Scholar]
- 50.Zittermann A, Frisch S, Berthold HK, Götting C, Kuhn J, Kleesiek K, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. 2009;89:1321–7. doi: 10.3945/ajcn.2008.27004. [DOI] [PubMed] [Google Scholar]
- 51.Sharifi N, Amani R, Hajiani E, Cheraghian B. Women may respond different from men to Vitamin D supplementation regarding cardiometabolic biomarkers. Exp Biol Med (Maywood) 2016;241:830–8. doi: 10.1177/1535370216629009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Golzarand M, Hollis BW, Mirmiran P, Wagner CL, Shab-Bidar S, Vitamin D. supplementation and body fat mass: A systematic review and meta-analysis. European Journal of Clinical Nutrition. 2018 doi: 10.1038/s41430-018-0132-z. In press. [DOI] [PubMed] [Google Scholar]
- 53.Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y, et al. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol Endocrinol. 2005;19:2685–95. doi: 10.1210/me.2005-0106. [DOI] [PubMed] [Google Scholar]
- 54.Can MŞ, Baykan H, Baykan Ö, Erensoy N, Karlıdere T. Vitamin D levels and Vitamin D receptor gene polymorphism in major depression. Psychiatr Danub. 2017;29:179–85. doi: 10.24869/psyd.2017.179. [DOI] [PubMed] [Google Scholar]