Abstract
Lower extremity ulcers represent the most ominous, feared, and costly complications of diabetes mellitus. The aim of this review is to highlight the role of daily life physical activities (PAs) and continuous movement monitoring (CMM) in the prevention of foot ulcers. Peripheral neuropathy and peripheral vascular disease are the main causes of foot ulceration and contribute, in turn, to the development of additional risk factors such as foot deformities and/or joint and muscular alterations. Moreover, a deficit of balance, posture abnormalities, followed by gait alterations, increases the risk of ulceration. PA can play a key role in the management of patients with diabetes and in the prevention of ulcers; however, even if it has been reported that some of these risk factors significantly improve after a few weeks of exercise therapy (ET), the real preventive role of ET has not yet been demonstrated. These uncertain results can occur due to some limitations in the management of the same relationship between PA and diabetic foot prevention. Technological advances during the last years enable timely management of overall daily PA. The use of these modern technologies and devices allows CMM assessment and description of daily PA even in the long term. The data collected from these devices can be used to properly manage patients’ PA and thus contribute to the prevention of foot ulcers.
Keywords: Continuous movement monitoring, daily lifestyle, diabetic foot ulcer, exercise therapy, physical activity, prevention
Introduction
Diabetes mellitus (DM) is a metabolic disorder which is drastically increasing, making it one of the most important public health problems around the globe. Recent studies have found that the incidence of DM is increasing faster than in the past, and it is estimated that by 2035, almost 592 million people will be affected worldwide.[1]
Of the long-term complications that can affect DM patients, diabetic foot ulcers are most ominous and feared because the ulcers affect not only the patients’ mobility and their overall well-being but can also increase morbidity and mortality in those with both type 1 and type 2 DM.[2,3,4]
The yearly incidence of diabetic foot ulcer in the DM population is around 4% in developed countries and even higher in developing countries where the lifetime risk of a patient to develop a foot ulcer rises to about 25%.[2,5]
The severity of this phenomenon is also accentuated by the fact that only two-thirds of foot ulcers will ever heal. In addition, about one-third of patients with a recent history (1 year) of foot ulcer or amputation have a high risk of recurrence.[5,6] Consequently, every 20 s, there is an amputation caused by diabetes somewhere in the world.[2]
The complexity of the multifactorial pathogenesis of diabetic foot ulcer makes patients difficult to treat.[5,6,7] The timely consideration of all this is important also in guaranteeing a proper treatment through physical activity (PA).
In this sense, since many years, several studies suggested that PA can concur in the prevention of diabetic foot by the treatment of its major risk factors.[8,9,10,11,12] In this article, “PA” means body movement generated by muscle contraction, whereas “exercise” means PA aimed at improving fitness or functional/motor deficits. Unstructured PA means “nonexercise” or daily living activities.[8,13] Both structured and unstructured PA can be performed in an adapted and scheduled way to prevent diabetic foot.
During the 20th century, and especially starting from the end of 1970s, the importance of investigating the qualitative or quantitative aspects of human movement for the prevention of foot ulcers has aroused more attention. The early studies were focused on analyzing foot plantar pressures and gait quality.[14,15] The study of such parameters was possible thanks to advances in technology and the availability of new devices for the evaluation of the patient's movement and functional alterations (i.e., gait biomechanics–foot plantar pressure in static-dynamic conditions).[14,15,16,17,18] Subsequently, the assessment of daily life PA performed by patients with and without risk of developing diabetic foot ulcer has been carried out with different methods (questionnaires, pedometer, and accelerometer) [Table 1].[19,20,21,22,23,24,25,26]
Table 1.
Physical activity monitoring for the prevention of diabetic foot ulcer
| Study (year) | General purpose | Study type | Sample size | Equipment and duration | Results and conclusion |
|---|---|---|---|---|---|
| Armstrong et al.[27] (2001) | To compare the effectiveness of three off-loading modalities to heal neuropathic foot ulcerations | Prospective longitudinal study | 63 patients with DM and plantar foot ulcers | 12 weeks. Pedometer | Patients treated with the total-contact casts were significantly less active than those treated with the half-shoe. There was not a significant difference in activity between patients treated with the total-contact casts and with the removable cast walkers |
| Armstrong et al.[24] (2001) | To evaluate the magnitude and location of patients’ activity level | Prospective longitudinal study | 20 DM patients at high risk | 1 week. Activity monitor | Patients were most active during the late morning and mid-afternoon hours. At home, the patients used the physician-approved shoes less than outside the home |
| Maluf and Mueller[19] (2003) | To compare the amount of weight-bearing activity and estimates of cumulative plantar tissue stress | Cross-sectional study with matched groups | 20 patients with DPN with and without a history of foot plantar ulcer, 10 nondiabetic control subjects | 1 week. Two-dimensional accelerometers and in-shoe pressure measurement | Patients with diabetes and a history of previous ulcers may be susceptible to plantar tissue injury even at relatively low levels of cumulative tissue stress |
| Lemaster et al.[21] (2003) | To determine whether weight-bearing activity increased the risk of foot ulcer | Prospective longitudinal cohort study | 400 patients with DM and a prior history of foot ulcer | 2 years. 24-h activity questionnaire | Increased weight-bearing activity did not increase the risk of foot reulceration |
| Armstrong et al.[23] (2004) | To evaluate the role of activity in the development of neuropathic foot ulceration | Prospective longitudinal study | 100 DM patients at high risk | >25 weeks (or until ulceration). Accelerometer/pedometer | Individuals with diabetes who develop ulceration may actually have a lower overall daily activity than their nonulcerated counterparts, but the quality of that activity may be more variable |
| Kanade et al.[28] (2006) | To explore plantar loading of the surviving foot following unilateral transtibial amputation | Cross-sectional study with matched groups | 21 patients with DPN and transtibial amputation; 21 patients with DPN without history of ulceration | 8 consecutive days. Step watch activity monitors and in-shoe pressure measurement system | Adaptations in gait and level of walking activity affect the plantar pressure distribution and ultimately the potential risk of ulceration to the surviving foot |
| Najafi et al.[26] (2010) | To monitor spontaneous daily PA and examine both walking and standing activities | Prospective longitudinal study | 13 patients with DPN | 2 days. Body-worn sensor | Patients with DPN spent 13.5% of time in standing and 6.1% in walking. Walking may cover as little of a person’s daily PA and hence might not be representative of what the subject is doing during daily living activities |
| Van Schie et al.[29] (2011) | To evaluate the validity of the “step activity monitor” for assessing PA and the relation with the self-reported PA | Prospective longitudinal study | 24 patients with DPN | 2 days. Step activity monitor, step watch 3, and international PA questionnaire | Step activity monitor was shown to be a valid tool to assess PA |
| Waaijman et al.[30] (2013) | To objectively assess adherence to wearing prescribed custom-made footwear | Randomized controlled trial | 107 DPN patients with a recently healed plantar foot ulcer | 7 consecutive days. Temperature-based monitor and ankle-worn activity monitor | Adherence to wearing custom-made footwear is insufficient, particularly at home where patients exhibit their largest walking activity. This low adherence is a major threat for reulceration |
| Lim et al.[31] (2016) | To investigate the effect of an individualized multidisciplinary U-health care service combined with exercise monitoring and dietary feedback on glucose control | Randomized controlled trial | 100 patients with type 2 DM assigned to a self-monitored blood glucose group or U-healthcare group | 6 months. Glucometer and an activity monitor that automatically transferred test results to a hospital-based server | The HbA1c level was significantly decreased in the U-healthcare group compared with the self-monitored blood glucose group |
| Brazeau et al.[32] (2008) | To determine if there was an inverse relationship between sitting and step counts in a diabetes cohort | Prospective cohort study | The cohort included 198 T2DM adults | 14 days. Pedometer, international PA questionnaire | There was a low correlation between sitting time and step counts |
| Dasanayake et al.[33] (2015) | To develop a method to detect the onset and end of exercise | Research study | 16 adults with T1DM | 2 days. Diary, accelerometer, heart rate monitor, and continuous glucose monitor | The method identified the onset and end of exercise in approximately 5 min, with an average blood glucose change of only - 6 mg/dL |
| Crews et al.[34] (2016) | To evaluate the role and adherence to off-loading | Prospective, multicenter study | 79 patients with T2DM and active foot ulcer | 6 weeks. Two concealed activity monitors | The presence of an independent relationship between the level of adherence to off-loading devices and the amount of DFU healing that occurs was detected. Neuropathic postural instability was found to be the strongest barrier to off-loading adherence |
| Crews et al.[25] (2017) | To assess the feasibility of objectively, synchronously, and continuously monitoring finely detailed PA and its location of occurrence | Pilot study | 5 at risk and 5 actively ulcerated patients | 3 days. Tri-axial accelerometer and GPS monitors | For DFU participant’s weight-bearing activity was 188% higher at home than away from home. At risk participants showed similar weight-bearing activity at home as active DFU participants |
| Kluding et al.[35] (2017) | To determine the impact of an intense lifestyle intervention on neuropathy progression and quality of life | Randomized controlled trial | 140 type 2 DM patients with peripheral neuropathy | 18 months of supervised exercise training. 7 days of actigraphy based counseling to reduce sedentary behavior | An intensive lifestyle intervention may be a sustainable, clinically effective approach for people with DPN that improves patients outcomes and can have an immediate impact on patient care |
| Jao et al.[36] (2017) | To evaluate the accuracy of 2 PA monitors | Cross-sectional study | 31 patients with history of foot ulcer | 14 weight-bearing and nonweight-bearing activities. Two PA monitors | Between PA monitors, there was an important difference in accuracy of weight-bearing activities |
DM=Diabetes mellitus, DPN=Diabetic peripheral neuropathy, DFU=Diabetic foot ulcer, CMM=Continuous movement monitoring, HbA1c=Hemoglobin A1c, PA=Physical activity, GPS=Global Positioning System
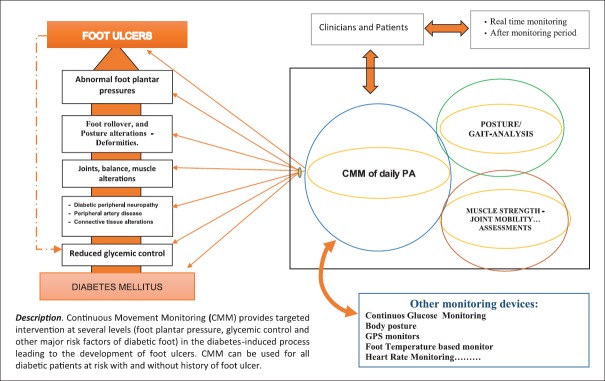
Nowadays, the use of even more advanced sensors, digital devices (sometimes similar to wristwatches), and their software allows a better patient's PA assessment and description along with continuous movement monitoring (CMM). CMM has been carried out with different devices, following different protocols, and provides targeted intervention at several levels in the process, leading to the development of foot ulcers in diabetic patients [Figure 1 and Table 1].[23,24,25,26,29] The set of information collected from gait analysis and CMM allows a level of management of patients’ PA not possible in the past.
Figure 1.
“Three cornerstones” of physical activity management; the possible role of continuous movement monitoring in the prevention of diabetic foot ulcer
The aim of this review is to highlight the role of the proper monitoring and management of structured and unstructured PAs as important methods of prevention against ulcers.
For this review, we searched the current medical literature through PubMed, MEDLINE, EMBASE, and Cochrane library databases. The topics searched were diabetic foot prevention, PA monitoring, exercise training, adapted PA published in English.
Physical Activity and Diabetic Foot Prevention
Diabetic foot prevention begins with proper care of the patient at the time of diagnosis through treatment aimed at achieving good metabolic control [Figure 1]. This treatment involves patient education sessions on the role and importance of an active lifestyle.[8,37] However, as well as nutrition, even PA can induce considerable variations in glycemic levels of patients with diabetes. This effect can limit metabolic control and become a barrier to exercise, especially in patients with type 1 diabetes (PA as risk factor for glycemic control).[32,38] As a result, the management of a patient's PA is also to improve blood glucose control in addition to a better peripheral insulin action and the maintenance of a good body mass index.[8,37]
CMM means that patients with diabetes have to be informed on PA performed and it provides indications on what has to be performed on the basis of data collected during the long-term monitoring.[8,38] As a result, patients are more aware of managing appropriate food–liquid intake and/or drug therapy to achieve good metabolic control.[13,20,38] It has also been suggested that the evaluation of PA performed between main meals, in addition to that during 24 h, can enable patients to better orient themselves in their choices regarding glycemic management.[39]
It is important to consider that vigorous or prolonged PA can have significant acute effects on glycemic fluctuations that can be difficult to manage.[13,37,38,40] All this hinders glycemic control.
In comparison to structured PA, daily life movement can usually be performed at light or moderate intensity so as not to excessively modify blood glucose values.[8,37,38,40]
The improvement in glycemic control over time can be attained with CMM, providing knowledge of type, duration, intensity, and distribution of the activity performed. Each of these parameters can be set up or modified to achieve proper glycemia.[38,41]
Exercise Therapy and Diabetic Foot Prevention
Even if movement and especially gait are a key element of therapy for diabetic patients, it is important to consider that PA is, at the same time, stressful for feet and can cause foot lesions. For this, PA should be accurately assessed, monitored, and managed.
The early studies of this paradoxical “risk factor” (PA) focused on analyzing foot plantar pressures and gait quality and providing new information on gait and foot rollover alterations that patients with diabetes can show.[14,15,16,42,43]
According to these results, during the last 20 years, research studies were aimed at verifying diabetic patients’ response to PA protocols [Table 2]. These studies demonstrated that targeted exercise therapy (ET) protocols can improve glycemic control, muscle strength, joint mobility, flexibility, and balance, in addition to gait abnormalities (gait speed and walking distance).[12,43,44,45,49,50,51,52,56,58,59]
Table 2.
Studies on the effect of exercise program on patients with diabetes and risk of developing foot ulcers
| Study (year) | General purpose | Study type | Sample size | Duration and tests | Results and conclusion |
|---|---|---|---|---|---|
| Dijs et al.[44] (2000) | To evaluate if physical therapy may result in improvement in the mobility of the ankle and foot joints | Pilot study | 11 patients with DM, LJM, and neuropathy | 2 sessions per week up to 20 sessions. Joint mobility was measured at the ankle and foot joints | Physical therapy may be useful in the prevention of plantar ulceration in diabetic patients with LJM and neuropathy |
| Richardson et al.[45] (2001) | To determine the effect of exercise regimen on clinical measures of postural stability and confidence | Prospective, controlled, single-blind study | 20 DM patients with DPN | 3-week intervention exercise. Unipedal stance time, functional reach, tandem stance time, score on the ABC | A brief, specific exercise regimen improved clinical measures of balance in patients with DPN |
| Goldsmith et al.[46] (2002) | To determine the effects of home exercise therapy on joint mobility and plantar pressures | Randomized controlled study | 19 DM patients | 1 month of exercise program. Joint stiffness and peak plantar pressures | An unsupervised range-of-motion exercise program can significantly reduce peak plantar pressures in diabetic patients within a relatively short period of time |
| Brandon et al.[47] (2003) | To determine the nature and duration of mobility and strength benefits associated with strength training intervention | Randomized controlled trial | 31 community-dwelling older adults with DM | 24 months of intervention. Strength and mobility | Moderate-intensity resistive-training program can improve mobility and strength for a duration of 24-month intervention in older adults with diabetes |
| Balducci et al.[48] (2006) | To examine the effects of long-term exercise training on the development of DPN | Prospective randomized intervention study | 78 patients without DPN. Intervention group (n=31); control group (n=47) | 4-year of intervention. Vibration perception threshold, nerve distal latency, nerve conduction velocity, nerve action potential amplitude | Long-term supervised mild aerobic exercise training may modify the natural history of DPN or even delay its onset |
| Smith et al.[9] (2006) | To evaluate intraepidermal nerve fiber density as a sensitive measure of neuropathy change | Multicenter research study | 40 subjects with neuropathy and impaired glucose tolerance | 1-year of intervention. 6-min walk test, nerve conduction studies, quantitative sensory testing, quantitative sudomotor axon reflex testing, visual analog pain scale | Diet and exercise counseling for impaired glucose tolerance results in cutaneous reinnervation and improved pain |
| Allet et al.[12] (2010) | To evaluate the effect of a specific training program on gait and balance | Randomized controlled trial | 71 patients with DM. Intervention group (n=35); control group (n=36) | 12 weeks, twice-weekly exercise program. Performance-oriented mobility assessment, outdoor gait assessment, dynamic balance test, and static balance test evaluating postural control | Specific training can improve gait speed, balance, muscle strength and joint mobility in diabetic patients. Intervention group participants partially lost their treatment benefit in the 6-month follow-up, but their performance level remained superior to that at baseline |
| Morrison et al.[49] (2010) | To assess the effects of balance/strength training on falls risk and posture | Research study | Intervention group (n=16); control group (n=21) | 6-week, thrice weekly exercise program. Postural stability and falls risk | Older DM patients had impaired balance, slower reactions and consequently a higher falls-risk than age-matched control subjects. All these variables improved after resistance/balance training |
| Otterman et al.[50] (2011) | To investigate the feasibility and preliminary effectiveness of an exercise program | Research study | 20 patients with diabetic complications | 12-week, twice weekly. Patient-specific functional scale and muscle strength | The prescribed exercise program (aerobic and resistance exercise) resulted in significant improvements of HbA1c, muscle strength and perceived limitations in functioning |
| Collins et al.[51] (2011) | To determine the efficacy of a home-based walking intervention to improve walking ability and quality of life | Randomized controlled trial cross-sectional study | 145 patients with diabetes and peripheral arterial disease | 6-month home-based walking program. Maximal treadmill walking distance, lower limb function, quality of life, exercise behaviors, depressive symptoms, and self-efficacy | A home-based walking intervention did not improve walking distance but did improve walking speed and quality of life in people with diabetes and peripheral arterial disease |
| Song et al.[52] (2011) | To assess the effects of an exercise program on balance and trunk proprioception | Randomized controlled trial | 38 older DPN. Intervention group (n=19) and control group (n=19) | 8 weeks, twice weekly. Multifunction force measuring plate, One-leg standing test, berg balance test, functional reach test, timed up and go test, and trunk repositioning errors | The balance exercise program improved balance and trunk proprioception |
| Kluding et al.[11] (2012) | To assess the effects of an exercise intervention on neuropathic symptoms, nerve function, and cutaneous innervation | Pilot study | 17 patients with DPN | 10-week. Pain measures, neuropathic symptoms, nerve function measures, intraepidermal nerve fiber density and branching in lower extremities skin biopsies | A supervised aerobic and strengthening exercise program significant reduces pain, neuropathic symptoms, and increases intraepidermal nerve fiber branching from proximal skin biopsy |
| Shah KM[53] (2012) | To determine the in-shoe plantar pressures during selected forms of weight-bearing and nonweight-bearing exercises | Randomized control study | 15 elderly in patients with DPN | In shoe foot planar pressure, weight-bearing exercises and nonweight-bearing exercises | Nonweight-bearing exercises provide greater reductions in plantar pressures than weight-bearing exercises |
| Melai et al.[54] (2013) | To evaluate whether lower-extremity strength training can reduce plantar pressures | Randomized controlled trial | 94 patients with DPN. Intervention group (n=48), control group (n=46) | 24 weeks. Plantar pressure platform | A lower extremity strength training program was not successful in reducing plantar loading of the forefoot for people with DPN |
| Mueller et al.[55] (2013) | To determine the effects of weight-bearing versus nonweight-bearing exercise | Randomized controlled trial | 29 patients with DPN. Weight-bearing (n=15); nonweight-bearing (n=14) exercise groups | 12-week, thrice weekly. 6-minute walk distance, daily step counts | The weight-bearing group showed greater gains than the nonweight-bearing group over time on the 6-min walk distance and average daily step count. The nonweight-bearing group showed greater improvements than the weight-bearing group over time in HbA1c values |
| Sartor et al.[43] (2014) | To determine the effects of training on foot rollover process during gait | Randomized controlled trial | 55 patients with DPN Intervention group (n=26), control group (n=29) | 12 weeks, twice a week. Foot rollover, in-shoe dynamic pressure measuring system | Intervention discreetly changed foot rollover toward a more physiological process, supported by improved plantar pressure distribution and better functional condition of the foot–ankle complex |
| Francia et al.[56] (2015) | To evaluate the effect of an experimental protocol of exercise therapy in a group | Pilot study | 26 patients with long-term DM (intervention group), 17 healthy controls | 12-week, thrice weekly exercise program. Joint mobility, muscular strength at the ankle and gait speed | A 12-week supervised program significantly improves joint mobility, muscular performance, and walking speed in DM patients |
| Kluding et al.[57] (2015) | To determine the impact of an intense lifestyle intervention on neuropathy progression and quality of life | Single-blinded randomized controlled trial | 140 type 2 DM patients with DPN | 18 months of supervised exercise training. Intraepidermal nerve fiber density in a distal thigh skin biopsy, Norfolk Quality Of Life, Diabetic Neuropathy Score, pain, gait, balance, and mobility | An intensive lifestyle intervention may be a sustainable, clinically effective approach for people with DPN that improves patients outcomes and can have an immediate impact on patient care |
DM=Diabetes mellitus, DPN: Diabetic peripheral neuropathy, LJM=Limited joint mobility, HbA1c=Hemoglobin A1c, ABC=Activities-specific balance confidence scale
These studies have thus provided preliminary information of positive effects on foot rollover and plantar pressure distribution.[43,46]
However, the limited use of PA/ET in the prevention of diabetic foot can be due to the lack of evidence on its preventive role. Since the results achieved seem to be temporary meaning that they are lost if the training is interrupted.[12,44,60,61]
Further factors limiting the regular use of PA in the prevention of foot ulcers are as follows: patients’ vulnerability and limited compliance, difficulty in performing the protocols routinely and for prolonged periods, feelings of tiredness, and fear of hypoglycemia.[12,31,32,43,44,62,63]
The lack of clear evidence on the preventive role of PA in diabetic foot requires further investigation.
Considering these problems in the use of PA, technologies available nowadays are possible solutions. Furthermore, thanks to the wide range of devices providing innumerable applications possibilities, such technologies monitoring daily PA by CMM can allow the definition of the proper treatment through structured or unstructured PA and clarify the preventive role of PA [Table 1].
Physical Activity and Diabetic Foot Risk Factors
Diabetic foot lesions frequently occur in patients who show two or more simultaneous risk factors.[4] More than half of the patients with type 2 DM are affected by diabetic peripheral neuropathy (DPN) that can progressively induce motor dysfunction preceded by sensory deficits.[7,10]
The neuromuscular problems (i.e., muscle weakness, reduced endurance, and loss of coordination) that may occur in patients with diabetes can worsen or lead to abnormalities in the biomechanics of the foot and of the whole body, in dynamic as well as in static postures.[42,43,64,65] These impairments can also result in an abnormal foot rollover and plantar pressures, which significantly increase the risk of painless foot ulcer.[7,35,42,43,54,57]
In addition to DPN, peripheral arterial disease plays an important role in the development of foot ulcers and can also negatively affect healing.[2,4,36] The presence of these complications indicates the patients at risk. It has been observed that about 50%–60% of all diabetic foot ulcers are ischemic or neuroischemic.[5] This condition can induce different functional limitations: minor gait speed, reduced walking distance, resting pain, and claudication.[51,61,66]
The presence of foot deformities and the importance of avoiding foot and leg trauma are other major risk factors to be considered in the PA management of diabetic patients.[2,4,7]
The same joint mobility of the lower limbs, usually evaluated at the ankle, and foot joints (subtalar and first metatarsophalangeal), can significantly decrease in patients with diabetes,[60,67,68,69,70] and this also contributes to the development of foot deformities and gait abnormalities.
Many factors concur to cause the development of structural abnormalities in diabetic patients. The presence of DPN, peripheral vascular disease (PVD), connective tissue alterations, and then deficit of balance, muscles strength, and biomechanics can trigger the development of foot deformities and ulcers due to the stress induced by the PA performed.[69,70]
Continuous Movement Monitoring and Major Risk Prevention
Even if PA can be fundamental in the prevention or treatment of major risk factors of the diabetic foot such as limited joint mobility or muscle weakness,[8,44,46,48,51,53,61] the presence of advanced DPN, in addition to PVD, seem cannot be significantly improved by ET and limit the possibility of full and long-lasting prevention or effective recovery from the other major risk factors (i.e., posture and biomechanical gait alterations).[12,43,61,54]
As a result, it is important to begin the management of patients’ PA as early as possible, starting from diabetes diagnosis. If, on the one hand, PA can have limited positive effects on DPN and PVD, on the other hand, their presence can explain the reason why, in individuals at risk, ET can have a limited positive effect on the maintenance of improvements over time.[12,20,60,61]
It is important to underline that all due precautions must be taken into consideration in the management of a patient's daily PA designed to prevent the occurrence or recovery of ulcerative risk factors.[50,56,57,61] In addition to leg trauma and falls, the disuse/overuse of muscles and connective tissues must be avoided.[56,61,71,72]
The abnormal balance, posture, and gait biomechanics that patients at risk can show, in addition to the presence of foot deformities, may lead to overuse of some lower limb structures (i.e., muscle and connective tissue), while others cannot be involved during daily PA (disuse).[61,72] In particular, the overuse of the foot and leg structures (i.e., Achilles tendon or plantar fascia) is mostly feared because it can contribute to the development of foot ulcer.[60,72,73]
In addition to this, the real effect of the improvement of patients’ functional and motor deficits on their biomechanics and foot plantar pressures distribution is not yet clear; the short term in which they can be achieved can be a paradoxical risk for patients, leading to abnormal foot stress and overuse condition.[43,46,71,72]
The patients’ functional abilities and their quality of movement have to be checked (as well as lifestyle changes) so that instructions can be promptly adapted to the new needs identified. These evaluations have to be periodically repeated according to the patients’ needs.
Once again, the results of postural and gait analysis can provide useful information on the management of daily PA. Such PA management based on the “three cornerstones” (CMM, posture–gait analysis, and muscle strength/joint mobility assessments) may ensure that the results achieved by an ET program are more long-lasting in patients [Figure 1].
Continuous Movement Monitoring and Foot Plantar Pressure
The abnormal distribution and concentration of foot plantar pressure are one of the most feared causes of ulcer. Therefore, historically, the risk of plantar ulcers has been associated with the amount and type of the force exerted on the patient's foot.
Consequently, patients who are more active were considered to be at higher risk.[27,28,30,34]
The CMM of daily lifestyle can be of great importance for its possible correlation with the total plantar pressure exerted on the feet. This estimation on long period foot pressures can be nowadays possible considering both the results of CMM and patients’ foot pressure in static and dynamic conditions evaluated by the use of devices such as baropodometry, in-shoe pressure sensors, or instrumental walkways with a force platform.[Table 1].[19,25,28,34,42]
CMM can also highlight potentially harmful lifestyles such as those tending to concentrate or increased plantar foot stresses up to a dangerous degree and help patients modify their daily lifestyle to prevent ulcers.[19,20,24,27]
However, it is worthy of attention that in the past decade, some studies, thanks to CMM, have not fully confirmed this correlation.[21,23] It has been reported that patients with a history of ulceration are less active; one explanation being that higher variability in daily PA in less active patients could make them at higher risk for ulcers.[22,23] These studies seem to suggest that patients’ weakness and the possibility of developing foot ulcers are inversely associated with the level of daily PA performed.
A further complication of CMM is that also, the time spent in standing and sitting positions should be assessed.[26,75] The amount of time spent in standing can correspond to a double walking activity in patients with DPN.[26] Therefore, the standing position is potentially dangerous in these patients because it could increase the risk of ulcer.[22,23,25,26]
In this complex picture, CMM evaluation of daily PA can detect alarming factors such as a decrease in the daily PA performance. The understanding of causal factors of the reduced daily PA helps define preventive interventions. Similarly, a significant increase/change in PA intensity, duration, or distribution has to be carefully considered in patients at risk.
Discussion
Currently, it is an important transitional moment for PA in the prevention of the diabetic foot. Timely attention should be given to the best uses for new PA assessment and monitoring devices in diabetes clinical practice.
To date, it has been demonstrated that most of the motor and functional deficits in DM patients significantly improve after short ET periods and patients can almost achieve the level of the healthy control group performance.[12,47,56,58]
Patients can perform most of these activities as home-based exercise programs[44,46,51,56] including weight-bearing and/or nonweight-bearing exercises according to the therapist's goals and the patient's needs.[12,44,55]
However, to date, the role of PA, mostly limited to ET in the prevention of foot ulcers, has not yet been fully clarified, and in-depth investigation is needed.[43,61,74]
In this context, the application possibilities of CMM open up new important perspectives and allow PA to be considered in its entirety.
However, on the one hand, if the 24-h monitoring of patients’ PA is now possible through the use of devices showing an accuracy not previously achieved,[25,33,34,75] on the other hand, it shows limitations that seem to hinder its application. Studies on CMM-involving patients with a history of foot ulcers or at risk have often been carried out with different devices providing several possibilities of evaluation.
CMM can help to evaluate the amount of daily foot stress, PA distribution, intensity, type, and duration and compare it to the lifestyle previously maintained.[19,28,29,31,75]
This approach may also be useful in determining what constitutes an unhealthy lifestyle, seasonal changes in leisure-time or working hours in addition to the location of activities to enhance patients’ management. A full comprehension of these parameters requires repeating measurements several times a year.[48,76]
Moreover, with the use of CMM, it can be possible to evaluate the effect of different treatment such as the use of protective foot devices. Indeed, CMM provides information on the amount and total time spent with the footwear and in-shoe devices worn.[27,30,34]
However, the use of devices for CMM evaluations involves costs, not only for buying the equipment but also for the involvement of specialized personnel and time for processing the data collected [Table 3].
Table 3.
Pros and cons of continuous movement monitoring
| Pros | Cons |
|---|---|
| Being informed of the lifestyle and daily PA performed[23,24,25,26] | Device availability and limitations[62] |
| Estimate foot plantar pressure and its daily distribution[19] | Time and cost for processing the data collected |
| Monitoring of the effects of the interventions carried out[26,29] | Patient’s compliance[30,34,33] |
| Willingness to wear the device for enough time and repeat the evaluation several times if necessary | |
| Does not change his/her own lifestyle during the monitoring | |
| Fills in a log book to record activities and also those performed without wearing CMM devices | |
| Synchronizes the devices for remote monitoring | |
| Remote patient monitoring[27,34] | Knowing patient’s functional, postural, and movement characteristics before modifying daily lifestyle |
| Being aware of seasonal and daily variations of PA level[23] | Differences in the results achieved by different devices[36] |
| Multi-parametric monitoring[28,31] | Limitations in the accurate recognition of each kind of complex movement (small steps, cycling, etc.)[26] |
| Indirect evaluation of foot plantar pressure | |
| Type of activities not allowing the use of CMM devices (i.e., immersions) |
PA=Physical activity, CMM=Continuous movement monitoring
Moreover, different devices can provide varying results on the PA monitored. This involves a difficulty in analyzing the results achieved unless the same type of device is always used.[36]
It is important that CMM covers all 24 h in patients at risk. In fact, irregular monitoring can adversely affect the possibility to analyze PA performed since only a few minutes is needed to significantly modify the daily PA evaluation.
Although patients cannot wear a device continuously, they can fill out a log-book or diary to record the activities performed when the device is not worn. Special forms can help the patient to accurately register the activities performed.[24,26,33,75]
Drawbacks to the use of new technology for CMM include the difficulty in assessing, understanding, and managing the lifestyle of patients at risk.
Therefore, to date, also in the case of CMM, the lack of prospective studies on larger sample size of patients at risk does not allow the definition of its real preventive impact. This can explain the currently limited use of such methods, despite their promising start.[20,24]
Limitations of the study
This article provides a narrative review of studies without following the PRISMA guidelines for a transparent and complete reporting of systematic reviews and meta-analyses.[77] Some studies on movement monitoring in diabetic patients may not have been considered.
Conclusions
Technological advances highlighted the application possibilities of devices for the evaluation and management of PAs. This allowed the proper management of daily life activities, well organized in quantity, intensity, type, and distribution, monitored by new CMM devices, to be a key element in the treatment of patients with diabetes. At the same time, further studies on the role of CMM and PA in the prevention of foot ulcers are needed to translate such type of monitoring into routine clinical practice. Currently, it is strongly recommended to define the role of CMM as cornerstone also in the prevention of diabetic foot.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank Mrs. G. Iannone for the technical and administrative support and Mrs. Mary Colonnelli for revising the English content.
References
- 1.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE, et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–49. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Bakker K, Apelqvist J, Lipsky BA, Van Netten., JJ International Working Group on the Diabetic Foot. The 2015 IWGDF guidance documents on prevention and management of foot problems in diabetes: Development of an evidence-based global consensus. Diabetes Metab Res Rev. 2016;32(Suppl 1):2–6. doi: 10.1002/dmrr.2694. [DOI] [PubMed] [Google Scholar]
- 3.van Schie CH. Neuropathy: Mobility and quality of life. Diabetes Metab Res Rev. 2008;24(Suppl 1):S45–51. doi: 10.1002/dmrr.856. [DOI] [PubMed] [Google Scholar]
- 4.Apelqvist J, Bakker K, van Houtum WH, Schaper NC. International Working Group on the Diabetic Foot (IWGDF) Editorial Board. Practical guidelines on the management and prevention of the diabetic foot: Based upon the international consensus on the diabetic foot (2007) prepared by the international working group on the diabetic foot. Diabetes Metab Res Rev. 2008;24(Suppl 1):S181–7. doi: 10.1002/dmrr.848. [DOI] [PubMed] [Google Scholar]
- 5.Prompers L, Schaper N, Apelqvist J, Edmonds M, Jude E, Mauricio D, et al. Prediction of outcome in individuals with diabetic foot ulcers: Focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE study. Diabetologia. 2008;51:747–55. doi: 10.1007/s00125-008-0940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pound N, Chipchase S, Treece K, Game F, Jeffcoate W. Ulcer-free survival following management of foot ulcers in diabetes. Diabet Med. 2005;22:1306–9. doi: 10.1111/j.1464-5491.2005.01640.x. [DOI] [PubMed] [Google Scholar]
- 7.Apelqvist J. Diagnostics and treatment of the diabetic foot. Endocrine. 2012;41:384–97. doi: 10.1007/s12020-012-9619-x. [DOI] [PubMed] [Google Scholar]
- 8.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care. 2010;33:147–67. doi: 10.2337/dc10-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith AG, Russell J, Feldman EL, Goldstein J, Peltier A, Smith S, et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. 2006;29:1294–9. doi: 10.2337/dc06-0224. [DOI] [PubMed] [Google Scholar]
- 10.Vinik AI. Clinical practice. Diabetic sensory and motor neuropathy. N Engl J Med. 2016;374:1455–64. doi: 10.1056/NEJMcp1503948. [DOI] [PubMed] [Google Scholar]
- 11.Kluding PM, Pasnoor M, Singh R, Jernigan S, Farmer K, Rucker J, et al. The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabetes Complications. 2012;26:424–9. doi: 10.1016/j.jdiacomp.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allet L, Armand S, de Bie RA, Golay A, Monnin D, Aminian K, et al. The gait and balance of patients with diabetes can be improved: A randomised controlled trial. Diabetologia. 2010;53:458–66. doi: 10.1007/s00125-009-1592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colberg SR The Benefits of Unstructured Physical Activity. [Last accessed on: 2016 May 06]. Available from: https://shericolberg.wordpress.com/2007/05/12/ unstructured-activities-count-too/
- 14.Stokes IA, Faris IB, Hutton WC. The neuropathic ulcer and loads on the foot in diabetic patients. Acta Orthop Scand. 1975;46:839–47. doi: 10.3109/17453677508989271. [DOI] [PubMed] [Google Scholar]
- 15.Ctercteko GC, Dhanendran M, Hutton WC, Le Quesne LP. Vertical forces acting on the feet of diabetic patients with neuropathic ulceration. Br J Surg. 1981;68:608–14. doi: 10.1002/bjs.1800680904. [DOI] [PubMed] [Google Scholar]
- 16.Mueller MJ, Minor SD, Sahrmann SA, Schaaf JA, Strube MJ. Differences in the gait characteristics of patients with diabetes and peripheral neuropathy compared with age-matched controls. Phys Ther. 1994;74:299–308. doi: 10.1093/ptj/74.4.299. [DOI] [PubMed] [Google Scholar]
- 17.Bauman JH, Brand PW. Measurement of pressure between foot and shoe. Lancet. 1963;1:629–32. doi: 10.1016/s0140-6736(63)91271-6. [DOI] [PubMed] [Google Scholar]
- 18.Cavanagh PR, Simoneau GG, Ulbrecht JS. Ulceration, unsteadiness, and uncertainty: The biomechanical consequences of diabetes mellitus. J Biomech. 1993;26:23–40. doi: 10.1016/0021-9290(93)90077-r. [DOI] [PubMed] [Google Scholar]
- 19.Maluf KS, Mueller MJ. Novel award 2002. Comparison of physical activity and cumulative plantar tissue stress among subjects with and without diabetes mellitus and a history of recurrent plantar ulcers. Clin Biomech (Bristol, Avon) 2003;18:567–75. doi: 10.1016/s0268-0033(03)00118-9. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong DG, Boulton AJ. Activity monitors: Should we begin dosing activity as we dose a drug? J Am Podiatr Med Assoc. 2001;91:152–3. doi: 10.7547/87507315-91-3-152. [DOI] [PubMed] [Google Scholar]
- 21.Lemaster JW, Reiber GE, Smith DG, Heagerty PJ, Wallace C. Daily weight-bearing activity does not increase the risk of diabetic foot ulcers. Med Sci Sports Exerc. 2003;35:1093–9. doi: 10.1249/01.MSS.0000074459.41029.75. [DOI] [PubMed] [Google Scholar]
- 22.Crews RT, Schneider KL, Yalla SV, Reeves ND, Vileikyte L. Physiological and psychological challenges of increasing physical activity and exercise in patients at risk of diabetic foot ulcers: A critical review. Diabetes Metab Res Rev. 2016;32:791–804. doi: 10.1002/dmrr.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armstrong DG, Lavery LA, Holtz-Neiderer K, Mohler MJ, Wendel CS, Nixon BP, et al. Variability in activity may precede diabetic foot ulceration. Diabetes Care. 2004;27:1980–4. doi: 10.2337/diacare.27.8.1980. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong DG, Abu-Rumman PL, Nixon BP, Boulton AJ. Continuous activity monitoring in persons at high risk for diabetes-related lower-extremity amputation. J Am Podiatr Med Assoc. 2001;91:451–5. doi: 10.7547/87507315-91-9-451. [DOI] [PubMed] [Google Scholar]
- 25.Crews RT, Yalla SV, Dhatt N, Burdi D, Hwang S. Monitoring location-specific physical activity via integration of accelerometry and geotechnology within patients with or at risk of diabetic foot ulcers: A Technological report. J Diabetes Sci Technol. 2017;11:899–903. doi: 10.1177/1932296816651631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Najafi B, Crews RT, Wrobel JS. Importance of time spent standing for those at risk of diabetic foot ulceration. Diabetes Care. 2010;33:2448–50. doi: 10.2337/dc10-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armstrong DG, Nguyen HC, Lavery LA, van Schie CH, Boulton AJ, Harkless LB, et al. Off-loading the diabetic foot wound: A randomized clinical trial. Diabetes Care. 2001;24:1019–22. doi: 10.2337/diacare.24.6.1019. [DOI] [PubMed] [Google Scholar]
- 28.Kanade RV, van Deursen RW, Price P, Harding K. Risk of plantar ulceration in diabetic patients with single-leg amputation. Clin Biomech (Bristol, Avon) 2006;21:306–13. doi: 10.1016/j.clinbiomech.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 29.van Schie CH, Noordhof EL, Busch-Westbroek TE, Beelen A, Nollet F. Assessment of physical activity in people with diabetes and peripheral neuropathy. Diabetes Res Clin Pract. 2011;92:e9–11. doi: 10.1016/j.diabres.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Waaijman R, Keukenkamp R, de Haart M, Polomski WP, Nollet F, Bus SA, et al. Adherence to wearing prescription custom-made footwear in patients with diabetes at high risk for plantar foot ulceration. Diabetes Care. 2013;36:1613–8. doi: 10.2337/dc12-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim S, Kang SM, Kim KM, Moon JH, Choi SH, Hwang H, et al. Multifactorial intervention in diabetes care using real-time monitoring and tailored feedback in type 2 diabetes. Acta Diabetol. 2016;53:189–98. doi: 10.1007/s00592-015-0754-8. [DOI] [PubMed] [Google Scholar]
- 32.Brazeau AS, Rabasa-Lhoret R, Strychar I, Mircescu H. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care. 2008;31:2108–9. doi: 10.2337/dc08-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dasanayake IS, Bevier WC, Castorino K, Pinsker JE, Seborg DE, Doyle FJ, 3rd, et al. Early detection of physical activity for people with type 1 diabetes mellitus. J Diabetes Sci Technol. 2015;9:1236–45. doi: 10.1177/1932296815592409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crews RT, Shen BJ, Campbell L, Lamont PJ, Boulton AJ, Peyrot M, et al. Role and determinants of adherence to off-loading in diabetic foot ulcer healing: A prospective investigation. Diabetes Care. 2016;39:1371–7. doi: 10.2337/dc15-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kluding PM, Singleton JR, Pasnoor M, Dimachkie MM, Barohn RJ, Smith AG, et al. Activity for diabetic polyneuropathy (ADAPT): Study design and protocol for a 2-site randomized controlled trial. Phys Ther. 2017;97:20–31. doi: 10.2522/ptj.20160200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jao YL, Gardner SE, Carr LJ. Measuring weight-bearing activities in patients with previous diabetic foot ulcers. J Wound Ostomy Continence Nurs. 2017;44:34–40. doi: 10.1097/WON.0000000000000270. [DOI] [PubMed] [Google Scholar]
- 37.Umpierre D, Ribeiro PA, Kramer CK, Leitão CB, Zucatti AT, Azevedo MJ, et al. Physical activity advice only or structured exercise training and association with hbA1c levels in type 2 diabetes: A systematic review and meta-analysis. JAMA. 2011;305:1790–9. doi: 10.1001/jama.2011.576. [DOI] [PubMed] [Google Scholar]
- 38.Colberg SR, Hernandez MJ, Shahzad F. Blood glucose responses to type, intensity, duration, and timing of exercise. Diabetes Care. 2013;36:e177. doi: 10.2337/dc13-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Francia P, Bianchi E, Gulisano M, Tedeschi A, Anichini R, De Bellis A. Evaluation of physical activity performed between the main meals for improving glucose control in women with gestational diabetes mellitus (GDM) Diabetes. 2016;65:A594. [Google Scholar]
- 40.Yardley JE, Kenny GP, Perkins BA, Riddell MC, Balaa N, Malcolm J, et al. Resistance versus aerobic exercise: Acute effects on glycemia in type 1 diabetes. Diabetes Care. 2013;36:537–42. doi: 10.2337/dc12-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loprinzi PD, Pariser G. Physical activity intensity and biological markers among adults with diabetes: Considerations by age and gender. J Diabetes Complications. 2013;27:134–40. doi: 10.1016/j.jdiacomp.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Sawacha Z, Gabriella G, Cristoferi G, Guiotto A, Avogaro A, Cobelli C, et al. Diabetic gait and posture abnormalities: A biomechanical investigation through three dimensional gait analysis. Clin Biomech (Bristol, Avon) 2009;24:722–8. doi: 10.1016/j.clinbiomech.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Sartor CD, Hasue RH, Cacciari LP, Butugan MK, Watari R, Pássaro AC, et al. Effects of strengthening, stretching and functional training on foot function in patients with diabetic neuropathy: Results of a randomized controlled trial. BMC Musculoskelet Disord. 2014;15:137. doi: 10.1186/1471-2474-15-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dijs HM, Roofthooft JM, Driessens MF, De Bock PG, Jacobs C, Van Acker KL, et al. Effect of physical therapy on limited joint mobility in the diabetic foot. A pilot study. J Am Podiatr Med Assoc. 2000;90:126–32. doi: 10.7547/87507315-90-3-126. [DOI] [PubMed] [Google Scholar]
- 45.Richardson JK, Sandman D, Vela S. A focused exercise regimen improves clinical measures of balance in patients with peripheral neuropathy. Arch Phys Med Rehabil. 2001;82:205–9. doi: 10.1053/apmr.2001.19742. [DOI] [PubMed] [Google Scholar]
- 46.Goldsmith JR, Lidtke RH, Shott S. The effects of range-of-motion therapy on the plantar pressures of patients with diabetes mellitus. J Am Podiatr Med Assoc. 2002;92:483–90. doi: 10.7547/87507315-92-9-483. [DOI] [PubMed] [Google Scholar]
- 47.Brandon LJ, Gaasch DA, Boyette LW, Lloyd AM. Effects of long-term resistive training on mobility and strength in older adults with diabetes. J Gerontol A Biol Sci Med Sci. 2003;58:740–5. doi: 10.1093/gerona/58.8.m740. [DOI] [PubMed] [Google Scholar]
- 48.Balducci S, Iacobellis G, Parisi L, Di Biase N, Calandriello E, Leonetti F, et al. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications. 2006;20:216–23. doi: 10.1016/j.jdiacomp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Morrison S, Colberg SR, Mariano M, Parson HK, Vinik AI. Balance training reduces falls risk in older individuals with type 2 diabetes. Diabetes Care. 2010;33:748–50. doi: 10.2337/dc09-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Otterman NM, van Schie CH, van der Schaaf M, van Bon AC, Busch-Westbroek TE, Nollet F, et al. An exercise programme for patients with diabetic complications: A study on feasibility and preliminary effectiveness. Diabet Med. 2011;28:212–7. doi: 10.1111/j.1464-5491.2010.03128.x. [DOI] [PubMed] [Google Scholar]
- 51.Collins TC, Lunos S, Carlson T, Henderson K, Lightbourne M, Nelson B, et al. Effects of a home-based walking intervention on mobility and quality of life in people with diabetes and peripheral arterial disease: A randomized controlled trial. Diabetes Care. 2011;34:2174–9. doi: 10.2337/dc10-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song CH, Petrofsky JS, Lee SW, Lee KJ, Yim JE. Effects of an exercise program on balance and trunk proprioception in older adults with diabetic neuropathies. Diabetes Technol Ther. 2011;13:803–11. doi: 10.1089/dia.2011.0036. [DOI] [PubMed] [Google Scholar]
- 53.Shah KM, Mueller MJ. Effect of selected exercises on in-shoe plantar pressures in people with diabetes and peripheral neuropathy. Foot (Edinb) 2012;22:130–4. doi: 10.1016/j.foot.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Melai T, Schaper NC, Ijzerman TH, de Lange TL, Willems PJ, Lima Passos V, et al. Lower leg muscle strengthening does not redistribute plantar load in diabetic polyneuropathy: A randomised controlled trial. J Foot Ankle Res. 2013;6:41. doi: 10.1186/1757-1146-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mueller MJ, Tuttle LJ, Lemaster JW, Strube MJ, McGill JB, Hastings MK, et al. Weight-bearing versus nonweight-bearing exercise for persons with diabetes and peripheral neuropathy: A randomized controlled trial. Arch Phys Med Rehabil. 2013;94:829–38. doi: 10.1016/j.apmr.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Francia P, Anichini R, De Bellis A, Seghieri G, Lazzeri R, Paternostro F, et al. Diabetic foot prevention: The role of exercise therapy in the treatment of limited joint mobility, muscle weakness and reduced gait speed. Ital J Anat Embryol. 2015;120:21–32. [PubMed] [Google Scholar]
- 57.Kluding PM, Pasnoor M, Singh R, D’Silva LJ, Yoo M, Billinger SA, et al. Safety of aerobic exercise in people with diabetic peripheral neuropathy: Single-group clinical trial. Phys Ther. 2015;95:223–34. doi: 10.2522/ptj.20140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nicolucci A, Balducci S, Cardelli P, Cavallo S, Fallucca S, Bazuro A, et al. Relationship of exercise volume to improvements of quality of life with supervised exercise training in patients with type 2 diabetes in a randomised controlled trial: The Italian diabetes and exercise study (IDES) Diabetologia. 2012;55:579–88. doi: 10.1007/s00125-011-2425-9. [DOI] [PubMed] [Google Scholar]
- 59.Flahr D. The effect of nonweight-bearing exercise and protocol adherence on diabetic foot ulcer healing: A pilot study. Ostomy Wound Manage. 2010;56:40–50. [PubMed] [Google Scholar]
- 60.Francia P, Anichini R, Seghieri G, De Bellis A, Gulisano M. History, prevalence and assessment of limited joint mobility: From stiff hand syndrome to diabetic foot ulcer prevention. Curr Diabetes Rev. 2018;14:411–26. doi: 10.2174/1573399813666170816142731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Francia P, Gulisano M, Anichini R, Seghieri G. Diabetic foot and exercise therapy: Step by step the role of rigid posture and biomechanics treatment. Curr Diabetes Rev. 2014;10:86–99. doi: 10.2174/1573399810666140507112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ding S, Schumacher M. Sensor monitoring of physical activity to improve glucose management in diabetic patients: A review. Sensors (Basel) 2016;16:589. doi: 10.3390/s16040589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thomas N, Alder E, Leese GP. Barriers to physical activity in patients with diabetes. Postgrad Med J. 2004;80:287–91. doi: 10.1136/pgmj.2003.010553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uccioli L, Giacomini PG, Monticone G, Magrini A, Durola L, Bruno E, et al. Body sway in diabetic neuropathy. Diabetes Care. 1995;18:339–44. doi: 10.2337/diacare.18.3.339. [DOI] [PubMed] [Google Scholar]
- 65.Toosizadeh N, Mohler J, Armstrong DG, Talal TK, Najafi B. The influence of diabetic peripheral neuropathy on local postural muscle and central sensory feedback balance control. PLoS One. 2015;10:e0135255. doi: 10.1371/journal.pone.0135255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stewart KJ, Hiatt WR, Regensteiner JG, Hirsch AT. Exercise training for claudication. N Engl J Med. 2002;347:1941–51. doi: 10.1056/NEJMra021135. [DOI] [PubMed] [Google Scholar]
- 67.Delbridge L, Perry P, Marr S, Arnold N, Yue DK, Turtle JR, et al. Limited joint mobility in the diabetic foot: Relationship to neuropathic ulceration. Diabet Med. 1988;5:333–7. doi: 10.1111/j.1464-5491.1988.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 68.Francia P, Seghieri G, Gulisano M, De Bellis A, Toni S, Tedeschi A, et al. The role of joint mobility in evaluating and monitoring the risk of diabetic foot ulcer. Diabetes Res Clin Pract. 2015;108:398–404. doi: 10.1016/j.diabres.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 69.Fernando DJ, Masson EA, Veves A, Boulton AJ. Relationship of limited joint mobility to abnormal foot pressures and diabetic foot ulceration. Diabetes Care. 1991;14:8–11. doi: 10.2337/diacare.14.1.8. [DOI] [PubMed] [Google Scholar]
- 70.Zimny S, Schatz H, Pfohl M. The role of limited joint mobility in diabetic patients with an at-risk foot. Diabetes Care. 2004;27:942–6. doi: 10.2337/diacare.27.4.942. [DOI] [PubMed] [Google Scholar]
- 71.Abate M, Schiavone C, Salini V, Andia I. Management of limited joint mobility in diabetic patients. Diabetes Metab Syndr Obes. 2013;6:197–207. doi: 10.2147/DMSO.S33943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Jonge S, Rozenberg R, Vieyra B, Stam HJ, Aanstoot HJ, Weinans H, et al. Achilles tendons in people with type 2 diabetes show mildly compromised structure: An ultrasound tissue characterisation study. Br J Sports Med. 2015;49:995–9. doi: 10.1136/bjsports-2014-093696. [DOI] [PubMed] [Google Scholar]
- 73.Giacomozzi C, D’Ambrogi E, Uccioli L, Macellari V. Does the thickening of achilles tendon and plantar fascia contribute to the alteration of diabetic foot loading? Clin Biomech (Bristol, Avon) 2005;20:532–9. doi: 10.1016/j.clinbiomech.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 74.De León Rodriguez D, Allet L, Golay A, Philippe J, Assal JP, Hauert CA, et al. Biofeedback can reduce foot pressure to a safe level and without causing new at-risk zones in patients with diabetes and peripheral neuropathy. Diabetes Metab Res Rev. 2013;29:139–44. doi: 10.1002/dmrr.2366. [DOI] [PubMed] [Google Scholar]
- 75.Brazeau AS, Hajna S, Joseph L, Dasgupta K. Correlates of sitting time in adults with type 2 diabetes. BMC Public Health. 2015;15:793. doi: 10.1186/s12889-015-2086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pivarnik JM, Reeves MJ, Rafferty AP. Seasonal variation in adult leisure-time physical activity. Med Sci Sports Exerc. 2003;35:1004–8. doi: 10.1249/01.MSS.0000069747.55950.B1. [DOI] [PubMed] [Google Scholar]
- 77.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]