Abstract
Objective:
There are some evidence that Vitamin D supplementation may be beneficial for patients with irritable bowel syndrome (IBS). The aim of this study was to evaluate the effects of Vitamin D supplementation on symptoms and quality of life (QOL) in patients with IBS.
Methods:
In a randomized, double-blind, placebo-controlled clinical trial, 116 patients with IBS were supplemented weekly with either a pearl of 50,000 IU Vitamin D or an identical pearl of placebo containing medium chain triglyceride for 6 weeks.
Results:
Mean age of patients was 42.24 ± 12.26, and 40.06 ± 13.37 in Vitamin D and placebo groups, respectively. Dietary intakes were similar between and within groups. Serum concentration of 25-hydroxy Vitamin D increased significantly from 21.10 ± 5.23 to 36.43 ± 12.34 in the Vitamin D group (P < 0.001), while it was not significantly different before and after the trial in placebo group. The IBS symptoms severity scores (SSSs), disease-specific QOL, and total score were evaluated at weeks 0 and 6. IBS-SSS, IBS-QOL, and the total score were improved significantly more in Vitamin D group in comparison to the placebo group (P < 0.05).
Conclusions:
This study indicates that Vitamin D therapy can improve the severity of symptoms and QOL in patients with IBS; however, the long-term effects remained to be elucidated. Trial registration at IRCT: IRCT201402234010N11 IRB Number: 116/3976
Keywords: Clinical trial, irritable bowel syndrome, quality of life, supplementation, Vitamin D
Introduction
Irritable bowel syndrome (IBS) is one of the most common gastrointestinal conditions, characterized by chronic abdominal pain, discomfort, bloating, and alteration of bowel habits without any organic cause.[1] There has not yet been found any effective cure for IBS; however, some remedies have shown promising effects in the management of it.[2,3] Recently, an analysis of social media (blogs/forums) reported that from 37 patients with IBS, 70% described improvements in their symptoms with Vitamin D supplementation and the majority of these individuals reported being Vitamin D deficient before supplementation.[4] This study suggested a role for Vitamin D in the management of IBS, which was supported by another recent study showing the high prevalence of Vitamin D deficiency in patients with IBS.[5] On the other hand, two recent clinical trials have shown promising effects of Vitamin D supplementation on IBS symptoms.[6,7] Furthermore, a recent review article has shown immune system activation is more frequent in IBS patients in comparison to healthy controls.[8] Given the immunomodulatory role of Vitamin D,[9,10] we hypothesized that patients with IBS who have low serum level of Vitamin D, might benefit from Vitamin D supplementation. Thus, we designed this randomized, double-blind, placebo-controlled clinical trial to evaluate the effects of Vitamin D supplementation on symptoms and quality of life (QOL) in patients with IBS.
Methods
Recruitment and eligibility screening
IBS patients were recruited from two gastroenterology clinics from October 2013 to January 2016. IBS was diagnosed according to the ROME III criteria.[11] Inclusion and exclusion criteria were the same as our previous study.[6] All participants were examined for serum Vitamin D, and those with serum Vitamin D level of >30 ng/ml were also excluded from the study.
Study design
The study protocol was explained for all the participants, and those who agreed to participate and signed the written informed consent were enrolled in the study. The study protocol was approved at our local ethics committee. The participants were randomly assigned to receive either 50,000 IU Vitamin D or identical placebo weekly for 6 weeks according to the available guidelines.[12] Randomization lists were computer-generated by a statistician, and given to the interviewer. The investigators and patients were not aware of the supplements content.
Intervention
The Vitamin D pearls (Zahravi co., Iran) consisted of 50,000 IU cholecalciferol that was taken one pearl weekly. The placebos were in the same shape and color of Vitamin D pearls and contained 10 mg medium-chain triglyceride oil.
Clinical, paraclinical, and dietary intake assessments
Data on the anthropometric characteristics and 3 days dietary recalls were measured at the baseline and the end of the study. The nutrients compositions of dietary intakes were calculated using the available composition tables.[13]
Validated IBS Symptoms Severity Score (IBS-SSS) questionnaire,[14] IBS-QOL questionnaire,[15] and total score of IBS (visual analog scale)[16] were filled out at the beginning and the end of the study period.
Patients were followed by phone call to ask the probable side effects and participants’ compliance. Furthermore, adherence to the study protocol was assessed using tablet count at the end of the study.
Statistical analysis
Data were analyzed using SPSS software (version 16; SPSS, Chicago, IL, USA). Continuous and categorical data were presented as means ± standard deviation and frequency, respectively. Demographic variables were analyzed using a Chi-square or t-test, as appropriate. Paired t-test and Student's t-test were used to compare variables between and within groups, respectively. If the distribution of variables were not normal, Wilcoxon and Mann–Whitney U-tests were used to compare quantitative variables between groups, respectively. The data were analyzed according to the intention-to-treat principle. To eliminate the effects of confounding factors, the analysis of covariance test was used.
Results
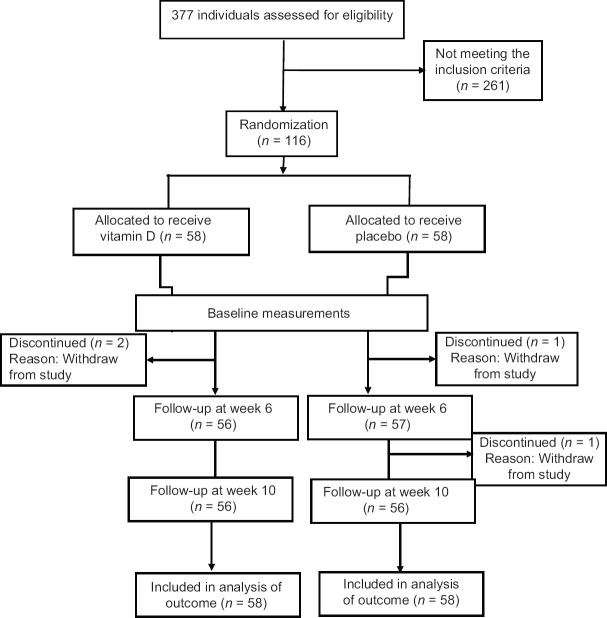
The consort flow chart of participants through the trial is presented in Figure 1. Two participants from each group were withdrawn from the study; however, they were included in the intention to treat analysis. General characteristics of the participants are shown in Table 1. There was no significant difference between two groups at the baseline characteristics. Table 2 shows the dietary intakes of two groups at the baseline of the study, which was not different between the two groups. The dietary intakes were not different between and within the groups at the end of the study too. Serum concentration of 25-hydroxy Vitamin D increased significantly from 21.10 ± 5.23 to 36.43 ± 12.34 in the Vitamin D group (P < 0.001), while it was not different before (21.33 ± 5.54) and after (21.25 ± 4.98) the trial in placebo group. IBS-SSS, IBS-QOL, and the total score were improved in both groups significantly (P < 0.001). As it is shown in Table 3, IBS-QOL and total score at week 6 were improved significantly more in Vitamin D group in comparison to placebo group; however, this significance was not observed in IBS-QOL before adjusting for the baseline values. IBS-SSS changes were not significantly different between two groups; however, this difference was near significant after adjustment for the baseline values (P = 0.05) [Table 3].
Figure 1.
Study flow chart
Table 1.
Demographic characteristics of the study participants
| Baseline characteristic | Vitamin D group | Placebo group | P |
|---|---|---|---|
| Age (mean±SD) | 42.24±12.26 | 40.06±13.37 | 0.571 |
| Weight (mean±SD) | 66.15±9.37 | 66.28±12.39 | 0.968 |
| BMI (mean±SD) | 25.85±3.78 | 25.27±4.07 | 0.619 |
| Smoking (%) | 0 | 2 (8.7) | 0.489 |
| Vitamin D consumption (%) | 0 | 0 | 1.00 |
| SSS (mean±SD) | 250.87±96.92 | 245.78±112.82 | 0.737 |
| IBS-QOL (mean±SD) | 57.35±27.96 | 46.70±31.37 | 0.231 |
| Total score (mean±SD) | 81.30±15.17 | 80.00±13.40 | 0.759 |
Significances are based on independent t-test for quantitative variables and Pearson’s Chi-squared test/Fisher exact test for qualitative factors. SSS=Symptoms severity score, QOL=Quality of life, SD=Standard deviation, IBS=Irritable bowel syndrome, BMI=Body mass index
Table 2.
Dietary intake of selected nutrients in the study participants at baseline
| Dietary intakes | Mean±SD | P | |
|---|---|---|---|
| Vitamin D group | Placebo group | ||
| Calorie | 1585.89±403.60 | 1538.25±504.98 | 0.725 |
| Protein | 71.72±23.40 | 62.17±32.72 | 0.261 |
| Carbohydrate | 198.50±66.36 | 202.16±70.63 | 0.857 |
| Total fat | 58.99±20.36 | 57.34±29.22 | 0.824 |
| Cholesterol | 261.08±170.83 | 227.81±266.28 | 0.617 |
| Vitamin C | 83.73±82.87 | 132.57±155.80 | 0.191 |
| Calcium | 658.30±339.98 | 634.78±281.25 | 0.803 |
| Vitamin D | 1.09±1.41 | 1.27±2.64 | 0.772 |
| Zinc | 5.92±3.88 | 7.45±4.32 | 0.212 |
| Magnesium | 177.98±125.30 | 220.03±100.30 | 0.216 |
| Phosphor | 889.59±409.13 | 965.47±495.98 | 0.580 |
| Fiber | 15.80±15.11 | 23.38±18.58 | 0.136 |
| Ferrous | 9.99±3.32 | 9.90±4.29 | 0.939 |
| Vitamin B2 | 1.20±0.43 | 1.22±0.59 | 0.882 |
Significances are based on independent t-test. SD=Standard deviation
Table 3.
The mean difference of the effect of Vitamin D versus placebo on symptoms severity score, irritable bowel syndrome-quality of life, and total score between the groups
| Characteristic | Vitamin D group | Placebo group | Crude (P) | Adjusted (P) | ||
|---|---|---|---|---|---|---|
| Crude | Adjusted | Crude | Adjusted | |||
| SSS (mean±SE)a | 60.23±12.67 | 61.46±14.54 | 34.45±13.76 | 35.55±13.54 | <0.05 | <0.05 |
| IBS-QOL (mean±SE)a | 44.17±6.98 | 48.13±4.24 | 39.13±4.87 | 35.18±4.24 | 0.556 | 0.039* |
| Total score (mean±SE)a | 66.52±5.35 | 66.69±4.08 | 26.30±3.30 | 26.14±4.08 | <0.001* | <0.001* |
Crude significances are based on independent t-test and adjusted significances are based on ANCOVA adjusting the effects of age, IBS-QOL baseline score, and baseline value of each factor as covariates, *Statistically significant, aOn week 6, bOn week 10. SSS=Symptoms severity score, QOL=Quality of life, SE=Standard error, IBS=Irritable bowel syndrome, ANCOVA=Analysis of covariance
Discussion
Our results have shown that 6 weeks supplementation with Vitamin D improves the symptoms and QOL in patients with IBS. It seems that Vitamin D supplementation improves the IBS characteristics through improving the factors involved in the development of IBS.
Although the exact pathophysiology of IBS has not yet been elucidated, it has been shown that alterations in the gut microbiome, intestinal permeability, gut immune function, visceral sensation, brain–gut interactions, and psychosocial status are involved in the development of this syndrome.[2,17] Furthermore, it has been shown previously that Vitamin D can modulate all of these probable mechanisms involved in IBS pathogenesis. Bashir et al.[18] have shown that 8-week Vitamin D supplementation changes the human gastrointestinal microflora with a reduction in opportunistic pathogens and an increase in bacterial richness. The effects of Vitamin D on the improvement of intestinal barrier function have been demonstrated in in vitro,[19,20] experimental,[21] and human studies.[22] Moreover, it has been shown that Vitamin D regulates immune cell trafficking and differentiation, gut barrier function, and antimicrobial peptide synthesis, all of which, has been shown that play a role in the development of IBS. Vitamin D regulates the innate immune response to the microbiota. Vitamin D is a critical regulator of T-cell function, and the expression of several pattern recognition receptors involved in intestinal inflammation is regulated by Vitamin D.[23] In the absence of Vitamin D, there are many effector T-cells that produce inflammatory cytokines in the intestine. Vitamin D promotes regulatory T-cell development and function to turn off the Th1 and Th17 cells and to control inflammation in the intestine. The ability of Vitamin D to inhibit Th1, Th17 cells, induce regulatory T-cells, and reduce inflammation resulted in a shift in the microbiome and maintenance of tolerance in the gut.[24,25,26] Furthermore, it has been shown that low Vitamin D levels are associated with increased central sensitivity, particularly augmented pain processing on mechanical stimulation in chronic pain,[27,28] depression,[29,30] and anxiety,[31] which all are related with the risk of IBS development. Thus, Vitamin D can improve the IBS symptoms through reduction in all known risk factors that are involved in the pathogenesis of this syndrome.
This study has several advantages; it was designed as a randomized, double-blind, placebo-controlled, clinical trial on the IBS patients who did not receive any medication for their problems, which shows the net effects of Vitamin D supplementation in the study participants. The study was continued 4 weeks after the end of supplementation; remaining effects 4 weeks after supplementation indicates that these beneficial effects are related to the treatment of Vitamin D deficiency, and it confirms the hypothesis that Vitamin D deficiency might be involved in the pathogenesis of IBS. Finally, the dietary intakes were similar in both the groups that reduced the effects of dietary confounders, which shows that our results are independent to the effects of dietary intakes of the study participants.
This study has some limitation; although the sample size was calculated with an accepted power for the study, the results need to be confirmed in larger studies. Moreover, since we recruited only women for this study, the results may not be implemented in men. Another limitation of this study is that we did not measure the sun exposure in participants; however, we measured the serum Vitamin D concentration, which is the most accurate assessment of the Vitamin D status.
Conclusions
This study indicates that Vitamin D therapy has some beneficial effects in the management of IBS in women; however, the long-term effects remained to be elucidated.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This study was financially supported by National Nutrition and Food Technology Research Institute, Tehran, Iran.
References
- 1.Flik CE, van Rood YR, de Wit NJ. Systematic review: Knowledge and educational needs of patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2015;27:367–71. doi: 10.1097/MEG.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 2.Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: A clinical review. JAMA. 2015;313:949–58. doi: 10.1001/jama.2015.0954. [DOI] [PubMed] [Google Scholar]
- 3.Ford AC, Talley NJ. Irritable bowel syndrome. BMJ (Clinical research ed) 2012;345:e5836. doi: 10.1136/bmj.e5836. [DOI] [PubMed] [Google Scholar]
- 4.Sprake EF, Grant VA, Corfe BM. Vitamin D3 as a novel treatment for irritable bowel syndrome: Single case leads to critical analysis of patient-centred data. BMJ Case Rep 2012. 2012 doi: 10.1136/bcr-2012-007223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khayyat Y, Attar S. Vitamin D deficiency in patients with irritable bowel syndrome: Does it exist? Oman Med J. 2015;30:115–8. doi: 10.5001/omj.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jalili M, Hekmatdoost A, Vahedi H, Poustchi H, Khademi B, Saadi M, et al. Co-administration of soy isoflavones and vitamin D in management of irritable bowel disease. PLoS One. 2016;11:e0158545. doi: 10.1371/journal.pone.0158545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbasnezhad A, Amani R, Hajiani E, Alavinejad P, Cheraghian B, Ghadiri A, et al. Effect of vitamin D on gastrointestinal symptoms and health-related quality of life in irritable bowel syndrome patients: A randomized double-blind clinical trial. Neurogastroenterol Motil. 2016;28:1533–44. doi: 10.1111/nmo.12851. [DOI] [PubMed] [Google Scholar]
- 8.Matricon J, Meleine M, Gelot A, Piche T, Dapoigny M, Muller E, et al. Review article: Associations between immune activation, intestinal permeability and the irritable bowel syndrome. Aliment Pharmacol Ther. 2012;36:1009–31. doi: 10.1111/apt.12080. [DOI] [PubMed] [Google Scholar]
- 9.Tiwari S, Pratyush DD, Gupta SK, Singh SK. Vitamin D deficiency is associated with inflammatory cytokine concentrations in patients with diabetic foot infection. Br J Nutr. 2014;112:1938–43. doi: 10.1017/S0007114514003018. [DOI] [PubMed] [Google Scholar]
- 10.Sharma P, Mitra P. Vitamin D: The need of the hour. Indian J Clin Biochem. 2016;31:243–4. doi: 10.1007/s12291-016-0578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drossman DA, Dumitrascu DL. Rome III: New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:237–41. [PubMed] [Google Scholar]
- 12.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 13.Ghaffarpour M, Houshiar-Rad A, Kianfar H. Tehran, Iran: National Institute of Nutrition and Food Technology; 1999. The Manual for HOUSEHOLD measures, Cooking Yields Factors and Edible Portion of Foods. [Google Scholar]
- 14.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 15.Andrae DA, Covington PS, Patrick DL. Item-level assessment of the irritable bowel syndrome quality of life questionnaire in patients with diarrheal irritable bowel syndrome. Clin Ther. 2014;36:663–79. doi: 10.1016/j.clinthera.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Ludidi S, Conchillo JM, Keszthelyi D, Van Avesaat M, Kruimel JW, Jonkers DM, et al. Rectal hypersensitivity as hallmark for irritable bowel syndrome: Defining the optimal cutoff. Neurogastroenterol Motil. 2012;24:729–33. doi: 10.1111/j.1365-2982.2012.01926.x. e345-6. [DOI] [PubMed] [Google Scholar]
- 17.Cashman MD, Martin DK, Dhillon S, Puli SR. Irritable bowel syndrome: A Clinical review. Curr Rheumatol Rev. 2016;12:13–26. doi: 10.2174/1573397112666151231110521. [DOI] [PubMed] [Google Scholar]
- 18.Bashir M, Prietl B, Tauschmann M, Mautner SI, Kump PK, Treiber G, et al. Effects of high doses of vitamin D3 on mucosa-associated gut microbiome vary between regions of the human gastrointestinal tract. Eur J Nutr. 2016;55:1479–89. doi: 10.1007/s00394-015-0966-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chirayath MV, Gajdzik L, Hulla W, Graf J, Cross HS, Peterlik M, et al. Vitamin D increases tight-junction conductance and paracellular ca2+transport in caco-2 cell cultures. Am J Physiol. 1998;274:G389–96. doi: 10.1152/ajpgi.1998.274.2.G389. [DOI] [PubMed] [Google Scholar]
- 20.Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294:G208–16. doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- 21.Zhu T, Liu TJ, Shi YY, Zhao Q. Vitamin D/VDR signaling pathway ameliorates 2,4,6-trinitrobenzene sulfonic acid-induced colitis by inhibiting intestinal epithelial apoptosis. Int J Mol Med. 2015;35:1213–8. doi: 10.3892/ijmm.2015.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raftery T, Martineau AR, Greiller CL, Ghosh S, McNamara D, Bennett K, et al. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in crohn's disease: Results from a randomised double-blind placebo-controlled study. United European Gastroenterol J. 2015;3:294–302. doi: 10.1177/2050640615572176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meeker S, Seamons A, Maggio-Price L, Paik J. Protective links between vitamin D, inflammatory bowel disease and colon cancer. World J Gastroenterol. 2016;22:933–48. doi: 10.3748/wjg.v22.i3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantorna MT. Why do T cells express the vitamin D receptor? Ann N Y Acad Sci. 2011;1217:77–82. doi: 10.1111/j.1749-6632.2010.05823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cantorna MT, McDaniel K, Bora S, Chen J, James J. Vitamin D, immune regulation, the microbiota, and inflammatory bowel disease. Exp Biol Med (Maywood) 2014;239:1524–30. doi: 10.1177/1535370214523890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ooi JH, Chen J, Cantorna MT. Vitamin D regulation of immune function in the gut: Why do T cells have vitamin D receptors? Mol Aspects Med. 2012;33:77–82. doi: 10.1016/j.mam.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Känel R, Müller-Hartmannsgruber V, Kokinogenis G, Egloff N. Vitamin D and central hypersensitivity in patients with chronic pain. Pain Med. 2014;15:1609–18. doi: 10.1111/pme.12454. [DOI] [PubMed] [Google Scholar]
- 28.McCabe PS, Pye SR, Beth JM, Lee DM, Tajar A, Bartfai G, et al. Low vitamin D and the risk of developing chronic widespread pain: Results from the european male ageing study. BMC Musculoskelet Disord. 2016;17:32. doi: 10.1186/s12891-016-0881-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stokes CS, Grünhage F, Baus C, Volmer DA, Wagenpfeil S, Riemenschneider M, et al. Vitamin D supplementation reduces depressive symptoms in patients with chronic liver disease. Clin Nutr. 2016;35:950–7. doi: 10.1016/j.clnu.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 30.von Känel R, Fardad N, Steurer N, Horak N, Hindermann E, Fischer F, et al. Vitamin D deficiency and depressive symptomatology in psychiatric patients hospitalized with a current depressive episode: A Factor analytic study. PLoS One. 2015;10:e0138550. doi: 10.1371/journal.pone.0138550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bičíková M, Dušková M, Vítků J, Kalvachová B, Řípová D, Mohr P, et al. Vitamin D in anxiety and affective disorders. Physiol Res. 2015;64(Suppl 2):S101–3. doi: 10.33549/physiolres.933082. [DOI] [PubMed] [Google Scholar]