Abstract
Background:
The aim of this study was to investigate the effect of hydroethanolic Nigella sativa L. extract on skin wound healing in diabetic male rats.
Methods
This experimental study was conducted on 49 male Wistar rats weighing 220–250 g divided into 7 groups of 7 each: control (nondiabetic untreated), sham (nondiabetic eucerin-treated), nondiabetic phenytoin (1%)-treated, diabetic untreated, and three diabetic groups treated independently with phenytoin 1%, hydroethanolic N. sativa extracts 20% or 40%. Diabetes was induced with 60 mg/kg streptozosin in one administration. After anesthesia, 2 × 1 cm2 wounds were made on the rats’ backs and each group was administered with its own respective treatment until the wounds were healed completely. Tissue specimens were prepared for histological examinations. The areas of the wounds were measured every 3 days. The data were analyzed by ANOVA and Tukey's post-hoc test.
Results:
The mean duration of wound healing was 27 and 24 days for diabetic untreated and diabetic phenytoin-treated groups, respectively. Wounds were healed completely in nondiabetic untreated, sham, and nondiabetic phenytoin-treated groups on days 23, 24, and 21, respectively. The shortest duration of wound healing was seen in diabetic N. sativa extract (40%)-treated group (15 days) followed by diabetic N. sativa (20%)-treated group (18 days). These two groups were found to have the lowest mean wound area during the study with a significant difference from mean wound area in the controls (P < 0.05).
Conclusions:
N. sativa extract significantly promoted wound healing in diabetic rats in comparison with control groups. Although the beneficial mechanism of the promotion of wound healing was not specifically studied, it is believed that the anti-inflammatory and antimicrobial properties of N. sativa would contribute to this enhanced wound healing.
Keywords: Diabetes, Nigella sativa L., rat, skin wound
Introduction
Wound healing refers to a set of processes that lead to replicating lost tissue as much as possible. Wound healing is a dynamic process consisting of five phases: stopping of bleeding, inflammation, cell proliferation, replacement, and reconstruction or scar formation. These phases should be fulfilled continuously and for a specific period so that wound is healed successfully. It is noteworthy that each phase starts immediately after the preceding phase and the durations of the phases vary depending on the type and site of the wound and injured tissue.[1] Meanwhile, diabetic wounds healing is particularly important because wound healing may be delayed and defective in people with diabetes because of disturbed inflammation phase, disturbed and reduced angiogenesis, excessive formation and long-term survival of granulation tissue, disturbed epithelialization, as well as disturbed restructuring and reconstruction.[2,3,4,5,6]
Therefore, patients with diabetes are likely to face certain complications such as wound infection, amputation, high treatment costs, and even death.[7] Shortening the duration of wound healing has long attracted medical researchers’ attention and different classes of pharmacological drugs have been recommended to achieve this purpose. With medical advances, plant-based drugs and compounds are being increasingly used in the hope for finding better treatments.[8,9,10,11,12,13,14,15,16]
Black cumin (Nigella sativa L.) is one of the plants that has been studied for treatment of diseases.[17] N. sativa is a plant in the family of Ranunculaceae. It is a herbaceous, annual, spontaneously growing plant covered with trichomes. N. sativa reaches a height of 40–90 cm. Its fruit is similar to a large and swollen capsule that bears 3–7 follicles. Each follicle contains a large number of seeds.[18] When the capsule of a fruit is ripened, it opens and its seeds fall out and then become black.[19] The medical use of N. sativa dates back to 2000 years ago. N. sativa use was started in Asia and then spread to Europe, Africa, and India.[20] This plant is used as a spice and also to treat certain diseases such as asthma, hypertension, diabetes, inflammation, headache, cough, bronchitis, eczema, fever, dizziness, and influenza.[21] Interestingly, N. sativa can exert anti-inflammatory, antimicrobial, antioxidant, antitumor, immune system-boosting, hypotensive, hypoglycemic, and hypolipidemic actions.[22] A comprehensive preclinical pharmacological review of the actions of N. sativa has recently been published (Dajani EZ, Shahwan T, Dajani N. Overview of the preclinical pharmacological properties of Nigella sativa (Black seeds): A complementary drug with historical and clinical significance. J Physiol Pharmacol 2016;67 (6):801–817). The objective of our study is to investigate the potential therapeutic actions of a hydroethanolic N. sativa extract in healing skin wound in diabetic male rats.
Methods
Laboratory animals
This experimental study was conducted on 49 male Wistar rats weighing 220–250 g. All rats were maintained in animal house of department of biology, faculty of basic sciences, Bu-Ali Sina University, Hamedan, Iran under controlled (23 ± 2°C) temperature and 12-h dark/12-h light cycle with free access to food and water. The rats were randomly assigned to seven groups consisting nondiabetic untreated, nondiabetic phenytoin (1%)-treated, sham or nondiabetic eucerin-treated, diabetic untreated, diabetic phenytoin (1%)-treated, diabetic N. sativa extract (20%)-treated, and diabetic N. sativa extract (40%)-treated.
Extraction and ointment preparation
After N. sativa was purchased and scientifically identified by the botanist of Bu-Ali Sina University, 500 g seeds were pulverized with an electric mill. To extract active compounds, maceration was used. Adequate amount of ethanol (2 l) 80% was added to 500 g N. sativa powder and the solution was refrigerated for a week. Then, the extract was separated with a filter paper and hopper and concentrated with a rotary instrument as much as possible. Next, eucerin (Pasteur Institute of Iran, Tehran, Iran)-based ointments with 20% and 40% weight/weight ratio were prepared.[8,23]
Induction of diabetes in the rats
To induce diabetes, 60 mg/kg streptozosin (Sigma-Aldrich, USA) was intraperitoneally administered to the rats in all groups in one injection.[24] Three days after streptozosin administration, blood sugar levels of the rats were measured with a glucometer to ensure induction of diabetes in them. The rats with glucose levels of over 200 mg/dl were considered to be diabetic.
Making and treating wounds
After the induction with intraperitoneal administration of combination of ketamine (50 mg/kg, alfasan, Woerden-Holland) and xylazine (10 mg/kg, alfasan, Woerden-Holland), the rats were placed prone on a surgery table.[8,24] Then, the hairs on back between the two shoulders were completely shaven with an electric shaver and a 1 × 2 rectangle was drawn on the skin. Then, a full-thickness specimen consisting of epiderm, derm, and hypodermic was taken from the skin with a scalpel. The day of surgery was considered to be day 0; all rats were left untreated.
One day after surgery (day 1) to the day when complete healing was observed and epiderm was reconstructed, the treatment was applied once daily with a clean cotton swab on wound surface completely every day. Before application of the treatments, the wounds’ surfaces were separately cleansed with physiological serum-soaked tampon slowly and closely. Throughout the study, the wounds were left undressed.[25]
Wound Area Measurement
Wound area was measured once every 3 days until the wounds were completely healed. To prevent rats from moving in measuring the wound areas, they were anesthetized with inhaled ether. Then, the rats were placed prone and wound areas were drawn using a transparent paper and measured using a 1-mm graph paper as described by Shoohani et al.[23] Measurements errors were minimized by measuring the wounds’ dimensions with a caliper and calculating the mean value of the measurements made by these two methods.
Histological examinations
To conduct histological examinations, hematoxylin and eosin staining was used. To do this, specimens of full-thickness skin were taken on day 21 in all groups. In N. sativa extract-treated groups, the specimens were taken on the day when wound healing was completed.
Then, the specimens underwent tissue processing. Formalin 10%-fixed skins tissues were embedded in paraffin and then cut into 5 μm thick sections by microtom (OSK, OGAWA SEIKI Co. Ltd. – Japan). The slides were stained with the conventional hematoxylin and eosin, mounted with neutral resin, and examined with optical microscope (ZEISS, Axiostar Pluss – Germany).[26] After completion of the passage, the specimens were molded and thin (5-μm) sections of them were prepared using a microtome and placed on a lam. The microscopic stained sections were examined by an optical microscope at magnifications 100×, 200×, and 400 × and then images were taken.
For data analysis, the wound areas of different groups were measured on the studied days. The data were analyzed by intersubject one-way ANOVA and Tukey's post-hoc test in SPSS 23.
Results
Wound area increased very markedly in diabetic untreated and diabetic phenytoin-treated groups on the first 3 days and decreased very slowly on subsequent days so that the wound was not completely healed even on day 23 [Table 1]. As treatment continued, the wounds were completely healed on days 27 and 24 in diabetic untreated and diabetic phenytoin ointment-treated rats, respectively. Wound healing occurred more rapidly in phenytoin-treated group than in untreated group yet with no significant difference (P > 0.05).
Table 1.
Wound healing of different groups in different days of treatment
| Group (n=7) | Days | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1st | 3rd | 6th | 9th | 12th | 15th | 18th | 21st | 23rd | |
| A | 2.67±0.08 | 2.34±0.4 | 2.00±0.01 | 1.6±0.05 | 0.89±0.06 | 0.62±0.13 | 0.22±0.12 | 0.02±0.00 | 0.00 |
| B | 2.66±0.05 | 2.18±0.09 | 1.72±0.02 | 1.24±0.02 | 0.69±0.02 | 0.27±0.11 | 0.05±0.01 | 0.00 | 0.00 |
| C | 2.67±0.09 | 2.37±0.04 | 2.03±0.02 | 1.64±0.04 | 1.02±0.05 | 0.58±0.06 | 0.26±0.01 | 0.16±0.01 | 0.04±0.02 |
| D | 2.96±0.04 | 3.45±0.06 | 2.95±0.04 | 2.60±0.05 | 1.17±0.05 | 1.24±0.10 | 0.91±0.06 | 0.39±0.04 | 0.17±0.02 |
| E | 2.95±0.04 | 3.33±0.10 | 2.70±0.06 | 2.23±0.04 | 1.84±0.05 | 1.24±0.07 | 0.74±0.05 | 0.10±0.03 | 0.02±0.005 |
| F | 2.92±0.06 | 2.10±0.04 | 1.28±0.04 | 0.70±0.03 | 0.23±0.02 | 0.04±0.02 | 0.00 | 0.00 | 0.00 |
| G | 2.93±0.06 | 1.93±0.02 | 1.09±0.04 | 0.23±0.02 | 0.07±0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
NS=Nigella sativa. Data are mean±SEM of wound area (cm2) in rats. (A) Nondiabetic with no treatment, (B) nondiabetic + phenytoin 1%, (C) nondiabetic + eucerin, (D) diabetic + no treatment, (E) diabetic + phenytoin 1%, (F) diabetic + NS ointment (20%), (G) diabetic + NS ointment (40%)
Wound area increased on day 1 and started to decrease on subsequent days in diabetic N. sativa extract (20%)-treated group so that the wound was completely healed on day 18. These findings were significantly different from the findings in diabetic untreated and diabetic phenytoin-treated groups (P < 0.001).
In diabetic N. sativa extract (40%)-treated group, wound area increased on day 1, but on subsequent days, it decreased rapidly so that the wound was completely healed on day 15. Mean wound area decreased significantly in this group compared to diabetic untreated and diabetic phenytoin-treated groups (P < 0.001). Although wound healing occurred more rapidly in N. sativa extract (40%)-treated group than in N. sativa extract (20%)-treated group, no statistically significant difference in mean wound area was noted between the two groups throughout treatment period (P > 0.05).
In all nondiabetic groups, wound area increased less markedly compared to diabetic groups such that a significant difference was seen between nondiabetic and diabetic groups (irrespective of the studied treatment as no group was treated on day 1 of measurement) (P < 0.05).
In untreated nondiabetic group, after this primary increase, wound area started to decrease so that the wound was completely healed on day 23. A significant difference in wound area was seen between untreated nondiabetic and untreated diabetic groups (P < 0.01) [Figure 1].
Figure 1.
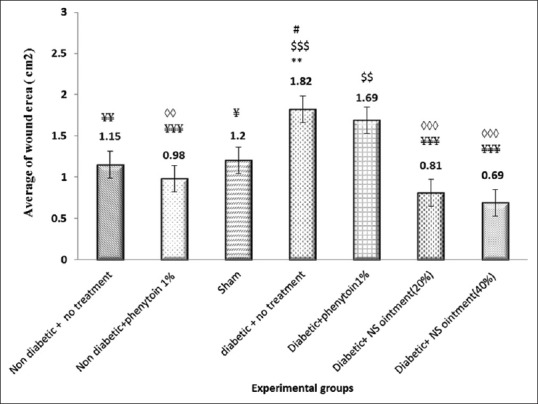
Mean wound area in different groups throughout wound healing; *significant compared to untreated nondiabetic group (**P < 0.01), $significant compared to nondiabetic phenytoin-treated group ($$,$$$P < 0.01 and 0.001, respectively), #significant compared to sham group (#P < 0.05), ¥significant compared to untreated diabetic group (¥, ¥¥,¥¥¥P < 0.05, 0.01, and 0.001, respectively), ◊significant compared to diabetic phenytoin-treated group (◊◊,◊◊◊0.01 and 0.001, respectively)
In sham group, after observing a primary increase in wound area, we found that wound area started to decrease so that the wounds were completely healed on day 25. No statistically significant difference was seen between sham and untreated nondiabetic groups (P > 0.05).
In nondiabetic phenytoin ointment-treated, the wound area increased slightly on day 1 and started to decrease on subsequent days. The wounds were healed on day 21 [Figure 2]. The findings of this group and the controls (untreated nondiabetic) were not significantly different (P > 0.05), but significant difference was noted between the findings of nondiabetic phenytoin ointment-treated and diabetic phenytoin-treated groups (P < 0.01).
Figure 2.

The status of wound healing on day 12. (a) Diabetic untreated, (b) diabetic phenytoin-treated, (c) diabetic Nigella sativa extract (20%)-treated, (d) diabetic N. sativa extract (40%)-treated, (e) full-thickness wound made on the rat's back on day 0 (the day of surgery)
In the N. sativa extract-treated groups, the epidermal thickness was significantly greater the untreated and even phenytoin-treated groups. Overall, the epidermal thickness was lower in the untreated group than in other groups (A).
Figure 3 illustrates N. sativa extract (40%)-treated group (D), which has the most epidermal thickness. This finding indicates that the wound healing was better in this group. Moreover, the collagen fibers are thicker and are arranged more regularly in N. sativa extract-treated wounds, which represent natural process of wound healing in these groups. A large number of fibroblasts were seen in the groups treated with the extract, which represents the natural process of wound healing because of the main role of fibroblasts in producing collagen fibers.
Figure 3.
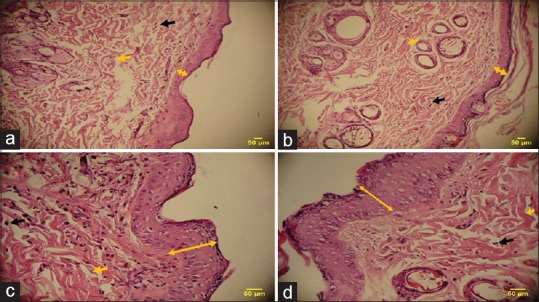
Hematoxylin–eosin-stained tissue section (magnification 400×): (a) diabetic untreated, (b) diabetic phenytoin-treated, (c) diabetic Nigella sativa extract (20%)-treated, (d) diabetic N. sativa extract (40%)-treated,  : epidermal thickness,
: epidermal thickness,  : collagen fibers,
: collagen fibers,  : fibroblasts
: fibroblasts
Discussion
Medicinal plants are considered a precious treasure and their extracts have been commonly used to heal skin wounds since old time.[27,28,29] Regarding the findings of the present study, we found hydroethanolic N. sativa extract (40%) to be the best studied treatment with the shortest period of wound healing in diabetic rats. N. sativa ointment was found to exert anti-inflammatory effects on the early days of healing period, and therefore, the healing effects of this ointment can be partly attributed to its anti-inflammatory effects. Aydin et al. study has reported N. sativa to exert anti-inflammatory effect.[30]
Moreover, Noorbar et al. review article has reported anti-inflammatory property to be one of the properties of N. sativa.[21] Besides that, according to Ashrafi et al. study, anti-inflammatory drugs, particularly nonsteroidal ones, are one of the most commonly used drugs after surgery to relieve pain, reduce inflammation, and improve general conditions of the patients in order to accelerate the process of wound healing.[8] Therefore, medicinal plants, such as N. sativa, which have anti-inflammatory properties and can positively affect the phases of wound healing accelerate this process.[22,30]
A study found that thymoquinone (one of the N. sativa-active compounds) could prevent oxidative damage in vitro and in vivo.[30] N. sativa is thought to speed up wound healing through scavenging free radicals.[30] According to Zareian et al. study, modulating inflammation and using antioxidants contribute to speeding up wound healing.[31] N. sativa extract has antioxidant property that is even more potent than the antioxidant properties of synthetic antioxidants such as BHA and BHT.[32] These findings are consistent with the results of the present study.
Khaksari et al. study found that preventing wound infection accelerated wound healing. According to Khaksari et al. study, antibiotic drugs can accelerate wound healing through controlling wound infection.[33] Consistently, Noorbar et al. review article has reported that N. sativa has antibacterial property.[21]
This plant has been demonstrated to exert antibacterial effect on both gram-negative and gram-positive bacteria.[34] Morsi and Aydin et al. studies have confirmed antimicrobial action of N. sativa.[30,35] Accordingly, it can be argued that one of the reasons for speeding up wound healing is antimicrobial and antiseptic effects. None of N. sativa extract-treated rats acquired infection in this study, which is consistent with Morsi and Aydin et al. studies.[30,35]
Thymol is one of the active compounds of N. sativa.[20] Thymol is considered to be a constituent of plant-based compounds and essential oils that is used as a nature-based, potent antibacterial compound alone or in combination with other antibacterial compounds.[36] Antibacterial, antioxidant, and antifungal actions of thymol have been confirmed.[37]
Because N. sativa contains large amounts of thymol that has antibacterial property, hydroethanolic N. sativa extract is able to control skin infection in the wound and, therefore, contributes to speeding up the wound healing. Large amounts of thymol in Mentha piperita essential oil caused the wounds to be healed more rapidly in the rats treated with this essential oil.[36]
Moreover, wound healing occurred much more optimally and rapidly in both N. sativa extract ointment-treated groups than in phenytoin ointment-treated group with a significant difference. Therefore, it is likely that certain mechanisms that have been recommended to explain phenytoin effect in speeding up wound healing can also be considered to be the mechanisms of N. sativa's even more potently and efficient action in this process. Increased angiogenesis and development of highly stretch resistance have been suggested to be phenytoin's repair mechanisms for wound healing.[38] As a result, N. sativa may use these mechanisms even more potently in wound healing process.
Both diabetic phenytoin-treated and sham (healthy eucerin-treated) groups reached complete wound healing on day 24. The wounds were healed on day 23 in healthy untreated group. Regarding this finding and the results, we can argue that there is no significant difference between healthy eucerin-treated and healthy untreated groups. Therefore, eucerine alone cannot affect wound healing process and can be surely used as the ointment base to produce N. sativa extract ointment, and therefore, the effect of the produced ointment can be exclusively attributed to N. sativa. Ashrafi et al. study confirms this argument.[8]
Comparison of healthy and diabetic untreated and phenytoin ointment (1%)-treated groups demonstrated a significant difference between similarly treated groups with more pleasant healing process in diabetic groups than in healthy ones. Abdollahzadeh Fard et al. have reported consistent findings. A mechanism proposed for diabetes-induced adverse effects on wound healing is that changes in the release time and expression levels of growth factors can affect normal process of wound healing.[39]
Comparison of healthy untreated and healthy phenytoin-treated groups in this study demonstrated that despite the fact that the wounds were healed more quickly in healthy phenytoin-treated group than in healthy untreated group, the difference was not found to be statistically significant. Mirnezami et al. study on the effects of phenytoin, estrogen, and silver sulfadiazine on wound healing demonstrated that phenytoin 1% decreased duration of healing compared to estrogen-treated and control groups yet with no significant difference. Therefore, the effects of these drugs can be considered to be virtually similar.[40] Shamseddini et al. found that phenytoin-treated male rats exhibited more pleasant effects in healing the wounds compared to the control group yet with no significant difference.[41]
These findings are in agreement with the present study. Lack of significant effect of phenytoin 1% ointment on wound healing can be explained by the fact that phenytoin exerts healing effects on chronic wounds while the studied wounds in the present study are acute, and therefore, phenytoin fails to affect their healing. This finding is consistent with Asadbegi et al. study.[42] Besides that, Asadbegi et al., however, argued that the 21-day treatment was not long enough to observe the effects of phenytoin ointment.[42] In contrast to this argument, in the present study, the treatment continued until the wounds were healed completely. Therefore, the length of treatment cannot be considered a reason for lack of significant effect of phenytoin compared to the untreated group in this study.
Density of inflammatory cells and fibroblasts as well as vascular density are other parameters that were investigated in this study. These parameters represent angiogenesis rate. Besides that, density of collagen fibers and their arrangements were studied in this study.
Wound healing is a complex process, which should be deliberately considered from histological perspective. Immediately after the induction of the wound, blood clotting is started by the production of thrombine. Subsequent natural subprocesses of wound healing can occur throughout three independent processes: inflammation, proliferation of connective tissue cells, and maturation.
In the first phase, some hours after incidence of the wound, inflammation phase cells (immune system multinuclear cells or neutrophils) migrate to the wound site to scavenge. It has been demonstrated that increased number of neutrophils leads to increased inflammation in the wound. Therefore, the smaller the number of neutrophils is, the more quickly wound is healed. Afterward, in the middle of the first phase, the presence of immune system multinuclear cells becomes less marked and the number of mononuclear immune system cells increases. The presence of inflammatory mononuclear cells is necessary for release of different cytokines that are effective in healing the wound and retrieval of fibroblasts and migration of them to the wound site. Moreover, in the late stages of the first phase of wound healing, as infectious agents and wound inflammation decrease, vascular regeneration is started for formation of epithelial tissue.[43] The histological examinations in this study indicated that N. sativa extract-treated groups had a very small number of inflammatory multinuclear cells (neutrophils). Therefore, inflammation rate of the extract-treated groups was lower than other groups (diabetic untreated and diabetic phenytoin-treated), and therefore, the extract-treated groups were found to have more rapid wound healing. This finding is consistent with Farahpor et al. study. According to Farahpor et al. study, antibacterial compounds in and anti-inflammatory property of Hypericum perforatum flower extract caused a significant decrease in inflammation severity and decrease in inflammatory multinuclear cells in the wounds treated with a combination ointment of H. perforatum and cotton oil.[36] Because N. sativa has antibacterial and anti-inflammatory properties, we can argue that these properties have caused decrease in inflammation and inflammatory cells in the present study (Aydin et al. 2015).
Throughout wound healing, as infectious agents and wound inflammation decrease, angiogenesis is started for formation of granulation tissue.[44] Examination of the tissue specimens indicated a significant difference in the number of vascular epithelium between N. sativa extract-treated and untreated groups such that this parameter was higher in N. sativa extract-treated group than in untreated group. Therefore, topical application of N. sativa can cause increase in angiogenesis or vascular regeneration. This is consistent with previous studies.[36]
The second stage of wound healing is the phase of reproduction or proliferation of connective tissue cells, which begins from the third day after incidence of the wound. Throughout this phase, angiogenesis, collagen deposition, epithelialization, and granulation tissue formation occur to restore the integrity of skin tissue. Naturally, throughout this phase, on the one hand, immune cells decrease in the wound site and on the other hand, fibroblasts migration to the wound site increases.[44]
In this study, comparison of the number of fibroblasts in tissue specimens of N. sativa extract-treated group with this parameter in untreated and phenytoin-treated groups indicated that topical application of N. sativa extract caused a significant increase in fibroblasts migration to the wound site. Farahpor et al. study reported that changes observed in maturation phase can be explained by antioxidant property of the pharmaceutical agent.[36] Because the antioxidant property of N. sativa has been confirmed, we can argue that this plant can help the second stage, proliferation stage, progress rapidly by exerting antioxidant property, and therefore the wound be completely healed within a shorter duration. In this regards, other plants with antioxidant activity (Sepahvand et al. 2016) may also have the same effects.
The third phase of wound healing is maturation. Throughout this phase, collagen synthesis increases and collagen bundles are further organized, granulation tissue evolves into scar tissue, other cells are eliminated by apoptosis, and wound area is further reduced.[44] According to comparison of the specimens, in the groups treated with hydroethanolic N. sativa extract ointment, collagen fibers had a more organized arrangement and epidermis diameter increased more markedly than other groups. Because microscopic examinations in this study were conducted in the late days of wound healing, it could be clearly observed that collagen fibers were organized and epidermis formed in them.
This study not only confirmed the findings of previous studies on N. sativa properties but also demonstrated that N. sativa extract could clinically and histologically cause mitigation of inflammation and acceleration of wound healing after it was topically applied on full-thickness skin wounds.
However, before N. sativa can be recommended for clinical use in diabetic patients, it is necessary to conduct prospective clinical trials to investigate its safety, efficacy, and possible mechanisms of action on wound healing.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors gratefully thank Ramtin Pakzad, the expert at plant physiology laboratory, and Samineh Noori at plant systematic laboratory. This article was financially supported by Bu-Ali Sina University (Grant No: 94-379).
References
- 1.Tatiana N, Michael R, Ira M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care.Adv. Skin Wound Care. 2012;25:304–14. doi: 10.1097/01.ASW.0000416006.55218.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loots MA, Lamme EN, Zeegelaar J, Mekkes JR, Bos JD, Middelkoop E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J Invest Dermatol. 1998;111:850–7. doi: 10.1046/j.1523-1747.1998.00381.x. [DOI] [PubMed] [Google Scholar]
- 3.Baradaran A. The role of biomarkers to detect progression of diseases. J Negat Results Clin Exp Stud. 2018;1:e05. [Google Scholar]
- 4.Amiri M. Type 2 diabetes mellitus; an international challenge. Ann Res Dial. 2016;1:e04. [Google Scholar]
- 5.Baharvand-Ahmadi B, Bahmani M, Tajeddini P, Naghdi N, Rafieian-Kopaei M. An ethno-medicinal study of medicinal plants used for the treatment of diabetes. J Nephropathol. 2016;5:44–50. doi: 10.15171/jnp.2016.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tavafi M. Biomarkers of diabetic nephropathy detection, today and future. J Ren Endocrinol. 2018;4:e07. [Google Scholar]
- 7.Ahmadi R, Ghasemi N. Effect of local application and injection of cinnamomum zeylanicum on burn wound improvement in diabetic and non-diabetic male rats. Med Sci. 2015;25:27–32. [Google Scholar]
- 8.Ashrafi A, Rezaei A, Sohrabi hagh doost I, Mohajeri D, Nejad Borhan M, Ashrafi I, et al. Histometric and histophatologic evaluation of the effects of equistum arvense herbal extract versus zinc oxide in rabbit skin wound healing model. J Vet Clin Pathol. 2010;4:843–53. [Google Scholar]
- 9.Hajian S. Positive effect of antioxidants on immune system. Immunopathol Persa. 2015;1:e02. [Google Scholar]
- 10.Kafeshani M. Ginger, micro-inflammation and kidney disease. J Ren Endocrinol. 2015;1:e04. [Google Scholar]
- 11.Baradaran A. Herbal antioxidant to ameliorate vascular biology. Angiol Persica Acta. 2017;2:e01. [Google Scholar]
- 12.Asgari A. Herbal medicines and kidney; friends or foes? J Nephropharmacol. 2014;3:5–6. [PMC free article] [PubMed] [Google Scholar]
- 13.Dehghan Shahreza F. From oxidative stress to endothelial cell dysfunction. J Prev Epidemiol. 2016;1:e04. [Google Scholar]
- 14.Hasanvand A, Mir S, Mohammadrezaei Khorramabadi R, Darabi S. Ameliorative effect of ferulic acid on gentamicin-induced nephrotoxicity in a rat model; role of antioxidant effects. J Renal Inj Prev. 2018;7:73–7. [Google Scholar]
- 15.Mohammadi A, Ahmadizadeh M. Effects of antioxidants on xenobiotics-induced nephrotoxicity. J Renal Inj Prev. 2018;7:56–7. [Google Scholar]
- 16.Jafari T. Antioxidants; helpful or harmful? Ann Res Antioxid. 2016;1:e13. [Google Scholar]
- 17.Nasri H. Herbal drugs and new concepts on its use. J Prev Epidemiol. 2016;1:e01. [Google Scholar]
- 18.Aftab A, Asif H, Mohd M, Shah AK, Abul KN, Nasir AS, et al. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac J Trop Biomed. 2013;3:337–52. doi: 10.1016/S2221-1691(13)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schleicher P, Saleh M. Vol. 90. Rochester, Vermont: Healing Arts Press; 1998. Black Seed Cumin: The Mafical Egyptian Herb for Allergies Asthma and Immune Disorders. [Google Scholar]
- 20.Marderosian A, Lawrence L, John A. 6th ed. U.S: Facts and Comparisons; 2011. Beutler, Review of Natural Products; pp. 789–93. [Google Scholar]
- 21.Noorbar E, Mirazi N. Study of Nigella sativa L.seed's hydroethanolic extract on skin wound healing in male diabetic rats. YUMSJ. 2017;22:419–30. [Google Scholar]
- 22.Mehri S, Shahi M, Razavi BM, Hassani FV, Hosseinzadeh H. Neuroprotective effect of thymoquinone in acrylamide-induced neurotoxicity in Wistar rats. Iran J Basic Med Sci. 2014;17:1007–11. [PMC free article] [PubMed] [Google Scholar]
- 23.Shoohani B, Hematti AA, Taheri Moghadam M. Effects of Scrop hularia Extract on Wound Healing in Rabbit. SJIUMS. 2010;17:9–16. [Google Scholar]
- 24.Babaei S, Darabi M, Bayat M, Nakhaei M, Bayat P, Baazm M, et al. Effect of pentoxifylline on biomechanical indices in acute phase of skin wound healing in diabetic rats. Arak Med Univ J (AMUJ) 2014;17:12–21. [Google Scholar]
- 25.Alahtavakoli M, Vazirinejad R, Ansari A, Negahban MS, Mashayekhi H, Nazari M, et al. Effect of Teucrium polium extract on skin wound healing in rat. Hormozgan Med Univ J. 2012:17–24. [Google Scholar]
- 26.Bancroft J, Marilyn G. England: Churchill Livingstone; 2008. Theory and Practice of Histological Techniques. [Google Scholar]
- 27.Bagheri S, Ahmadvand H, Mohammadrezaei Kkorramabadi R, Khosravi P. Amount of limonene in different parts of plants. Geriatr Persia. 2017;1:e03. [Google Scholar]
- 28.Baradaran A. Administration of herbal drugs in geriatric individuals; trends on its helps and hazards. Geriatr Persia. 2017;1:e01. [Google Scholar]
- 29.Dehghan Shahreza F. Oxidative stress, free radicals, kidney disease and plant antioxidants. Immunopathol Persa. 2017;3:e11. [Google Scholar]
- 30.Aydin MS, Kocarslan A, Kocarslan S, Kucuk A, Eser İ, Sezen H, et al. Thymoquinone protects end organs from abdominal aorta ischemia/reperfusion injury in a rat model. Rev Bras Cir Cardiovasc. 2015;30:77–83. doi: 10.5935/1678-9741.20140066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zareian P, Zahiri SH, Ketabchi F, Ruzmeh SH. Effect of local Tamarix monnifera on skin wound healing process in rabbit. J Mazand Univ Med Sci. 2007;17:48–57. [Google Scholar]
- 32.Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000;14:323–8. doi: 10.1002/1099-1573(200008)14:5<323::aid-ptr621>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 33.Khaksari M, Rezvani ME, Sajadi MA, Soleimani A. The effect of topically applied water extract of Rhazya stricta on cutaneous wound healing. J Semnan Univ Med Sci. 2000;1:1–10. [Google Scholar]
- 34.Hassanien MF, Assiri AM, Alzohairy AM, Oraby HF. Health-promoting value and food applications of black cumin essential oil: An overview. J Food Sci Technol. 2015;52:6136–42. doi: 10.1007/s13197-015-1785-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morsi N. M. Antimicrobial effect of crude extracts of Nigella sativa on multiple antibiotics-resistant bacteria. Acta Microbiol Polonica. 2000;49:63–74. [PubMed] [Google Scholar]
- 36.Farahpor M, Farhangi N, Neiriz M. Histopathological evaluation of the effect of Mentha piperita essential oil on cutaneous wound healing in rats infected with C. albicans. J Comp Pathobiol. 2015;5:1453–62. [Google Scholar]
- 37.Palaniappan K, Holley RA. Use of natural antimicrobials to increase antibiotic susceptibility of drug resistant bacteria. Int J Food Microbiol. 2010;140:164–8. doi: 10.1016/j.ijfoodmicro.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Mehvarz SH, Tahmasebi MH, Asgari AR. Effect of phenytoin powder on open wound healing process in rat skin. Kowsar Med J. 1998;3:177–82. [Google Scholar]
- 39.Abdollahzade Fard A, Zarifkar A, Dehghan GA, Ay J. Effects of systemic administration of estrogen on the process of wound healing in excisional wounds in diabetic rat. Urmia Med J. 2009;20:26–33. [Google Scholar]
- 40.Mirnezami M, Ebrahimi Fakhar H R, Rezaei K, Rahimi H. Comparing the healing effects of topical phenytoin, conjugated estrogen and silver sulfadiazine on skin wounds in male rats. KAUMS J Feyz. 2011;15:11–4. [Google Scholar]
- 41.Shamseddini S, Yavar Zadeh M, Shamseddinia Comparison of the healing effects of topical phenytoin, estrogen and silver sulfadiazine on skin wound in male rats. Iran J Dermatol. 2006;8:488–2. [Google Scholar]
- 42.Asadbegi M, Mirazi N, Vatanchian M. Comparative study of Lotus corniculatus L. hydroethanolic extract and phenytoin ointment effects on rat skin wound healing: Morphometrical and histopathological studies. J Cell Tissue. 2011;2:213–24. [Google Scholar]
- 43.Khodadadi S, Rafieian-Kopaei M. Herbs, health and hazards; a nephrology viewpoint on current concepts and new trends. Ann Res Antioxid. 2016;1:e05. [Google Scholar]
- 44.Beldon P. Basic science of wound healing. Surgery. 2010;28:409–12. [Google Scholar]


