Abstract
Background
Circular RNAs are important regulators in human cancers, including thyroid carcinoma. The circ_0067934 RNA is reported to participate in hepatocellular carcinoma, esophageal squamous cell carcinoma, and lung cancer. Whether it regulates thyroid carcinoma remains unclear. The purpose of this study was to research potential mechanisms of circ_0067934 in thyroid tumors to provide potential new diagnostic and treatment targets.
Material/Methods
The expression level of circ_0067934 in thyroid tumors, adjacent tissues, and cell lines was measured by qRT-PCR. The Kaplan-Meier survival curve analysis was used to explore the relationship between circ_0067934 level and survival time of patients. Circ_0067934 was knocked down to research its functional role in thyroid tumors. Cell proliferation was detected by CCK-8 (cell counting kit-8) assay. Migration and invasion were analyzed by Transwell assay. Western blot was applied to analyze the expression of epithelial-mesenchymal-transition (EMT) and PI3K/AKT related proteins.
Results
Compared with adjacent tissue, circ_0067934 was highly expressed in thyroid tumors. Circ_0067934 expression level was highly expressed in thyroid tumor cell lines. Patients with high expression of circ_0067934 showed lower survival rates. Knockdown of circ_0067934 inhibited cell proliferation, migration, and invasion and also promoted apoptosis. In addition, circ_0067934 knockdown inhibited EMT and PI3K/AKT signaling pathways.
Conclusions
circ_0067934 could improve the development of thyroid carcinoma by promoting EMT and PI3K/AKT signaling pathways.
MeSH Keywords: Phosphatidylinositol 3-Kinases; RNA, Untranslated; Thyroid Neoplasms
Background
Thyroid carcinoma is the common thyroid malignancy [1]. Thyroid cancer accounts for 1% of systemic malignancies [2]. Except for medullary carcinoma, most thyroid cancers originate from follicular epithelial cells [3]. In the early stages, patients may experience swallowing and breathing difficulties, hoarse voice, and there may be blockage when swallowing [4]. The current effective treatment is surgical resection combined with radiotherapy. However, according to a World Health Organization survey, the 5-year cure rate is about 57%, the mortality rate is 43%, and the 10-year survival rate of differentiated thyroid cancer is more than 90% [5]. Undifferentiated mortality is higher with a survival period of only 4 to 8 months [6]. Thus, there is an urgent need to investigate new diagnostic and treatment targets and related mechanisms.
Currently, circular RNAs (circRNAs) have been confirmed to be suitable molecular biomarkers for human cancers. Because of their closed structure, circRNAs have high stability and strong resistant to RNA degradative pathways [7]. Many reports have identified differentially expressed circRNAs in thyroid cancer [8,9]. Peng et al. bioinformatics analysis found that circRNA_100395/miR-141-3p/miR-200a-3p axis might participate in pathogenesis of papillary thyroid carcinoma tumors [10]. Similarly, circZFR participates in miR-1261 sponge and regulation of C8orf4, and then effects the invasion and proliferation of papillary thyroid cancer cells [11]. In addition, circRNA_NEK6 has been confirmed to target miR-370-30, participate in Wnt signaling pathway, and regulate invasion and proliferation of thyroid cancer [12]. In the past several years, circ_0067934 has been screened as a new target for various cancers, such as non-small cell lung cancer [13], esophageal squamous cell carcinoma [14], and hepatocellular carcinoma [15]. However, the molecular mechanism of circRNA in thyroid tumor has not yet been revealed.
In our study, in order to research the molecular mechanism of circ_0067934 in thyroid tumors, the expression of circ_0067934 was studies and related pathways and mechanism were explored. This study may identify new targets for the diagnosis and treatment of thyroid cancer.
Material and Methods
Clinical samples
A total of 57 thyroid cancer tissue samples and paracancer tissue samples were collected at Thyroid Department of our hospital between May 2014 and May 2017. All patients were diagnosed with thyroid cancer and underwent thyroidectomy. Data on the general condition of the patients included: age, gender, tumor size, lymph node metastasis, and American Joint Committee on Cancer (AJCC) stage. The study experiment was approved by the Ethics Committee of our hospital.
Cell lines and transfection
Human thyroid tumor cell lines (K1 and SW579) and a normal thyroid cell line (Nthy-ori3-1) were obtained from Shanghai Cell Biochemical Institute. The cells were cultured in DMEM (Invitrogen, CA, USA) with 10% fetal bovine serum (FBS), 1% penicillin (100 units/mL) and streptomycin (100 μg/mL) in a humidified incubator containing 5% CO2 at 37°C.
K1 and SW579 cells were cultured on 6-well plates, and transfected with siRNA and control by Lipofectamine 2000 (Invitrogen, CA, USA) based on the manufacturer’s instructions. Circ_0067934 siRNA plasmids were obtained from RiboBio Company. The success of the transfection was confirmed by quantitative real-time (qRT)-PCR. The siRNA sequences for hsa_circ_0067934 were as follows: sense 5′-UGUUGAUUGGGAUAUGUUAUU-3′; antisense: 5′-UAACAUAUCCCAAUCAACAUU-3′.
RNA isolation and qRT-PCR
Total mRNA was isolated from thyroid tumor tissue and cell lines by TRIzol RNA isolation kit (Qiagen, USA). cDNA was synthesized by reverse transcription system. SYBR green kit was used for qRT-PCR. An iQ5 Real-time PCR Detection System (Bio-Rad, Hercules, CA, USA) was used to detect the expression of circ_0067934. The conditions for the PCRs were as follows: 10-minute pre-denaturation at 50°C, 40 cycles of 95°C for 10 seconds, and at 60°C for 60 seconds. GAPDH was chosen as the control. The primers used were: circ_0067934, F: TAGCAGTTCCCCAATCCTTG, R: CACAAATTCCCATCATTCCC; GAPDH, F: GAAGGTGAAGGTCGGAGTC, R: GAAGATGGTGATGGGATTTC.
Cell viability assay
The transfected cells were seeded into 96-well plates. After cultured for 24, 48, 72, and 96 hours, the cell numbers were detected. Cell counting kit-8 was used to detect the cell proliferation according to the operation manual. The absorbance selected at 490 nm.
Apoptosis assay by flow cytometer
At 48 hours after transfection, the cells were collected and stained with Annexin V-FITC and PI. Then, flow cytometer (Becton Dickinson, USA) was used to evaluate the apoptosis of transfected cells. The data were analyzed by cell quest software version 3.3.
Migration and invasion assay
For the migration assay, the transfected cells was collected, and 4×104 cells were applied to the upper chamber of the plates. The medium in upper chamber was without FBS, while the medium in upper chamber was with 10% FBS. Then, the plates were cultured for 24 hours.
For the invasion assay, the steps were similarly as used for the migration assay. In addition, 200 mg/mL Matrigel was coated on the plate inserts.
After 48 hours of transfection, the cells in the lower chamber were stained using 0.1% crystal violet. A phase contrast microscope was used to photograph and record the cell staining.
Western blot
The expression of N-cadherin, E-cadherin, p-AKT, and total-AKT was detected by western blot. The transfected cells were washed with pre-cooled phosphate buffered saline (PBS) 3 times. The whole protein lysate was added, and the protein was collected by lysis on ice for 10 minutes. The aspirated fractions were placed in a 0.5 mL centrifuge tube and stored at −70°C. After extracting the protein by bicinchoninic acid assay (BCA), 50 μg was used in the 100 V lane. Then, the protein was transferred to the PVDF membrane at 90 V for 90 minutes. 5% TBS milk was used for blocking at room temperature for 1 to 2 hours. N-cadherin, E-cadherin, p-AKT, and total-AKT antibodies (Cell Signaling, Boston, USA) were incubated at 1: 1000 at 4°C overnight and GAPDH was used as an internal reference. The TBST solution was used to wash the membrane for 10 minutes, 3 times. Then, the nitrocellulose membrane was incubated with horseradish peroxidase-labeled goat anti-mouse (1: 5000) for 1 hour at room temperature, and then washed twice with TBST solution for 10 minutes each time. DAB was used for dyeing. The experiments were repeated 3 times.
Statistical analysis
SPSS statistics 22 (IBM Corp, Armonk USA) was performed for statistical analysis. Student’s t-test was used to calculate the differences between 2 groups. The Kaplan-Meier method was used to estimate circ_0067934 and survival period of thyroid carcinoma patients. The threshold of significance was P<0.05.
Results
General condition of involved patients
As shown in Table 1, all involved patients were divided into low and high expression of circ_0067934 groups. The age and gender ratio between the 2 groups showed no significant difference. The patients in the high expression group had higher tumor size, stronger lymph node metastasis, and higher AJCC stage (P<0.05).
Table 1.
Association between circ_0067934 level and clinicopathologic characteristics in the validated cohort.
| Characteristics | Low (n=29) | High (n=28) | P-value |
|---|---|---|---|
| Age | 0.289 | ||
| ≤45 | 19 | 14 | |
| >45 | 10 | 14 | |
| Gender | 0.167 | ||
| Female | 22 | 16 | |
| Male | 7 | 12 | |
| Tumor size | 0.016 | ||
| ≤10 mm | 17 | 7 | |
| >10 mm | 12 | 21 | |
| Lymph node metastasis | 0.035 | ||
| Yes | 10 | 18 | |
| No | 19 | 10 | |
| AJCC stage | 0.031 | ||
| I+II | 22 | 13 | |
| III+IV | 7 | 15 |
Chi-square test for significant difference.
Circ_0067934 was upregulated in thyroid tumor tissues and cells
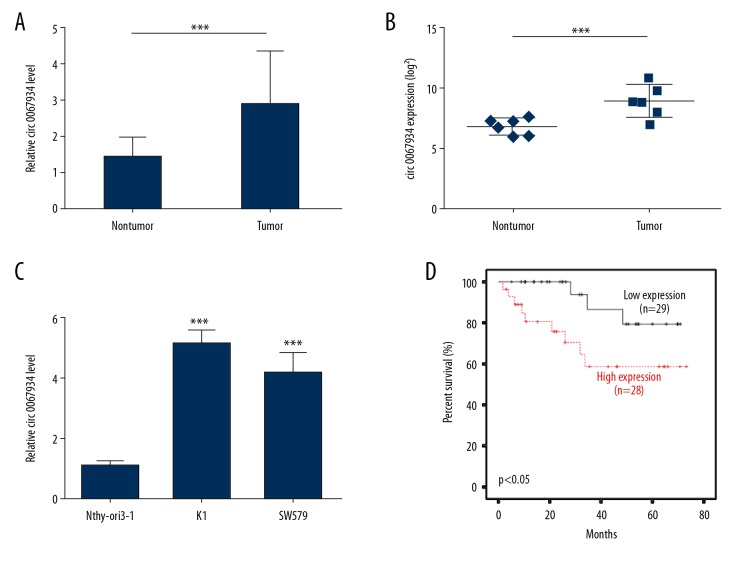
The expression of circ_0067934 in 57 thyroid tumor tissues and matched adjacent tissues were detected by qRT-PCR. The results showed that the expression level of circ_0067934 was significantly higher in thyroid tumor tissues and cell lines than normal (P<0.01, Figure 1A). The upregulated expression of circ_0067934 in thyroid tumor tissues was also observed in Peng’s dataset (GSE93522) (Figure 1B). Moreover, its expression in Nthy-ori3-1, K1, and SW579 were also detected. The circ_0067934 level was increased in these thyroid cancer cell lines (Figure 1C). In addition, patients with high expression of circ_0067934 showed lower survival rates (Figure 1D). Importantly, Cox proportional hazards regression model analysis also indicated that circ_0067934 was an independent risk factor for prognosis (Table 2). The results indicated that high level of circ_0067934 expression might participate in thyroid tumor development and affect the survival period of patients.
Figure 1.
Circ_0067934 upregulates in thyroid tumor tissues and cells. (A) Expression level of circ_0067934 in thyroid tumor and para-carcinoma tissues detected by qRT-PCR. (B) The expression level of circ_0067934 according to Peng’s dataset (GSE93522). (C) Expression level of circ_0067934 in in thyroid tumor and normal cell lines detected by qRT-PCR. (D) Survival curve analysis by Kaplan-Meier method. ** P<0.01 and *** P<0.001.
Table 2.
Cox proportional hazards regression model analysis of risk factors for poor prognosis of OS in thyroid cancer.
| Variables | RR | 95% CI | P-value |
|---|---|---|---|
| circ_0067934 expression | 4.385 | 1.087–17.544 | 0.038 |
| Age | 2.660 | 0.777–9.091 | 0.119 |
| Lymph node metastasis | 2.618 | 0.759–9.010 | 0.128 |
RR – relative risk; 95% CI – 95% confidence interval.
Circ_0067934 knockdown inhibited proliferation and promoted apoptosis in thyroid tumor cells
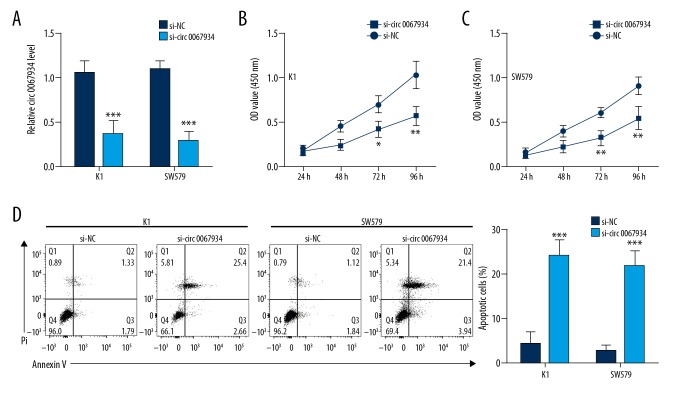
In order to explore the effects of circ_0067934 in cell growth and apoptosis of thyroid tumor cells, CCK8 and flow cytometry were undertaken. As shown in Figure 2A, the expression of circ_0067934 was significantly downregulated by siRNA-circ_0067934 transfection, which indicated the transfection was successful. After transfection, the cell growth in K1 and SW579 cell lines was significantly reduced (Figure 2B, 2C), while apoptosis was increased (P<0.01, Figure 2D).
Figure 2.
Circ_0067934 knockdown inhibits proliferation and promotes apoptosis in thyroid tumor cells. (A) Analysis of circ_0067934 expression level in K1 and SW579 cells after transfection by qRT-PCR. (B, C) Cell counting kit-8 assay indicated that circ_0067934 silencing inhibited proliferation. (D) Flow cytometry assay indicated that circ_0067934 silencing promoted apoptosis. * P<0.05, ** P<0.01 and *** P<0.001.
Circ_0067934 knockdown inhibited migration and invasion in thyroid tumor cells
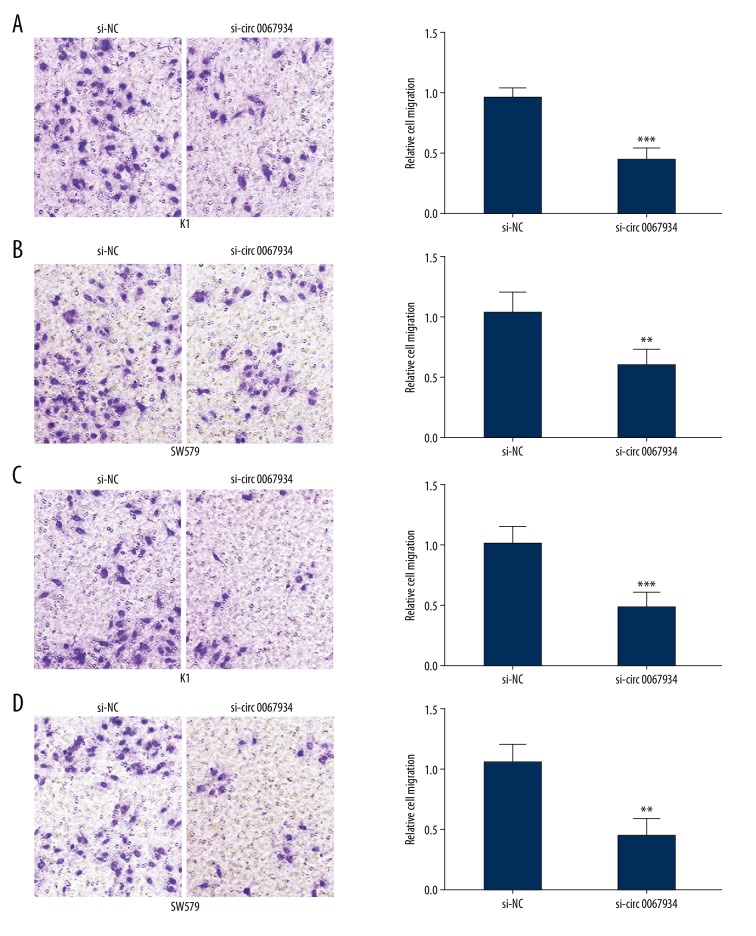
Migration and invasion assays were used to research the mechanism of circ_0067934 in K1 and SW579 cell lines. Similar to the trend for proliferation, the migration and invasion ability of K1 and SW579 cell lines were significantly decreased after siRNA transfection (Figure 3A–3D). The results confirmed that circ_0067934 knockdown induced lower migration and invasion of thyroid tumor cells.
Figure 3.
Circ_0067934 knockdown inhibits migration and invasion in thyroid tumor cells. (A, B) Transwell migration assay in K1 and SW579 cell lines indicated that circ_0067934 silencing inhibited migration. (C, D) Transwell invasion assay in K1 and SW579 cell lines indicated that circ_0067934 silencing inhibited invasion. ** P<0.01 and *** P<0.001
Circ_0067934 knockdown inhibited EMT and PI3K/AKT signaling pathways
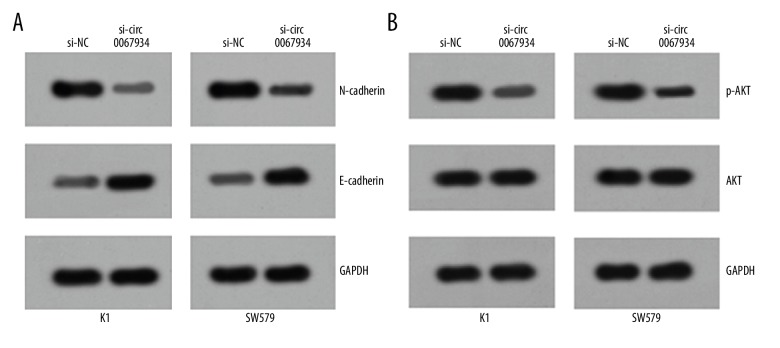
The aforementioned results confirmed that circ_0067934 was a critical factor for proliferation, migration, invasion, and apoptosis of thyroid tumor cells. In order to explore potential molecular mechanism, related pathways were explored. As shown in Figure 4A, 4B, the expression of N-cadherin and p-AKT were significantly lower, while the expression of E-cadherin was significantly upregulated. EMT (epithelial-mesenchymal-transition) and PI3K/AKT signaling pathways were both important for tumor development. Thereby, the result confirmed that circ_0067934 could affect EMT and PI3K/AKT signaling pathways in thyroid tumor progression.
Figure 4.
Circ_0067934 knockdown inhibits EMT and PI3K/AKT signaling pathway. (A) Western blot assay showed that circ_0067934 silencing inhibited EMT. (B) Western blot assay showed that circ_0067934 silencing suppressed PI3K/AKT signaling pathways in tumor cell lines.
Discussion
In this study, circ_0067934 was found to be highly expressed in thyroid tumors than adjacent tissue, and also highly expressed in thyroid tumor cell lines. Patients with high expression of circ_0067934 showed lower survival period. Interestingly, downregulation of circ_0067934 inhibited EMT signaling pathways and PI3K/AKT signaling pathways. More importantly, circ_0067934 knockdown inhibited proliferation, migration, and invasion, and also promoted apoptosis.
In previous studies, circ_0067934 has been confirmed to be a critical circRNA in various cancers. In esophageal squamous cell carcinoma, circ_0067934 was upregulated, and also participated in cell cycle, proliferation, and migration [14]. In addition, circ_0067934 was found to regulate miR-1324/FZD5/Wnt/β-catenin axis, and further promote metastasis and growth of hepatocellular cancer [15]. In non-small cell lung cancer (NSCLC), circ_0067934 could regulate the process of epithelial-to-mesenchymal transition, and then suppress proliferation, migration, and invasion of NSCLC cells [16]. In this study, circ_0067934 knockdown inhibited EMT and PI3K/AKT signaling pathways.
EMT have been confirmed to participate in various biological processes, such as wounding healing, embryonic development, and cancer progression [17]. As shown in previous studies, EMT was found to participate in ATCs development and local invasion of thyroid cancer [18]. More importantly, it was closely related to tumor treatment and recurrence [19]. EMT related proteins, such as p53, affected invasion and metastatic movement, and also maintained transcriptional programs [20]. In addition, the EMT and PI3K/AKT pathways could be involved in thyroid tumor development. Han et al. [21] confirmed that mammalian study it could inhibit mTOR signaling pathways, then decrease the expression of c-Myc and Cyclin D1, and further inhibit growth of thyroid cancer cells. More importantly, HPIP could activate PI3K/AKT signaling pathway, and further improve growth, migration, and EMT of thyroid cancer cells [22]. Similarly, upregulated ING5 could decrease HGF-induced EMT, invasion, and proliferation by regulating Met/PI3K/Akt signaling pathway in development of thyroid cancer [23].
Interestingly, various genetic alterations in PI3K/AKT signaling pathway was abnormally regulated in numerous diseases, including breast cancer, lung cancer, and thyroid cancer. Liu et al. [24] confirmed that genetic alterations, such as PIK3 mutations, was detected in thyroid carcinoma. Perifosine, a genetic-based target for PI3K/AKT signaling pathway, was shown to be a promising treatment strategy for thyroid cancers [23]. Similarly, combretastatin A4 could decrease the expression of PI3K/AKT signaling pathway related genes, such as Vimentin, Twist1, and ZEB1, and then inhibit migration and proliferation and promote apoptosis of thyroid cancer cells [25]. Besides, leptin could regulate PI3K/AKT signaling pathway, and improve the migration of papillary thyroid cancer [26].
There were some limitations in our study. Because of limitation at our hospital, the number of study patients was relatively small. In addition, downstream pathways of EMT and PI3K/AKT signaling pathway have not been researched. Thereby, more patients will be involved in further study. Additionally, how circ_0067934 affects the PI3K/AKT signaling pathway remains unclear in our study. Previously, a study showed that circ_0067934 serves as a sponge for miRNA in hepatocellular carcinoma [15]. Thus, circ_0067934 might be utilized by some miRNAs to regulate PI3K/AKT signaling pathway; this will require further investigation in the future.
Conclusions
In conclusion, circRNA circ_0067934 promoted development of thyroid carcinoma by regulating EMT and PI3K/AKT signaling pathways. Circ_0067934 might be a new target for thyroid tumors treatment. This study might provide a theoretical basis for the prevention and treatment of thyroid cancer.
Footnotes
Source of support: Departmental sources
Conflict of interests
None.
References
- 1.Hughes DT, Haymart MR, Miller BS, et al. The most commonly occurring papillary thyroid cancer in the United States is now a microcarcinoma in a patient older than 45 years. Thyroid. 2011;21:231–36. doi: 10.1089/thy.2010.0137. [DOI] [PubMed] [Google Scholar]
- 2.Gong L, Chen P, Liu X, et al. Expressions of D2-40, CK19, galectin-3, VEGF and EGFR in papillary thyroid carcinoma. Gland Surg. 2012;1:25–32. doi: 10.3978/j.issn.2227-684X.2012.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlumberger MJ. Papillary and follicul thyroid carcinoma. N Engl J Med. 1998;338:297–306. doi: 10.1056/NEJM199801293380506. [DOI] [PubMed] [Google Scholar]
- 4.Wei L. [Discussion on operation modes to thyroid tiny papillary carcinoma (with clinical analysis of 176 cases)]. Journal of Zhejiang Chinese Medical University. 2013 [in Chinese] [Google Scholar]
- 5.Nagahara K, Iwai H, Konishi J, Takahashi K. Usefulness of needle biopsy in diagnosis of thyroid disease. Practotol. 1976;69:860–65. [Google Scholar]
- 6.De FV, Guarino V, Avilla E, et al. Biological role and potential therapeutic targeting of the chemokine receptor CXCR4 in undifferentiated thyroid cancer. Cancer Res. 2007;67:11821–29. doi: 10.1158/0008-5472.CAN-07-0899. [DOI] [PubMed] [Google Scholar]
- 7.Cai D, Liu Z, Kong G. Molecular and bioinformatics analyses identify 7 circular RNAs involved in regulation of oncogenic transformation and cell proliferation in human bladder cancer. Med Sci Monit. 2018;24:1654–61. doi: 10.12659/MSM.908837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teng H, Mao F, Liang J, et al. Transcriptomic signature associated with carcinogenesis and aggressiveness of papillary thyroid carcinoma. Theranostics. 2018;8:4345–58. doi: 10.7150/thno.26862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lan X, Xu J, Chen C, et al. The landscape of circular RNA expression profiles in papillary thyroid carcinoma based on RNA sequencing. Cell Physiol Biochem. 2018;47:1122–32. doi: 10.1159/000490188. [DOI] [PubMed] [Google Scholar]
- 10.Peng N, Shi L, Zhang Q, et al. Microarray profiling of circular RNAs in human papillary thyroid carcinoma. PLoS One. 2017;12:e0170287. doi: 10.1371/journal.pone.0170287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei H, Pan L, Tao D, Li R. Circular RNA circZFR contributes to papillary thyroid cancer cell proliferation and invasion by sponging miR-1261 and facilitating C8orf4 expression. Biochem Biophys Res Commun. 2018;503(1):56–61. doi: 10.1016/j.bbrc.2018.05.174. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Chen Z, Yu S, et al. Long noncoding RNA LINC01234 functions as a competing endogenous RNA to regulate CBFB expression by sponging miR-204-5p in gastric cancer. Clin Cancer Res. 2018;24(8):2002–14. doi: 10.1158/1078-0432.CCR-17-2376. [DOI] [PubMed] [Google Scholar]
- 13.Zou Q, Wang T, Li B, et al. Overexpression of circ-0067934 is associated with increased cellular proliferation and the prognosis of non-small cell lung cancer. Oncol Lett. 2018;16(5):5551–56. doi: 10.3892/ol.2018.9357. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia W, Qiu M, Rui C, et al. Circular RNA has_circ_0067934 is upregulated in esophageal squamous cell carcinoma and promoted proliferation. Sci Rep. 2016;6:35576. doi: 10.1038/srep35576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Q, Lu G, Luo Z, et al. CircRNA circ_0067934 promotes tumor growth and metastasis in hepatocellular carcinoma through regulation of miR-1324/FZD5/Wnt/β-catenin axis. Biochem Biophys Res Commun. 2018;497(2):626–32. doi: 10.1016/j.bbrc.2018.02.119. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Li H. CircRNA circ_0067934 silencing inhibits the proliferation, migration and invasion of NSCLC cells and correlates with unfavorable prognosis in NSCLC. Eur Rev Med Pharmacol Sci. 2018;22:3053–60. doi: 10.26355/eurrev_201805_15063. [DOI] [PubMed] [Google Scholar]
- 17.Richards EJ, Zhang G, Li ZP, et al. Long non-coding RNAs (LncRNA) regulated by transforming growth factor (TGF) β, lncRNA-hit-mediated TGF-induced epithelial to mesenchymal transition in mammary epithelia. J Biol Chem. 2015;290:6857–67. doi: 10.1074/jbc.M114.610915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braun J, Hoang VCH, Huttelmaier S. Downregulation of microRNAs directs the EMT and invasive potential of anaplastic thyroid carcinomas. Oncogene. 2010;29:4237–44. doi: 10.1038/onc.2010.169. [DOI] [PubMed] [Google Scholar]
- 19.Qin W, Kang P, Xu Y, et al. Long non-coding RNA HOTAIR promotes tumorigenesis and forecasts a poor prognosis in cholangiocarcinoma. Sci Rep. 2018;8(1):12176. doi: 10.1038/s41598-018-29737-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen H, Takahara M, Xie L, et al. Levels of the EMT-related protein snail/slug are not correlated with p53/p63 in cutaneous squamouscell carcinoma. J Cutan Pathol. 2013;40:651–56. doi: 10.1111/cup.12142. [DOI] [PubMed] [Google Scholar]
- 21.Han B, Cui H, Kang L, et al. Metformin inhibits thyroid cancer cell growth, migration, and EMT through the mTOR pathway. Tumor Biol. 2015;36:1–10. doi: 10.1007/s13277-015-3315-4. [DOI] [PubMed] [Google Scholar]
- 22.Wang SC, Chai DS, Chen CB, et al. HPIP promotes thyroid cancer cell growth, migration and EMT through activating PI3K/AKT signaling pathway. Biomed Pharmacother. 2015;75:33–39. doi: 10.1016/j.biopha.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 23.Lei G, Xu M, Xu Z, et al. Overexpression of ING5 inhibits HGF-induced proliferation, invasion and EMT in thyroid cancer cells regulation of the c-Met/PI3K/Akt signaling pathway. Biomed Pharmchother. 2018;98:265–70. doi: 10.1016/j.biopha.2017.12.045. [DOI] [PubMed] [Google Scholar]
- 24.Liu D, Hou P, Liu Z, et al. Genetic alterations in the PI3K/Akt signaling pathway confer sensitivity of thyroid cancer cells to therapeutic targeting of Akt and mTOR. Cancer Res. 2009;69:7311. doi: 10.1158/0008-5472.CAN-09-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang W, Lai Y, Zhu M, et al. Combretastatin A4 regulates proliferation, migration, invasion, and apoptosis of thyroid cancer cells via PI3k/aAKT signaling pathway. Med Sci Monit. 2016;22:4911–17. doi: 10.12659/MSM.898545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng SP, Yin PH, Hsu YC, et al. Leptin enhances migration of human papillary thyroid cancer cells through the PI3K/AKT and MEK/ERK signaling pathways. Oncol Rep. 2011;26:1265. doi: 10.3892/or.2011.1388. [DOI] [PubMed] [Google Scholar]