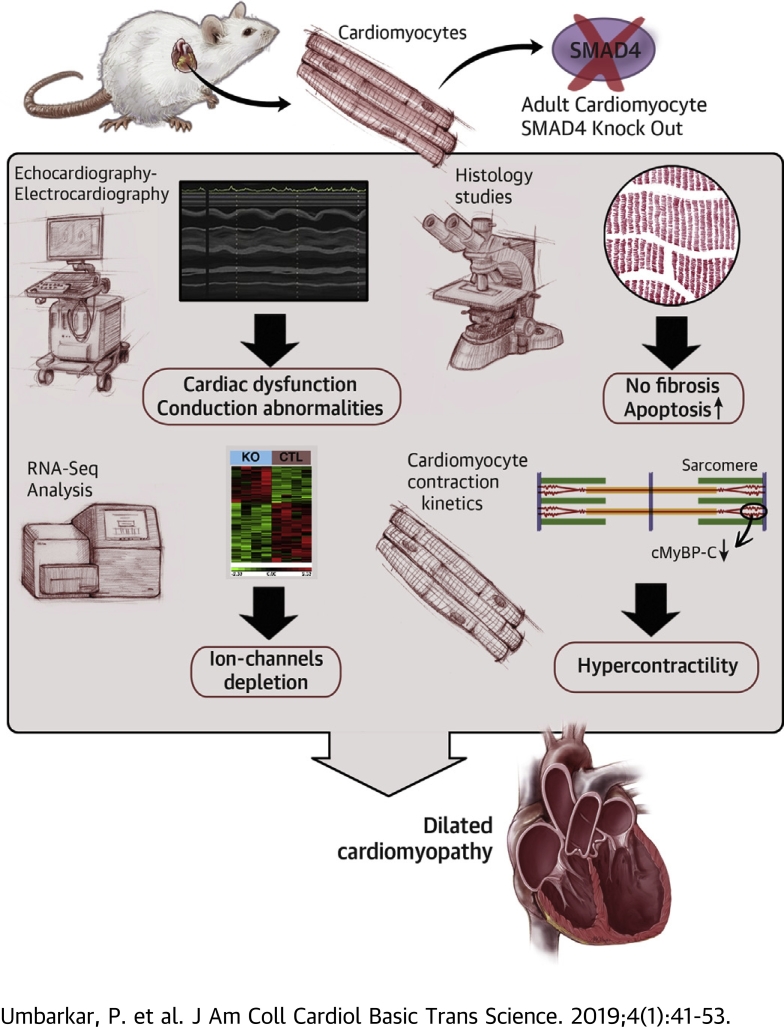
Visual Abstract
Key Words: cardiomyocyte, cardiomyopathy, fibrosis, heart failure, SMAD4, TGF-β
Abbreviations and Acronyms: CM, cardiomyocyte; cMyBP-C, cardiac myosin-binding protein C; CSA, cross-sectional area; CTL, control; DCM, dilated cardiomyopathy; KO, knockout; LV, left ventricle/ventricular; MAPK, mitogen-activated protein kinase; MCM, MerCreMer; PI3K, phosphoinositide-3 kinase; RNA-Seq, RNA sequencing; TAK1, transforming growth factor beta–activated kinase 1; TAM, tamoxifen; TGF, transforming growth factor
Highlights
-
•
SMAD4 is the central intracellular mediator of TGF-β pathway.
-
•
CM-specific loss of SMAD4 causes cardiac dysfunction independent of fibrotic remodeling.
-
•
Deletion CM-SMAD4 affects CM survival.
-
•
CM-SMAD4 loss leads to down-regulation of several ion channels’ genes, resulting in cardiac conduction abnormalities.
-
•
CM-SMAD4 deletion alters sarcomere shortening kinetics, in parallel with reduction in cardiac myosin-binding protein C levels.
-
•
These results demonstrate a fundamental role for CM-SMAD4–dependent TGF-β signaling in adult heart homeostasis.
Summary
The role of the transforming growth factor (TGF)-β pathway in myocardial fibrosis is well recognized. However, the precise role of this signaling axis in cardiomyocyte (CM) biology is not defined. In TGF-β signaling, SMAD4 acts as the central intracellular mediator. To investigate the role of TGF-β signaling in CM biology, the authors deleted SMAD4 in adult mouse CMs. We demonstrate that CM-SMAD4–dependent TGF-β signaling is critical for maintaining cardiac function, sarcomere kinetics, ion-channel gene expression, and cardiomyocyte survival. Thus, our findings raise a significant concern regarding the therapeutic approaches that rely on systemic inhibition of the TGF-β pathway for the management of myocardial fibrosis.
Heart failure is a major health problem worldwide. At present, approximately 6.5 million people in the United States experience heart failure, and this number is expected to grow to more than 8 million by 2030 1, 2. Heart failure has increased spending on health care services, resulting in an economic burden of about $31 billion/year (2). Although diagnosis and treatments have improved, a poor survival rate in heart failure patients suggests a critical need for research in cardiac pathophysiology.
Transforming growth factor (TGF)-β are cytokines that play pleiotropic roles in a wide range of cellular processes during embryonic development, tissue homeostasis, and disease 3, 4. The TGF-β superfamily consists of more than 30 ligands that belong to subfamilies like the TGF-β, bone morphogenetic proteins, activins, inhibins, growth differentiation factors, and Nodal and anti-Müllerian hormones. In canonical TGF-β signaling, TGF-β ligands bind to specific Ser/Thr kinase receptors (type I and type II), which mediate activation of receptor-specific SMADs. Activated receptor-specific SMADs form heteromeric complexes with a common mediator-SMAD, SMAD4. This SMAD complex translocates into the nucleus, where it regulates transcription of various genes via other transcriptional cofactors 5, 6. In addition to SMAD-dependent canonical signaling, TGF-β can regulate the activity of other signaling pathways, such as TGF-β-activated kinase 1 (TAK1), mitogen-activated protein kinases (MAPKs), phosphoinositide-3 kinase (PI3K), Ras homolog family member A, protein phosphatase 2A, and nuclear factor κB 7, 8, 9, 10, 11, 12, 13. Collectively, these are referred to as noncanonical or SMAD-independent pathways. The extent of interactions among these canonical and noncanonical signaling molecules varies considerably depending on the context, but are essential to deliver spatially and temporally specific TGF-β signaling outputs (14).
TGF-βs are often chronically overexpressed in diseased hearts and are instrumental in eliciting multiple, even opposing cellular responses 15, 16, 17, 18, 19, 20. For instance, excessive TGF-β signaling contributes to cardiac dysfunction in muscular dystrophy, whereas blockade of TGF-β signaling using neutralizing Abs (N-Abs) increases left ventricular (LV) dilatation and mortality after myocardial infarction 21, 22. Moreover, disruption of cardiomyocyte (CM) TGF-β signaling by deletion of the TGF-β receptor mitigates pressure overload–induced maladaptive remodeling via noncanonical TAK1 signaling (23). An elegant study by Kong et al. (24) demonstrated that TGF-β-SMAD3 signaling activation in cardiac fibroblasts is required for cardiac repair following myocardial infarction. Despite studies suggesting the importance of TGF-β signaling to cardiac pathophysiology, the exact role of CM canonical TGF-β signaling in cardiac homeostasis has remained enigmatic.
In the present study, we examined the role of CM canonical TGF-β signaling in maintaining adult heart homeostasis. As SMAD4 is a central intracellular mediator of canonical TGF-β signaling, we examined the effects of CM-specific SMAD4 deletion on cardiac function. A previous study has shown that CM-specific SMAD4 deletion results in developmental cardiac defects and embryonic lethality (25). Hence, we designed a model in which SMAD4 is conditionally deleted in adult mouse CMs using tamoxifen (TAM)-inducible alpha-myosin heavy chain promoter–driven Cre recombinase. We report that CM-specific knockdown of SMAD4 significantly alters the cardiac contractile function at cellular as well as organ level. Moreover CM-specific SMAD4 deficiency is associated with a reduction in gene expression of several ion channels and cardiac conduction abnormalities. These findings provide the first evidence that endogenous CM canonical TGF-β signaling is essential for maintaining adult heart homeostasis.
Methods
A detailed description of the Methods is provided in the Supplemental Appendix.
Mice
To achieve conditional deletion of SMAD4 specifically in CM, SMAD4fl/fl mice (Stock No. 017462, The Jackson Laboratory, Bar Harbor, Maine) were crossed with mice carrying the MerCreMer (MCM) transgene driven by the alpha-myosin heavy chain promoter (Myh6-Cre+/+) (Stock No. 005657, The Jackson Laboratory). Further details of generation and characterization of the CM-specific SMAD4 knockout (KO) mice are described in the Results section. The Institutional Animal Care and Use Committee of Vanderbilt University Medical Center approved all animal procedures and treatments used in this study (protocol #M1700133-00).
Statistical analysis
Analyses were performed using GraphPad Prism (version 7.02, GraphPad Software, La Jolla, California). Differences between 2 data groups (SMAD4 protein expression, ion-channel gene expression, morphometric, and electrocardiography parameters) were evaluated for significance by the Mann-Whitney test. For comparisons of more than 2 groups (echocardiographic and CM-contractility parameters, protein expression studies), analysis of variance followed by Tukey’s multiple comparison test was applied. All data are expressed as mean ± SEM. For all tests, a p value <0.05 was considered statistically significant.
Results
Generation and characterization of CM-specific SMAD4 KO mice
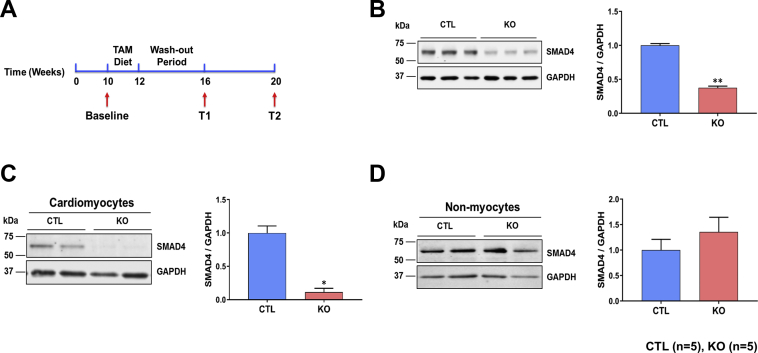
To investigate the role of CM TGF-β signaling in the adult heart, we generated a mouse model in which SMAD4 is conditionally deleted in CM-specific manner. SMAD4fl/fl mice were crossed with Myh6-Cre+/+ mice to generate SMAD4fl/flCre+/–. Also, Myh6-Cre+/+ transgenic mice were crossed with C57BL/6 mice (Stock No. 000664, The Jackson Laboratory) to generate Myh6-Cre+/–. A well-established TAM chow diet protocol was used to induce the CM-specific expression of Cre recombinase, as we previously reported 26, 27. Briefly, at 10 weeks of age, when physiological development is largely complete, all male mice were placed on a TAM chow diet (400 mg/kg, TD.130859, Harlan Sprague-Dawley, Indianapolis, Indiana) for 2 weeks followed by regular chow for an additional 4 weeks (to allow the clearance of TAM from the mice) (Figure 1A). SMAD4fl/fl/Cre+/−/TAM mice were the conditional KO mice, whereas littermates SMAD4fl/fl/Cre–/–/TAM represented control (CTL) animals. Myh6-Cre+/–/TAM mice were used as no-flox Cre CTL animals (MCM). There were no differences in mortality between CTL and SMAD4 KO mice up to the termination of the study (i.e., 8 weeks after TAM treatment). Western blot analysis of LV tissue lysates confirmed that TAM treatment led to a ∼60% reduction in SMAD4 protein levels in KO (Figure 1B). To confirm CM-specific SMAD4 deletion, Western blotting was performed with protein extracted from isolated CMs and nonmyocytes. SMAD4 protein levels were significantly low (∼90% deletion) in CMs, as expected, nonmyocytes displayed no change (Figures 1C and 1D).
Figure 1.
Generation and Characterization of CM-Specific SMAD4 KO Mice
(A) Experimental design showing 10-week-old mice were fed a tamoxifen (TAM) diet for 2 weeks followed by a 4-week wash-out period. T1 and T2 represent time points selected for studies. (B) Representative Western blot and quantification of SMAD4 expression in control (CTL) and knockout (KO) hearts at 4 weeks after TAM treatment. (C) Representative Western blot quantification of SMAD4 expression in cardiomyocytes (CMs) isolated from CTL and KO hearts at 4 weeks after TAM treatment. (D) Representative Western blot quantification of SMAD4 expression in non-CMs isolated from CTL and KO hearts at 4 weeks after TAM treatment. *p = 0.0286, **p = 0.0079. All p values are versus CTL. GAPDH = glyceraldehyde 3-phosphate dehydrogenase.
CM-specific deletion of SMAD4 causes dilated cardiomyopathy
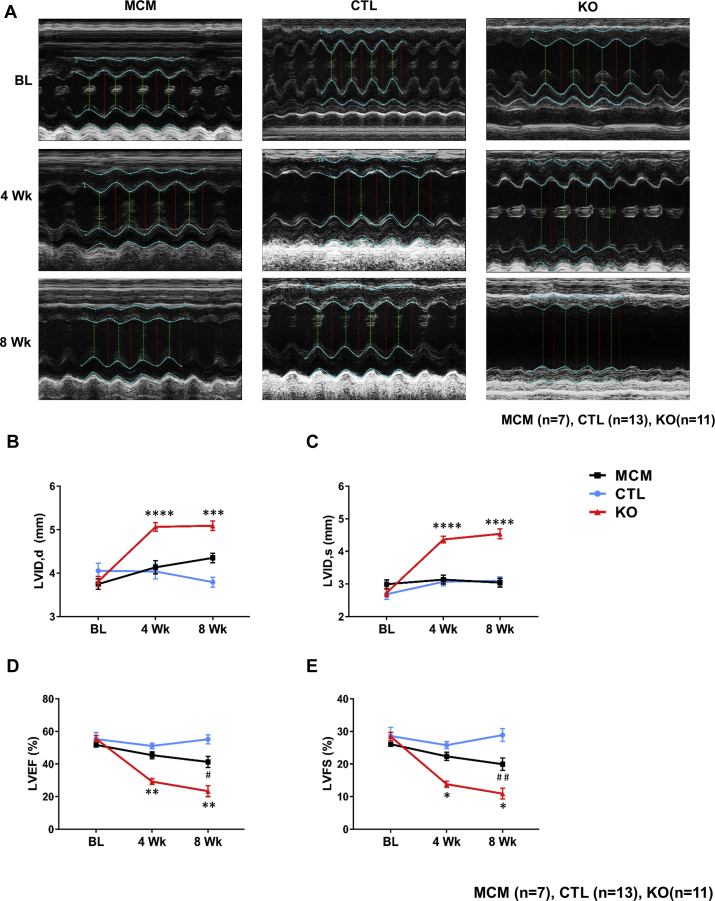
We performed serial M-mode echocardiography to assess the effect of CM-SMAD4 deletion on cardiac function. Importantly, to account for any confounding effects of Cre overexpression or TAM on cardiac function, these critical controls were also included in the study (Figure 2A, Supplemental Table 1). At baseline, MCM, CTL, and SMAD4 KO hearts had comparable chamber dimensions and ventricular function. At 4 weeks after TAM treatment, SMAD4 KO animals had a substantial increase in LV internal dimension in diastole and systole in comparison with CTL animals, indicating dilative remodeling of the LV (Figures 2B and 2C). These changes were associated with marked LV dysfunction as reflected by a significant decline in LV ejection fraction and LV fractional shortening (Figures 2D and 2E). At 8 weeks after TAM treatment, MCM displayed a modest but significant reduction in LV ejection fraction and LV fractional shortening as compared with the CTL group. Considering the fact that SMAD4 KO mice had a significant decline in cardiac function parameters as compared with both the CTL and MCM groups, our findings strongly indicate that cardiac dysfunction developed in SMAD4 KO animals is the specific effect of deletion of CM-canonical TGF-β signaling.
Figure 2.
CM-Specific Deletion of SMAD4 Causes Cardiac Dysfunction
CTL and SMAD4 KO mice underwent baseline transthoracic echocardiographic examination and then were subjected to a TAM treatment for 2 weeks. After a 4-week wash-out period, mice were then followed with serial echocardiography assessment at the time points shown. (A) Representative images of serial echocardiographic measurements. (B) Left ventricular internal dimension in diastole (LVID,d). ****p < 0.0001 versus MerCreMer (MCM) group, ***p = 0.0008 versus MCM group. (C) LVID in systole (LVID,s). ****p < 0.0001 versus MCM. (D) LV ejection fraction (LVEF). **p = 0.0035 versus MCM group (time = 4 weeks), **p = 0.0014 versus MCM group, #p = 0.0161 versus CTL group (time = 8 weeks). (E) LV fractional shortening (LVFS). *p = 0.0220 versus MCM group (time = 4 weeks), *p = 0.0184 versus MCM group, ##p = 0.0096 versus CTL (time = 8 weeks). BL = baseline; Wk = weeks; other abbreviations as in Figure 1.
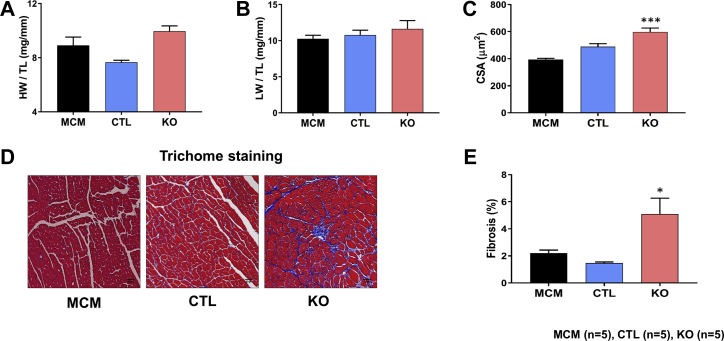
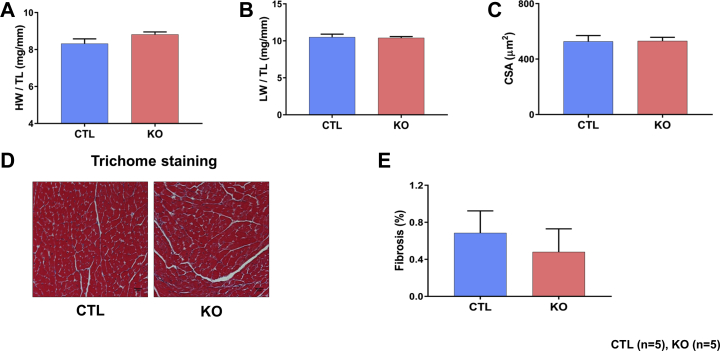
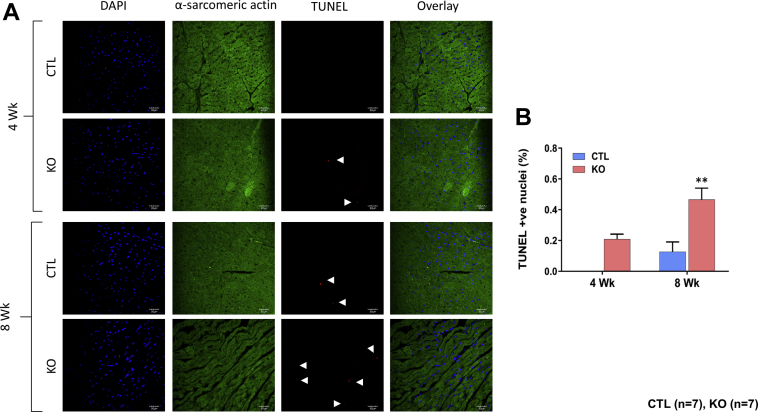
At the end of each time point, heart weight to tibia length and lung weight to tibia length ratios were compared. These parameters were comparable between the SMAD4 KO and CTL groups at both time points (Figures 3A, 3B, 4A, and 4B). For the assessment of cardiac hypertrophy, CM cross-sectional area (CSA) was measured. We found no significant difference in CSA between the SMAD4 KO and CTL groups at 4 weeks after TAM treatment (Figure 4C). However, at 8 weeks, CSA was significantly higher in SMAD4 KO mice than respective CTL animals (Figure 3C). Cardiac fibrosis was assessed by performing Masson’s trichome staining. Examination of trichome-stained heart sections demonstrated that fibrosis was absent in SMAD4 KO mice at 4 weeks after TAM treatment (Figures 4D and 4E) and was prominently seen in these animals at 8 weeks (Figures 3D and 3E). To confirm the histology data, we analyzed expression levels of molecular markers for fibrotic remodeling by the quantitative polymerase chain reaction method. In agreement with the trichome staining results, we saw an increasing trend in the levels of col1a1, col1a2, and col3a1 in the KO group (Supplemental Figure 1). CM dropout due to cell death causes remodeling of the heart. Hence, we also examined cardiac sections for the presence of DNA fragmentation, a classical marker of cell death, by terminal deoxynucleotidyl transferase dUTP nick-end labeling staining. Cell death was significantly evident at 8 weeks after TAM treatment (Figures 5A and 5B). Considered together, these results demonstrate that CM-specific disruption of canonical TGF-β signaling via deletion of the critical intermediate regulator SMAD4 leads to dilated cardiomyopathy (DCM) development. Thus, CM-SMAD4 is indispensable for adult heart homeostasis.
Figure 3.
CM-Specific SMAD4 Deletion Induces Cardiac Remodeling
Morphometric studies were performed at the end of 8 weeks after TAM treatment. (A) Heart weight (HW) to tibia length (TL). (B) Lung weight (LW) to TL. (C) Quantification of cardiomyocyte cross-sectional area (CSA). ***p = 0.0002 versus MCM group. (D) Masson’s trichome staining. Representative trichome-stained LV regions. (E) Quantification of LV fibrosis. *p = 0.0165 versus MCM group. Scale bar = 30 μm. Abbreviations as in Figures 1 and 2.
Figure 4.
CM-Specific SMAD4 Deletion Induces Cardiac Remodeling
Morphometric studies were performed at the end of 4 weeks after TAM treatment. (A) HW to TL. (B) LW to TL. (C) Quantification of cardiomyocyte CSA. (D) Masson’s trichome staining. Representative trichome-stained LV regions. (E) Quantification of LV fibrosis. Scale bar = 30 μm. Abbreviations as in Figures 1, 2, and 3.
Figure 5.
CM-Specific SMAD4 Deletion Leads to Cardiac Cell Death
(A) Representative images show terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)–positive cardiomyocytes from the CTL and KO hearts. (B) Quantification shows significantly increased number of TUNEL-positive myocytes in KO mice at 8 weeks after TAM treatment. **p = 0.0069 versus CTL group. Scale bar = 30 μm. Abbreviations as in Figures 1, 2, and 3.
CM-specific SMAD4 deletion does not affect MAPK and PI3K-AKT signaling pathways
Previous studies showed that the TGF-β could activate MAPK and PI3K-AKT signaling pathway independently of SMADs 7, 8, 12. Thus, to verify whether any aberrant changes in the MAPK or PI3K-AKT pathway contributed to the observed cardiac phenotype in SMAD4 KO mice, we examined the protein levels of phosphorylated ERK1/2, p38, and AKT by Western blotting at both time points. The phosphorylation levels of ERK1/2, p38, and AKT were comparable in the LV lysates from SMAD4 KO and CTL mice (Supplemental Figures 2A to 2C). These results rule out the possible causal role of the MAPK and PI3K-AKT signaling pathways in the development of cardiac dysfunction in SMAD4 KO.
CM contractility is affected by CM-specific SMAD4 deletion
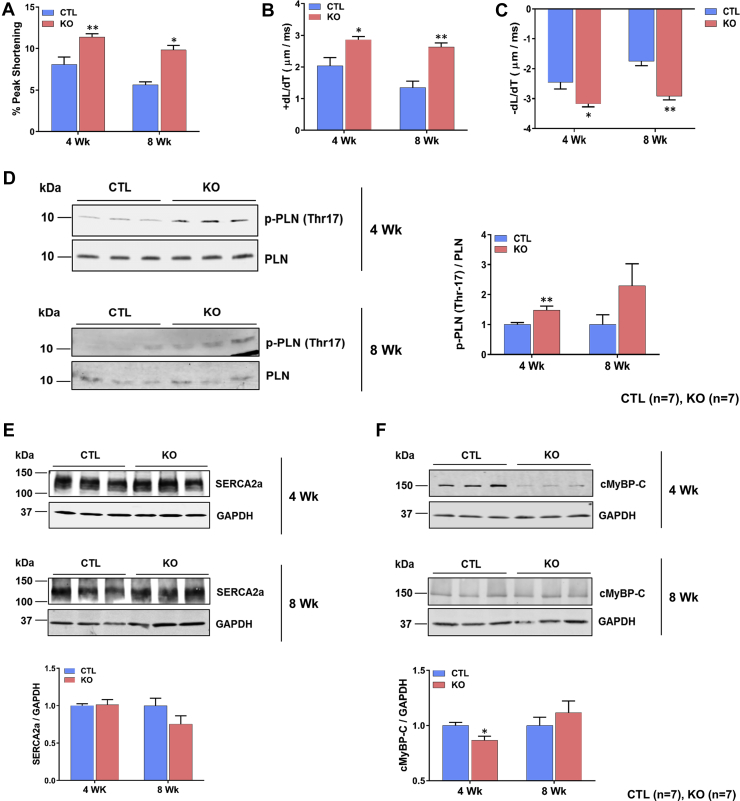
To determine whether the observed in vivo LV systolic dysfunction was attributable to defects in contractility of CMs, we assessed sarcomere contractility of CMs isolated from SMAD4 KO and CTL mice. Surprisingly, SMAD4 KO CMs displayed significantly higher sarcomere peak shortening, as well as sarcomere shortening and relengthening velocities compared with CMs isolated from CTL mice (Figures 6A to 6C).
Figure 6.
Effect of CM-Specific SMAD4 Deletion on CM Contraction Kinetics and on Contractility-Regulating Proteins
CMs were isolated from the hearts of experimental mice at the end of each time point and sarcomere shortening parameters assessed (n = 10 to 20 CMs/heart and 8 to 9 hearts/group). (A) Sarcomere peak shortening normalized to resting sarcomere length (% peak shortening). *p = 0.0104, **p = 0.0033 versus CTL group. (B) Maximal relengthening (+dL/dT) velocity. *p = 0.0101, **p = 0.0035. (C) Maximal shortening (–dL/dT) velocity. *p = 0.0102, **p = 0.0041. Western blot analysis of proteins that regulate calcium homeostasis and cardiomyocyte contractility. Representative Western blots and quantification. (D) Phospholamban (PLN). **p = 0.0099. (E) Sarco/endoplasmic reticulum Ca2+-ATPase 2a (SERCA2a). (F) Cardiac myosin binding protein C (cMyBP-C). *p = 0.0317. All p values are versus the CTL group. Abbreviations as in Figures 1, 2, and 3.
Calcium homeostasis is a key factor in regulating CM contractility. Therefore, we next examined the effect of SMAD4 deletion on calcium handling proteins. Total protein levels of sarco- or endoplasmic reticulum Ca2+-ATPase 2a (SERCA2a) and its inhibitor phospholamban were comparable in SMAD4 KO and CTL hearts. However, phospholamban was hyperphosphorylated in SMAD4 KO hearts, indicating a reduction in its inhibitory effect on SERCA2a pump activity (Figures 6D and 6E).
The observed increase in CM contraction kinetics suggested that there might be alterations in myofilament calcium sensitivity and cross-bridge cycling rates. As cardiac myosin-binding protein C (cMyBP-C) is known to regulate these processes, we examined levels of these proteins by Western blotting. Indeed, cMyBP-C was significantly down-regulated in the SMAD4 KO hearts at 4 weeks after TAM treatment (Figure 6F). These results support our observation of improved CM contractility in SMAD4 KO mice.
RNA sequencing results reveal down regulation in cardiac ION channels’ genes expression in SMAD4 KO mice
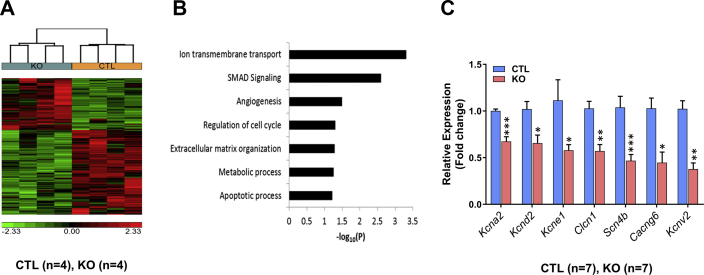
To examine the primary consequences of CM-SMAD4 deletion on the cardiac transcriptome, RNA sequencing (RNA-Seq) analysis was performed at 4 weeks after TAM treatment. Comparison of SMAD4 KO versus littermate CTL animals resulted in the identification of 166 transcripts with significant differential expression (shown in Cluster), including 151 characterized gene products, and 15 uncharacterized transcripts (complementary DNAs, Expressed sequence tags, or long noncoding RNAs). Of the 151 altered genes, only 55 were up-regulated, with the majority (96 genes) down-regulated by at least 1.5-fold with p < 0.05 (Figure 7A).
Figure 7.
RNA-Seq Analysis Reveals Alteration in Ion Channel Genes in SMAD4 KO Mice
RNA sequencing (RNA-Seq) analysis was performed at 4 weeks after TAM treatment. (A) Hierarchical clustering of 161 genes detected as significantly differential (at least 1.5-fold, p value <0.05) between the CTL and SMAD4 KO groups. (B) Gene Ontology analysis. (C) Quantitative polymerase chain reaction analysis of ion-channel genes: kcna2 (***p = 0.0006), kcnd2 (*p = 0.0291), kcne1 (*p = 0.0111), clcn1 (**p = 0.0016), scn4b (***p = 0.0006), cacng6 (*p = 0.0130), and kcnv2 (**p = 0.0012). All p values are versus the CTL group. Abbreviations as in Figures 1 and 2.
Gene Ontology analysis identified ion transmembrane transport as the top over-represented biological process in SMAD4 KO hearts (Figure 7B). Based on this finding, we identified the ion-channel genes that were significantly down-regulated (Clcn1, Kcnd2, Kcna2, Cacng6, Kcne1, Scn4b, Kcnv2). We validated their gene expression by quantitative polymerase chain reaction and consistent with the RNA-Seq results, these genes showed significant down-regulation in the hearts of SMAD4 KO mice, compared with littermate CTL animals (Figure 7C).
SMAD4 KO mice exhibit electrocardiographic abnormalities
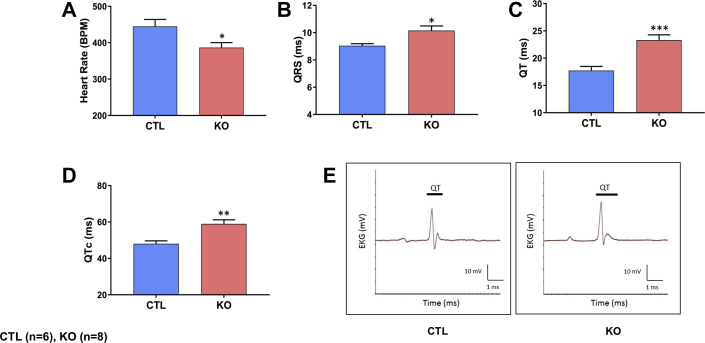
The identification of ion transmembrane transport as the top biological process in RNA-Seq analysis prompted us to examine whether CM-specific SMAD4 deletion causes any electrophysiological defects in SMAD4 KO mice. Thus, electrocardiography analysis was performed on SMAD4 KO and littermate CTL mice at 4 weeks after TAM. Indeed, slower heart rates were evident in SMAD4 KO than littermate CTL animals (Figure 8A). Furthermore, SMAD4 KO mice displayed prolongation of QRS, QT, and QTc intervals (Figures 8B to 8E).
Figure 8.
CM-Specific SMAD4 Deletion Causes Cardiac Conduction Abnormalities
Electrocardiography (EKG) analysis was performed on mice at 4 weeks after TAM treatment and the following ECG parameters were examined: (A) heart rate (*p = 0.0426), (B) QRS interval (*p = 0.0127), (C) QT interval (***p = 0.0007), (D) QTc interval (**p = 0.0027), and (E) representative ECG traces. All p values are versus the CTL group. BPM = beats per minute; other abbreviations as in Figure 1.
Discussion
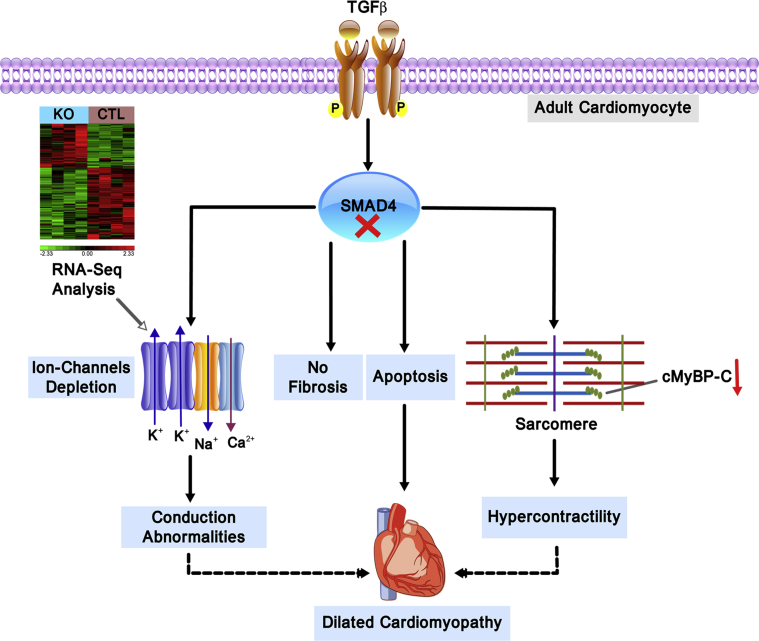
Herein we examined the contribution of CM canonical TGF-β signaling to the heart function. We demonstrated that CM-specific SMAD4 deletion in the fully mature heart causes DCM. Interestingly, cardiac dysfunction in SMAD4 KO mice was seen despite improved CM contractility and preceded by the development of pathological features of DCM such as hypertrophy and fibrosis. Furthermore, we also observed down-regulation of ion channels' gene expression and cardiac conduction abnormalities in SMAD4 KO mice. These results highlight a fundamental role for CM-specific canonical TGF-β signaling in the regulation of adult heart function (Figure 9).
Figure 9.
CM Canonical TGF-β Signaling is Essential to Maintain Adult Heart Homeostasis
Deletion of canonical TGF-β signaling in adult CMs by targeting SMAD4 leads to down-regulation of several ion-channel genes and a key sarcomeric protein, cMyBP-C. These adverse effects of SMAD4 deletion results in cardiac conduction abnormalities and hyper contractile phenotype and are at least in part responsible for dilated cardiomyopathy development. Importantly, CM-specific canonical TGF-β signaling does not seem to be a significant contributor of fibrosis in adult hearts. Abbreviations as in Figures 1, 6, and 7.
In SMAD4 KO mice, development of marked cardiac dysfunction preceded the fibrotic remodeling indicating that fibrosis was not a cause of observed phenotype. It is well established that canonical TGF-β signaling is critical to fibroblast biology and fibrosis 24, 28, 29, 30, 31. Additionally, the previous study by the Kass lab (23) demonstrated that the activation of the noncanonical TGF-β pathway in CMs contributes to maladaptive fibrotic remodeling in pressure-overloaded hearts. Thus, it is possible that fibrosis observed at a later point could be a maladaptive response evoked by fibroblasts with intact TGF-β signaling or the effect of activation of noncanonical TGF-β signaling spared in CMs. Furthermore, a study pertaining to muscle-specific SMAD4 deletion in muscular dystrophy models revealed that functional enhancement of dystrophic muscle occurs without a significant reduction in fibrosis (21). In line with this report, our study also provides valuable evidence that CM-specific canonical TGF-β signaling is not a significant contributor to fibrotic remodeling and highlights its ability to alter cardiac performance by targeting CM-specific pathways.
Wang et al. (32) demonstrated that targeted disruption of SMAD4 in CMs results in the early onset of cardiac hypertrophy and implicated the excessive activation of MEK1-ERK1/2 as the possible underlying mechanism. In stark contrast, herein we demonstrate that CM-SMAD4 does not regulate MEK1-ERK1/2 signaling or cardiac hypertrophy in the adult heart. This discrepancy might be due to differences in the timing of CM-specific SMAD4 deletion. Wang et al. (32) deleted SMAD4 in mouse embryos using alpha-myosin heavy chain promoter–driven Cre. Considering the essential role of SMAD4 in cardiogenesis (25), the phenotype of the animals used in the previous study might have been compromised by developmental defects. However, in our model, SMAD4 deletion was done when the physiological development of mice is complete, thus excluding the causal role of developmental defects in the observed cardiac phenotype. We believe that inducible loss-of-function approaches are likely of greater biological relevance in identifying the true targets of SMAD4 in the fully mature heart compared with transgenesis, embryonic knockout, or cell culture approaches.
To our complete surprise, SMAD4 KO mice displayed enhanced CM contractility. This finding contrasted with decreased cardiac function seen in these animals. However, further studies performed to ascertain these intriguing observations revealed that there was a remarkable reduction in cMyBP-C in SMAD4 KO hearts. MyBP-C is a crucial sarcomeric protein that has structural as well as regulatory functions in muscles. Genetic studies have identified MYBPC3 (cardiac isoform) as one of the major mutated genes that results in the development of hypertrophic and dilated cardiomyopathies 33, 34. Several mouse models bearing such mutations showed haploinsufficiency and reduction in cMyBP-C levels 35, 36, 37, 38. These models had increased myofilament calcium sensitivity and faster cross-bridge cycling rate, and developed a hypercontractile phenotype. Additionally, pathological features such as fibrosis, hypertrophy, and arrhythmogenesis were seen in these models. Thus, considering the phenotypic similarities in MYBPC3 mutants and SMAD4 KO mice, we speculate that reduction in cMyBP-C protein in SMAD4 KO heart may, at least in part, be responsible for development of cardiac abnormalities in these animals.
The disagreement between contractile function assessed in isolated myocytes and intact hearts of SMAD4 KO suggests that there might be impaired muscle force transduction, increased myocyte death, or compromised electrical activation of the myocardium. To examine whether CM-specific SMAD4 deletion has any effect on key proteins involved in muscle force transmission, we assessed levels of intercalated disc and costameric proteins by Western blotting. The expression of these proteins was not different in CTL and KO animals (Supplemental Figures 3A to 3D). However, analysis of terminal deoxynucleotidyl transferase dUTP nick-end labeling data suggested that in SMAD4 KO there is a progressive increase in cardiac cell death. Considering the prosurvival effect of TGF-β superfamily members on CM, it is not surprising that CM-specific SMAD4 deletion resulted in the CMs death in SMAD4 KO mice 39, 40, 41. Together, these evidences support our hypothesis that cardiac cell death might have contributed to the development of cardiac dysfunction in SMAD4 KO mice.
The most striking finding from RNA-Seq analysis was the significant down-regulation of several ion-channel genes in hearts from SMAD4 KO mice. Of these, the pivotal role of Kcnd2 is demonstrated in encoding primary repolarizing current in rodents and when selectively eliminated in mice resulted in QT prolongation (42). Additionally, a mutation in gene SCN4B which codes for sodium channel β4 subunit has been implicated in congenital long-QT syndrome type 10 in humans (43). In keeping with these reports and RNA-Seq results, electrocardiography analysis revealed that SMAD4 KO mice exhibit QT prolongation. These observations suggest that down-regulation in the expression of ion-channel genes could possibly account for the alteration in the electrocardiography parameters of SMAD4 KO animals. However, given the possibility that QRS and QT prolongation may have resulted from the developing pathology, additional studies with more comprehensive electrophysiological assessments are necessary to explain whether SMAD4 KO mice exhibit causal relationship between conduction and contractility defects. To our knowledge, only a few reports have provided evidence for a direct role of TGF-β signaling in modulating ion channels essential for cardiac electrophysiology. For instance, Kaur et al. (44) showed that TGF-β-1 increases the transcription and activity of sodium channels in adult rat CMs. On the other hand, Ramos-Mondragon et al. (45) reported reduction in the expression and activity of sodium channel in TGF-β1–treated neonatal rat atrial myocytes. It is important to note that these studies are performed in isolated CMs, which differ in biophysical and electrophysiological properties compared with the entire organ. With that said, our study is the first to provide evidence for the significant role of CM-specific SMAD4-dependent TGF-β signaling in regulating gene expression of ion channels that are essential for cardiac conduction.
Conclusions
In summary, we report that CM-specific deletion of canonical TGF-β signaling in fully mature CMs leads to cardiac dysfunction and dilated cardiomyopathy. Although canonical TGF-β signaling has been suggested as a critical target for the management of adverse fibrotic remodeling, our findings strongly suggest that cell-specific TGF-β responses should be considered when developing anti–TGF-β strategies for treating cardiac diseases to avoid interference with its beneficial actions.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Vast published data has established the central role of canonical TGF-β signaling in fibroblast activation, wound healing, and myocardial fibrosis. However, the role of canonical TGF-β signaling in fully mature functional CMs is not clear. Our studies establish that the CMs canonical TGF-β signaling is essential to preserve adult heart homeostasis. We also establish that this signaling axis in CMs is critical for maintaining cMyBP-C levels, sarcomere kinetics, and expression of key ion channels. Thus, the role of CM canonical TGF-β signaling in myocardial physiology appears to be substantial.
TRANSLATIONAL OUTLOOK: Pharmacological targeting of canonical TGF-β signaling is highly implicated for the management of multiple organ fibrosis including the myocardial fibrosis. In light of our finding presented herein, we suggest caution in going forward with the drugs that target canonical TGF-β signaling in the heart. Strategies for such drug development may include the cell-specific targeting or delineation of potential downstream targets. Thus, our finding identifies what we believe to be a new paradigm for pharmacological targeting of canonical TGF-β1 signaling and raises a significant concern regarding the approaches relies on systemic inhibition.
Acknowledgments
The authors would like to thank Dr. Sakthivel Sadayappan, University of Cincinnati, for providing expert advice and antibodies to detect cardiac myosin binding protein C.
Appendix
References
- 1.Benjamin E.J., Blaha M.J., Chiuve S.E. Heart Disease and Stroke Statistics-2017 Update: a report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidenreich P.A., Albert N.M., Allen L.A. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu M.Y., Hill C.S. TGF-beta superfamily signaling in embryonic development and homeostasis. Dev Cell. 2009;16:329–343. doi: 10.1016/j.devcel.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Akhurst R.J., Hata A. Targeting the TGFbeta signalling pathway in disease. Nat Rev Drug Discov. 2012;11:790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmierer B., Hill C.S. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 6.Derynck R., Zhang Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 7.Lee M.K., Pardoux C., Hall M.C. TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J. 2007;26:3957–3967. doi: 10.1038/sj.emboj.7601818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamashita M., Fatyol K., Jin C., Wang X., Liu Z., Zhang Y.E. TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-beta. Mol Cell. 2008;31:918–924. doi: 10.1016/j.molcel.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaguchi K., Nagai S., Ninomiya-Tsuji J. XIAP, a cellular member of the inhibitor of apoptosis protein family, links the receptors to TAB1-TAK1 in the BMP signaling pathway. EMBO J. 1999;18:179–187. doi: 10.1093/emboj/18.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhowmick N.A., Ghiassi M., Bakin A. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12:27–36. doi: 10.1091/mbc.12.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petritsch C., Beug H., Balmain A., Oft M. TGF-beta inhibits p70 S6 kinase via protein phosphatase 2A to induce G(1) arrest. Genes Dev. 2000;14:3093–3101. doi: 10.1101/gad.854200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakin A.V., Tomlinson A.K., Bhowmick N.A., Moses H.L., Arteaga C.L. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem. 2000;275:36803–36810. doi: 10.1074/jbc.M005912200. [DOI] [PubMed] [Google Scholar]
- 13.Freudlsperger C., Bian Y., Contag Wise S. TGF-beta and NF-kappaB signal pathway cross-talk is mediated through TAK1 and SMAD7 in a subset of head and neck cancers. Oncogene. 2013;32:1549–1559. doi: 10.1038/onc.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massague J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li R.K., Li G., Mickle D.A. Overexpression of transforming growth factor-beta1 and insulin-like growth factor-I in patients with idiopathic hypertrophic cardiomyopathy. Circulation. 1997;96:874–881. doi: 10.1161/01.cir.96.3.874. [DOI] [PubMed] [Google Scholar]
- 16.Hein S., Arnon E., Kostin S. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107:984–991. doi: 10.1161/01.cir.0000051865.66123.b7. [DOI] [PubMed] [Google Scholar]
- 17.Gramley F., Lorenzen J., Koellensperger E., Kettering K., Weiss C., Munzel T. Atrial fibrosis and atrial fibrillation: the role of the TGF-beta1 signaling pathway. Int J Cardiol. 2010;143:405–413. doi: 10.1016/j.ijcard.2009.03.110. [DOI] [PubMed] [Google Scholar]
- 18.Deten A., Holzl A., Leicht M., Barth W., Zimmer H.G. Changes in extracellular matrix and in transforming growth factor beta isoforms after coronary artery ligation in rats. J Mol Cell Cardiol. 2001;33:1191–1207. doi: 10.1006/jmcc.2001.1383. [DOI] [PubMed] [Google Scholar]
- 19.Li J.M., Brooks G. Differential protein expression and subcellular distribution of TGFbeta1, beta2 and beta3 in cardiomyocytes during pressure overload-induced hypertrophy. J Mol Cell Cardiol. 1997;29:2213–2224. doi: 10.1006/jmcc.1997.0457. [DOI] [PubMed] [Google Scholar]
- 20.Bujak M., Frangogiannis N.G. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldstein J.A., Bogdanovich S., Beiriger A. Excess SMAD signaling contributes to heart and muscle dysfunction in muscular dystrophy. Hum Mol Genet. 2014;23:6722–6731. doi: 10.1093/hmg/ddu390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frantz S., Hu K., Adamek A. Transforming growth factor beta inhibition increases mortality and left ventricular dilatation after myocardial infarction. Basic Res Cardiol. 2008;103:485–492. doi: 10.1007/s00395-008-0739-7. [DOI] [PubMed] [Google Scholar]
- 23.Koitabashi N., Danner T., Zaiman A.L. Pivotal role of cardiomyocyte TGF-beta signaling in the murine pathological response to sustained pressure overload. J Clin Invest. 2011;121:2301–2312. doi: 10.1172/JCI44824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong P., Shinde A.V., Su Y. Opposing actions of fibroblast and cardiomyocyte smad3 signaling in the infarcted myocardium. Circulation. 2018;137:707–724. doi: 10.1161/CIRCULATIONAHA.117.029622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song L., Yan W., Chen X., Deng C.X., Wang Q., Jiao K. Myocardial smad4 is essential for cardiogenesis in mouse embryos. Circ Res. 2007;101:277–285. doi: 10.1161/CIRCRESAHA.107.155630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad F., Lal H., Zhou J. Cardiomyocyte-specific deletion of Gsk3alpha mitigates post-myocardial infarction remodeling, contractile dysfunction, and heart failure. J Am Coll Caridol. 2014;64:696–706. doi: 10.1016/j.jacc.2014.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupte M., Tumuluru S., Sui J.Y. Cardiomyocyte-specific deletion of GSK-3beta leads to cardiac dysfunction in a diet induced obesity model. Int J Cardiol. 2018;259:145–152. doi: 10.1016/j.ijcard.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khalil H., Kanisicak O., Prasad V. Fibroblast-specific TGF-beta-Smad2/3 signaling underlies cardiac fibrosis. J Clin Invest. 2017;127:3770–3783. doi: 10.1172/JCI94753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo Y., Gupte M., Umbarkar P. Entanglement of GSK-3beta, beta-catenin and TGF-beta1 signaling network to regulate myocardial fibrosis. J Mol Cell Cardiol. 2017;110:109–120. doi: 10.1016/j.yjmcc.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lal H., Ahmad F., Zhou J. Cardiac fibroblast glycogen synthase kinase-3beta regulates ventricular remodeling and dysfunction in ischemic heart. Circulation. 2014;130:419–430. doi: 10.1161/CIRCULATIONAHA.113.008364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biernacka A., Cavalera M., Wang J. Smad3 signaling promotes fibrosis while preserving cardiac and aortic geometry in obese diabetic mice. Circ Heart Fail. 2015;8:788–798. doi: 10.1161/CIRCHEARTFAILURE.114.001963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J., Xu N., Feng X. Targeted disruption of Smad4 in cardiomyocytes results in cardiac hypertrophy and heart failure. Circ Res. 2005;97:821–828. doi: 10.1161/01.RES.0000185833.42544.06. [DOI] [PubMed] [Google Scholar]
- 33.Daehmlow S., Erdmann J., Knueppel T. Novel mutations in sarcomeric protein genes in dilated cardiomyopathy. Biochem Biophys Res Commun. 2002;298:116–120. doi: 10.1016/s0006-291x(02)02374-4. [DOI] [PubMed] [Google Scholar]
- 34.Richard P., Charron P., Carrier L. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003;107:2227–2232. doi: 10.1161/01.CIR.0000066323.15244.54. [DOI] [PubMed] [Google Scholar]
- 35.van Dijk S.J., Dooijes D., dos Remedios C. Cardiac myosin-binding protein C mutations and hypertrophic cardiomyopathy: haploinsufficiency, deranged phosphorylation, and cardiomyocyte dysfunction. Circulation. 2009;119:1473–1483. doi: 10.1161/CIRCULATIONAHA.108.838672. [DOI] [PubMed] [Google Scholar]
- 36.Cheng Y., Wan X., McElfresh T.A. Impaired contractile function due to decreased cardiac myosin binding protein C content in the sarcomere. Am J Physiol Heart Circ Physiol. 2013;305:H52–H65. doi: 10.1152/ajpheart.00929.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fraysse B., Weinberger F., Bardswell S.C. Increased myofilament Ca2+ sensitivity and diastolic dysfunction as early consequences of Mybpc3 mutation in heterozygous knock-in mice. J Mol Cell Cardiol. 2012;52:1299–1307. doi: 10.1016/j.yjmcc.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carrier L., Knoll R., Vignier N. Asymmetric septal hypertrophy in heterozygous cMyBP-C null mice. Cardiovasc Res. 2004;63:293–304. doi: 10.1016/j.cardiores.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Kempf T., Eden M., Strelau J. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res. 2006;98:351–360. doi: 10.1161/01.RES.0000202805.73038.48. [DOI] [PubMed] [Google Scholar]
- 40.Zaidi S.H., Huang Q., Momen A., Riazi A., Husain M. Growth differentiation factor 5 regulates cardiac repair after myocardial infarction. J Am Coll Cardiol. 2010;55:135–143. doi: 10.1016/j.jacc.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y., Alexander P.B., Wang X.F. TGF-beta family signaling in the control of cell proliferation and survival. Cold Spring Harbor Perspect Biol. 2017;9:a022145. doi: 10.1101/cshperspect.a022145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oudit G.Y., Kassiri Z., Sah R., Ramirez R.J., Zobel C., Backx P.H. The molecular physiology of the cardiac transient outward potassium current (I(to)) in normal and diseased myocardium. J Mol Cell Cardiol. 2001;33:851–872. doi: 10.1006/jmcc.2001.1376. [DOI] [PubMed] [Google Scholar]
- 43.Medeiros-Domingo A., Kaku T., Tester D.J. SCN4B-encoded sodium channel beta4 subunit in congenital long-QT syndrome. Circulation. 2007;116:134–142. doi: 10.1161/CIRCULATIONAHA.106.659086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaur K., Zarzoso M., Ponce-Balbuena D. TGF-beta1, released by myofibroblasts, differentially regulates transcription and function of sodium and potassium channels in adult rat ventricular myocytes. PLoS One. 2013;8:e55391. doi: 10.1371/journal.pone.0055391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramos-Mondragon R., Vega A.V., Avila G. Long-term modulation of Na+ and K+ channels by TGF-beta1 in neonatal rat cardiac myocytes. Pflugers Archiv. 2011;461:235–247. doi: 10.1007/s00424-010-0912-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.