 Novel primaquine–cell penetrating peptide conjugates were synthesised and tested in vitro against liver stage Plasmodium berghei parasites, showing that generally the conjugates were more active than the parent peptides and, in some cases, than the parent drug.
Novel primaquine–cell penetrating peptide conjugates were synthesised and tested in vitro against liver stage Plasmodium berghei parasites, showing that generally the conjugates were more active than the parent peptides and, in some cases, than the parent drug.
Abstract
Novel primaquine–cell penetrating peptide conjugates were synthesised and tested in vitro against liver stage Plasmodium berghei parasites. Generally, the conjugates were more active than the parent peptides and, in some cases, than the parent drug. These are unprecedented findings that may open a new route towards antimalarial drug rescuing.
Malaria is one of the world's deadliest diseases and poses a severe threat to many billion people living in endemic areas. Malaria is caused by parasites of the Plasmodium genus that encompasses many different species, five of which are known to infect humans: P. falciparum, P. vivax, P. ovale, P. malariae and P. knowlesi. The most predominant and severe forms of human malaria are caused by P. falciparum and P. vivax, the former being the deadliest parasite species.1,2 The parasite's life cycle is extremely complex: upon transmission to a human host through the bite of an infected Anopheles mosquito, the Plasmodium sporozoites infect the liver, where they initiate a clinically silent asexual reproduction stage.3 This multiplication phase results in the formation of thousands of merozoites that are subsequently released into the bloodstream. Parasites then invade red blood cells and undergo another asexual reproduction stage that culminates in the rupture of infected erythrocytes (schizonts), with concomitant release of new merozoites ready to infect healthy red blood cells. In each parasite replication cycle in the blood the stage is responsible for disease symptoms, a small proportion of parasites differentiate into sexual forms – gametocytes. These can be taken up by mosquitos feeding on the blood of the infected human host, initiating the parasite's sexual reproduction stage. This phase culminates in the accumulation of sporozoites in the mosquito's salivary glands, ready to infect a new human host.3 Thus, blocking gametocyte formation or uptake by mosquitoes will block disease transmission from the host to the vector. Another aspect that contributes to malaria's high morbidity results from the fact that P. vivax and P. ovale, are able to form hypnozoites, parasite forms that may remain dormant for years or even decades in the liver, and may lead to disease relapses.1,2
Although the worldwide incidence of malaria has been falling since 2010, the rate of decline has stalled and even reversed in some regions since 2014.2 In fact, eradication of this disease is constantly delayed by the (i) complexity of the malaria parasite's life cycle, (ii) widespread resistance to less expensive and most popular antimalarials such as chloroquine, (iii) toxicity of some classical antimalarial drugs, e.g., primaquine, (iv) lack of effective vaccines, and (v) scarcity of multi-stage antimalarials that are able to efficiently eliminate liver- and blood-stage forms of Plasmodium parasites, including gametocytes, given their role for the vector-mediated dissemination of the disease.4 Nevertheless, primaquine (PQ) remains a useful weapon against malaria on three different fronts: (i) primary prophylaxis against all species of malaria parasites, (ii) presumptive anti-relapse therapy (radical cure) for people exposed to P. vivax or P. ovale, as it is able to kill hypnozoites,5–8 and (iii) transmission-blocking action, due to its gametocytocidal activity towards all species of Plasmodium, thus halting the spread of the disease.1,9 Moreover, clinically relevant parasite resistance to PQ has not been reported. Altogether, these features explain why, 70 years after its discovery, PQ remains the only hypnozoiticidal and transmission-blocking antimalarial drug in clinical use worldwide. Yet, PQ is not devoid of relevant drawbacks: it is rapidly metabolized to carboxyprimaquine, which has no significant antimalarial activity,10 and it triggers the development of haemolytic anemia in people who present a glucose-6-phosphate dehydrogenase (G6PD) deficiency, which is a relatively common genetic trait in areas where malaria is endemic.11,12 In view of this, several strategies to improve PQ's pharmacokinetics and safety have been pursued, not only by medicinal chemists in academic settings, but also by big pharma, in some cases leading to the discovery of highly promising candidates, like tafenoquine;13 this investigational drug has been recently submitted for approval by the Food and Drug Administration (FDA) as a single-dose therapy against P. vivax, but only for patients 16 years old or older, meaning that an efficient and safe PQ surrogate or derivative is still needed to treat young children, which are most vulnerable to malaria.14
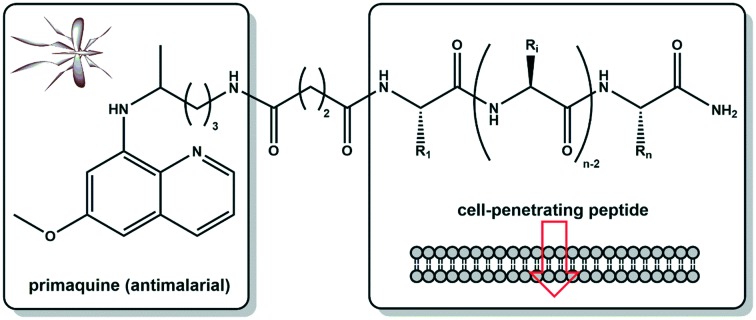
Our group has been working over the past two decades in the so-called “recycling” of classical antimalarial pharmacophores,9 with a particular, although not exclusive, focus on PQ.15–19 Following the widely reported benefits of cell-penetrating peptides (CPP) as promising shuttles for intracellular drug delivery as a consequence of their low toxicity, their ability to be taken up by diverse cell types, and their compatibility with different cargo sizes or types,20 we hypothesized that PQ conjugation to CPP might enhance its antiplasmodial activity, while possibly reducing its adverse effects, through a better targeting of the drug into infected cells. In other words, PQ conjugation to CPP through the drug's primary amine might increase its concentration at site of action, due not only to a targeted delivery effect, but also because PQ conjugation to amino acids, oligopeptides and peptidomimetics has been reported to delay or even block oxidative deamination into carboxyprimaquine, which in turn would allow the use of lower drug doses.10,19,21–23 In view of this, we conjugated several CPP (Table 1) to PQ, as depicted in Scheme 1, and the conjugates obtained were tested in vitro against liver stage P. berghei parasites. This unprecedented approach delivered encouraging preliminary results as herein reported.
Table 1. Selected CPP, produced by SPPS in both the unconjugated and PQ-conjugated form (cf.Scheme 1).
| CPP | Amino acid sequence | Molecular weight (Da) | Net charge |
| TAT | RKKRRQRRRPPQ | 1661 | +9 |
| PasTAT | FFLIPKGGRKKRRQRRRPPQ | 2521 | +10 |
| Transportan | GWTLNSAGYLLGKINLKALAALAKKIL | 2840 | +5 |
| Transportan 10 (TP10) | AGYLLGKINLKALAALAKKIL | 2182 | +5 |
| IDR-1018 | VRLIVAVRIWRR | 1535 | +5 |
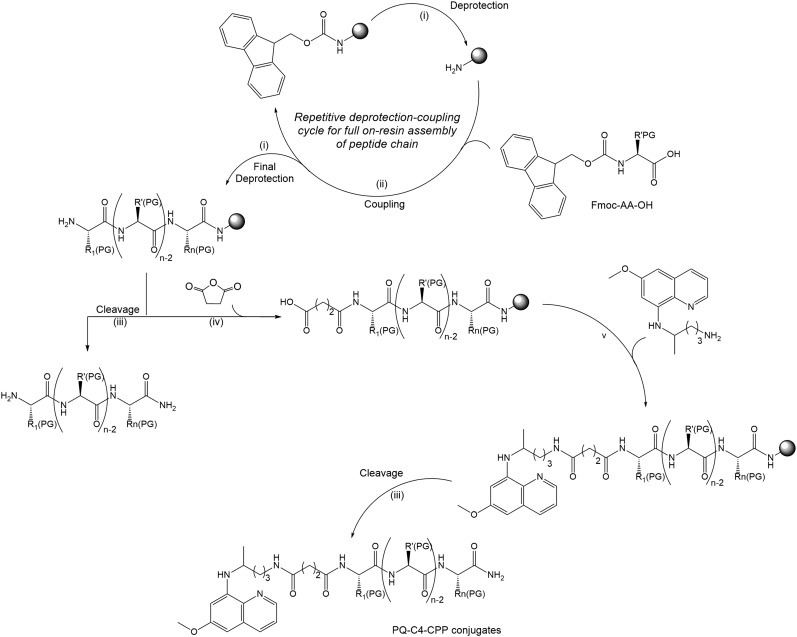
Scheme 1. Synthetic route towards PQ-C4-CPP conjugates: (i) 20% piperidine in N,N-dimethylformamide (DMF), r.t., 20 min; (ii) 5 molar equivalents (eq.), respective to resin reactive groups, of O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HBTU) and of Fmoc-protected amino acid (Fmoc-AA-OH), 10 eq. of N-ethyl-N,N-diisopropylamine (DIEA) in DMF, r.t., 60 min; (iii) trifluoroacetic acid (TFA)/water/triisopropylsilane cocktail in 95 : 2.5 : 2.5 volumetric proportion (1 mL/100 mg of resin), r.t., 120 min; (iv) 20 eq. of succinic anhydride and of DIEA in DMF, r.t., 60 min; (v) 5 eq. of PQ and of HBTU, 10 eq. DIEA in DMF, r.t., 60 min. Grey sphere stands for the polymer support used for peptide chain assembly, which was a Rink-amide resin in this case.
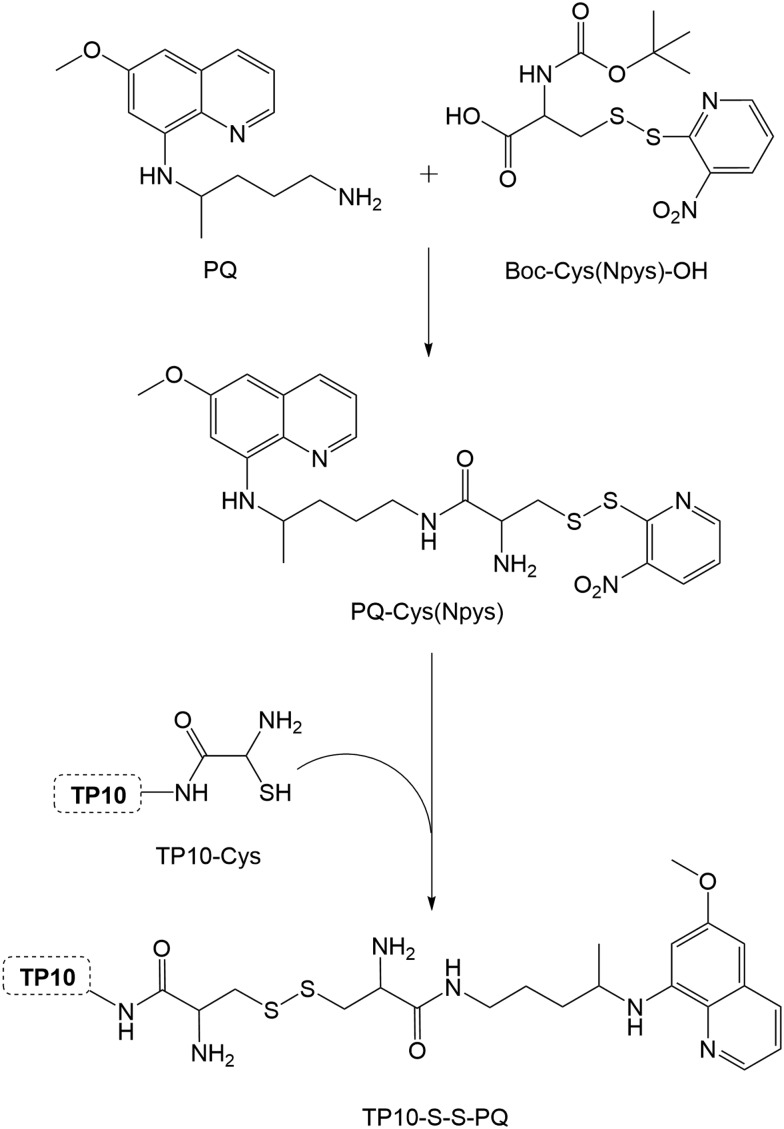
Five different CPP (Table 1) were chosen on the basis of either their (i) well-known cell-penetrating properties (TAT, PasTAT),23,24 (ii) wide-range antimicrobial and immunomodulatory action (IDR-1018),25 and/or (iii) intrinsic antiparasitic activity (transportan 10, or TP10).26 The peptides were assembled by standard solid-phase peptide synthesis (SPPS) methods,27 and used in their unconjugated form in subsequent in vitro assays, for comparison with both the parent drug (PQ) and the new drug-CPP conjugates. These were produced by on-resin coupling, onto the pre-assembled full peptide sequence, of succinic anhydride followed by PQ (Scheme 1), after which the resulting conjugates (PQ-C4-CPP) were released from the resin by means of standard acidolytic cleavage with a trifluoroacetic acid (TFA)-based cocktail.27 In order to assess the relevance of the drug's relative position within the conjugate, an analogue was synthesized, TP10-S2-PQ,28 where the drug was coupled to the C-terminus of the peptide. This coupling was promoted via disulfide bond formation between TP10-Cys and PQ-Cys(Npys) (Scheme 2), also prepared in-house.28 Once cleaved from the resin, all conjugates were purified by preparative high-performance liquid chromatography (HPLC), and their structures confirmed by liquid chromatography coupled to a mass detector (LC-MS).30
Scheme 2. Synthesis of TP10-S2-PQ: TP10-Cys, pre-assembled by SPPS,28 was reacted with PQ-Cys(Npys), previously prepared by coupling PQ to Boc-Cys(NPys)-OH.29.
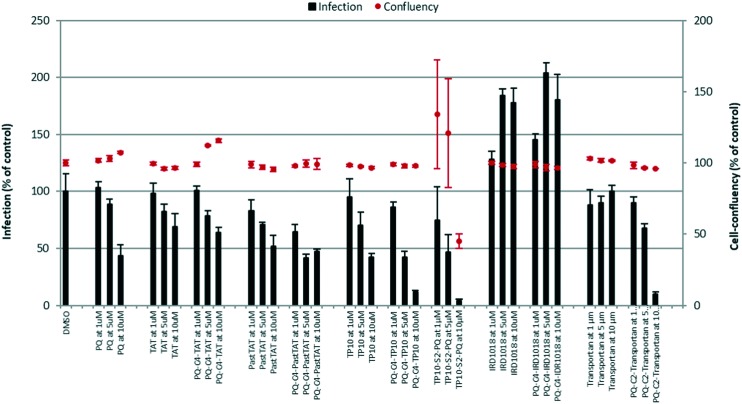
The synthetic PQ-C4-CPP conjugates, and the corresponding free CPP, were screened for their ability to inhibit in vitro hepatic infection by rodent P. berghei parasites at 1, 5 and 10 μM concentrations. Parasite load was assessed by luminescence measurements in lysates of Huh-7 cells31 infected 48 h earlier with sporozoites of a firefly luciferase-expressing P. berghei parasite line, as previously described,32 using PQ as the reference drug (Fig. 1).
Fig. 1. In vitro screening of the activity of the selected CPP and their respective PQ-C4-CPP conjugates against liver-stage P. berghei parasites, according to a previously reported method:32 infection burden (bars) and host cell confluency (dots) are expressed as % of control. The compounds were assayed at 1, 5 and 10 μM, and PQ was included as the reference parent drug, for comparison. The graph shows representative data from 3 independent experiments, each of which employing 3 replicates for each experimental condition.
Our results indicate that:
• all the test compounds (CPP and corresponding conjugates), except for TP10-S2-PQ, were not cytotoxic, as they did not affect confluency (dots in Fig. 1) of Huh-7 cells at any of the concentrations employed;
• with the exception of TP10, whose activity was only slightly lower than that of the reference drug, PQ, all other free (unconjugated) CPP ranged from inactive (IDR-1018, transportan) to poorly active (TAT, PasTAT) against liver stage P. berghei parasites;
• PQ-C4-CPP conjugates where the peptide building block was either transportan (PQ-C4-transportan) or the related peptide TP10 (PQ-C4-TP10) were more active than the respective free CPP and, more relevantly, than the reference drug, PQ;
• coupling PQ to TP10's C-terminus, as in TP10-S2-PQ, did not significantly improve activity, while leading to a notable increase in cytotoxicity; hence, drug conjugation to the CPP's N-terminus appears to be the best choice;
• CPP size and net charge do not seem to be key factors for activity, as (i) PQ-C4-TP10 and PQ-C4-transportan showed similar activities, despite their different size; (ii) PQ-C4-IDR1018 was inactive, despite having the same net charge as TP10, and (iii) TAT and PasTAT, as well as the corresponding PQ conjugates, did not dramatically differ from each other, despite the quite different molecular weights of these two CPP.
Collectively, it is shown that the activity of classical antimalarial drugs can be enhanced upon their conjugation with selected CPP. Specific amino acid sequence, rather than size or net charge of the CPP carriers, seems to determine whether the activity of the final conjugate will or will not be improved over that of the free parent drug. Ongoing work with the most promising TP10–PQ conjugates is focused on (i) confirmation of their higher metabolic stability towards oxidative deamination into carboxyprimaquine, and (ii) evaluation of their gametocytocidal, i.e., transmission-blocking activity. Expectations on the outcome of these studies are high, considering earlier reports on peptide and peptidomimetic conjugates of PQ.10,16,17,19,23 In addition, we are currently working on the chemical synthesis and in vitro screening of a wider panel of PQ–CPP conjugates, all of which share TP10 as the CPP carrier, but differ in the length, flexibility, and chemo/bio-lability of the drug-peptide linker employed. This will widen the chemical space available, to enable drawing relevant structure–activity relationships that may guide future development of CPP conjugates with other classical antimalarial drugs.
Conclusions
Altogether, this is an unprecedented report regarding PQ conjugation to CPP as a possible means to improve the drug's activity. Our encouraging findings warrant further pursuit of this approach, especially considering PQ's unique antiplasmodial properties, and the obvious advantages of acting against malaria at the initial liver stage of infection. Moreover, since resistance against artemisinin-based therapies is growing, and cases of re-sensitization to chloroquine have been reported,2 we are already applying the same concept to this and other classical blood-stage antimalarials, namely, mepacrine. Results thereof will be timely reported, and hopefully contribute for the establishment of drug conjugation to selected CPP carriers as an attractive strategy for the rescuing of known antimalarial drugs.
Conflicts of interest
There are no conflicts to declare.
Acknowledgments
The authors are indebted to Fundação para a Ciência e Tecnologia (FCT, Portugal) for funding LAQV-REQUIMTE research unit through project UID/QUI/50006/2013. NV thanks FCT and FEDER (European Union) for funding project IF/00092/2014/CP1255/CT0004 and IF position. LA thanks FCT and the Medicinal Biochemistry and Biochemistry International Doctoral Programme (M2B-PhD) for PhD grant PD/BD/106035/2015.
References
- Baird J. K. Expert Opin. Emerging Drugs. 2012;17:439–444. doi: 10.1517/14728214.2012.720252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Malaria Report 2017, World Health Organization, Geneva, 2017, ISBN 978–92–4-156552-3.
- Prudêncio M., Rodríguez A., Mota M. M. Nat. Rev. Microbiol. 2006;4:849–856. doi: 10.1038/nrmicro1529. [DOI] [PubMed] [Google Scholar]
- Gomes A., Machado M., Lobo L., Nogueira F., Prudêncio M., Teixeira C., Gomes P. ChemMedChem. 2015;10:1344–1349. doi: 10.1002/cmdc.201500164. [DOI] [PubMed] [Google Scholar]
- Ashley E. A., Recht J., White N. J. Malar. J. 2014;13:1–7. doi: 10.1186/1475-2875-13-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruah U. K., Gowthamarajan K., Ravisankar V., Karri V. V. S. R., Simhadri P. K., Singh V., Babu P. P. Artif. Cells, Nanomed., Biotechnol. 2017;0:1–21. doi: 10.1080/21691401.2017.1394870. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Baird J. K., Parise M. E., Lewis L. S., Ryan E. T., Magill A. J. Am. J. Trop. Med. Hyg. 2006;75:402–415. [PubMed] [Google Scholar]
- Vale N., Moreira R., Gomes P. Eur. J. Med. Chem. 2009;44:937–953. doi: 10.1016/j.ejmech.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Teixeira C., Vale N., Pérez B., Gomes A., Gomes J. R. B., Gomes P. Chem. Rev. 2014;114:11164–11220. doi: 10.1021/cr500123g. [DOI] [PubMed] [Google Scholar]
- Constantino L., Paixão P., Moreira R., Portela M. J., do Rosário V. E., Iley J. Exp. Toxicol. Pathol. 1999;51:299–303. doi: 10.1016/S0940-2993(99)80010-4. [DOI] [PubMed] [Google Scholar]
- Beutler E. Blood. 1994;84:3613–3636. [PubMed] [Google Scholar]
- Cappellini M. D., Fiorelli G. Lancet. 2008;371:64–74. doi: 10.1016/S0140-6736(08)60073-2. [DOI] [PubMed] [Google Scholar]
- Frampton J. E. Drugs. 2018;78:1517–1523. doi: 10.1007/s40265-018-0979-2. [DOI] [PubMed] [Google Scholar]
- Bhagavathula A. S., Elnour A. A., Shehab A. Infect. Dis. Poverty. 2016;5:1–12. doi: 10.1186/s40249-016-0196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraz R., Noronha J., Murtinheira F., Nogueira F., Machado M., Prudencio M., Parapini S., D'Alessandro S., Teixeira C., Gomes A., Prudencio C., Gomes P. RSC Adv. 2016;6:56134–56138. [Google Scholar]
- Matos J., Vale N., Collins M. S., Gut J., Rosenthal P. J., Cushion M. T., Moreira R., Gomes P. MedChemComm. 2010;1:199–201. [Google Scholar]
- Pérez B., Teixeira C., Albuquerque I. S., Gut J., Rosenthal P. J., Prudêncio M., Gomes P. MedChemComm. 2012;3:1170–1172. [Google Scholar]
- Matos J., da Cruz F. P., Cabrita É., Gut J., Nogueira F., do Rosário V. E., Moreira R., Rosenthal P. J., Prudêncio M., Gomes P. Antimicrob. Agents Chemother. 2012;56:1564–1570. doi: 10.1128/AAC.05345-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale N., Prudêncio M., Marques C. A., Collins M. S., Gut J., Nogueira F., Matos J., Rosenthal P. J., Cushion M. T., Mota M. M., Moreira R., Gomes P. J. Med. Chem. 2009;52:7800–7807. doi: 10.1021/jm900738c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copolovici D. M., Langel K., Eriste E., Langel Ü. ACS Nano. 2014;8:1972–1994. doi: 10.1021/nn4057269. [DOI] [PubMed] [Google Scholar]
- Baker J. K., Yarber R. H., Nanayakkara N. P., McChesney J. D., Homo F., Landau I. Pharm. Res. 1990;7:91–95. doi: 10.1023/a:1015899928897. [DOI] [PubMed] [Google Scholar]
- Vale N., Matos J., Gut J., Nogueira F., do Rosário V. E., Rosenthal P. J., Moreira R., Gomes P. Bioorg. Med. Chem. Lett. 2008;18:4150–4153. doi: 10.1016/j.bmcl.2008.05.076. [DOI] [PubMed] [Google Scholar]
- Vale N., Fernandes I., Moreira R., Mateus N., Gomes P. Drug Metab. Lett. 2012;6:15–25. doi: 10.2174/187231212800229273. [DOI] [PubMed] [Google Scholar]
- Brooks H., Lebleu B., Vives E. Adv. Drug Delivery Rev. 2005;57:559–577. doi: 10.1016/j.addr.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Takayama K., Nakase I., Michiue H., Takeuchi T., Tomizawa K., Matsui H., Futaki S. J. Controlled Release. 2009;138:128–133. doi: 10.1016/j.jconrel.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Mansour S. C., de La Fuente-Núñez C., Hancock R. E. W. J. Pept. Sci. 2015;21:323–329. doi: 10.1002/psc.2708. [DOI] [PubMed] [Google Scholar]
- Arrighi R. B. G., Ebikeme C., Jiang Y., Ranford-Cartwright L., Barrett M. P., Langel Ü., Faye I. Antimicrob. Agents Chemother. 2008;52:3414–3417. doi: 10.1128/AAC.01450-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solid-phase peptide synthesis (SPPS): all reactions were carried out in a Liberty1 Microwave Peptide Synthesizer (CEM Corporation). Generally, the peptide (C-terminal amide) was assembled by Fmoc/tBu SPPS methodologies, assisted with microwave (MW) energy. The resin was preconditioned for 15 min in DMF and then transferred into the MW-reaction vessel. The initial Fmoc deprotection step was carried out using 20% piperidine in DMF containing 0.1 M of 1-hydroxybenzotriazole (HOBt) in two MW irradiation pulses: 30 s at 24 W plus 3 min at 28 W, in both cases temperature being no higher than 75 °C. The C-terminal amino acid was then coupled to the deprotected Rink amide resin, using 5 molar equivalents (eq.) of the Fmoc-protected amino acid in DMF (0.2 M), 5 eq. of 0.5 M with HBTU/HOBt in DMF, and 10 eq. of 2 M DIEA in N-methylpyrrolidone (NMP); the coupling step was carried out for 5 min at 35 W MW irradiation, with maximum temperature reaching 75 °C. The remaining amino acids were sequentially coupled in the C → N direction by means of similar deprotection and coupling cycles, except for the incorporation of (i) Fmoc-Arg(Pbf)-OH, whose coupling was done in two steps, 25 min with no MW irradiation (room temperature) followed by 5 min coupling at 25 W; (ii) Fmoc-Cys(Trt)-OH, coupled also in two steps, first with 2 min coupling without MW irradiation (room temperature), then followed by 4 min coupling at 25 W, with maximum temperature reaching 50 °C. Following completion of the sequence assembly, the peptide was released from the resin with concomitant removal of side-chain protecting groups, by a 3 h acidolysis at room temperature using a trifluoroacetic acid (TFA)-based cocktail containing thioanisole, ethane-1,2-dithiol, and anisole as scavengers (TFA/thioanisole/ethane-1,2-dithiol/anisole 90 : 5 : 3 : 2 v/v/v/v). The crude peptides were precipitated and washed with cold diethyl ether
- Cleaved TP10-Cys was dissolved in a minimum volume of DMF and 1 eq. of PQ-Cys(Npys) was added. The reaction was stirred at room temperature and monitored by HPLC. Once the reaction was complete, the mixture was purified by preparative HPLC. Synthesis of the precursor PQ-Cys(Npys): Boc-Cys(Npys)-OH (1.2 eq.) was activated with HBTU (1.2 eq.) in the presence of DIEA (2.4 eq.), in DMF and the mixture was stirred in an ice-water bath for 10 minutes. Then, primaquine (1.0 eq.) was added, and the reaction was kept in an ice-water bath for 2 h. The mixture was diluted with ethyl acetate and extracted 4 times with an aqueous solution of Na2CO3 at 30%. The organic phase was dried over anhydrous MgSO4 and evaporated to dryness. The residue was then submitted to column chromatography on silica-gel, using DCM/methanol mixtures as eluents. The resulting compound, PQ-Cys(Npys)-Boc, was dissolved in neat trifluoroacetic acid (TFA) and the deprotection reaction allowed to proceed for 1 h in an ice-water bath. The mixture was evaporated to dryness and the residue was then submitted to column chromatography on silica-gel, using DCM/methanol mixtures as eluents, to isolate PQ-Cys(Npys)
- Peptide analysis by HPLC was done using a Hitachi-Merck LaChrom Elite system equipped with a quaternary pump, a thermostatted automated sampler, and a diode-array detector (DAD). Purification of peptides was done using preparative HPLC system (LaPrep Sigma), with LP1100 Quaternary LPG pump injection with fractionation valve. High resolution mass spectrometer attached to a liquid chromatography system (LC/MS/DAD) with electrospray (ESI) ion source from Thermo Scientific LTQ Orbitrap XL was also used for peptide analysis
- Huh7 cells employed in this work were obtained as a generous gift to Instituto de Medicina Molecular by Cenix Bioscience GmBH (Dresden, Germany), who, in turn, obtained an early passage of these cells from Bayer AG (Leverkusen, Germany)
- Ploemen I. H. J., Prudêncio M., Douradinha B. G., Ramesar J., Fonager J., van Gemert G. J., Luty A. J. F., Hermsen C. C., Sauerwein R. W., Baptista F. G., Mota M. M., Waters A. P., Que I., Lowik C. W. G. M., Khan S. M., Janse C. J., Franke-Fayard B. M. D. PLoS One. 2009;4:1–12. doi: 10.1371/journal.pone.0007881. [DOI] [PMC free article] [PubMed] [Google Scholar]