Summary
Objective:
To model breastfed infant growth and body composition patterns over the first 4 months with multiple bioactive components of human milk (HM) and clinical factors (including maternal BMI status), which are related to growth.
Methods:
Longitudinal observation of infant growth and body composition from 0 to 4 months among 41 predominantly breastfed infants (25 mothers of Normal-weight and 16 mothers with overweight/obesity). Fasted morning HM samples were collected at 5 time-points. Macronutrients, leptin, adiponectin, ghrelin, insulin, cytokines and n-6:n-3 esterified fatty acid ratio were measured. Infant weight-for-length Z-score (WLZ) trajectory, fat-free mass (FFM) gain, fat mass gain and %fat gain were modelled controlling for clinical covariates.
Results:
HM insulin negatively associated with WLZ trajectory among infants of NW mothers (P = 0.028), but not associated with WLZ trajectory among infants of OW/Ob mothers. HM glucose (P < 0.001) was associated with slower rates of infant FFM gain. Infants of mothers with OW/Ob exhibited slower rates of FFM gain. HM protein, adiponectin and insulin concentrations, and n-6:n-3 ratio were all significant predictors in the model of infant fat mass gain (P < 0.03). Any amount of formula supplementation was associated with faster fat gain (P = 0.002). The model of %fat gain was similar to that of fat mass gain, excepting HM adiponectin was not a significant covariate, and a trend for maternal OW/Ob to correlate with faster %fat gain (P = 0.056).
Conclusions:
Bioactive components in HM may contribute to regulation of partitioning of body composition, and these contributions may differ between mothers of normal-weight vs. with OW/Ob.
Introduction
The first months of life are a critical period of development, when nutritional cues provided by breastmilk can exert long-lasting programming effects (1). Exclusive breastfeeding is universally recommended due to the immunologic protection it provides the recipient infant (2,3). Exclusive breastfeeding also imparts a modest protective effect against later obesity (4–7) and type 2 diabetes (8,9). This protective effect is likely partly mediated by a reduction in rapid and excessive weight gain specifically during early infancy (1). However, the biological mechanisms remain controversial and may differ depending on maternal BMI status (5,10,11).
Human milk (HM) is a dynamic and complex substance that delivers a milieu of hormones and other bioactive components that support infant development and optimize health (12,13). Previous studies have characterized many individual components within HM known to modulate weight regulation in adults with obesity and rodent models, including leptin (14,15), adiponectin (14,16–18), insulin (14,19,20), cytokines (14) and fatty acids (21); all of which vary in HM according to maternal BMI. However, their role in regulation of infant body composition over time has not been well-studied. Furthermore, most studies of HM composition consider these constituents individually, and not acting in combination, as they are ingested by the breastfed infant.
Historical studies of infant growth focused on anthropometric measurements of infant size and growth characteristics and were unable to differentiate between programming influences that may have impacted the lean and fat mass compartments individually. Disproportionate postnatal accumulation of adipose (vs. lean tissue – as seen in ‘catch up growth’) is associated with later obesity (22,23). Understanding independent drivers of the adipose and fat-free mass (FFM) compartments in healthy breastfed infants may provide valuable insight into early postnatal programming events that could contribute to later risk of excess adiposity. This is particularly relevant among offspring born to mothers with elevated BMI.
We devised a longitudinal study of breastfed infants that incorporates extensive characterization of HM constituents, infant growth and infant body composition. This study builds upon our previous publication which detailed HM composition between mothers of normal weight (NW) vs. overweight/obesity (OW/Ob) (24). In the present work, we constructed holistic models of infant growth to test the hypothesis that HM factors are associated with the partitioning of infant FFM vs. fat mass accumulation during the first 4 months of life. Our extensive characterization of HM composition and maternal characteristics allowed us to control for the simultaneous delivery of multiple bioactive components within HM, as well as maternal factors such as gestational weight gain. Secondly, we were able to test if any relationships differed among offspring of NW vs. OW/Ob mothers. In this way, we have identified factors in HM that are associated with infant growth and may contribute to regulation of FFM or fat-mass accumulation independently during this critical window of postnatal programming.
Methods
Participants
All aspects of this study were approved by the Colorado Multiple Institutional Review Board (clinicaltrials.gov: NCT01693406). Women between 20 and 36 years of age with a pre-pregnancy BMI <40.0 kg/m2, carrying a singleton fetus and planning to exclusively breastfeed for at least 4 months were recruited and consented during pregnancy. Exclusion criteria included renal disease or diabetes, development of gestational diabetes, pregnancy induced hypertension or delivery <37 weeks gestation. All women delivered their infants at the University of Colorado Hospital (UC Health) at the Anschutz Medical Campus (Aurora, CO). Maternal pre-pregnancy BMI was based on self-report of pre-pregnant weight and measured height. Normal weight (NW) was defined as a pre-pregnant BMI <25 kg/m2; OW/Ob was defined as a pre-pregnant BMI ≥ 25 kg/m2.
Breast milk collections
A fasted mid-feed breast milk sample was collected at 2 weeks, 1, 2, 3 and 4 months, as previously described (24). Milk was immediately placed on ice and transported to the laboratory for processing. Skim milk was generated by spinning milk at 10 000 g at 4°C for 10 min. Aliquots of skim and whole milk were then stored at −80°C until analysis. Milk macronutrients (carbohydrate, protein and fat), caloric density, insulin, free glucose, leptin, adiponectin, 8-hydroxydeoxyguansoine (8OHdG) and inflammatory cytokines IL-6, IL-8 and TNF-α were measured as previously reported (24). Total esterified fatty acid composition in HM at 2 weeks and 4 months was quantified by lipid mass spectrometry as previously described (21). The total omega-6 (n-6) to omega-3 (n-3) ratio (n-6:n-3) was calculated as the quantitative sum (nmoles per nmole of triglyceride) of all n-6 fatty acids divided by the sum of all n-3 fatty acids (21).
Breastfeeding exclusivity
A breastfeeding exclusivity score was calculated at each study visit as the percentage of feeds in the past week that were breast milk. At 4 months, total breastfeeding exposure was calculated to reflect exclusivity over time (24). For example, a total breastfeeding exposure of 50% could indicate either exclusive breastfeeding for 2 months and then no breastfeeding for months 2 to 4, or 50% of feeds from breastmilk over the entire 4-month study. This study only included infants who were still receiving HM at 4 months and had a total breastfeeding exposure of ≥70%. A total of seven mother–infant dyads did not meet this feeding criterion and were excluded from the original cohort of 48 (24). These seven dyads exhibited an average breastfeeding exclusivity score of 22% (range: 16–37%).
Anthropometry and body composition
Study personnel visited mothers in the hospital within 48 h of delivery. Infant sex, birth weight and mode of delivery were obtained from medical records. Birth length was measured in triplicate by research staff using an infantometer (Ellard Instrumentation Ltd, Monroe, WA) that was accurate to 0.1 cm. At 2 weeks, 1, 2, 3 and 4 months, participants were seen for follow-up visits. At each visit, maternal height and weight were measured, and mothers were administered a modified version of the Infant Feeding Practices II questionnaire which queried about current feeding practices and breastfeeding exclusivity (25).
At each visit, infant tricep and subscapular skinfold thickness were measured in triplicate using skinfold callipers (Lange Skinfold Callipers, Cambridge Scientific Industries, Cambridge MD). Naked infant weight was measured in triplicate on a Sartorius Research Scale (Sartorius, Bradford, MA) that was accurate to 0.1 g and was calibrated at each visit. Infant length was measured in triplicate on an infantometer. All anthropometry measurements were performed by one of four research personnel who all underwent standardized training for infant anthropometry.
At 2 weeks and 4 months, infant %body fat was measured by air displacement plethysmography (PEAPOD; COSMED USA, Inc., Concord, CA), by the same investigator.
Calculations
Infant weight for length Z-scores (WLZ) were calculated according to the WHO standards for breastfed infants 0–24 months (26,27). At each follow-up measurement, an estimate of infant %body fat from skinfolds (SF %fat) was calculated based on infant sex, and tricep and subscapular skinfold measures (28). Differences in infant growth characteristics and HM composition between maternal BMI groups (NW vs. OW) were tested for using t-tests or non-parametric tests.
All variables were tested for normality using the Shapiro–Wilks test. Non-normal variables were log-transformed to ensure normality (HM insulin and cytokines) in multivariable models. HM insulin was considered in models as either a log-transformed linear variable or categorized into quintiles (based on the findings of Chan et al. (20)). The insulin variable that contributed the most parsimony to the model was included when relevant.
A HM inflammatory score was calculated as the standardized mean of IL-6, IL-8 and TNF-α at each time point, such that a score > 0 indicates higher than cohort-average inflammatory cytokine concentrations and a score < 0 indicates lower than cohort-average concentrations.
Models of infant WLZ
Linear mixed models were used to determine the association between infant WLZ change over time (trajectory) with longitudinal measures of HM analytes. Milk analytes were measured over time and were separated into two variables in the models. One variable represented the mean of the analyte level across all 5 time points (2 weeks, 1, 2, 3, 4 months) allowing for assessment of the differences between individuals. The second variable was the difference at each individual time point between a participant’s 5-time point mean and the measured analyte at that time point, allowing for assessment of the pattern of change for the milk analyte over time (differences within individuals). Because the n-6:n-3 ratio in HM was only measured a two time points (2 weeks and 4 months), and because the fatty acid composition of HM reflects maternal dietary intake (21), the 2-time point average n-6:n-3 ratio was used in the longitudinal models as an estimate of cumulative infant exposure over the 4-month study period. Final models were determined based on univariate statistical significance of HM analytes and non-milk factors identified as potential covariates. Potential covariates considered included maternal pre-pregnancy BMI group, birth weight, ponderal index, birth SF %fat, gestational age at delivery, gestational weight gain, maternal age, breastfeeding exclusivity, infant sex and mode of delivery. A potential interaction between each initially identified covariate and maternal BMI group was considered in the initial model.
Models of infant body composition (PEAPOD)
Infant rate of change in FFM (g/day) and %fat (%/day) was calculated from the change in infant fat mass (estimated by PEAPOD) from 2 weeks to 4 months, divided by the time in days elapsed. To test the primary hypothesis that HM factors are associated with the partitioning of infant FFM vs. fat mass accumulation during the first 4 months of life, multivariable regression was utilized to model these two outcomes as dependent variables. Potential covariates and predictors considered included maternal BMI group (NW vs. OW/Ob), birth weight, ponderal index, birth SF %fat, gestational age at delivery, gestational weight gain, maternal age, total breastfeeding exposure, infant sex, delivery type and average HM composition (lactose, fat, calories, insulin, glucose, leptin, adiponectin, 8OHdG and inflammatory score). Variables that were marginally statistically associated with the outcome in a univariate manner (P < 0.25) were included in an initial model, and backwards stepwise regression was utilized to reach the most parsimonious model. A potential interaction between each initially identified covariate and maternal BMI group was considered in the initial model.
Data analyses were performed using SAS 9.4 and JMP Pro13 (SAS Institute Inc, Cary, NC). Data are presented as mean ± SD, unless otherwise specified.
Results
Cohort characteristics
This cohort of predominantly breastfeeding dyads included 41 mother-infant pairs (25 NW and 16 OW/Ob). Characteristics of the cohort as a whole and by BMI group separately are presented in Table 1. Formula supplementation rates were low (minimum total breastfeeding exposure =70%). Only six of the 41 mothers (14.6%) were providing any supplementation at 4 months; these six dyads had an average total breastfeeding exposure of 86%. Thus, total breastfeeding exposure was dichotomized for entry into models of growth – separating women who provided any amount of formula supplementation vs. those exclusively breastfeeding through 4 months (85.4% of cohort).
Table 1.
Cohort characteristics
| Characteristic | Whole cohort1 | NW (n = 25)1 | OW/Ob (n = 16)1 | P1,2 |
|---|---|---|---|---|
| Maternal age (years) | 30.8 ±3.1 | 30.6 ± 2.6 | 31.0 ± 3.9 | 0.78 |
| Pre-pregnancy BMI (kg/m2) | 24.6 ±5.1 | 21.2 ± 1.9 | 29.9 ± 3.8 | <0.0001 |
| Maternal gestational weight gain (lb) | 31.1 ±11.2 | 32.5 ± 9.2 | 28.8 ± 13.9 | 0.31 |
| Gestational age (weeks) | 39.9 ± 0.9 | 39.9 ± 0.7 | 39.9 ± 1.0 | 0.79 |
| Delivery type (% vaginal) | 78% | 84% | 69% | 0.25 |
| Infant sex (%male) | 56.1% | 44% | 75% | 0.051 |
| Total breastfeeding exposure | 98 ± 7% | 97 ± 8% | 99 ± 2% | 0.288 |
NW, normal weight pre-pregnant maternal BMI.
OW/Ob, overweight/obese pre-pregnant maternal BMI.
Mean ± SD.
P-value for comparison between BMI groups.
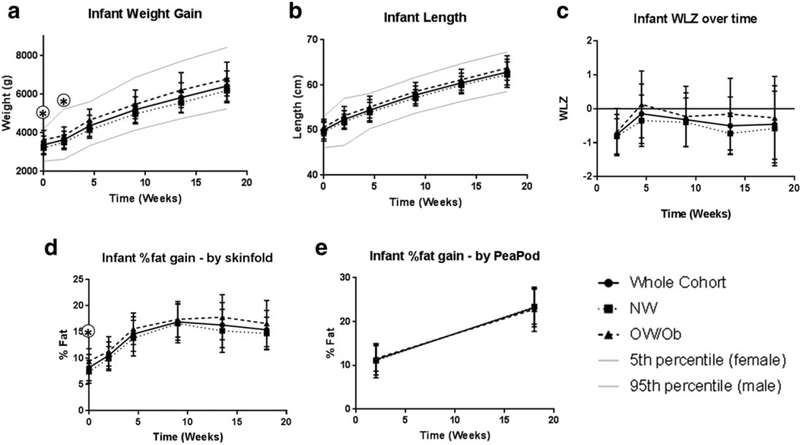
All infants were born at term (range for gestational age: 37.6–41.9 weeks) and birth weight ranged from 2665 to 4465 g. Infant growth and body composition characteristics in the cohort as a whole and by BMI groups separately are presented in Fig. 1. Controlling for sex, infants born to OW/Ob mothers were larger (3218 ± 354 g vs. 3639 ± 493 g; P = 0.01) and had a higher SF %fat (7.4 ± 2.2% vs. 9.4 ± 2.5% P = 0.02) at birth. This difference in weight remained significant at 2 weeks (P = 0.04), but thereafter there were no differences between groups in weight (controlling for sex), or any measures of growth or adiposity.
Figure 1.
Predominantly and exclusively breastfed infants were followed over the first 4 months of life. Anthropometry (weight, length and skinfolds) was measured at birth, 2 weeks, 1, 2, 3 and 4 months. Body composition was measured via air displacement plethysmography (PEAPOD) at 2 weeks and 4 months. Results are presented as mean ± SD for the cohort as a whole (solid line), in the normal weight (NW) group (dashed line) and the overweight/obese (OW/Ob) group (dotted line) individually. (A) Longitudinal infant weight (g). (B) Longitudinal infant length (cm). (C) Infant weight for length Zscore (WLZ) over time. (D) Infant %body fat gain as estimated by tricep and subscapular skinfold thicknesses 28. (E) Infant %body fat gain as measured by air displacement plethysmography (PEAPOD). * P-value for comparison between groups < 0.05. All comparisons were controlled for sex, with the exception of WLZ
Milk composition
Description of the HM composition of the entire cohort has been previously published (21,24). The milk macronutrients and bioactive molecule composition of this predominantly breastfed sub-set was representative of the previously published cohort and is presented in Table S1.
Infant growth modelling – longitudinal modelling of infant WLZ
The longitudinal model of infant WLZ trajectory is presented in Table 2A. A positive parameter estimate indicates a positive relationship with WLZ trajectory (an upwards trajectory). Insulin was the only HM component remaining significant in this model and the effect differed by maternal BMI. The association of HM insulin with WLZ was significantly different between the NW and OW/Ob mothers (P = 0.019), it was negatively associated with WLZ trajectory (P = 0.028) among infants of NW mothers, but not associated with WLZ trajectory among infants of OW/Ob mothers. As WLZ trajectory can be driven by shifts in either the FFM or fat mass compartments, we therefore pursued multivariable modelling of these individual compartments, as detailed below.
Table 2.
Longitudinal and multivariable models of infant WLZ trajectory, fat-free mass gain and fat mass gain
| 2 A. Longitudinal model of infant weight for length Z-score (WLZ) trajectory | |||
|---|---|---|---|
| Parameter | Estimate | SE | P |
| Intercept | −0.1725 | 0.2724 | 0.530 |
| Time | 0.001 | 0.001 | 0.278 |
| Maternal BMI group (0 = NW) | −0.316 | 0.307 | 0.306 |
| HM insulin differences between individuals | −0.042 | 0.019 | 0.028 |
| HM insulin differences within individuals | 0.003 | 0.003 | 0.237 |
| HM insulin differences between individuals × BMI group | 0.045 | 0.019 | 0.019 |
| 2 B. Multivariable model of fat-free mass deposition (g/day; by PEAPOD) | |||
| Parameter | Estimate | SE | P |
| Intercept | 24.4 | 2.3 | <.0001 |
| Infant sex (0 = male) | −2.355 | 0.810 | 0.007 |
| Maternal BMI group (0 = NW) | −10.27 | 3.10 | 0.002 |
| Birth SF %fat | −0.192 | 0.212 | 0.373 |
| Birth SF %fat × maternal BMI group | 1.226 | 0.351 | 0.001 |
| HM glucose concentration | −0.265 | 0.069 | 0.001 |
| HM ghrelin concentration | −0.003 | 0.001 | 0.021 |
| 2 C. Multivariable model of fat mass deposition (g/day; by PEAPOD) | |||
| Parameter | Estimate | SE | P |
| Intercept | 85.3 | 19.3 | 0.0002 |
| Gestational age at delivery | −1.97 | 0.49 | 0.0003 |
| Total breastfeeding exposure (0 = exclusive) | 1.83 | 0.055 | 0.002 |
| Average HM n-6:n-3 ratio | 1.29 | 0.33 | 0.0005 |
| Average HM protein | −5.63 | 1.65 | 0.002 |
| Average HM adiponectin | −0.11 | 0.05 | 0.027 |
| Quintile of average HM insulin1 | N/A | 0.010 | |
| Insulin quintile 2−1 | 0.293 | 1.18 | 0.806 |
| Insulin quintile 3−2 | −1.43 | 1.17 | 0.228 |
| Insulin quintile 4−3 | 4.40 | 1.28 | 0.002 |
| Insulin quintile 5−4 | −4.90 | 1.41 | 0.002 |
Bimodal distribution.
Infant growth modelling – rate of infant FFM accumulation/day
The multivariable model results for rate of infant FFM accumulation (g/day, as estimated by PEAPOD) are presented in Table 2B. As expected, male infants exhibited faster FFM accrual (P = 0.007) (29). Infants born to OW/Ob mothers showed significantly slower FFM accumulation than infants born to NW mothers (P = 0.002). Furthermore, a higher SF %fat birth was associated with a faster rate of FFM deposition only among infants of OW/Ob mothers (‘infant SF %fat at birth × Maternal BMI Group’ interaction term P = 0.001). Both HM glucose and HM ghrelin were associated with a slower rate of FFM deposition, but the effect size of HM glucose was of clinical significance (–0.27 g/day); this estimate indicated that for every 6 mg/dL increase in HM glucose (the standard deviation of this measurement), the infant lays down 178 fewer grams of FFM over the study interval. All the predictor variables presented in Table 2B together explained 52% of the variability in rate of FFM gain (Model R2 = 0.52).
Infant growth modelling – modelling of infant fat mass gain/day
The multivariable model of rate of infant fat gain (g/day, as estimated by PEAPOD) is presented in Table 2C. As expected in the context of ‘catch-up growth’, earlier gestational age was associated with faster fat deposition (P < 0.001). The total breastfeeding exposure parameter in the model demonstrates that any degree of formula supplementation was associated with a higher rate of fat deposition (P < 0.001) that would be equivalent to roughly 200 g more body fat by 4 months. We have reported that HM n-6:n-3 ratio was associated with a faster %fat gain in our larger cohort. HM n-6:n-3 ratio was associated with a faster fat gain (P < 0.001) indicating that a 1.5 unit increase in HM n-6:n-3 ratio (the approximate standard deviation of this measure) would correlate with roughly 217 g more body fat over the study period. HM protein concentrations were inversely associated with the rate of fat gain indicating that a 0.3 unit increase in HM protein (the approximate standard deviation of this measure) would correlate with roughly 189 g less body fat over the study period. HM adiponectin concentrations were inversely associated with the rate of fat gain indicating that a 13.6 unit increase in HM protein (the approximate standard deviation of this measure) would correlate with roughly 168 g less body fat over the study period. The nature of the relationship between HM insulin quintiles and %fat gain/day was a complex bimodal distribution (an ‘M-shaped’ curve). All the predictor variables presented in Table 2C together explained 47% of the variation in infant %fat gain /day (Model R2 = 0.47).
Infant growth modelling – modelling of infant %fat gain/day
The multivariable model of rate of infant %fat gain (%/day, as estimated by PEAPOD) is presented in Table S2. The results were similar to the model of fat mass gain. Differences included that the model of %fat gain/day indicated a trend for maternal pre-pregnancy OW/Ob to be associated with a greater rate of %fat increase (P = 0.056) that would be equivalent to a 1.2% higher %body fat at 4 months. An additional difference as that HM adiponectin did not remain a significant predictor in the model of %fat gain/day. The other predictor variables were the same between the model of %fat and fat mass g/day. All the predictor variables presented in Table S2 together explained 50% of the variation in infant %fat gain /day (Model R2 = 0.50).
Discussion
The first months of life are a critical period of accelerated growth of both the fat and FFM compartments. There is increasing evidence that the partitioning of early life nutrients into fat and/or FFM associates with the risk for later life obesity and associated metabolic diseases (22,23,30). The precise mechanisms driving this partitioning are unclear. Our results suggest that specific components present in HM may contribute differently to the way that breastfed infants partition FFM vs. fat mass and that this effect may differ depending on maternal weight status.
We were able to construct sophisticated models of infant WLZ trajectory utilizing all of the longitudinal growth data collected at 5 time points. In a model of WLZ trajectory, the effect of HM insulin varied by maternal BMI group. In NW women, HM insulin was associated with a downward WLZ trajectory, while in OW/Ob women HM insulin was not associated with WLZ trajectory. This interaction is consistent with ‘fetal programming’ indicating that infants who were gestated by an OW/Ob mother exhibit les of a response to HM insulin that infants gestated by NW women. However, the variability in WLZ trajectories observed was all within the normal range for the ‘gold standard’ of growth, i.e., that expected from healthy breastfed infants (Fig. 1). None of these infants exhibited excessive or poor weight gain and relationships with growth rates should be interpreted with caution. Our body composition models were used to provide insight into whether increased fat-mass, or limited length gains were underlying WLZ trajectory differences.
Our models of infant FFM accrual indicated that infants of OW/Ob mothers put on less overall FFM, but among these infants born to OW/Ob mothers, a higher %fat (by skinfold) at birth was associated with faster FFM gain. This insight is interesting because this interaction was not present in models of fat mass. Thus, infants of OW/Ob mothers particularly (who are potentially at genetic risk of later obesity) seem to ‘normalize’ %body fat by exhibiting faster FFM mass deposition, without any difference in fat mass deposition, when born with higher %body fat. All infants in this cohort exhibited normal growth and %body fat levels at 4 months, so variation observed was all within normal growth limits. This observation supports the benefit of exclusive breastfeeding to achieve healthy normalized growth and potentially mitigate effects of in-utero exposure.
These effects may be mediated by concentrations of HM glucose and ghrelin which were both associated with slower rates of FFM deposition; the effect size of HM glucose carried clinical significance (one standard deviation increase in HM glucose translated to 178 fewer grams of FFM between 2 weeks and 4 months). A small cross-sectional study (n = 19) reported that HM glucose was positively related to infant WLZ and a trend for positive relationship with FFM (P < 0.10) (19), contradicting our results. However, a subsequent publication of longitudinal data from the same cohort did not replicate the relationship with HM glucose (31). Furthermore, our analysis controlled for maternal prandial state during milk collection, which would be expected to reduce HM glucose variability. The mechanism whereby HM glucose may impact infant growth is unclear. The concentration of HM glucose is too low to contribute significantly to caloric variation in HM, and thus any impact on growth is likely indirect. It is possible that glucose in HM could impact gastric and upper gastro-intestinal hormonal signalling, indirectly affecting FFM accumulation over time.
In our models of fat mass gain over time, any degree of formula supplementation (total breastfeeding exposure) was associated with faster fat gain. This is notable because supplementation was minimal – the supplementing group (<15% of infants) was providing an average of 2.6 oz formula/day at 4 months. Our model also suggested that HM with a higher n-6:n-3 ratio was predictive of faster fat mass gain. This finding replicates a similar finding detected in the larger cohort that did not account for other factors of HM composition (21). Mean HM adiponectin concentrations were inversely correlated with fat mass gain. This finding has been detected in other studies of breast milk composition and infant growth (16,18). Mean HM insulin quintiles remained significant in the model of infant fat mass gain, which suggests that the role of insulin in the model of WLZ trajectory may be via regulation of the fat-mass compartment. Chan et al. recently reported a U-shaped relationship between HM insulin and infant WLZ in a large cross-sectional study (20). Our data were longitudinal, included body fat measures and milk sampling controlled for maternal prandial state, which may have contributed to our detection of a more complicated relationship. The complicated nature of the relationship between HM insulin quintile and infant %fat gain may be due to the multifactorial impact of HM insulin on infant intestinal maturation, gene expression, blood glucose regulation, microbiome and potential interactive effects of insulin with other components of HM (32,33). Layering of these various effects of HM insulin atop individual fetal programming and HM hormonal milieu likely underlies the complexity of the relationship detected. This is an area where more research is clearly called for.
Our models of infant %fat gain over time provided very similar results to the model of fat mass gain. A notable exception was HM adiponectin, which was not identified as a predictor of %fat gain. Secondly, elevated maternal BMI (Ow/Ob) tended (P = 0.056) to be associated with a faster rate of %fat gain even among this predominantly breastfed cohort. It is important to note that this cohort exhibited normal growth rates and body composition; thus, faster %fat gain in the OW/Ob group does not necessarily imply increased obesity risk. However, it is noteworthy that maternal obesity status possibly contributes to infant %fat gain (but not fat mass) in the context of predominant breastfeeding, while controlling for HM composition.
The novelty of this study lies in our ability to model holistic growth (WLZ) as well as the %fat and FFM compartments of growth while considering the multiple bioactive contributors in HM as potential regulators of growth simultaneously. Our milk sampling methodology was well-controlled and rigorous. The minimal formula supplementation rates and longitudinal data design are additional strengths. Limitations include lack of measurements of HM volume intake. Furthermore, our sample size limits our ability to detect mores subtle relationships that may be underlying these complex physiological outcomes. It also limits the power we have to construct models of this nature, and larger cohorts are necessary to con-firm replication of findings.
Together, these data indicated that individual bioactive components of HM may regulate different compartments of infant weight gain separately. Specifically, HM protein, n-6:n-3 ratio and insulin may contribute to adiposity, while HM glucose may contribute to accumulation of lean mass. Of note, these data suggest that breastfeeding and HM may mitigate the risk imposed by in-utero exposure to maternal OW/Ob, strengthening recommendations for exclusive breastfeeding, especially in infants at risk for later obesity.
Supplementary Material
Milk composition (mean ± SD)
Multivariable model of infant %fat gain (%/day; by PEAPOD)
Acknowledgements
We wish to deeply thank the mothers and infants who participated in this research. We also wish to thank Claire Westcott, BS; Catherine Chartier-Logan, MPH; Melanie Reece, PhD; Becky de La Houssaye, MS; and Zachary Patinkin, MPH for support with study execution. We wish to acknowledge the Mass Spectrometry Lipidomics Core Facility for kindly providing use of instruments.
Funding
This study was supported by:
• NIH NICHD F32-HD0978068 (PI: B.E.Y.);
• NIH NIDDK: K24-DK083772 (PI: N.F.K.), T32-DK007658–21 (PI: N.F.K.) and K01-DK109079 (PI: M.C.R.);
• Thrasher Research Fund Early Career Award (PI: B.E.Y.);
• Center for Women’s Health Research at the University of Colorado Anschutz Medical Campus (PI: B.E.Y.);
• Colorado Clinical and Translational Sciences Institute (CCTSI) with the Development and Informatics Service Center (DISC) grant support (NIH/NCRR Colorado CTSI Grant Number UL1 RR025780; PI: B.E.Y.);
• NIH/NCATS Colorado CTSA Grant UL1 TR001082 and Colorado Clinical and Translational Science Institute grants UL1 RR025780;
• BIRCWH Scholar Award K12-HD057022 (M.C.R.);
• University of CO NORC Pilot award P30-DK048520 (M.C.R.).
Footnotes
Conflicts of Interest
All authors have no conflict of interest to disclose.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.Young BE, Johnson SL, Krebs NF. Biological determinants linking infant weight gain and child obesity: current knowledge and future directions. Adv Nutr 2012; 3: 675–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breastfeeding ASo. Breastfeeding and the use of human milk. Pediatrics 2012; 129: e827–e841. [DOI] [PubMed] [Google Scholar]
- 3.Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev 2012;(8): CD003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan J, Liu L, Zhu Y, Huang G, Wang PP. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health 2014; 14: 1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer-Davis EJ, Rifas-Shiman SL, Zhou L, Hu FB, Colditz GA, Gillman MW. Breast-feeding and risk for childhood obesity: does maternal diabetes or obesity status matter? Diabetes Care 2006; 29: 2231–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hancox RJ, Stewart AW, Braithwaite I, et al. Association between breastfeeding and body mass index at age 6–7 years in an international survey. Pediatr Obes 2015; 10: 283–287. [DOI] [PubMed] [Google Scholar]
- 7.Modrek S, Basu S, Harding M, et al. Does breastfeeding duration decrease child obesity? An instrumental variables analysis. Pediatr Obes 2017; 12: 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. Am J Clin Nutr 2006; 84: 1043–1054. [DOI] [PubMed] [Google Scholar]
- 9.Williams DM, Martin RM, Davey Smith G, Alberti KG, Ben-Shlomo Y, McCarthy A. Associations of infant nutrition with insulin resistance measures in early adulthood: evidence from the Barry-Caerphilly Growth (BCG) study. PloS one 2012; 7: e34161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C, Kaur H, Choi WS, Huang TT, Lee RE, Ahluwalia JS. Additive interactions of maternal prepregnancy BMI and breast-feeding on childhood overweight. Obes Res 2005; 13: 362–371. [DOI] [PubMed] [Google Scholar]
- 11.Buyken AE, Karaolis-Danckert N, Remer T, Bolzenius K, Landsberg B, Kroke A. Effects of breastfeeding on trajectories of body fat and BMI throughout childhood. Obesity 2008; 16: 389–395. [DOI] [PubMed] [Google Scholar]
- 12.Garofalo R Cytokines in human milk. J Pediatr 2010; 156: S36–S40. [DOI] [PubMed] [Google Scholar]
- 13.Groer MW, Shelton MM. Exercise is associated with elevated proinflammatory cytokines in human milk. JObstetGynecolNeonatal Nurs 2009; 38: 35–41. [DOI] [PubMed] [Google Scholar]
- 14.Fields DA, Schneider CR, Pavela G. A narrative review of the associations between six bioactive components in breast milk and infant adiposity. Obesity (Silver Spring) 2016; 24: 1213–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miralles O, Sanchez J, Palou A, Pico CA. physiological role of breast milk leptin in body weight control in developing infants. Obesity (Silver.Spring) 2006; 14: 1371–1377. [DOI] [PubMed] [Google Scholar]
- 16.Woo JG, Guerrero ML, Altaye M, et al. Human milk adiponectin is associated with infant growth in two independent cohorts. BreastfeedMed 2009; 4: 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weyermann M, Brenner H, Rothenbacher D. Adipokines in human milk and risk of overweight in early childhood: a prospective cohort study. Epidemiology 2007; 18: 722–729. [DOI] [PubMed] [Google Scholar]
- 18.Woo JG, Guerrero ML, Guo F, et al. Human milk adiponectin affects infant weight trajectory during the second year of life. J Pediatr Gastroenterol Nutr 2012; 54: 532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fields DA, Demerath EW. Relationship of insulin, glucose, leptin, IL-6 and TNF-alpha in human breast milk with infant growth and body composition. PediatrObes 2012; 7: 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan D, Goruk S, Becker AB, et al. Adiponectin, leptin and insulin in breast milk: associations with maternal characteristics and infant body composition in the first year of life. Int J Obes (Lond) 2018; 42(1): 36–43. [DOI] [PubMed] [Google Scholar]
- 21.Rudolph MC, Young BE, Lemas DJ, et al. Early infant adipose deposition is positively associated with the n-6 to n-3 fatty acid ratio in human milk independent of maternal BMI. Int J Obes (Lond) 2017; 41: 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dulloo AG, Jacquet J, Seydoux J, Montani JP. The thrifty ‘catch-up fat’ phenotype: its impact on insulin sensitivity during growth trajectories to obesity and metabolic syndrome. Int J Obes (Lond) 2006; 30: S23–S35. [DOI] [PubMed] [Google Scholar]
- 23.Ay L, Van Houten VA, Steegers EA, et al. Fetal and postnatal growth and body composition at 6 months of age. J Clin Endocrinol Metab 2009; 94: 2023–2030. [DOI] [PubMed] [Google Scholar]
- 24.Young BEPZ, Palmer C, de la Houssaye B, et al. Human milk insulin is related to maternal plasma insulin and BMI – but other components of human milk do not differ by BMI. Eur J Clin Nutr 2017; 71: 1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fein SB, Labiner-Wolfe J, Shealy KR, Li R, Chen J, Grummer-Strawn LM. Infant Feeding Practices Study II: study methods. Pediatrics 2008; 122: S28–S35. [DOI] [PubMed] [Google Scholar]
- 26.Group WHOMGRS. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr 2006; 450: 76–85. [DOI] [PubMed] [Google Scholar]
- 27.Center for Disease Control and Prevention (CDC). Growth Charts. In: WHO Growth Standards Are Recommended for Use in the U.S. for Infants and Children 0 to 2 Years of Age.: Atlanta, GA, 2010. [Google Scholar]
- 28.Dauncey MJ, Gandy G, Gairdner D. Assessment of total body fat in infancy from skinfold thickness measurements. Arch Dis Child 1977; 52: 223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Organization WH. The WHO child growth standards. In
- 30.Dulloo AG. Thrifty energy metabolism in catch-up growth trajectories to insulin and leptin resistance. Best Pract Res Clin Endocrinol Metab 2008; 22: 155–171. [DOI] [PubMed] [Google Scholar]
- 31.Fields DA, George B, Williams M, et al. Associations between human breast milk hormones and adipocytokines and infant growth and body composition in the first 6 months of life. Pediatr Obes 2017; Suppl 1: 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemas DJ, Young BE, Baker PR 2nd, et al. Alterations in human milk leptin and insulin are associated with early changes in the infant intestinal microbiome. Am J Clin Nutr 2016; 103: 1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shehadeh N, Sukhotnik I, Shamir R. Gastrointestinal tract as a target organ for orally administered insulin. J Pediatr Gastroenterol Nutr 2006; 43: 276–281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Milk composition (mean ± SD)
Multivariable model of infant %fat gain (%/day; by PEAPOD)