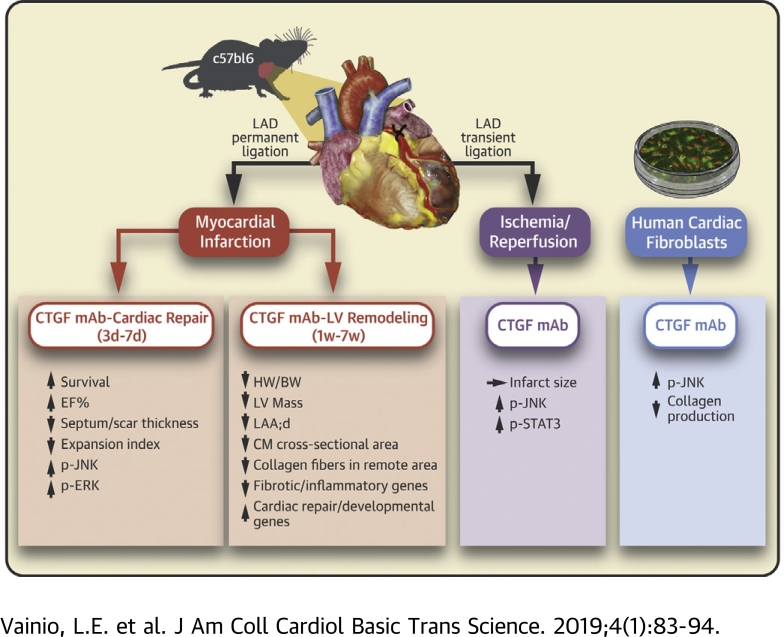
Visual Abstract
Key Words: connective tissue growth factor monoclonal antibody, fibrosis, heart failure, ischemia−reperfusion injury, left ventricle, myocardial infarction
Abbreviations and Acronyms: CTGF, connective tissue growth factor; ECM, extracellular matrix; ERK, extracellular signal-regulated kinase; FB, fibroblast; I/R, ischemia−reperfusion; HF, heart failure; Ig, immunoglobulin; JNK, c-Jun N-terminal kinase; LV, left ventricular; mAb, monoclonal antibody; MI, myocardial infarction; TGF, transforming growth factor
Highlights
-
•
To study the role of CTGF in post-MI cardiac repair and LV remodeling, we antagonized the function of CTGF with a mAb.
-
•
Treatment of mice with CTGF mAb during post-MI cardiac repair improved survival and resulted in better preserved LV systolic function.
-
•
Treatment with CTGF mAb during post-MI LV remodeling reduced the heart weight to body weight ratio, LV mass, cardiomyocyte hypertrophy, and fibrosis in the remote nonischemic myocardium.
-
•
CTGF mAb treatment induced c-Jun N-terminal kinase phosphorylation in ischemic hearts in vivo and in cultured human cardiac fibroblasts.
-
•
In conclusion, treatment of mice with CTGF mAb in a model of MI enhances cardiac repair and reduces adverse post-MI LV remodeling.
Summary
Myocardial infarction (MI)−induced cardiac fibrosis attenuates cardiac contractile function, and predisposes to arrhythmias and sudden cardiac death. Expression of connective tissue growth factor (CTGF) is elevated in affected organs in virtually every fibrotic disorder and in the diseased human myocardium. Mice were subjected to treatment with a CTGF monoclonal antibody (mAb) during infarct repair, post-MI left ventricular (LV) remodeling, or acute ischemia−reperfusion injury. CTGF mAb therapy during infarct repair improved survival and reduced LV dysfunction, and reduced post-MI LV hypertrophy and fibrosis. Mechanistically, CTGF mAb therapy induced expression of cardiac developmental and/or repair genes and attenuated expression of inflammatory and/or fibrotic genes.
Heart disease is the leading cause of death in the western world with almost one-half of those deaths attributable to coronary heart disease (1). In response to cardiac stresses, such as myocardial infarction (MI), the heart undergoes structural and functional remodeling, with cardiomyocyte hypertrophy and excessive production of the extracellular matrix (ECM) as typical features (2). Molecular mechanisms that underlie cardiac fibrotic disorders are still mostly unclear, and no specific therapies exist for treatment of myocardial fibrosis.
Connective tissue growth factor (CTGF/CCN2) belongs to the CCN family (Connective tissue growth factor [CTGF], Cystein rich protein [CYR61], and Nephroblastoma overexpressed [NOV]) of matricellular proteins that consists of 6 homologous cysteine-rich proteins (3). Dysregulation of CCN protein expression or activities takes place in chronic inflammation or tissue injury, such as fibrosis, atherosclerosis, restenosis after vascular injury, arthritis, cancer, diabetic nephropathy, and retinopathy 3, 4. CTGF expression is elevated in human fibrotic diseases of virtually every organ or tissue (4). Patients with heart failure (HF) show elevated levels of plasma CTGF, which correlates with the severity of the disease (5). Plasma levels of CTGF are also useful in differentiating acute HF patients from patients with other causes of dyspnea and peripheral edema (6). CTGF expression in the myocardium is also induced in various animal models of myocardial fibrosis (for review, see Daniels et al. [7] and Leask [8]). Cardiomyocyte-specific overexpression of CTGF in transgenic mice alone did not induce fibrosis but did enhance pressure overload−induced cardiac fibrosis (9). On the other hand, pressure overload induced fibrosis was not attenuated in mice where CTGF was deleted in cardiomyocytes and cardiac fibroblasts (10), but not from other cell types in which CTGF may have been produced (11). However, no data are available from studies in which the function of CTGF was antagonized in the ischemic heart or during post-MI fibrotic remodeling.
FG-3019 (pamrevlumab) is a human monoclonal antibody (mAb) against CTGF that has shown efficacy in a randomized, placebo-controlled phase 2 clinical trial in subjects with idiopathic pulmonary fibrosis (12), as well as in phase 2 clinical trials for treatment of pancreatic cancer and Duchenne muscular dystrophy (NCT02210559 and NCT02606136, respectively). A chimeric antibody, designated FG-3149, has the binding motif of FG-3019 and a mouse IgG2a constant region. FG-3149 binds CTGF with similar affinity as FG-3019 but is less immunogenic in rodents than the human antibody. FG-3149 has shown activity in animal models of bronchopulmonary dysplasia (13), pressure overload−induced HF (14), and genetic cardiomyopathy 15, 16. In the present study, we aimed to investigate the role of CTGF in cardiac repair following MI, in post-MI cardiac fibrosis, and in acute ischemia−reperfusion (I/R) injury.
Methods
Study design
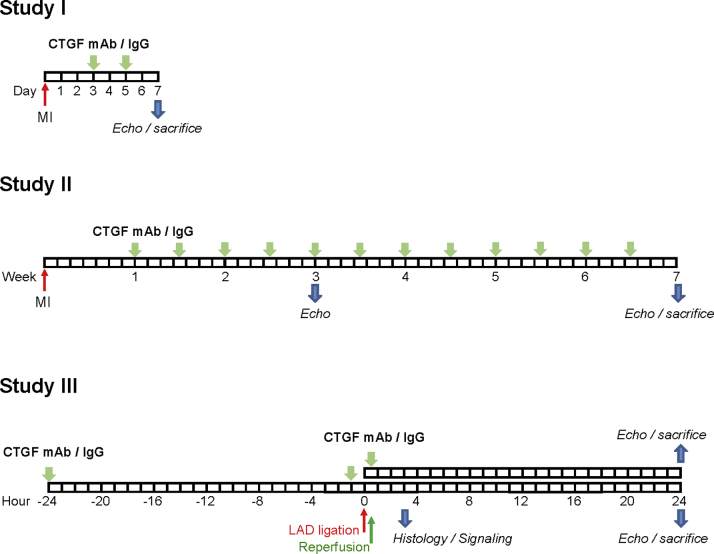
The experimental design was approved by Animal Experiment Committee in State Provincial Office of Southern Finland, and the methods were carried out in accordance with the national regulations of the usage and welfare of laboratory animals. Mice were subjected to MI by permanent ligation of the left anterior descending coronary artery or to I/R injury by transient ligation of the left anterior descending coronary artery, and treated with either CTGF mAb or control mouse immunoglobulin-G (IgG). The protocols are shown in Figure 1. A more detailed description of Methods is available in the Supplemental Material.
Figure 1.
Summary of Experimental Protocols
Study I, protocol for inhibition of connective tissue growth factor (CTGF) during post—myocardial infarction (MI) cardiac repair (treatment initiation on day 3, treatment completion on day 7). Study II, protocol for CTGF inhibition during post-MI cardiac remodeling (treatment initiation on day 7, treatment completion at the end of week 7). Study III, protocol for CTGF inhibition during and/or after acute ischemia (treatment 24 and 1 h before ischemia or only at reperfusion). IgG = immunoglobulin-G; LAD = left anterior descending; mAb = monoclonal antibody.
RNA sequencing analysis
RNAseq analysis was performed via single-end sequencing chemistry at a 75 base-pair read length (Illumina NextSeq, Illumina, Inc., San Diego, California). Sequences were de-multiplexed, and FASTQ generation was performed (Basespace, Illumina). Sequences were aligned to 10 mm, annotated using the RefSeq Gene 2013.04.01 build, and gene expression levels were quantitated using reads per kilobase of transcript, per million mapped reads (RPKM, Strand NGS, Strand Life Sciences, Bengaluru, India). Genes with a raw read count of >20 in at least 1 sample were used for further analysis. Altered transcripts were defined as having a >1.5-fold difference in expression at p < 0.05 (t-test). Gene ontology analysis was performed using gene ontology consortium software 17, 18. To identify common upstream regulators, gene sets were loaded into Pathway Studio MammalPlus 12.0.1.9 (Elsevier, Amsterdam, the Netherlands), and links to common regulators were identified.
Statistical analysis
Statistical analysis was performed with SPSS software (IBM, Armonk, New York). When 2 groups were compared, Student’s t-test or Mann-Whitney U test was performed. To compare multiple groups, 1-way analysis of variance was used, followed by Tukeys’s post hoc test to compare all the groups or Dunnett’s post hoc test to compare other groups with the control IgG-treated MI or I/R group. The Kruskall-Wallis test was performed when data did not represent normal distribution. Survival analysis was calculated by the Kaplan-Meier method, and groups were compared by the log-rank (Mantel-Cox) test. Data are shown as mean ± SD. Differences were considered statistically significant at p < 0.05.
Results
Therapy with CTGF mAb during post-MI cardiac repair improves survival (Study I)
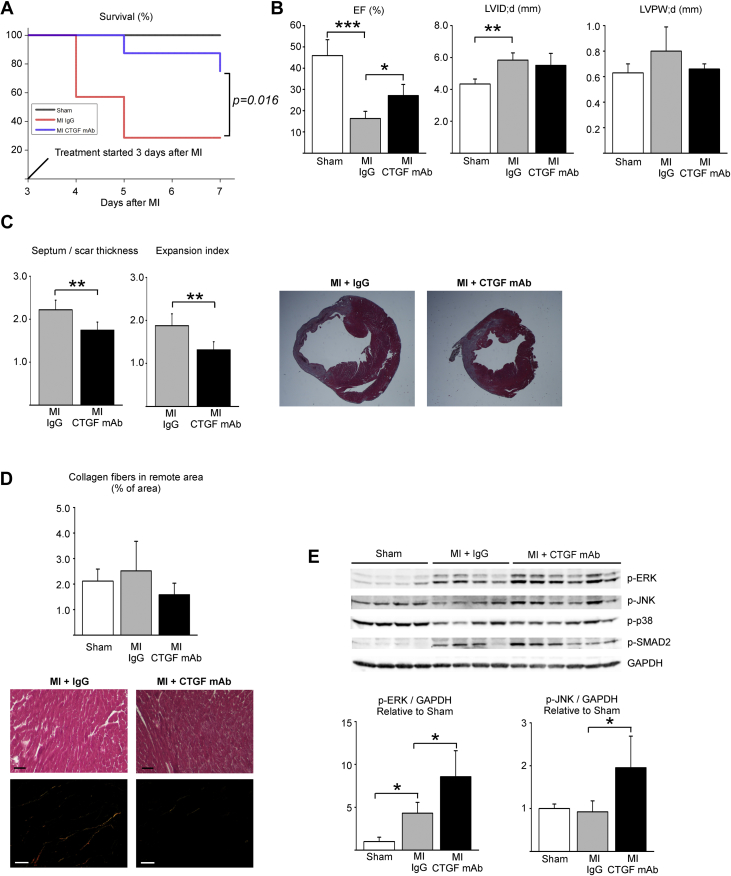
To investigate for the effect of CTGF mAb during cardiac repair after MI, mice were subjected to experimental MI, and 3 days after ligation, treatment began with either IgG or CTGF mAb for 4 days (Study I) (Figure 1). CTGF mAb significantly improved post-MI survival (p < 0.05) (Figure 2A). Echocardiography analysis at 7 days post-MI showed better preserved LV systolic function in mice treated with CTGF mAb compared with mice treated with control IgG (ejection fraction: 27.1 ± 5.2% vs. 16.3 ± 3.4%; p < 0.05) (Figure 2B, Table 1). No difference was observed in LV diameter or LV posterior wall thickness (Figure 2B, Table 1). Analysis for inflammation in the infarcted area by CD45 staining showed no difference between MI groups, and no difference was observed in expression of inflammatory genes in the remote LV between the MI groups (Supplemental Figure 1). Histological analysis of LV sections showed a decrease in septum thickness versus the scar thickness ratio in CTGF mAb−treated mice compared with control IgG-treated mice, which resulted in an observed decrease in the infarct expansion index (Figure 2C). Analysis for collagen content in the remote zone showed no difference between the experimental groups (Figure 2D). Analysis for central signaling pathways from the infarct scar showed induction of extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase 2 (JNK2) phosphorylation in MI hearts treated with CTGF mAb (Figure 2E). No difference was observed in p38 or SMAD2 signaling between the MI groups (Figure 2E).
Figure 2.
CTGF mAb Enhances LV Function (Study I)
Mice were subjected to MI, and 3 days after surgery randomly divided to receive either IgG vehicle or CTGF mAb for 4 days. (A) Survival of animals during the experiment. (B) Left ventricular (LV) ejection fraction (EF), end-diastolic dimension (LVID;d), and posterior wall thickness (LVPW;d) were analyzed by echocardiography at 7 days after MI injury. (C) Ratio of thickness of septum versus thickness of infarct and the infarct expansion index. (D) Analysis of interstitial fibrosis from picrosirius red−stained LV sections under polarized light. Masson’s trichrome-stained sections from the same tissue block are also shown. Scale bar: 50 μm. (E) Western blot analysis of LV samples from infarct areas for phosphorylated extracellular signal-regulated kinase (p-ERK), c-Jun N-terminal kinase (p-JNK), p38, and SMAD2. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as loading control. Data are presented as mean ± SD; number of animals was sham (n = 4), IgG (n = 5), and CTGF mAb (n = 8). *p < 0.05; **p < 0.01; ***p < 0.001. Abbreviations as in Figure 1.
Table 1.
CTGF mAb Therapy During Post-MI Cardiac Repair Enhances LV Function (Study I)
| Sham (n = 4) | MI + IgG (n = 5) | MI + CTGF mAb (n = 8) | |
|---|---|---|---|
| LVEDD (mm) | 4.34 ± 0.31 | 5.84 ± 0.45∗ | 5.51 ± 0.75∗ |
| LVESD (mm) | 3.43 ± 0.31 | 5.41 ± 0.50† | 4.81 ± 0.74∗ |
| LVEDPW (mm) | 0.63 ± 0.07 | 0.80 ± 0.19 | 0.66 ± 0.04 |
| LVESPW (mm) | 0.91 ± 0.12 | 0.98 ± 0.13 | 0.97 ± 0.11 |
| LVED Vol (μl) | 85.5 ± 14.0 | 170 ± 30.0∗ | 151 ± 47.7∗ |
| LVES Vol (μl) | 49.1 ± 12.1 | 143 ± 30.1† | 111 ± 40.0∗ |
| EF (%) | 45.9 ± 7.46 | 16.3 ± 3.36‡ | 27.1 ± 5.22†§ |
| FS (%) | 22.8 ± 4.27 | 7.46 ± 1.57‡ | 12.8 ± 2.61†§ |
| HR (beats/min) | 465 ± 45 | 488 ± 49 | 453 ± 43 |
| SV (μl) | 36.0 ± 8.08 | 28.0 ± 3.42 | 39.4 ± 9.57 |
| CO (ml/min) | 17.0 ± 5.47 | 13.8 ± 2.82 | 17.9 ± 5.30 |
| E/E′ | −44.0 ± 6.54 | −40.9 ± 14.2 | −31.7 ± 6.17 |
| IVRT (ms) | 14.6 ± 1.28 | 14.8 ± 3.68 | 15.6 ± 2.84 |
| LV mass (mg) | 84.5 ± 15.2 | 157 ± 48.8∗ | 117 ± 27.5 |
| HW/BW (mg/g) | 5.34 ± 0.59 | 7.27 ± 1.75 | 6.07 ± 0.66 |
Values are mean ± SD.
The mice were subjected to myocardial infarction (MI), and 3 days after surgery randomly divided to receive either immunoglobulin (IgG) vehicle or connective tissue growth factor (CTGF) monoclonal antibody (mAb) for 4 days, and subjected to echocardiography analysis.
LV mass and heart weight versus body weight (HW/BW) were analyzed.
CO = cardiac output; E/E′ = mitral E/E′ ratio; EF = ejection fraction; FS = fractional shortening; HR = heart rate; IVRT = isovolumic relaxation time; LV = left ventricular; LVEDD = LV end-diastolic dimension; LVESD = end-systolic dimension; LVEDPW = LV end-diastolic posterior wall thickness; LVESPW = LV end-systolic posterior wall thickness; LVED Vol = LV end-diastolic volume; LVES Vol = LV end-systolic volume, SV = stroke volume.
p < 0.05.
p < 0.01.
p < 0.001 versus sham.
p < 0.05 versus MI + IgG.
CTGF mAb reduces post-MI LV hypertrophy and fibrosis (Study II)
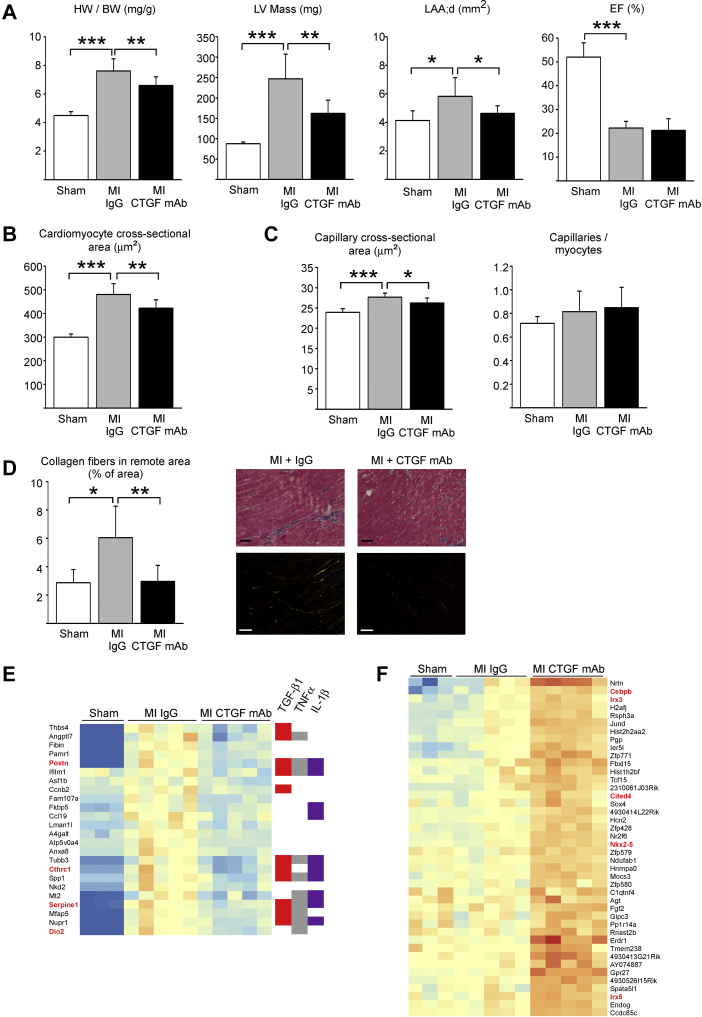
To investigate for the potential of CTGF mAb in post-MI remodeling, mice were subjected to experimental MI, and 1 week later treatment began with either control IgG or CTGF mAb for 6 weeks (Study II) (Figure 1). There was no difference in overall survival in MI groups because only 1 mouse was lost in the IgG group and none were lost in CTGF mAb group during the treatments. Two weeks after initiating treatment, echocardiography analysis showed no difference in LV structure or function between the MI groups (Supplemental Table 1). Analysis of harvested hearts after 6 weeks showed that CTGF mAb treatment resulted in a reduced heart weight to body weight ratio compared with the IgG-treated mice (p < 0.01) (Figure 3A). Echocardiography analysis at 6 weeks showed that mice treated with CTGF mAb had significantly lower LV mass and left atrial size (p < 0.01 and p < 0.05, respectively) (Figure 3A). No difference was observed in LV systolic function. Full echocardiography data are listed in Supplemental Table 2.
Figure 3.
CTGF mAb Protects Against Post-MI LV Hypertrophy and Fibrosis (Study II)
Mice were subjected to MI and 1 week after surgery randomly divided to receive IgG vehicle or CTGF mAb for 6 weeks. (A) Analysis for heart weight to body weight (HW/BW) ratio, LV mass, left atrial end-diastolic area (LAA;d), and EF. (B) Analysis of cardiomyocyte cross-sectional area from Masson trichrome−stained LV sections. (C) Analysis of mean capillary cross-sectional size and the number of capillaries per cardiomyocyte in the nonischemic myocardium from CD31 staining, (D) Analysis of interstitial fibrosis from picrosirius red−stained LV sections under polarized light. Scale bar: 50 μm. (A to D) Data are presented as mean ± SD; number of animals was sham (n = 5), IgG (n = 7), and CTGF mAb (n = 8). *p < 0.05; **p < 0.01; ***p < 0.001. (E) Hierarchical clustering of RNAseq data for transcripts that were altered by MI and at least partially normalized by CTGF mAb, indicating that many of these genes are regulated by transforming growth factor-β1 (TGF-β1), tumor necrosis factor (TNF)-α, or interleukin (IL)-1β. Red highlights indicate genes associated with various fibrotic disorders. (F) Hierarchical clustering of RNAseq data showing transcripts whose expression was increased in hearts of mice treated with CTGF mAb. Known cardiac development and/or repair related genes are highlighted (red). (E and F) Number of animals was sham (n = 3), IgG (n = 5), and CTGF mAb (n = 5). Abbreviations as in Figures 1 and 2.
Cardiomyocyte size was increased in both MI groups compared with sham-operated mice, but treatment with CTGF mAb significantly reduced the MI-induced increase in cardiomyocyte hypertrophy (Figure 3B). Histological analysis of hearts subjected to experimental MI showed that CTGF mAb treatment significantly attenuated the increase in capillary size in the LV (p < 0.05) without changing the capillary density (Figure 3C). Picrosirius red staining showed reduction in MI-induced fibrosis in the remote, nonischemic myocardium in mice treated with CTGF mAb compared with MI mice treated with IgG (Figure 3D) (p < 0.01). Determination of length of infarction showed no difference between the MI groups (Supplemental Figure 2).
CTGF mAb regulates genes related to fibrosis and/or inflammation and cardiac repair (Study II)
To explore potential mechanisms that mediated the cardioprotective effects of CTGF mAb, we performed RNA sequencing analysis of samples from mice subjected to chronic MI and treated with either control IgG (n = 5) or CTGF mAb (n = 5) for 6 weeks. We identified >1,000 genes with significantly altered expression after MI (fold change [FC] >1.5; p < 0.05) (Data Set 1 in the Supplemental Material). CTGF mAb treatment significantly affected expression of 72 transcripts in MI hearts (FC >1.5; p < 0.05) (Supplemental Table 3), 60 of which were also MI-altered. Gene ontology enrichment analysis indicated that 24 of 72 transcripts affected by CTGF mAb treatment were related to fibrosis and/or inflammation. An investigation of shared upstream regulators indicated that many transcripts are known to be co-regulated by multiple inflammatory factors, such as transforming growth factor (TGF)-β1, tumor necrosis factor-α, and interleukin-1β (Figure 3E). Normalized RNAseq data showed downregulation of selected MI-induced genes, and reduced expression of PAI-1 (Serpine1) was also confirmed by Western blotting (Supplemental Figure 3).
CTGF mAb treatment upregulated expression of 42 transcripts related to cardiac development and/or repair, including Nkx2-5 and Cited4 (Cbp/P300 interacting transactivator with Glu/Asp rich carboxy-terminal domain 4) in post-MI hearts (Figure 3F, Supplemental Figure 4). In addition, although it did not meet fold-change cutoffs, RNAseq analysis revealed significant induction of GATA binding protein 4 (GATA-4) expression in CTGF mAb−treated hearts compared with hearts treated with control IgG (Supplemental Figure 4).
CTGF mAb has no effect on infarct size following myocardial I/R injury (Study III)
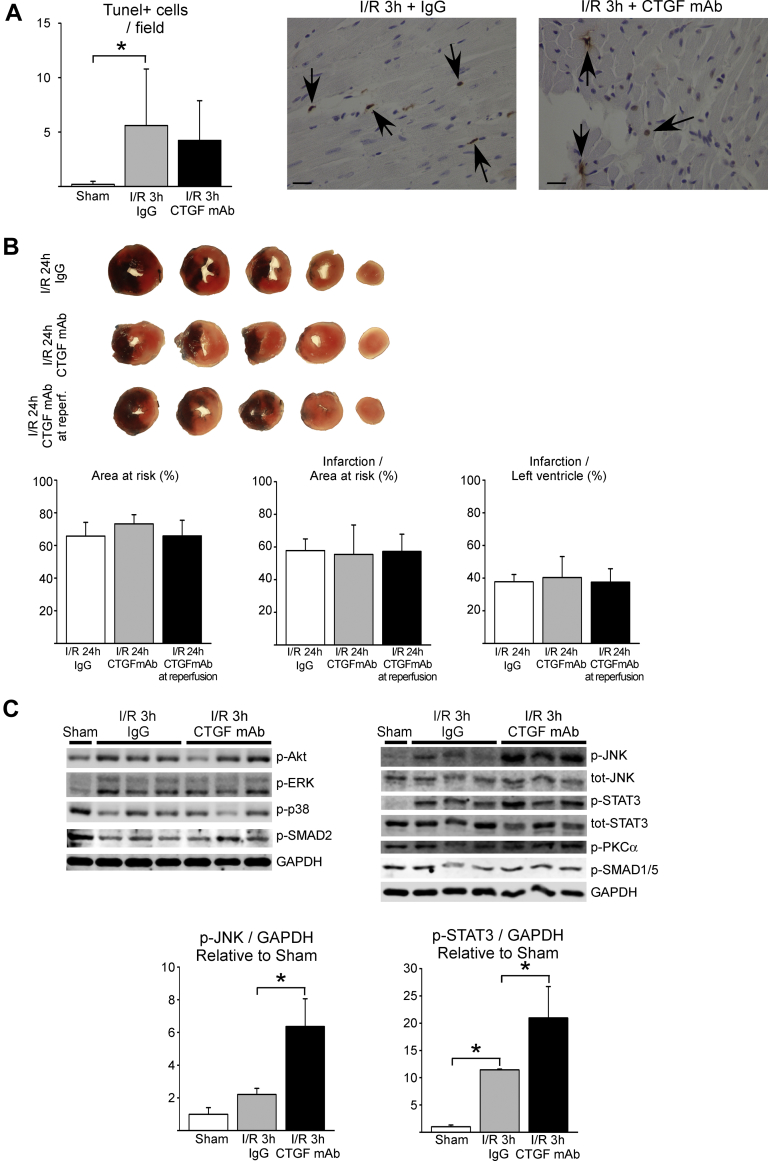
To assess if antagonizing the function of CTGF affected acute cardiac I/R injury, wild-type mice were subjected to 30 min of ischemia followed by reperfusion (Study III) (Figure 1). Analysis for cardiac injury by determination of apoptosis at 3 h after reperfusion showed no difference between the control IgG and CTGF mAb treatments (Figure 4A). When assessed 24 h after reperfusion, CTGF mAb treatment had no effect on the size of the area at risk (Figure 4B). Analysis of infarct size revealed no difference between the groups, which suggested that CTGF mAb had no effect on cardiomyocyte viability following I/R injury (Figure 4B). Echocardiography analysis at 24 h after reperfusion showed no difference in LV structure or function between the groups (Supplemental Table 4).
Figure 4.
Antagonizing the Function of CTGF mAb During Cardiac I/R Injury (Study III)
Mice were treated with IgG vehicle or CTGF mAb and subjected to ischemia−reperfusion injury (I/R). (A) Quantitative analysis of TUNEL-positive cells in hearts subjected to 30 min of ischemia and 3 h of reperfusion is shown. TUNEL-positive cells are marked with arrows; scale bar: 20 μm. (B) Mice were treated with IgG vehicle or CTGF mAb 24 h before ischemia and at reperfusion, or with CTGF mAb at reperfusion only. Infarct size and area at risk were analyzed from triphenyl tetrazolium chloride (TTC)−stained myocardial sections. (C) Western blot analysis of samples from infarct area 3 h after I/R injury. Analysis of phosphorylated protein kinase B (Akt), ERK, JNK, signal transducer and activator of transcription 3 (STAT3), p38, protein kinase C alpha (PKCα), SMAD2, and SMAD1/5 is shown. GAPDH was used as a loading control. Ratio of p-JNK to GAPDH and p-STAT3 to GAPDH data in the bar graphs are presented as mean ± SD. Number of animals in 3-h I/R experiment, including TUNEL labeling, was sham (n = 3), IgG (n = 6), and CTGF mAb (n = 6). Number of animals in 24-h I/R experiment including TTC staining was IgG (n = 11), CTGF mAb (n = 12), and CTGF in reperfusion (n = 12). TUNEL = terminal deoxynucleotidyl transferase dUTP nick end labeling; other abbreviations as in Figures 1 and 2.
The effect of CTGF inhibition on central signaling mechanisms in the ischemic heart was assessed 3 h after reperfusion with Western blotting. Analysis for reperfusion injury salvage kinase pathways in the ischemic area showed no difference in phosphorylation of protein kinase B (Akt) or ERK between the mice treated with IgG or CTGF mAb (Figure 4C). In contrast, phosphorylation of JNK2 and the signal transducer and activator of transcription 3 were significantly increased in hearts of mice treated with CTGF mAb (Figure 4C). CTGF mAb had no effect on SMAD, p38, or protein kinase C (PKC)-α phosphorylation (Figure 4C).
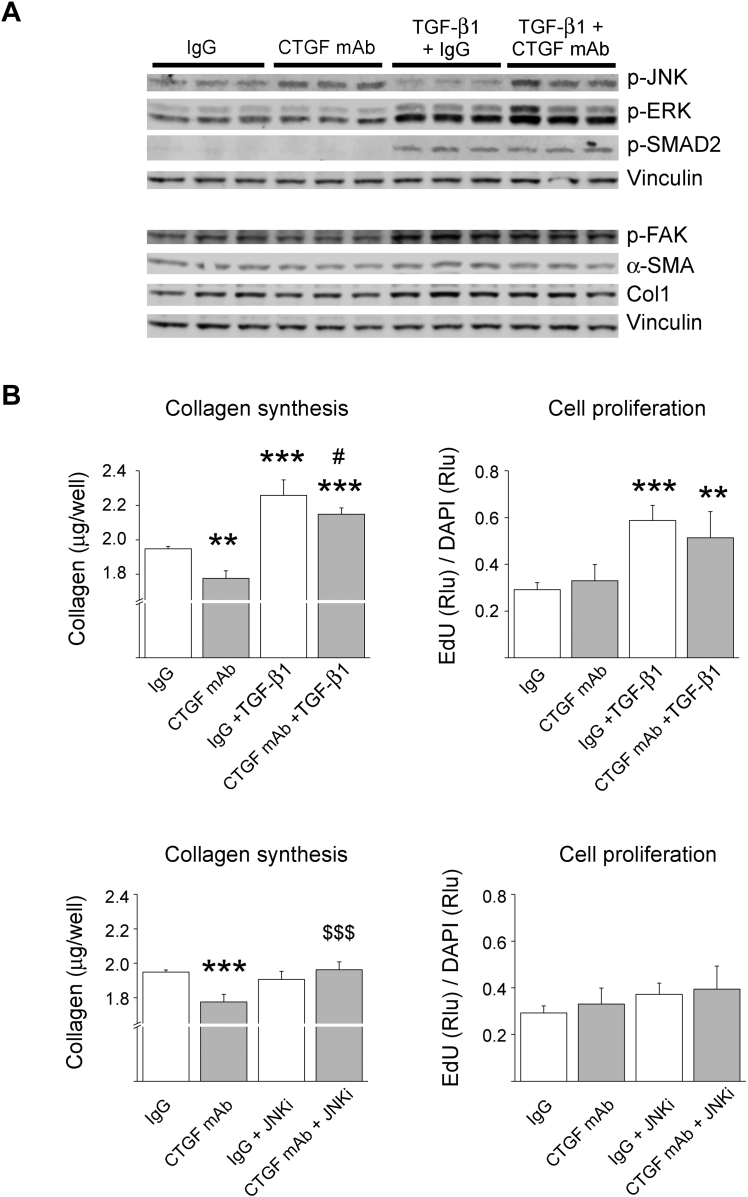
JNK pathway mediates effects of CTGF mAb
To more directly investigate the molecular function of CTGF mAb, we treated human cardiac FBs with CTGF mAb. Immunoblotting showed that treatment with CTGF mAb modestly reduced both basal and TGF-β1–induced αSMA and collagen-1 expression (Figure 5A). Examination of the affected signaling pathways showed that similar to findings in vivo, CTGF mAb induced JNK2 phosphorylation (Figure 5A). Antagonizing the function of CTGF also modestly reduced focal adhesion kinase (FAK) phosphorylation, but had no effect on ERK or SMAD2 phosphorylation (Figure 5A).
Figure 5.
CTGF mAb Activates JNK and Reduces Collagen Production in Cultured Human Cardiac Fibroblasts
Cultured human fibroblasts were treated with 10 μg/ml CTGF mAb or control IgG, and co-treated with TGF-β1 (1 ng/ml) or inhibitor of JNK inhibitor (JNKi) [(L)-Form, 2 μM)], where indicated. (A) Western blot analysis for phosphorylated JNK2, p-ERK, phosphorylated SMAD2, phosphorylated focal adhesion kinase (p-FAK), smooth muscle alpha actin (α-SMA), and collagen 1 (Col1) is shown. Vinculin was used as loading control. (B) Quantitative analysis for collagen production and fibroblast proliferation. Data are presented as mean ± SD. **p < 0.01; ***p < 0.001 versus IgG; #p < 0.05 versus IgG + TGF-β1; $$$p < 0.001 versus CTGF mAb. N = 5 per group. Abbreviations as in Figures 1, 2, and 3.
Quantitative analysis for the effect of CTGF mAb on collagen production showed that CTGF mAb significantly reduced both basal and TGF-β1–induced collagen production, but had no effect on basal or TGF-β1–induced FB proliferation (Figure 5B). We then investigated if JNK played a role in mediating the effects of CTGF mAb. Treatment of human cardiac FBs with JNK inhibitor I abrogated the reduced collagen production in CTGF mAb−treated cells, but had no effect on collagen production in control IgG-treated cells (Figure 5B). In contrast, FB proliferation was not affected by JNK inhibition (Figure 5B).
Discussion
ECM forms the structural backbone of the heart, and provides efficient mechanical and electrical coupling during contraction. In HF, excessive accumulation of ECM not only increases ventricular stiffness, but also disrupts normal electrical coupling, which predisposes to conduction abnormalities, arrhythmias, and sudden cardiac death. However, fibrotic wound healing is necessary to form a stable infarct scar to prevent cardiac wall rupture. Therapy for HF patients with angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, and mineralocorticoid receptor antagonists have been shown to attenuate the development of cardiac fibrosis 19, 20. In addition, novel approaches, such as inhibition of fibronectin polymerization, may provide novel therapeutic approaches (21). In the present study, we investigated the potential of CTGF mAb therapy in protecting the heart from MI-induced injury and fibrosis.
Treatment with CTGF mAb during cardiac repair
Repair of the infarcted heart can be divided into 3 overlapping phases: the inflammatory, proliferative, and maturation phases (22). The inflammatory phase in mouse hearts subjected to I/R injury lasts up to 72 hours after injury and is likely longer in hearts undergoing permanent MI. This is followed by a proliferative phase (days 3 to 7), which is characterized by FB proliferation, differentiation of FBs to myofibroblasts, and angiogenesis. Following repair of the infarct, activation of FBs occurs in both the peri-infarct area and the remote myocardium, which contribute to adverse remodeling of the LV. It is well documented that CTGF expression is elevated during wound healing in different tissues (23). In our study, treatment with CTGF mAb during the proliferative phase of cardiac repair (starting at day 3 after MI) resulted in better preserved ejection fraction at 1 week after MI and also improved survival. We also found that CTGF mAb treatment started at day 3 after MI reduced infarct scar thinning and infarct expansion. Infarct expansion is associated with a decrease in LV systolic function and increased infarct rupture, which most often occurs at the infarct border zone during the first week after MI 24, 25. In the present study, CTGF mAb−treated mice had less thinning of the infarct scar, which might have resulted in better preserved LV systolic function and provided protection from infarct rupture, and led to better post-MI survival. RNA sequencing data of samples from MI hearts treated with IgG or CTGF mAb showed that treatment with CTGF mAb induced expression of a number of genes involved in cardiac repair and/or development. Clear induction of Nkx2-5, a key nodal transcription factor in cardiogenesis, was noted, and further data mining revealed significant induction of GATA-4 expression in CTGF mAb−treated hearts. GATA-4 and Nkx2-5 physically interact and drive expression of a number of genes in cardiomyocytes (26), and are key mediators of cardiac repair and regeneration in the adult heart 27, 28.
Cited4 encodes for the CREB-binding protein (CBP)/p300-interacting transactivator. Cardiomyocyte-specific overexpression of Cited4 in mice has also been shown to induce an increase in heart weight and cardiomyocyte size with normal systolic function, and to induce functional recovery and reduction in fibrosis long term after I/R injury (29). Induction of cardioprotective genes in hearts of CTGF mAb−treated mice might have contributed to better post-MI survival and better preserved LV systolic function after MI.
CTGF mAb in development of myocardial fibrosis following MI
In the present study, we found that CTGF mAb treatment for 6 weeks post-MI resulted in reduced fibrosis in the remote, nonischemic myocardium. In addition, CTGF mAb treatment during post-MI LV remodeling reduced the MI-induced increase in cardiomyocyte size and LV mass. RNA sequencing analysis of samples from surviving myocardium at 7 weeks after MI identified downregulation of MI-induced expression of inflammatory and fibrotic genes in hearts of CTGF mAb−treated mice. Previously, treatment with CTGF mAb in a genetic model of dilated cardiomyopathy showed that CTGF mainly regulated the genes related to ECM structural proteins and remodeling enzymes (15). These data, together with our present data, thus suggested that the antifibrotic effect of CTGF mAb in MI hearts arises from downregulation of inflammatory and fibrotic genes. The effect of CTGF mAb on cardiomyocyte hypertrophy in the present study probably also stemmed from altered FB function and altered release of local growth factors and cytokines from activated FBs.
Investigation of the mechanisms of CTGF action in cultured human FBs indicated that CTGF mAb reduced collagen production, but had no effect on FB proliferation. Recent studies indicated that the increase in LV fibrosis in the remote myocardium during post-MI LV remodeling was dependent on activation of existing FBs rather than FB proliferation, which mainly occurs 2 to 7 days post-MI in the infarct region (30). It is known that CTGF can modulate many signaling pathways independently of TGF-β (31). However, with respect to TGF-β signaling, it was previously reported that CTGF did not modulate canonical signaling (i.e., via SMAD 2/3), but instead modulated at least 2 non-canonical TGF-β signaling pathways, SMAD 1 and ERK (32). We found that CTGF mAb consistently activated the JNK2 isoform both in vivo and in vitro, but had no effect on SMAD signaling. Although there are previous data that showed that JNK2 is a negative regulator of FB proliferation (33), we found that inhibition of JNKs abrogated the antifibrotic effect of CTGF mAb in cultured human FBs.
Effect of CTGF mAb on Acute I/R injury
Previous data from studies that used transgenic mice with cardiac-restricted overexpression of rat CTGF suggested that CTGF protects the myocardium from acute I/R injury (34). In the present study, we used strategies to administer CTGF mAb before I/R injury or immediately at reperfusion. Our data showed that treatment with CTGF mAb did not increase infarct size or compromise the recovery of LV systolic function at 24 h after I/R injury, as might be expected if CTGF were cardioprotective in acute I/R.
Study limitations
There were some possible limitations to this study. Experimental MI surgery itself might have resulted in inflammatory effects (35) that could have affected the analysis of post-MI inflammation at 7 days after MI. However, our model for MI surgery, which included pericardial incision without open-chest surgery, resulted in shortened recovery and reduced inflammation compared with conventional open-chest models (36). Unfortunately, experimental MI in rodents and open-chest cardiac surgery in patients can damage the pericardium and may induce pericardial adhesions. CTGF mAb therapy during the proliferative phase of infarct repair might have had an effect on the development of the post-operative pericardial adhesions, which possibly contributed to increased survival and better preserved ejection fraction. Similar limitations should be considered when investigating any antifibrotic intervention during infarct repair in rodent MI models. In addition, analysis for infarct size at 24 h after I/R injury did not rule out the possibility that CTGF mAb could have had an effect on I/R injury analyzed at a later time point.
Conclusions
We found that therapy with CTGF mAb during the proliferative phase of post-MI cardiac repair attenuated infarct expansion, improved survival, and attenuated the decrease in LV systolic function. Intervention with CTGF mAb during post-MI LV remodeling reduced LV fibrosis and attenuated the MI-induced cardiomyocyte hypertrophy and increase in LV mass. Mechanistically, therapy with CTGF mAb attenuated the MI-induced increase in inflammatory and pro-fibrotic genes and enhanced expression of genes related to cardiac development and/or repair. In addition, studies with cultured human FBs indicated a role for JNK in reducing the collagen production by CTGF mAb. Further studies in large animal models are needed to establish if CTGF mAb provides a novel therapy for MI patients.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In a pre-clinical model of MI, therapy with CTGF mAb improved post-MI survival and attenuated development of LV fibrosis.
TRANSLATIONAL OUTLOOK: Further work is needed to assess whether CTGF mAb therapy provides benefit in large animal models of myocardial infarction.
Acknowledgments
The authors thank Marja Arbelius, Kirsi Salo, Esa Kerttula, and Sirpa Rutanen for technical assistance.
Appendix
References
- 1.Writing Group M. Mozaffarian D., Benjamin E.J. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Heusch G., Libby P., Gersh B. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet. 2014;383:1933–1943. doi: 10.1016/S0140-6736(14)60107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leask A., Abraham D.J. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 4.Jun J.I., Lau L.F. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10:945–963. doi: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koitabashi N., Arai M., Niwano K. Plasma connective tissue growth factor is a novel potential biomarker of cardiac dysfunction in patients with chronic heart failure. Eur J Heart Fail. 2008;10:373–379. doi: 10.1016/j.ejheart.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Behnes M., Brueckmann M., Lang S. Connective tissue growth factor (CTGF/CCN2): diagnostic and prognostic value in acute heart failure. Clin Res Cardiol. 2014;103:107–116. doi: 10.1007/s00392-013-0626-6. [DOI] [PubMed] [Google Scholar]
- 7.Daniels A., van Bilsen M., Goldschmeding R., van der Vusse G.J., van Nieuwenhoven F.A. Connective tissue growth factor and cardiac fibrosis. Acta Physiol (Oxf) 2009;195:321–338. doi: 10.1111/j.1748-1716.2008.01936.x. [DOI] [PubMed] [Google Scholar]
- 8.Leask A. Getting to the heart of the matter: new insights into cardiac fibrosis. Circ Res. 2015;116:1269–1276. doi: 10.1161/CIRCRESAHA.116.305381. [DOI] [PubMed] [Google Scholar]
- 9.Yoon P.O., Lee M.A., Cha H. The opposing effects of CCN2 and CCN5 on the development of cardiac hypertrophy and fibrosis. J Mol Cell Cardiol. 2010;49:294–303. doi: 10.1016/j.yjmcc.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Accornero F., van Berlo J.H., Correll R.N. Genetic analysis of connective tissue growth factor as an effector of transforming growth factor beta signaling and cardiac remodeling. Mol Cell Biol. 2015;35:2154–2164. doi: 10.1128/MCB.00199-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pi L., Fu C., Lu Y. Vascular endothelial cell-specific connective tissue growth factor (CTGF) is necessary for development of chronic hypoxia-induced pulmonary hypertension. Front Physiol. 2018;9:138. doi: 10.3389/fphys.2018.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorina E., Richeldi L., Raghu G. PRAISE, a randomized, placebo-controlled, double-blind Phase 2 clinical trial of pamrevlumab (FG-3019) in IPF patients. Eur Respir J. 2017;50:OA3400. [Google Scholar]
- 13.Alapati D., Rong M., Chen S. Connective tissue growth factor antibody therapy attenuates hyperoxia-induced lung injury in neonatal rats. Am J Respir Cell Mol Biol. 2011;45:1169–1177. doi: 10.1165/rcmb.2011-0023OC. [DOI] [PubMed] [Google Scholar]
- 14.Szabo Z., Magga J., Alakoski T. Connective tissue growth factor inhibition attenuates left ventricular remodeling and dysfunction in pressure overload-induced heart failure. Hypertension. 2014;63:1235–1240. doi: 10.1161/HYPERTENSIONAHA.114.03279. [DOI] [PubMed] [Google Scholar]
- 15.Koshman Y.E., Sternlicht M.D., Kim T. Connective tissue growth factor regulates cardiac function and tissue remodeling in a mouse model of dilated cardiomyopathy. J Mol Cell Cardiol. 2015;89:214–222. doi: 10.1016/j.yjmcc.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chatzifrangkeskou M., Le Dour C., Wu W. ERK1/2 directly acts on CTGF/CCN2 expression to mediate myocardial fibrosis in cardiomyopathy caused by mutations in the lamin A/C gene. Hum Mol Genet. 2016;25:2220–2233. doi: 10.1093/hmg/ddw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashburner M., Ball C.A., Blake J.A. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Gene Ontology Consortium Expansion of the Gene Ontology knowledge base and resources. Nucleic Acids Res. 2017;45:D331–D338. doi: 10.1093/nar/gkw1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gourdie R.G., Dimmeler S., Kohl P. Novel therapeutic strategies targeting fibroblasts and fibrosis in heart disease. Nat Rev Drug Discov. 2016;15:620–638. doi: 10.1038/nrd.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talman V., Ruskoaho H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res. 2016;365:563–581. doi: 10.1007/s00441-016-2431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valiente-Alandi I., Potter S.J., Salvador A.M. Inhibiting fibronectin attenuates fibrosis and improves cardiac function in a model of heart failure. Circulation. 2018;138:1236–1252. doi: 10.1161/CIRCULATIONAHA.118.034609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinde A.V., Frangogiannis N.G. Fibroblasts in myocardial infarction: a role in inflammation and repair. J Mol Cell Cardiol. 2014;70:74–82. doi: 10.1016/j.yjmcc.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi-wen X., Leask A., Abraham D. Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev. 2008;19:133–144. doi: 10.1016/j.cytogfr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Gao X.M., Xu Q., Kiriazis H., Dart A.M., Du X.J. Mouse model of post-infarct ventricular rupture: time course, strain- and gender-dependency, tensile strength, and histopathology. Cardiovasc Res. 2005;65:469–477. doi: 10.1016/j.cardiores.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Kelley S.T., Malekan R., Gorman J.H., 3rd Restraining infarct expansion preserves left ventricular geometry and function after acute anteroapical infarction. Circulation. 1999;99:135–142. doi: 10.1161/01.cir.99.1.135. [DOI] [PubMed] [Google Scholar]
- 26.Durocher D., Charron F., Warren R., Schwartz R.J., Nemer M. The cardiac transcription factors Nkx2-5 and GATA-4 are mutual cofactors. EMBO J. 1997;16:5687–5696. doi: 10.1093/emboj/16.18.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pikkarainen S., Tokola H., Kerkela R., Ruskoaho H. GATA transcription factors in the developing and adult heart. Cardiovasc Res. 2004;63:196–207. doi: 10.1016/j.cardiores.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 28.Oka T., Maillet M., Watt A.J. Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circ Res. 2006;98:837–845. doi: 10.1161/01.RES.0000215985.18538.c4. [DOI] [PubMed] [Google Scholar]
- 29.Bezzerides V.J., Platt C., Lerchenmuller C. CITED4 induces physiologic hypertrophy and promotes functional recovery after ischemic injury. JCI Insight. 2016;1 doi: 10.1172/jci.insight.85904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu X., Khalil H., Kanisicak O. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J Clin Invest. 2018;128:2127–2143. doi: 10.1172/JCI98215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipson K.E., Wong C., Teng Y., Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012;5:S24. doi: 10.1186/1755-1536-5-S1-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakerakanti S.S., Bujor A.M., Trojanowska M. CCN2 is required for the TGF-beta induced activation of Smad1-Erk1/2 signaling network. PLoS One. 2011;6:e21911. doi: 10.1371/journal.pone.0021911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabapathy K., Hochedlinger K., Nam S.Y., Bauer A., Karin M., Wagner E.F. Distinct roles for JNK1 and JNK2 in regulating JNK activity and c-Jun-dependent cell proliferation. Mol Cell. 2004;15:713–725. doi: 10.1016/j.molcel.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed M.S., Gravning J., Martinov V.N. Mechanisms of novel cardioprotective functions of CCN2/CTGF in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2011;300:H1291–H1302. doi: 10.1152/ajpheart.00604.2010. [DOI] [PubMed] [Google Scholar]
- 35.Nossuli T.O., Lakshminarayanan V., Baumgarten G. A chronic mouse model of myocardial ischemia-reperfusion: essential in cytokine studies. Am J Physiol Heart Circ Physiol. 2000;278:H1049–H1055. doi: 10.1152/ajpheart.2000.278.4.H1049. [DOI] [PubMed] [Google Scholar]
- 36.Gao E., Lei Y.H., Shang X. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res. 2010;107:1445–1453. doi: 10.1161/CIRCRESAHA.110.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.