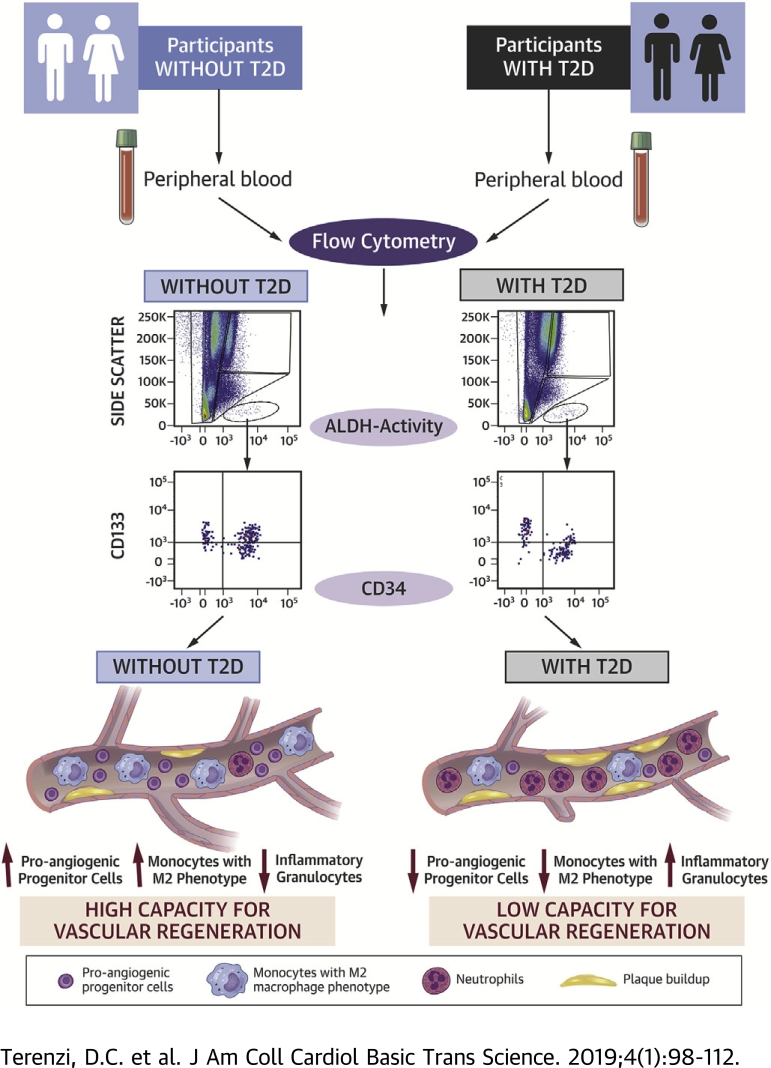
Visual Abstract
Key Words: aldehyde dehydrogenase, angiogenesis, ischemia, progenitor cells, type 2 diabetes
Abbreviations and Acronyms: ALDH, aldehyde dehydrogenase; BM, bone marrow; HbA1c, glycosylated hemoglobin; ROS, reactive oxygen species; SSC, side scatter; T2D, type 2 diabetes mellitus; Wnt, wingless related integration site
Highlights
-
•
This study combined ALDH activity with cell surface marker expression to develop a multiparametric flow cytometry assay to assess proangiogenic progenitor and proinflammatory cell content in the peripheral blood of patients with T2D compared with age-matched control subjects.
-
•
Patients with T2D exhibited an increased frequency of proinflammatory ALDHhi cells with granulocyte side scatter properties and a decreased frequency of circulating monocytes with an M2 phenotype that is associated with proangiogenic and anti-inflammatory functions.
-
•
Patients with T2D exhibited significant depletion of circulating provascular ALDHhiCD34+ progenitor cells with primitive, migratory, endothelial, and pericyte phenotypes.
-
•
Subgroup analyses that stratified patients with T2D according to age, duration of T2D, insulin requirement, and glycosylated hemoglobin levels revealed that only the duration of T2D correlated with vascular progenitor cell depletion.
-
•
Flow cytometric assessment of circulating ALDHhi cell subsets represents a promising translational approach for identifying patients with T2D at increased risk for cardiovascular comorbidities.
Summary
Detection of vascular regenerative cell exhaustion is required to combat ischemic complications during type 2 diabetes mellitus (T2D). We used high aldehyde dehydrogenase (ALDH) activity and surface marker co-expression to develop a high-throughput flow cytometry–based assay to quantify circulating proangiogenic and proinflammatory cell content in the peripheral blood of individuals with T2D. Circulating proangiogenic monocytes expressing anti-inflammatory M2 markers were decreased in patients with T2D. Individuals with longer duration of T2D exhibited reduced frequencies of circulating proangiogenic ALDHhiCD34+ progenitor cells with primitive (CD133) and migratory (CXCR4) phenotypes. This approach consistently detected increased inflammatory cell burden and decreased provascular progenitor content in individuals with T2D.
Approximately 400 million individuals worldwide experience type 2 diabetes (T2D), and this number is expected to rise to >600 million by 2045 1, 2, 3. Although various mechanisms have been suggested to mediate the vascular complications of diabetes, there is growing interest in the theory that diabetes may lead to chronic inflammation, which in turn increases oxidative stress on vascular regenerative cells, inciting a state of vasculopenia. This damaging microenvironment also contributes to the death and dysfunction of bone marrow (BM)-derived and circulating proangiogenic progenitor cells, leading to an inability to respond to vessel damage (4). Thus, ongoing endothelial damage combined with reduced blood vessel regenerative capacity in patients with T2D culminates in a 2- to 5-fold increased risk for the development of ischemic cardiovascular diseases, including critical limb ischemia, myocardial infarction, and stroke 1, 3. Although newer antihyperglycemic agents reportedly improve cardiovascular outcomes in diabetes 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, the unmet need and residual risk remain prohibitively high in T2D (16).
To minimize the risks associated with reduced blood flow causing ischemia, multiple endogenous mechanisms can be activated to reverse vascular dysfunction (4). These multicellular processes include vasculogenesis, the creation of de novo vessels from endothelial progenitor cells; angiogenesis, the sprouting of new blood vessels from pre-existing vessels; and arteriogenesis, the beneficial remodeling of pre-existing collateral vessels to form a “natural bypass” toward the ischemic region 4, 17. Although angiogenesis and postnatal vasculogenesis have been widely studied, both processes can be limited in adults by the scarcity of circulating provascular progenitor cells of hematopoietic and endothelial lineages 18, 19. Although arteriogenesis is not as well understood, accessory immune cells (including monocytes and macrophages) are recruited to pre-existing collateral vessels and participate in vessel remodeling to activate blood flow 4, 18, 20, 21. Thus, these processes rely on structural and secretory contributions from circulating hematopoietic and endothelial cells that originate from the BM 22, 23. In the context of T2D, the impact of glucotoxicity and increased oxidative stress on the frequency and function of these regenerative progenitor cells is not well understood.
Aldehyde dehydrogenase (ALDH) is an intracellular detoxification enzyme highly expressed in progenitor cells with documented proangiogenic secretory function (17). ALDH acts to protect long-lived cells from oxidative stress by metabolizing toxic alkylating aldehyde agents, which can lead to cellular damage. In addition, ALDH is the rate-limiting enzyme in the intracellular production of retinoic acid, a potent morphogen. Thus, as progenitor cells differentiate toward a mature phenotype, ALDH-activity is reduced. Our group and others have previously documented the proangiogenic signaling capacity of ALDHhi progenitor cells from BM and umbilical cord blood 17, 24, 25.
BM cells of patients with T2D exhibit reduced expression of markers associated with proangiogenic progenitor cells (CD34 and CD133) due to premature differentiation accelerated by hyperglycemia and increased oxidative stress 18, 23, 26. The T2D BM microenvironment also exhibits increased cell turnover, lending to heightened inflammatory responses and inhibited distribution of provascular progenitor cells to ischemic tissues 23, 27. The amplified inflammation leads to increased NADPH oxidase-1 function, which significantly elevates intracellular reactive oxygen species (ROS) formation (28). The examination of circulating progenitor cell content in the peripheral circulation may confirm the extent of this process (termed “regenerative cell exhaustion”) and illuminate the therapeutic implications of BM dysfunction on vascular regeneration.
The goal of this study was to assess the balance between circulating vascular regenerative progenitor cells and inflammatory cells in patients with T2D. We used the detection of high ALDH-activity according to flow cytometry to quantify the prevalence of circulating progenitor cells in the peripheral blood of patients with T2D and age-matched control subjects. High ALDH-activity in conjunction with 6-color cell surface marker analyses allowed us to quantify the frequencies of proangiogenic and inflammatory cell types that affect the repair of ischemic injury in patients with T2D. Patients with T2D exhibited a significant decrease in circulating cells with hematopoietic and endothelial progenitor cell phenotype. In addition, circulating monocytes with an anti-inflammatory M2 phenotype were decreased in patients with T2D, and primitive granulocytes with proinflammatory function were significantly increased in patients with T2D, suggesting a shift toward a proinflammatory phenotype (29). These studies provide a foundation to assess vascular regenerative cell content during the progression of T2D and may be developed as a surrogate assay to estimate the capacity to mitigate ischemia via a provascular regenerative response.
Methods
Patient characteristics
A total of 30 individuals >40 years of age with established T2D of >5 years were age- and sex-matched with 30 individuals without T2D. Written informed consent was provided, and all studies were approved prior to study initiation by the Advarra central institutional review board.
Isolation of peripheral blood mononuclear cells
Up to 50 ml of peripheral blood was drawn from each patient into ethylenediaminetetraacetic acid–lined blood collection tubes. Cells were layered on Hypaque-Ficoll solution placed in SepMate tubes (STEMCELL Technologies, Vancouver, British Columbia, Canada) to aid in the removal of red blood cells. Any red blood cells remaining were lysed with ammonium chloride and washed in phosphate-buffered saline to remove cellular debris.
Analyses of progenitor cells
Peripheral blood mononuclear cells were examined for ALDH-activity by using Aldefluor reagent (STEMCELL Technologies) following the manufacturer’s instructions 17, 24, 25. Briefly, cells were incubated at 37°C for 30 min with Aldefluor reagent. Subsequently, cells were centrifuged, washed with phosphate-buffered saline, and resuspended in ALDH buffer to block the efflux of the fluorescent substrate via adenosine triphosphate–binding cassette transporters. Next, cells were labeled with fluorochrome-conjugated, anti-human antibodies to surface markers marking primitive (CD34 and CD133) and more mature (CD33 and CD45) hematopoietic cells, endothelial cells (CD31, CD146, and CD144), monocytes (CD14), M1/M2 phenotype (CD68, CD80, and CD163), and granulocyte (CD15, CD16b, and CD66b) phenotypes. Antibodies were from Becton Dickinson, Miltenyi, and BioLegend, as specified in Supplemental Table 1. Cells were incubated with the antibodies for 30 min at 2°C to 8°C and washed in phosphate-buffered saline to ensure that excess antibodies were removed from the sample. Circulating progenitor cell content was assessed via 6-color, multiparametric flow cytometry on a BD LSRFortessa X-20 cytometer (BD Biosciences (Franklin Lakes, New Jersey) and analyzed with the FlowJo version 10 software (FlowJo, LLC, Ashland, Oregon). Side scatter (SSC) property, a measure of light scatter due to intracellular complexity or granularity, was used to further identify cells with low, intermediate, or high intracellular complexity. A minimum of 106 events was collected for every sample, assuring the analysis of >500 cells in the rare ALDHhiSSClow population.
Statistical analysis
Statistical analyses were performed with the Student's t-test for comparison of results from the group with T2D versus the age-matched control individuals and for analyses of patients with T2D (n = 30) stratified into subgroups for assessment of correlations with sex (male, n = 12; female, n = 18), HbA1c values (HbA1c ≤7, HbA1c >7, n = 15), insulin use (no insulin, n = 18; on insulin, n = 12), age (≤70 years, n = 16; >70 years, n = 14), and duration of T2D (≤13 years, n = 15; >13 years, n = 15) on relevant circulating cellular subpopulations. Data from all 30 patients with T2D were included in the subgroup analyses. The use of nonparametric tests or permutation tests was not required.
Results
Demographic and biochemical characteristics
Baseline patient characteristics as well as their clinical histories are shown in Table 1. As expected, HbA1c levels were higher in patients with T2D. The average duration of diabetes in this cohort was 14.0 ± 0.9 years, and the average age was 71.4 ± 1.7 years. The age and percent ratio of male to female patients (40:60) were balanced between the T2D cohort and matched control subjects. The frequency of patients taking an antihypertensive agent was equivalent in both populations. High-density lipoprotein cholesterol levels were similar between the groups, although total cholesterol and low-density lipoprotein cholesterol levels were significantly lower in patients with T2D. The lower total cholesterol and low-density lipoprotein cholesterol levels are likely due to greater use of statins within the T2D cohort (22 of 30) compared with the control group (15 of 30). The majority of the patients with T2D (77%) were taking metformin, and insulin was used by 12 (40%) of 30 patients with T2D.
Table 1.
Baseline Characteristics of the Study Population
| Control Subjects (n = 30) | Patients With T2D (n = 30) | |
|---|---|---|
| Age, yrs | 72.3 ± 1.6 | 71.4 ± 1.7 |
| Male/female | 12 (40)/18 (60) | 12 (40)/18 (60) |
| Duration of T2D, yrs | NA | 14.0 ± 0.9 |
| HbA1c, % | 5.5 ± 0.1 | 7.2 ± 0.2∗ |
| LDL-C, mmol/L | 2.5 ± 0.2 | 1.5 ± 0.1∗ |
| HDL-C, mmol/L | 1.4 ± 0.1 | 1.3 ± 0.1 |
| Total cholesterol, mmol/L | 4.5 ± 0.2 | 3.5 ± 0.2∗ |
| Hypertensive therapy | 24 (80) | 26 (87) |
| Statin therapy | 15 (50) | 22 (73) |
| Metformin | 0 (0) | 23 (77) |
| Insulin | 0 (0) | 12 (40) |
Values are mean ± SEM or n (%).
HbA1c = glycosylated hemoglobin; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; NA = not applicable; T2D = type 2 diabetes mellitus.
p < 0.001 with the Student's t-test.
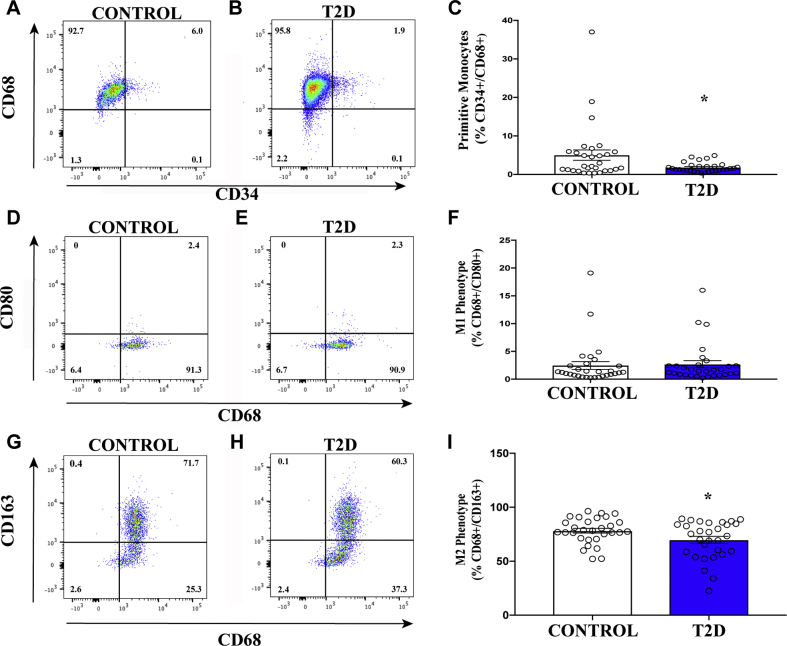
Circulating cells with proangiogenic monocyte and anti-inflammatory M2 phenotypes were decreased in patients with T2D
We first examined the relative expression of cell surface markers associated with hematopoietic, endothelial, monocyte, and granulocyte phenotypes. Importantly, previous studies have shown the functional relevance of these cell types in the coordination of proangiogenic responses after transplantation 30, 31, 32, 33. When gated on total cellular events and analyzed for single-cell surface marker expression, the frequency of circulating cells expressing primitive hematopoietic and endothelial cell–associated surface markers was equivalent in patients with T2D and the matched control subjects (Table 2, Supplemental Figures 1A to 1H). In contrast, the frequencies of circulating cells expressing the monocyte marker CD14 or the M2 polarization marker CD163 were significantly decreased in patients with T2D (Table 2, Supplemental Figures 1I to 1L); the frequency of cells expressing CD80, a marker associated with the M1 phenotype, was increased in patients with T2D. Collectively, the diminished frequency of provascular CD14+ circulating monocytes combined with a shift from the M2 to M1 phenotype suggested increased inflammation in patients with T2D. In addition, more sensitive analyses using multiple markers combined with ALDH-activity was required to accurately detect differences in circulating proangiogenic progenitor cell frequencies.
Table 2.
Circulating Monocytes With Anti-inflammatory M2 Phenotypes Were Decreased in Patients With T2D
| Marker | Control | T2D | p Value | Description/Expression | |
|---|---|---|---|---|---|
| Hematopoietic | CD45 | 91.1 ± 2.2 | 90.4 ± 2.2 | 0.82 | Pan-leukocyte marker or leukocyte common antigen
|
| CXCR4/CD184 | 83.8 ± 1.3 | 82.4 ± 2.4 | 0.61 | CXC chemokine receptor type 4 or fusin
|
|
| CD33 | 49.4 ± 2.5 | 49.2 ± 2.4 | 0.95 | Sialic acid binding IgG-like lectin 3 of Siglec-3
|
|
| CD34 | 3.7 ± 0.7 | 2.9 ± 0.6 | 0.38 | Sialomucin, adhesion to matrix and stromal cells in the bone marrow
|
|
| Endothelial | CD31 | 73.0 ± 1.9 | 72.3 ± 1.9 | 0.74 | Platelet endothelial cell adhesion molecule (PECAM-1)
|
| CD144 | 44.0 ± 2.8 | 43.6 ± 2.5 | 0.91 | Cadherin 5, type 2, or vascular endothelial–cadherin
|
|
| CD146 | 1.4 ± 0.3 | 1.1 ± 0.2 | 0.40 | Melanoma cell adhesion molecule (MCAM) or mucin 18
|
|
| CD133 | 1.5 ± 0.4 | 1.3 ± 0.3 | 0.57 | Prominin-1, pentaspan transmembrane protein
|
|
| Monocyte | CD14 | 10.1 ± 1.7 | 5.9 ± 0.8 | 0.05 | Co-receptor for bacterial lipopolysaccharide
|
| CD68 | 42.2 ± 3.0 | 43.6 ± 2.6 | 0.73 | Macrosialin, scavenger receptor class D, member 1
|
|
| M1 phenotype | CD80 | 3.6 ± 0.6 | 5.2 ± 0.5 | 0.04 | B7-1, ligand for CD28 and CTLA-4
|
| M2 phenotype | CD163 | 14.5 ± 1.0 | 10.8 ± 0.7 | 0.003 | Low-affinity scavenger receptor for hemoglobin-haptoglobin
|
The frequency of cells expressing mature and primitive hematopoietic and endothelial markers was equal in patients with type 2 diabetes mellitus (T2D) compared with age, gender, and sex-matched control subjects. The frequency of cells expressing the monocyte/macrophage marker CD14 was decreased in patients with T2D compared with control subjects. The frequency of cells expressing the M1 macrophage marker CD80 (pro-inflammatory phenotype) was increased whereas the frequency of cells expressing the M2 macrophage marker CD163 (anti-inflammatory phenotype) was decreased in patients with T2D compared with control subjects. Values are mean ± SEM.
CTLA-4 = cytotoxic T-lymphocyte associated protein 4; IgG = immunoglobulin G.
Statistical comparisons were conducted with the Student's t-test.
ALDHhiSSChi granulocytic cells were increased in patients with T2D
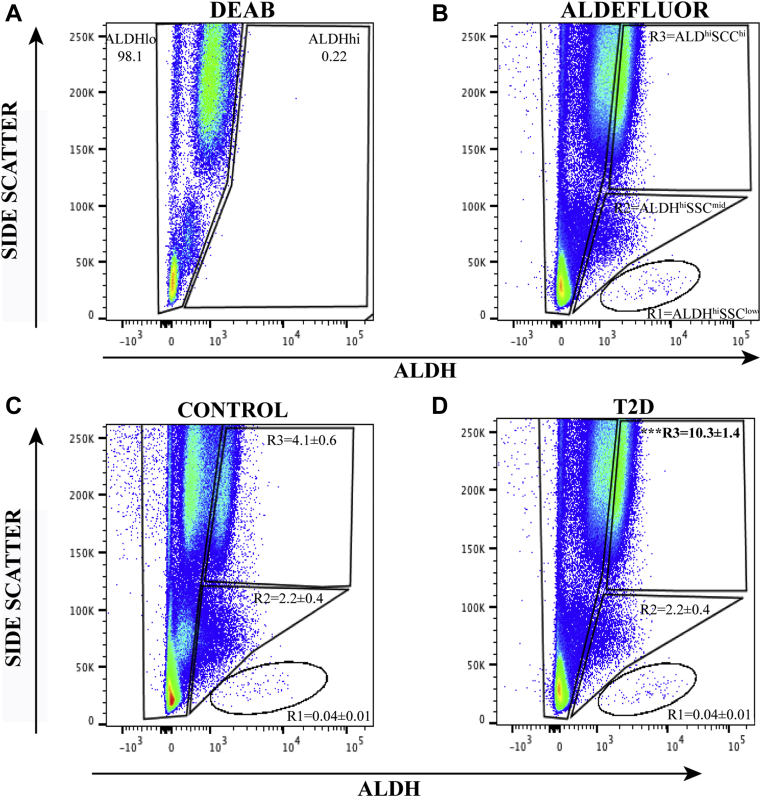
To more definitively assess circulating proangiogenic progenitor cell frequencies in the peripheral blood of patients with T2D, the Aldefluor assay was used to detect circulating cells with high ALDH-activity, a conserved characteristic in multiple progenitor cell lineages. We have previously documented the robust proangiogenic secretory function of ALDHhi cells from human umbilical cord blood and BM 17, 24, 25, 34. We identified three distinct cell populations with high ALDH-activity segregated further according to SSC properties representing cells with increasing intracellular complexity. A reversible inhibitor of ALDH-activity, N,N-diethylaminobenzaldehyde, was used to discern cells with low versus high ALDH-activity (Figure 1A), alongside low (R1) versus intermediate (R2) versus high (R3) SSC as shown in Figure 1B. Importantly, the frequency of cells with ALDHhiSSClow (progenitor cells) and ALDHhiSSCmid (primarily monocytes) phenotypes were equal in patients with T2D compared with control subjects (Figures 1C and 1D). In contrast, cells with the ALDHhiSSChi (granulocytes) phenotype were >2-fold increased in patients with T2D. These ALDHhiSSChi cells expressed neutrophil markers, including CD15, CD16b, and CD66b; some were positive for the monocyte marker CD14, and all cells were negative for CD34 co-expression. These findings confirm that the ALDHhiSSChi population primarily comprised granulocytes that can propagate inflammatory processes, again marking increased circulating inflammatory cell content in patients with T2D.
Figure 1.
Circulating Progenitor Cell Subpopulations Are Discerned According to High ALDH-Activity and SSC Properties
(A and B) Representative flow cytometry plots using N,N-diethylaminobenzaldehyde (DEAB) to inhibit aldehyde dehydrogenase (ALDH) activity establishing gates for low versus high ALDH-activity. Without inhibition, cells with high ALDH-activity exhibit increased fluorescence intensity (right shift) and detect primitive cells with a self-protective progenitor cell phenotype. High ALDH-activity combined with side scatter (SSC) properties selects for a progenitor cell subpopulation with low intracellular complexity (R1 = ALDHhiSSClow cells), a monocyte subpopulation with intermediate intracellular complexity (R2 = ALDHhiSSCmid cells), and a granulocytic subpopulation with high intracellular complexity (R3 = ALDHhiSSChi cells). (C and D) Representative flow cytometry plots showing the frequencies of each population in control subjects and patients with type 2 diabetes mellitus (T2D). Compared with control subjects, patients with T2D exhibited an increased frequency of ALDHhi cells within the granulocyte subpopulation (R3) and an equal frequency of circulating ALDHhi cells with low (R1) and intermediate (R2) complexity. Values are mean ± SEM. ***p < 0.001 with the Student's t-test.
ALDHhiSSClow cells with progenitor cell surface marker co-expression were decreased in patients with T2D
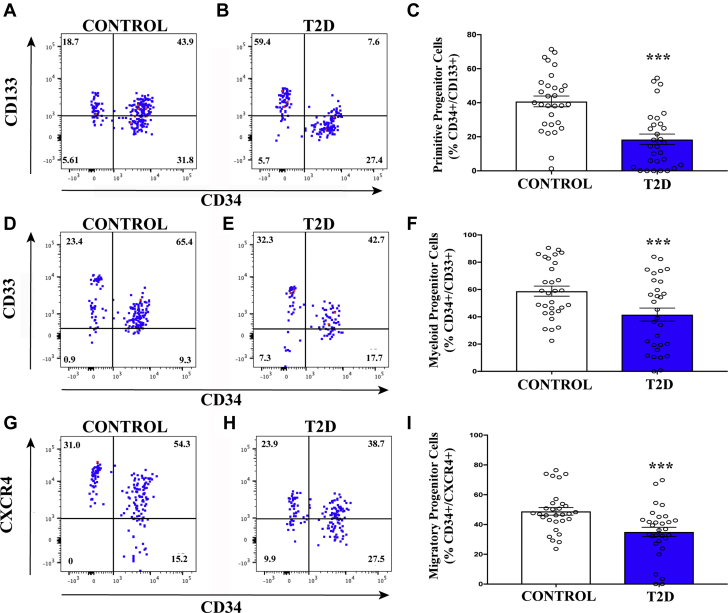
ALDHhiSSClow cells can be described as a heterogeneous progenitor cell population comprising primarily hematopoietic (>90%) and endothelial (<10%) cell lineages (17), and they have both been shown to support angiogenic blood vessel formation in immunodeficient mice with femoral artery ligation. Although these cells are extremely rare in the peripheral circulation (<0.1%), they possess a robust proangiogenic signaling profile and also contain rare endothelial precursor cells with the capacity to integrate into sprouting vessels 35, 36, 37, 38. Thus, detection of circulating ALDHhiSSClow cells is critical for the assessment of provascular regenerative capacity. Although we detected no significant differences in the overall frequency of ALDHhiSSClow or ALDHhiSSCmid cells between patients with T2D and matched control subjects, ALDHhiSSClow cells were further assessed for primitive hematopoietic and endothelial cell surface expression (CD34+/CD133+), early myeloid cell surface marker expression (CD34+/CD33+), and primitive migratory progenitor cells (CD34+/CXCR4+ cells). Both proangiogenic hematopoietic and vessel-integrating endothelial progenitor cells commonly express CD34 (36). The frequency of cells expressing CD34 alone was not different in the ALDHhiSSClow population (Supplemental Table 2) but patients with T2D exhibited significantly decreased frequencies of primitive progenitor (Figures 2A to 2C), early myeloid (Figures 2D to 2F), and migratory progenitor (Figures 2G to 2I) cells compared with control subjects; these findings indicate reduced vascular regenerative progenitor cell representation in patients with T2D 23, 39.
Figure 2.
Circulating ALDHhiSSClow Cells With Primitive, Myeloid, and Migratory Phenotypes Are Decreased in Patients with T2D
(A–C) The frequency of circulating ALDHhiSSClow progenitor cells with primitive cell phenotype (CD34+CD133+) was reduced in patients with T2D compared with control subjects. (D–F) The frequency of circulating ALDHhiSSClow progenitor cells with early myeloid cell phenotype (CD34+CD33+) was reduced in patients with T2D compared with control subjects. (G–I) The frequency of circulating ALDHhiSSClow progenitor cells with migratory phenotype (CD34+CXCR4+) was reduced in patients with T2D compared with control subjects. Values are mean ± SEM. ***p < 0.01 with the Student's t-test. Abbreviations as in Figure 1.
ALDHhiSSClow cells with endothelial-associated phenotypes were decreased in patients with T2D
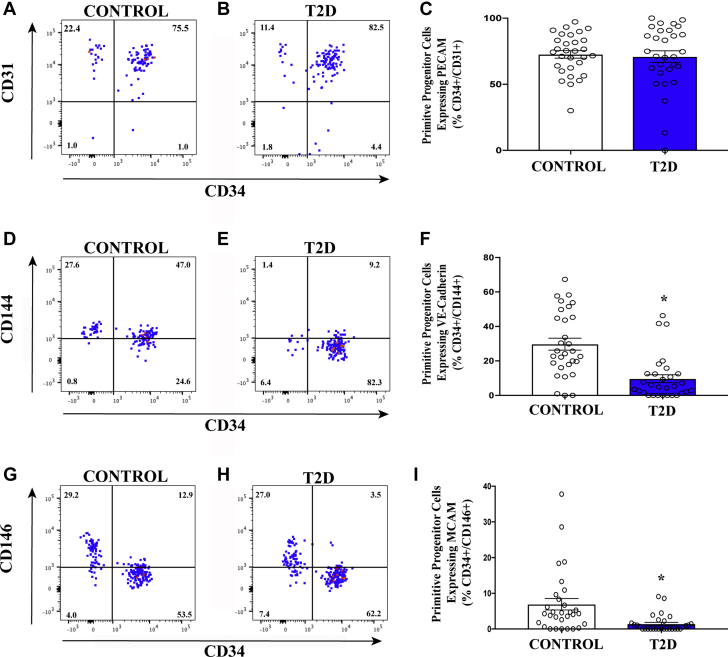
We next compared the ALDHhiSSClow population for the expression of endothelial cell–associated and pericyte-associated adhesion molecules. Although the frequency of cells co-expressing CD34 and platelet endothelial cell adhesion molecule-1 (CD31) was equivalent between patients with T2D and control subjects (Figures 3A to 3C), primitive (CD34+) cells expressing vascular endothelial–cadherin and the pericyte marker CD146 were decreased in patients with T2D compared with control subjects (Figures 3D to 3I). Cells expressing these markers are required for vasculogenic vessel formation (40). These findings were also consistent with the depletion of proangiogenic circulating cell content, potentially leading to a dysfunctional vascular regenerative response in patients with T2D.
Figure 3.
Circulating ALDHhiSSClow Cells With Endothelial Cell and Pericyte-associated Adhesive Phenotypes Are Decreased in Patients With T2D
(A–C) The frequency of circulating ALDHhiSSClow progenitor cells co-expressing CD34 with hematopoietic/endothelial cell marker platelet endothelial cell adhesion molecule (PECAM) (CD31) was equivalent in patients with T2D compared with control subjects. (D–F) The frequency of circulating ALDHhiSSClow cells in progenitor cells co-expressing CD34 with the endothelial cell–associated marker vascular endothelial (VE)-cadherin (CD144) was decreased in patients with T2D compared with control subjects. (G–I) The frequency of circulating ALDHhiSSClow progenitor cells co-expressing CD34 with the endothelial/pericytes marker melanoma cell adhesion molecule (MCAM) (CD146) was decreased in patients with T2D compared with control subjects. Values are mean ± SEM. ∗p < 0.001 with the Student's t-test. Other abbreviations as in Figure 1.
ALDHhiSSCmid cells with primitive and M2 phenotypes were decreased in patients with T2D
Primitive circulating monocytes with proinflammatory and anti-inflammatory cytokine secretion patterns 33, 41, 42 were further assessed by analyses of CD68+ cells co-expressing CD34 or CD80 versus CD163 (M1/M2 marker) specifically within the ALDHhiSSCmid cell subset. These cells can best be described as monocytes that possess either anti-inflammatory or proinflammatory secretory activities characterized by co-expression of M1/M2 polarization markers, respectively 33, 43. M2-polarized monocytes and tissue-resident M2 macrophages can contribute toward arteriogenic processes through secretion of cytokines and metalloproteinases that remodel pre-existing collateral vessels (44). In contrast, M1 macrophages generally contribute toward inflammatory processes that may impede new vessel progression 18, 43, 45. Although we detected no significant differences in the frequency of ALDHhiSSCmid cells with M1 (CD68+/CD80+) phenotypes (Figures 4D to 4F), patients with T2D exhibited significantly reduced frequency of ALDHhiSSCmid cells with primitive (CD34+/CD68+) (Figure 4A to 4C) or M2 (CD34+/CD163+) (Figures 4G to 4I) phenotypes compared with control subjects. These findings validated the reduction in circulating monocytes with M2 polarization phenotype shown in Table 1 and suggest that patients with T2D may also exhibit reduced capacity to mediate arteriogenic vessel remodeling.
Figure 4.
Circulating ALDHhiSSCmid Cells With M2 Phenotype Are Decreased in Patients With T2D
(A to C) The frequency of circulating ALDHhiSSCmid cells co-expressing CD34 with the macrophage scavenger receptor (CD68) was decreased in patients with T2D compared with control subjects. (D to F) The frequency of circulating ALDHhiSSCmid cells co-expressing CD68 with the M1 macrophage–associated marker CD80 was equal in patients with T2D compared with control subjects. (G to I) The frequency of circulating ALDHhiSSCmid progenitor cells co-expressing CD68 with the M2 macrophage–associated marker CD163 was decreased in patients with T2D compared with control subjects. Values are mean ± SEM. *p < 0.05 with the Student's t-test. Other abbreviations as in Figure 1.
Insulin administration correlated with reduced circulating granulocyte frequency
To determine whether factors such as sex, insulin use, or HbA1c status played a correlative role in the frequency of circulating provascular progenitor cells in patients with T2D, these patients were divided into 2 groups based on the median value for each category. The frequency of cell subsets with high ALDH-activity with primitive (CD34+/CD133+) and migratory (CD34+/CXCR4+) cell surface phenotype was equivalent in male (n = 12) and female (n = 18) patients with T2D (Supplemental Figure 2). Surprisingly, patients with HbA1c values ≤7.0% (n = 15) or >7.0% (n = 15) also showed no significant difference in the frequencies of ALDHhi cell subpopulations or in ALDHhiSSClow progenitor cells that expressed primitive or migratory phenotypes (Supplemental Figure 3). These data suggest that higher HbA1c levels did not correlate with a reduction in circulating progenitor cell content during T2D.
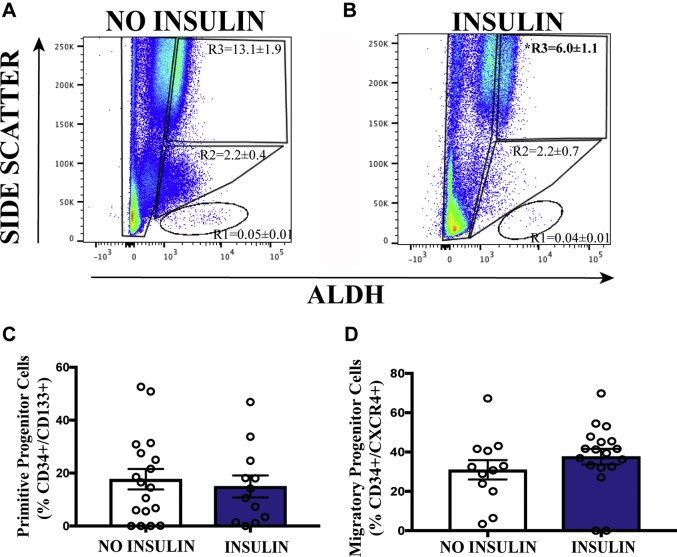
We next segregated patients according to their use of daily insulin injections, a general indication of more advanced T2D whereby glycemia is not controlled by diet, exercise, and medication. There was a significant decrease in the frequency of circulating pro-inflammatory ALDHhiSSChi granulocytes in patients receiving insulin therapy (Figures 5A and 5B). These data suggested reduced inflammation in patients who received insulin therapy; however, circulating primitive progenitor cell frequencies were not affected by insulin use (Figures 5C and 5D).
Figure 5.
Circulating ALDHhiSSChi Inflammatory Cells Are Decreased in Patients Taking Insulin
(A and B) The frequency of cells with high ALDH-activity and high SSC properties was decreased in patients with T2D taking insulin. (C and D) The frequency of primitive and migratory progenitor cells was equivalent in patients with T2D despite insulin therapy. Values are mean ± SEM. *p < 0.05 with the Student's t-test. Abbreviations as in Figure 1.
Provascular progenitor cell exhaustion correlated with increased duration of T2D
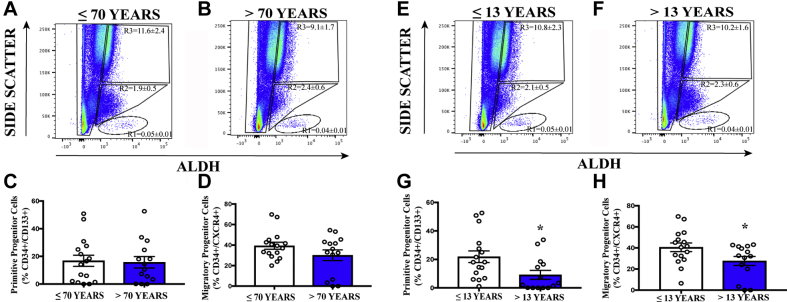
To further assess regenerative cell exhaustion during T2D, a process through which stem and progenitor cell frequency is reduced due to chronic disease, patients with T2D were subdivided based on chronological age or duration of T2D. Although increased age (≤70 years, n = 14; >70 years, n = 16) did not alter ALDH expression levels (Figures 6A and 6B) or primitive progenitor (CD34+/CD133+) or migratory (CD34+/CXCR4+) cell surface marker expression (Figures 6C and 6D), patients with increased duration of T2D (≤13 years, n = 15; >13 years, n = 15) demonstrated no difference in ALDH expression levels (Figure 6E and 6F), but did, however, exhibit significantly decreased frequency of primitive (CD34+/CD133+) and migratory (CD34+/CXCR4+) progenitor cells (Figures 6G and 6H). These data suggested that patients with prolonged T2D duration was the only analyzed subgroup that exhibited significantly reduced circulating proangiogenic progenitor cell content 23, 46, 47, 48.
Figure 6.
Circulating ALDHhiSSClow Cells With Primitive and Migratory Progenitor Cell Phenotypes Decreased With Longer Duration of Diabetes
(A and B) In patients with T2D, the frequency of cells with high ALDH-activity was equivalent in patients ≤70 years of age compared with patients >70 years of age with T2D. (C and D) The frequency of circulating primitive progenitor cells (CD34+/CD133+) and migratory progenitor cells (CD34+/CXCR4+) was equivalent in patients ≤70 years of age compared with patients >70 years of age. (E and F) In patients with T2D, the frequency of cells with high ALDH-activity was equivalent in patients with diabetes duration ≤13 years compared with patients with diabetes duration >13 years. (G and H) However, the frequency of circulating primitive progenitor cells (CD34+/CD133+) and migratory progenitor cells was decreased in patients with diabetes duration ≤13 years compared with patients with diabetes duration >13 years. Values are mean ± SEM. *p < 0.05 with the Student's t-test. Abbreviations as in Figure 1.
Discussion
The current study presents a novel diagnostic flow cytometry assay, using high ALDH-activity, a functional measure for a conserved progenitor cell function, combined with selected primitive and mature cell surface marker analyses, to characterize the frequency of cellular subsets with proangiogenic versus proinflammatory phenotypes from the peripheral blood of human patients with T2D compared with individuals without diabetes. The use of cell surface markers independently (e.g., CD34, CD133), as previously reported in several studies 35, 39, 49, revealed few differences in circulating cell frequencies between groups. However, by first detecting cells with high ALDH-activity, combined with SSC properties to discern granulocytic/neutrophil (SSChi cells), monocyte (SSCmid cells), or primitive progenitor cell (SSClow cells) subpopulations, allowed for additional comparison of primitive, progenitor cell markers (CD34, CD133) previously associated with proangiogenic secretory functions 37, 38, 50. By using this combined functional and phenotypic strategy, patients with T2D consistently exhibited the following unique characteristics: 1) an increased frequency of ALDHhiSSChi granulocytes predicted to propagate inflammatory burden 51, 52; 2) a reduced frequency of circulating ALDHhiSSCmid monocytes with CD14+ co-expression 33, 43; 3) a shift in ALDHhiSSCmid cell M1/M2 balance toward the pro-inflammatory M1 phenotype 31, 53; and 4) a decreased frequency of rare circulating ALDHhiSSClow progenitor cells that co-expressed CD34 and primitive (CD133), early myeloid (CD33), migratory (CXCR4), endothelial adhesion (CD144), or pericyte (CD146) cell surface markers. Collectively, these data suggest that a prolonged duration of T2D promotes a pro-inflammatory milieu, affecting both granulocytes and monocytes, in addition to depletion of rare progenitor cells shown previously to coordinate proangiogenic blood vessel repair in animal models 23, 35, 48, 54, 55.
The overall frequency of ALDHhiSSClow progenitor cells or CD34-expressing cells was surprisingly not decreased in patients with T2D compared with the control subjects. Selection for cells with high ALDH-activity 4, 56 or CD34 expression 30, 32, 57, 58 has been used in clinical trials as highly purified cell populations transplanted from autologous BM to combat ischemic disease. In contrast, a significant reduction in the frequency of circulating monocytes 33, 42, 43 with anti-inflammatory M2 phenotype 31, 59, 60 was easily detected in patients with T2D. These observations may be attributed to differences in the relative frequencies of circulating cells in the peripheral blood of patients with T2D and nondiabetic control subjects. The frequency of ALDHhiSSClow cells in the peripheral blood of both cohorts was exceedingly low, comprising <0.1% of peripheral blood mononuclear cells, whereas CD14+ monocytes were >100-fold more abundant. Thus, careful analyses of the rare ALDHhiSSClow cell subset required multicolor assessment of CD34 co-expression in addition to multiple cell surface molecules with functional significance to quantify the depletion of circulating proangiogenic progenitor cells. The expression of primitive (CD133), early myeloid (CD33), chemokine (CXCR4), and cellular adhesion (CD144) molecules was consistently reduced in patients with T2D compared with nondiabetic control subjects. These findings suggest that ALDHhi progenitor cells in the circulation of patients with T2D may exhibit deficits in cell adhesion and migration capacity toward ischemic endothelium. Therefore, direct comparison of ALDHhiSSClow cells for colony formation 24, 25, 34, cytokine secretion patterns (24), and migratory function in patients with T2D and control subjects are next required to assess potential functional deficits in circulating provascular cell populations.
Generalized inflammatory excess combined with circulating provascular progenitor cell depletion may contribute to an underlying issue affecting adult hematopoietic and endothelial progenitor cell maintenance in patients with T2D. This phenomenon, termed regenerative cell exhaustion, documents the loss of vascular regenerative capacity due to premature progenitor cell maturation and a reduction in the number of undifferentiated cells within the BM reservoir 27, 28, 46, 61, 62. Increased inflammation associated with chronic T2D is also known to induce increased expression of NADPH oxidase-1, which regulates the formation of ROS 28, 63, 64. Conceptually, although progenitor cells possess defense mechanisms such as elevated ALDH-activity to reduce oxidative stress and prevent premature apoptosis, excessive ROS may contribute to aberrant differentiation and maturation regulation within progenitor cells, resulting in the premature departure of vascular regenerative precursors from the endosteal niche in the BM 64, 65, 66. In the peripheral circulation, without the influence of developmental factors (e.g., Wnt [wingless related integration site], Notch) in the BM stem cell niche 67, 68, 69, 70, cells are expected to demonstrate aberrant differentiation, generating dysfunctional cells with reduced contribution toward blood vessel repair and regeneration 71, 72. Thus, further measurements of cell frequencies using this approach in the BM and other tissues may help correlate circulating cell deficiencies with compromised function in tissues.
To further show the utility of this assay, we stratified the T2D cohort based on patient sex, age, duration of diagnosed T2D, HbA1c value, and the requirement for insulin. Notably, only the duration of T2D correlated with reduced proangiogenic progenitor cell frequency. Indeed, regenerative cell depletion became more prominent with extended duration of T2D.
To our knowledge, the current study is the first to clearly document the depletion of circulating provascular progenitor cell content by using ALDH-activity during established T2D in human subjects. Furthermore, patients with T2D exhibited a departure from an anti-inflammatory M2 phenotype toward a pro-inflammatory M1 phenotype compounded by an increase in circulating granulocytes. Throughout these analyses, we documented changes in the frequency of circulating cell phenotypes implicated in the restoration of vascular regenerative function in patients with T2D. In addition, this research provides a starting point for development of novel therapeutic approaches to combat ischemic vascular disease progression during T2D. It is evident that regenerative cell depletion and heightened inflammation during T2D generates a harsh microenvironment for functional revascularization 23, 27, 48, 62, 63. By reducing inflammation and limiting ROS, development of therapeutic strategies tailored to the restoration of the vascular regenerative cell generation and function may aid in the prevention of ischemic vascular comorbidities that are so devastating during the progression of T2D.
Study limitations and future directions
Care must be taken when extending the utility of these studies toward potential clinical application. First, the detection of very rare circulating cell populations by using flow cytometry provides a diagnostic tool to measure altered cell frequencies during T2D. Future long-term clinical studies should incorporate multiple assessments of circulating cell subpopulations as T2D progresses. With mindful trial design, this assay may reveal the sensitivity required to correlate changes in circulating cell frequencies with specific outcomes such as adverse cardiovascular events. Second, circulating cell subpopulations were not assayed for relevant proangiogenic function in this study. In future studies, we intend to assess colony formation as well as secretory and migratory functions of relevant cell populations as T2D progresses. Third, analyses of tissue-resident progenitor cell or macrophage frequencies were not conducted in this study and are needed to determine how circulating cell content correlates with cell frequencies in the BM or other tissues affected by ischemia such as the heart or skeletal muscle. Finally, disease comorbidities such as obesity, atherosclerotic burden, previous ischemic events, and drug use need to be carefully controlled between groups when interpreting the relevance of these measurements on the potential alteration of T2D progression.
Conclusions
Circulating cells with pro-inflammatory phenotype were more abundant in patients with T2D, and rare ALDH-expressing progenitor cells previously associated with vascular regenerative function were markedly reduced. In addition, adhesive and migratory cell surface marker co-expression associated with homing to areas of ischemia and secretion of proangiogenic effectors was deficient on circulating ALDHhi progenitor cells in patients with T2D. Collectively, alterations in these circulating cell phenotypes may presumably contribute to the gradual loss of the capacity for vascular repair. Although we can clearly detect differences in the frequencies of rare circulating progenitor cells by using high ALDH-activity, further studies are warranted to assess the vascular regenerative functions of these circulating cell populations. Functional analyses relevant to ischemic disease include the formation of myeloid hematopoietic and endothelial cell colonies, tubule formation, migration to areas of ischemia, and secretion of proangiogenic cytokines that coordinate vascular regenerative processes. Furthermore, functional testing of the capacity of these cell types to contribute to perfused neovessel formation in vivo is still required. Nonetheless, potential reversal of this “exhausted” vascular regenerative cell phenotype during T2D, through regenerative medicine strategies or by administration of therapeutic agents with documented cardiovascular protective effects, represents an exciting avenue to be tested in future studies.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Individuals with T2D are at a heightened risk of developing cardiovascular disorders and often endure poor outcomes after a cardiovascular event. This study shows, for the first time, that compared with individuals who are normoglycemic, those with established T2D exhibit depleted circulating vascular regenerative progenitor cell content measured in the circulation by using ALDH-activity, a conserved protective function demonstrated by proangiogenic endothelial and hematopoietic progenitor cells. In contrast, circulating monocytes exhibit a migration from a protective anti-inflammatory phenotype to one that is proinflammatory, collectively discouraging functional revascularization.
TRANSLATIONAL OUTLOOK: Using a combination of ALDH-activity measurement and cell surface marker expression, we have developed a novel diagnostic flow cytometry assay to evaluate the balance between circulating proangiogenic progenitor and proinflammatory cell content in peripheral blood. This study provides a critical translational perspective by the suggestion that the balance in these cells during the progression of T2D is critical to the development of and recovery from ischemic vascular comorbidities. The potential of developing this assay into a diagnostic tool to estimate the capacity to mitigate ischemia via a provascular regenerative response represents an exciting avenue for exploration and will need to be investigated.
Contributor Information
Subodh Verma, Email: vermasu@smh.ca.
David A. Hess, Email: dhess@robarts.ca.
Appendix
References
- 1.American Diabetes Association 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018;41:S13–S27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Canada Clinical Practice Guidelines Expert Committee. Punthakee Z., Goldenberg R., Katz P. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes. 2018;42 Suppl 1:S10–S15. doi: 10.1016/j.jcjd.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 3.International Diabetes Federation . International Diabetes Federation; Brussels, Belgium: 2017. IDF Diabetes Atlas—Eight Edition. [Google Scholar]
- 4.Qadura M., Terenzi D.C., Verma S., Al-Omran M., Hess D.A. Concise review: cell therapy for critical limb ischemia: an integrated review of preclinical and clinical studies. Stem Cells. 2018;36:161–171. doi: 10.1002/stem.2751. [DOI] [PubMed] [Google Scholar]
- 5.Zinman B., Wanner C., Lachin J.M. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 6.Verma S., Mazer C.D., Fitchett D. Empagliflozin reduces cardiovascular events, mortality and renal events in participants with type 2 diabetes after coronary artery bypass graft surgery: subanalysis of the EMPA-REG OUTCOME(R) randomised trial. Diabetologia. 2018;61:1712–1723. doi: 10.1007/s00125-018-4644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verma S., Mazer C.D., Al-Omran M. Cardiovascular outcomes and safety of empagliflozin in patients with type 2 diabetes mellitus and peripheral artery disease: a subanalysis of EMPA-REG OUTCOME. Circulation. 2018;137:405–407. doi: 10.1161/CIRCULATIONAHA.117.032031. [DOI] [PubMed] [Google Scholar]
- 8.Verma S., Bhatt D.L., Bain S.C. Effect of liraglutide on cardiovascular events in patients with type 2 diabetes mellitus and polyvascular disease: results of the LEADER trial. Circulation. 2018;137:2179–2183. doi: 10.1161/CIRCULATIONAHA.118.033898. [DOI] [PubMed] [Google Scholar]
- 9.Mann J.F., Orsted D.D., Brown-Frandsen K. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839–848. doi: 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 10.Marso S.P., Daniels G.H., Brown-Frandsen K. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verma S., Leiter L.A., Mazer C.D. Liraglutide reduces cardiovascular events and mortality in type 2 diabetes mellitus independently of baseline low-density lipoprotein cholesterol levels and statin use. Circulation. 2018;138:1605–1607. doi: 10.1161/CIRCULATIONAHA.118.036862. [DOI] [PubMed] [Google Scholar]
- 12.Verma S., Poulter N.R., Bhatt D.L. Effects of liraglutide on cardiovascular outcomes in patients with type 2 diabetes with or without history of myocardial infarction or stroke: a post hoc analysis from the LEADER trial. Circulation. 2018;137:2179–2183. doi: 10.1161/CIRCULATIONAHA.118.033898. [DOI] [PubMed] [Google Scholar]
- 13.Sherman S.E., Bell G.I., Teoh H. Canagliflozin improves the recovery of blood flow in an experimental model of severe limb ischemia. J Am Coll Cardiol Basic Trans Science. 2018;3:327–329. doi: 10.1016/j.jacbts.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verma S., Rawat S., Ho K.L. Empagliflozin increases cardiac energy production in diabetes. Novel translational insights into the heart failure benefits of SGLT2 inhibitors. J Am Coll Cardiol Basic Trans Science. 2018;3:575–587. doi: 10.1016/j.jacbts.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wanner C., Inzucchi S.E., Lachin J.M. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 16.Prospective Studies Collaboration, Asia Pacific Cohort Studies Collaboration Sex-specific relevance of diabetes to occlusive vascular and other mortality: a collaborative meta-analysis of individual data from 980 793 adults from 68 prospective studies. Lancet Diabetes Endocrinol. 2018;6:538–546. doi: 10.1016/S2213-8587(18)30079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess D.A., Meyerrose T.E., Wirthlin L. Functional characterization of highly purified human hematopoietic repopulating cells isolated according to aldehyde dehydrogenase activity. Blood. 2004;104:1648–1655. doi: 10.1182/blood-2004-02-0448. [DOI] [PubMed] [Google Scholar]
- 18.Chambers S.E., O'Neill C.L., O'Doherty T.M., Medina R.J., Stitt A.W. The role of immune-related myeloid cells in angiogenesis. Immunobiology. 2013;218:1370–1375. doi: 10.1016/j.imbio.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Norgren L., Hiatt W.R., Dormandy J.A. Inter-society consensus for the management of peripheral arterial disease. Int Angiol. 2007;26:81–157. [PubMed] [Google Scholar]
- 20.Bergers G., Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buschmann I., Heil M., Jost M., Schaper W. Influence of inflammatory cytokines on arteriogenesis. Microcirculation. 2003;10:371–379. doi: 10.1038/sj.mn.7800199. [DOI] [PubMed] [Google Scholar]
- 22.King A., Balaji S., Keswani S.G., Crombleholme T.M. The role of stem cells in wound angiogenesis. Adv Wound Care (New Rochelle) 2014;3:614–625. doi: 10.1089/wound.2013.0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fadini G.P., Ferraro F., Quaini F., Asahara T., Madeddu P. Concise review: diabetes, the bone marrow niche, and impaired vascular regeneration. Stem Cells Transl Med. 2014;3:949–957. doi: 10.5966/sctm.2014-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Putman D.M., Liu K.Y., Broughton H.C., Bell G.I., Hess D.A. Umbilical cord blood-derived aldehyde dehydrogenase-expressing progenitor cells promote recovery from acute ischemic injury. Stem Cells. 2012;30:2248–2260. doi: 10.1002/stem.1206. [DOI] [PubMed] [Google Scholar]
- 25.Putman D.M., Cooper T.T., Sherman S.E. Expansion of umbilical cord blood aldehyde dehydrogenase expressing cells generates myeloid progenitor cells that stimulate limb revascularization. Stem Cells Transl Med. 2017;6:1607–1619. doi: 10.1002/sctm.16-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel R.S., Li Q., Ghasemzadeh N. Circulating CD34+ progenitor cells and risk of mortality in a population with coronary artery disease. Circ Res. 2015;116:289–297. doi: 10.1161/CIRCRESAHA.116.304187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bigarella C.L., Liang R., Ghaffari S. Stem cells and the impact of ROS signaling. Development. 2014;141:4206–4218. doi: 10.1242/dev.107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mangialardi G., Spinetti G., Reni C., Madeddu P. Reactive oxygen species adversely impacts bone marrow microenvironment in diabetes. Antioxid Redox Signal. 2014;21:1620–1633. doi: 10.1089/ars.2014.5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loomans C.J., van Haperen R., Duijs J.M. Differentiation of bone marrow-derived endothelial progenitor cells is shifted into a proinflammatory phenotype by hyperglycemia. Mol Med. 2009;15:152–159. doi: 10.2119/molmed.2009.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackie A.R., Losordo D.W. CD34-positive stem cells: in the treatment of heart and vascular disease in human beings. Tex Heart Inst J. 2011;38:474–485. [PMC free article] [PubMed] [Google Scholar]
- 31.Jetten N., Verbruggen S., Gijbels M.J., Post M.J., De Winther M.P., Donners M.M. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;17:109–118. doi: 10.1007/s10456-013-9381-6. [DOI] [PubMed] [Google Scholar]
- 32.Mathiyalagan P., Liang Y., Kim D. Angiogenic mechanisms of human CD34(+) stem cell exosomes in the repair of ischemic hindlimb. Circ Res. 2017;120:1466–1476. doi: 10.1161/CIRCRESAHA.116.310557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urbich C., Heeschen C., Aicher A., Dernbach E., Zeiher A.M., Dimmeler S. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–2516. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- 34.Capoccia B.J., Robson D.L., Levac K.D. Revascularization of ischemic limbs after transplantation of human bone marrow cells with high aldehyde dehydrogenase activity. Blood. 2009;113:5340–5351. doi: 10.1182/blood-2008-04-154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asahara T., Murohara T., Sullivan A. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 36.Asahara T., Masuda H., Takahashi T. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 37.Hess D.A., Wirthlin L., Craft T.P. Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood. 2006;107:2162–2169. doi: 10.1182/blood-2005-06-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siemerink M.J., Klaassen I., Vogels I.M., Griffioen A.W., Van Noorden C.J., Schlingemann R.O. CD34 marks angiogenic tip cells in human vascular endothelial cell cultures. Angiogenesis. 2012;15:151–163. doi: 10.1007/s10456-011-9251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fadini G.P., Miorin M., Facco M. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 40.Yoder M.C., Mead L.E., Prater D. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Travnickova J., Tran Chau V., Julien E. Primitive macrophages control HSPC mobilization and definitive haematopoiesis. Nat Commun. 2015;6:6227. doi: 10.1038/ncomms7227. [DOI] [PubMed] [Google Scholar]
- 42.Jaipersad A.S., Lip G.Y., Silverman S., Shantsila E. The role of monocytes in angiogenesis and atherosclerosis. J Am Coll Cardiol. 2014;63:1–11. doi: 10.1016/j.jacc.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 43.Dalton H.J., Armaiz-Pena G.N., Gonzalez-Villasana V., Lopez-Berestein G., Bar-Eli M., Sood A.K. Monocyte subpopulations in angiogenesis. Cancer Res. 2014;74:1287–1293. doi: 10.1158/0008-5472.CAN-13-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heil M., Schaper W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis) Circ Res. 2004;95:449–458. doi: 10.1161/01.RES.0000141145.78900.44. [DOI] [PubMed] [Google Scholar]
- 45.Peiser L., Gordon S. The function of scavenger receptors expressed by macrophages and their role in the regulation of inflammation. Microbes Infect. 2001;3:149–159. doi: 10.1016/s1286-4579(00)01362-9. [DOI] [PubMed] [Google Scholar]
- 46.Kovacic J.C., Moreno P., Hachinski V., Nabel E.G., Fuster V. Cellular senescence, vascular disease, and aging: part 1 of a 2-part review. Circulation. 2011;123:1650–1660. doi: 10.1161/CIRCULATIONAHA.110.007021. [DOI] [PubMed] [Google Scholar]
- 47.Dykstra B., de Haan G. Hematopoietic stem cell aging and self-renewal. Cell Tissue Res. 2008;331:91–101. doi: 10.1007/s00441-007-0529-9. [DOI] [PubMed] [Google Scholar]
- 48.Fadini G.P., Ciciliot S., Albiero M. Concise review: perspectives and clinical implications of bone marrow and circulating stem cell defects in diabetes. Stem Cells. 2017;35:106–116. doi: 10.1002/stem.2445. [DOI] [PubMed] [Google Scholar]
- 49.Werner N., Kosiol S., Scheigel T. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;355:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 50.Barcelos L.S., Duplaa C., Krankel N. Human CD133+ progenitor cells promote the healing of diabetic ischemic ulcers by paracrine stimulation of angiogenesis and activation of Wnt signaling. Circ Res. 2009;104:1095–1102. doi: 10.1161/CIRCRESAHA.108.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosales C. Neutrophil: a cell with many roles in inflammation or several cell types? Front Physiol. 2018;9:113. doi: 10.3389/fphys.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong S.L., Demers M., Martinod K. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med. 2015;21:815–819. doi: 10.1038/nm.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinez F.O., Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sata M. Role of circulating vascular progenitors in angiogenesis, vascular healing, and pulmonary hypertension: lessons from animal models. Arterioscler Thromb Vasc Biol. 2006;26:1008–1014. doi: 10.1161/01.ATV.0000206123.94140.f3. [DOI] [PubMed] [Google Scholar]
- 55.Xu H., Barnes G.T., Yang Q. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perin E.C., Murphy M., Cooke J.P. Rationale and design for PACE: patients with intermittent claudication injected with ALDH bright cells. Am Heart J. 2014;168:667–673. doi: 10.1016/j.ahj.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gupta R., Losordo D.W. Cell therapy for critical limb ischemia: moving forward one step at a time. Circ Cardiovasc Interv. 2011;4:2–5. doi: 10.1161/CIRCINTERVENTIONS.110.960716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Losordo D.W., Henry T.D., Davidson C. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109:428–436. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee W.J., Tateya S., Cheng A.M. M2 macrophage polarization mediates anti-inflammatory effects of endothelial nitric oxide signaling. Diabetes. 2015;64:2836–2846. doi: 10.2337/db14-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Y.C., Zou X.B., Chai Y.F., Yao Y.M. Macrophage polarization in inflammatory diseases. Int J Biol Sci. 2014;10:520–529. doi: 10.7150/ijbs.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tchkonia T., Zhu Y., van Deursen J., Campisi J., Kirkland J.L. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Freund A., Orjalo A.V., Desprez P.Y., Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Porto M.L., Rodrigues B.P., Menezes T.N. Reactive oxygen species contribute to dysfunction of bone marrow hematopoietic stem cells in aged C57BL/6 J mice. J Biomed Sci. 2015;22:97. doi: 10.1186/s12929-015-0201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shao L., Li H., Pazhanisamy S.K., Meng A., Wang Y., Zhou D. Reactive oxygen species and hematopoietic stem cell senescence. Int J Hematol. 2011;94:24–32. doi: 10.1007/s12185-011-0872-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shinohara A., Imai Y., Nakagawa M., Takahashi T., Ichikawa M., Kurokawa M. Intracellular reactive oxygen species mark and influence the megakaryocyte-erythrocyte progenitor fate of common myeloid progenitors. Stem Cells. 2014;32:548–557. doi: 10.1002/stem.1588. [DOI] [PubMed] [Google Scholar]
- 66.Rauscher F.M., Goldschmidt-Clermont P.J., Davis B.H. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108:457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 67.Williams A.R., Hare J.M. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kfoury Y., Scadden D.T. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell. 2015;16:239–253. doi: 10.1016/j.stem.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 69.Caplan A.I., Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Colter D.C., Sekiya I., Prockop D.J. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci U S A. 2001;98:7841–7845. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zoungas S., Woodward M., Li Q. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia. 2014;57:2465–2474. doi: 10.1007/s00125-014-3369-7. [DOI] [PubMed] [Google Scholar]
- 72.Duncan A.W., Rattis F.M., DiMascio L.N. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6:314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.