Abstract
Background
One of the major causes of perioperative mortality of patients undergoing major hepatic resections is post-hepatectomy liver failure (PHLF). For preoperative appraisal of the risk of PHLF it is important to accurately predict resectate volume and future liver remnant volume (FLRV). The objective of our study is to prospectively evaluate the accuracy of hemihepatectomy resectate volumes that are determined by computed tomography volumetry (CTV) when compared with intraoperatively measured volumes and weights as gold standard in patients undergoing hemihepatectomy.
Methods
Twenty four patients (13 women, 11 men) scheduled for hemihepatectomy due to histologically proven primary or secondary hepatic malignancies were included in our study. CTV was performed using a semi-automated module (S, hereinafter) (syngo.CT Liver Analysis VA30, Siemens Healthcare, Germany). Conversion factors between CT volumes on the one side and intraoperative volumes and weights on the other side were calculated using the method of least squares. Absolute and relative disagreements between CT volumes and intraoperative volumes were determined.
Results
A conversion factor of c = 0.906 most precisely predicted intraoperative volumes of exsanguinated hemihepatectomy specimens from CT volumes in all patients with mean absolute and relative disagreements between CT volumes and intraoperative volumes of 57 ml and 6.3%. The use of operation-specific conversion factors yielded even better results.
Conclusions
CTV performed with S accurately predicts intraoperative volumes of hemihepatectomy specimens when applying conversion factors which compensate for exsanguination. This allows to precisely estimate the FLRV and thus minimize the risk of PHLF in patients undergoing major hepatic resections.
Keywords: Computed tomography volumetry, Hemihepatectomy, Hepatic malignancy
Background
Major hepatic resections are often necessary to achieve curative resection in patients with extensive liver invasion by primary or secondary malignancy [1]. Patients undergoing a major hepatic resection are at increased risk for peri- and postoperative complications [2]. Among these, one of the major causes of perioperative mortality is post-hepatectomy liver failure (PHLF), defined as the impaired ability of the liver to maintain its synthetic, excretory, and detoxifying functions [1]. Many factors influence the risk of PHLF, including patient-related factors such as diabetes and overweight, the presence of underlying parenchymal liver disease such as steatosis, cirrhosis, and chemotherapy effects [3]. However, the size of the future liver remnant volume (FLRV) is considered to be the most important correctable predictor of PHLF [4]. Smaller FLRVs also increase the risk of postoperative infection [5]. Although there is no uniform agreement on the minimum FLRV, most hepatic surgeons consider a FLRV of 25.0% as sufficient in patients without underlying parenchymal liver disease, and a FLRV of at least 40.0–50.0% in patients with severe parenchymal disease [6, 7]. In Western patients without underlying parenchymal disease, the right liver lobe constitutes about two thirds of the total liver volume [4]. However, Abdalla et al. found great interpatient variability of segmental hepatic volumes determined by computed tomography volumetry (CTV) in 102 patients without liver disease [8]. Therefore, precise measurement of liver volumes plays a critical role in preoperative assessment of patients who are planned for major hepatic resections due to primary hepatic tumors or hepatic metastases [1].
Multiple imaging modalities have been exploited to measure the volume of FLR, including computed tomography (CT), magnetic resonance imaging (MRI), as well as ultrasound [4]. Among these, most authors consider CTV to be the current gold standard [6]. However, several studies have reported a significant inaccuracy rate of preoperatively measured liver volumes by manual, semi-automated or automated methods of CTV [9]. Most of these studies focused on living-related liver transplantation (LRLT) and showed over- or underestimation of actual graft volumes by CTV of up to 53.0% compared with intraoperatively measured volumes and weights [10, 11]. One study compared CTV of hepatectomy specimens with intraoperatively measured volumes in patients who underwent partial liver resection for focal liver lesions, with the result that their semi-automated method of CTV overestimates the specimen volume by about 14.0%, on average [9]. However, in this study the segmentation of the resected hemihepatectomy specimens was performed retrospectively according to the resection border visible on the postoperative MR images [9].
With this background, the objective of our study was to evaluate the accuracy of hemihepatectomy resectate volumes that were determined by CTV using a semi-automated Analysis Module (S) (syngo.CT Liver Analysis VA30, Siemens Healthcare, Germany) when compared with intraoperatively measured volumes and weights as gold standard in patients undergoing hemihepatectomy for primary or secondary hepatic malignancy.
Methods
Patient characteristics
24 patients (13 women, 11 men; mean age: 63 years ± SD [standard deviation] 11 years; range: 30 to 84 years) scheduled for hemihepatectomy due to histologically proven primary or secondary hepatic malignancies were included in our study. Malignancies were cholangiocarcinoma (CC) in 14 cases, hepatocellular carcinoma (HCC) in 1 case, and metastases in 9 cases (6 from colorectal cancer (CRC), 1 from thyroid cancer (TC), 1 from granulosa cell tumor (GCT), and 1 from malignant melanoma (MM)). Histopathologic analysis of the non-neoplastic tissue parts of the hemihepatectomy specimens showed no evidence of steatosis, portal fibrosis with formation of septa or parenchymal necrosis in 14 patients. The resected non-neoplastic liver parenchyma of the other 10 patients showed evidence of steatosis, portal fibrosis with formation of septa and/or parenchymal necrosis. None of the patients had liver cirrhosis. Patient demographics, performed operations, tumor diameters, and histopathological diagnoses are summarized in Table 1. All patients underwent preoperative CT examination for surgical planning. On average, the preoperative CT scan was performed 33 days prior to the operation (range: 1–226 days). CTV was performed using a semi-automated module (S) (syngo.CT Liver Analysis VA30, Siemens Healthcare, Germany) in all patients. Left hemihepatectomy was performed in 6 patients, right hemihepatectomy was performed in 11 patients and extended right hemihepatectomy was performed in 7 patients. The middle hepatic vein (MHV) was resected in 11 patients. Volumes (intraoperative volume) and weights (intraoperative weight) of the resected hemihepatectomy specimens were determined in the operating theatre.
Table 1.
Patient demographics, performed operations, tumor diameters, and histopathological diagnoses
| operation | ID | Age range | Resection of MHV | Tumor entity | Intrahepatic tumor diameter(s) (CT) | Histopathological diagnosis of resected non-neoplastic liver parenchyma |
|---|---|---|---|---|---|---|
| left hemi-hepatectomy | 1 | 55–59 yrs. | no | CC | 1.8 cm | portal fibrosis with formation of septa |
| 2 | 80–84 yrs. | no | CC | 1.4 cm | extensive parenchymal necrosis | |
| 3 | 65–69 yrs. | yes | CC | 3.2 cm | n.e. | |
| 4 | 60–64 yrs. | yes | CC | 11.0 cm | n.e. | |
| 5 | 65–69 yrs. | yes | CC | 5.0 cm | portal fibrosis with formation of septa | |
| 6 | 75–79 yrs. | no | CC | 1.8 cm | portal fibrosis with formation of septa | |
| right hemi-hepatectomy | 7 | 60–64 yrs. | no | 3 metastases (CRC) | 2.2 cm to 5.7 cm | n.e. |
| 8 | 70–74 yrs. | no | CC | 6.4 cm | portal fibrosis with formation of septa | |
| 9 | 50–54 yrs. | no | 6 metastases (CRC) | 1.2 cm to 3.3 cm | n.e. | |
| 10 | 65–69 yrs. | no | 5 metastasis (MM) | 1.2 cm to 6.0 cm | n.e. | |
| 11 | 60–64 yrs. | yes | CC | 2.2 cm | n.e. | |
| 12 | 75–79 yrs. | no | 1 metastasis (CRC) | 5.9 cm | n.e. | |
| 13 | 45–49 yrs. | no | 9 metastases (GCT) | 1.4 cm to 3.2 cm | n.e. | |
| 14 | 65–69 yrs. | no | HCC | 7.7 cm | n.e. | |
| 15 | 60–64 yrs. | no | CC (2 foci) | 1.4 cm to 10.1 cm | portal fibrosis with formation of septa | |
| 16 | 70–74 yrs. | no | CC | 1.7 cm | n.e. | |
| 17 | 50–54 yrs. | no | 6 metastases (TC) | 1.3 cm to 2.9 cm | hepatic steatosis | |
| extended right hemi-hepatectomy | 18 | 30–34 yrs. | yes | CC | 2.9 cm | n.e. |
| 19 | 60–64 yrs. | yes | 3 metastases (TC) | 1.8 cm to 13.8 cm | extensive parenchymal necrosis | |
| 20 | 60–64 yrs. | yes | CC | 11.8 cm | n.e. | |
| 21 | 70–74 yrs. | yes | 3 metastases (CRC) | 5.1 cm to 9.5 cm | n.e. | |
| 22 | 55–59 yrs. | yes | 5 metastases (CRC) | 3.8 cm to 6.7 cm | n.e. | |
| 23 | 65–69 yrs. | yes | CC | 1.0 cm | extensive parenchymal necrosis | |
| 24 | 55–59 yrs. | yes | CC | 1.5 cm | portal fibrosis with formation of septa |
f female, m male, yrs. years, MHV middle hepatic vein CC cholangiocarcinoma, GCT granulosa cell tumor, HCC hepatocellular carcinoma, MM malignant melanoma, TC thyroid carcinoma, n.e. no evidence of steatosis, portal fibrosis with formation of septa or parenchymal necrosis
CT scanning
CT examinations of the abdomen were performed on the following CT scanners: Siemens Definition Flash, Siemens Somatom Definition AS 40, Siemens Sensation 40, and Siemens Emotion 16 (256 rows, 40 rows, 40 rows, and 16 rows, Siemens Healthcare, Germany); Philips Brilliance iCT 256 (256 rows, Philips Healthcare, Hamburg, Germany). The CT scan protocol consisted of, at least, a portal-venous phase. Optionally, non-enhanced, arterial and late phases were performed. Accurate timing of the optional arterial phase was ensured by automated bolus tracking in the suprarenal aorta. The portal-venous phase was obtained with a delay of 60 s. Optionally, with an additional delay of 180 s, a late phase was acquired.
All images were reconstructed using a soft tissue convolution Kernel (either B30f, B30s, I30f, or B41s). Slice thicknesses of the reconstructed images were 2 mm in 1 patient, 3 mm in 16 patients, 4 mm in 1 patient, and 5 mm in 6 patients.
CT volumetry (CTV)
Axial images of the portal-venous phase were used for CTV, hence obtaining the CT volume. CTV was performed with a semi-automated Analysis Module (S) (syngo.CT Liver Analysis VA30, Siemens Healthcare, Germany). The liver outline was segmentated automatically, based on a hierarchical, learning-based approach [12]. After detection of the liver outline, hepatic veins (HV) and the portal vein (PV) were segmented semiautomatically. After setting seed points into the hepatocaval confluence and the main stem of the PV, the system automatically segments the HV and PV. Finally, the transection plane was defined in consensus by a medical student (MG), a radiologist with 12 years of experience in abdominal imaging (MK) and an experienced liver surgeon with 21 years of experience (PS). The volumes of the intrahepatic vessels in the liver area marked for resection were included in the CT volume. Surrounding extrahepatic vessels (e.g. extrahepatic PV), extrahepatic bile ducts, and the gallbladder were excluded.
Measurement of intraoperative weights and volumes
Intraoperative weights of hemihepatectomy specimens were measured using an electric table scale (CKW6R55-M, Ohaus Corporation, Pine Brook, NJ USA). Intraoperative volumes of the resected hemihepatectomy specimens were determined by water displacement based on the principle of Archimedes. A smaller bowl filled to the brim with 25 °C sterile physiologic saline was placed into a sufficiently larger bowl. The specimen was placed into the smaller inner bowl and overflowing saline was collected by the larger bowl. After removing the smaller bowl together with the specimen the overflow was accurately measured to 5 ml using a measuring cup.
Before intraoperative volume/ weight measurements the specimens were flushed with physiological saline to remove the blood.
Comparison of preoperatively defined transection planes with actual transection planes according to the resection border visible on postoperative CT images
In 18 out of 24 patients postoperative CT scans were available. The average time interval between surgery and postoperative CT scans was 55 days (range: 5–272 days). The maximum discrepancies between the preoperatively defined transection planes and the actual transection planes according to the resection border visible on postoperative CT images (taking into account the course of the PV and HV braches close to the resection border) were rated as ≤1.0 cm, 1.1 cm to 2.0 cm, or > 2.0 cm in consensus by two radiologists with 5 and 12 years of experience in abdominal imaging (PM and MK).
Statistical analysis
Statistical analysis was performed using GraphPad Prism Version 7.03 (GraphPad Software, La Jolla, CA, USA). CT volume measurements were compared to intraoperative weights and volumes. Correlation coefficients (r) were calculated between CT volumes on the one side and intraoperative volumes and weights on the other side, respectively. Conversion factors (c) between CT volumes on the one side and intraoperative volumes and weights on the other side were calculated using the method of linear least squares. This linear least squares fitting technique is a commonly applied form of linear regression and aims to minimize the sum of squared errors. A general conversion factor was determined for all specimens (coverall) and operation-specific conversion factors were determined for the specific type of operation performed (cspecific).
Differences (expressed in cm3) between CT volumes (with and without conversion factors) and intraoperative volumes were calculated using the following formula:
Mean values for differences were calculated for all specimens and separately for the specific type of operation performed.
Absolute disagreements (expressed in cm3) between CT volumes (with and without conversion factors) and intraoperative volumes were calculated using the following formulas:
Relative disagreements (expressed in %) between CT volumes (with and without conversion factors) and intraoperative volumes were calculated similarly as previously described using the following formulas [13]:
Mean values for absolute and relative disagreements were calculated for all specimens and separately for the specific type of operation performed.
Please note that differences between CT volumes and intraoperative volumes can be positive (+) or negative (−) depending on whether the CT volume or intraoperative volume is bigger. In contrast, absolute and relative disagreements represent moduli (|x|) of volumes and are always positive.
CT volumes and intraoperative volumes were compared using a two-tailed Wilcoxon signed-rank test in all specimens (n = 24), in left hemihepatectomy specimens (n = 6), in right hemihepatectomy specimens (n = 11), and in extended right hemihepatectomy specimens (n = 7).
Using a two-tailed Mann-Whitney U test, relative disagreements (with and without conversion factors) were compared between a) groups with with slice thickness ≤ 3 mm (n = 17) and with slice thickness > 3 mm (n = 7), b) between groups with resected (n = 11) or non-resected MHV (n = 13), as well as c) between groups with (n = 10) or without (n = 14) evidence of steatosis, portal fibrosis with formation of septa and/or parenchymal necrosis of the resected non-neoplastic liver parenchyma.
P values < 0.05 were considered significant.
Results
CT volumes
The mean CT volumes of the hemihepatectomy specimens determined by S were 1056 cm3 overall (range: 138–2245 cm3, n = 24), 281 cm3 in left hemihepatectomy specimens (range: 138–569 cm3, n = 6), 1070 cm3 in right hemihepatectomy specimens (range: 587–1512 cm3, n = 11), and 1697 cm3 in extended right hemihepatectomy specimens (range: 1144–2245 cm3, n = 7). The CT volumes included the volumes of the liver vessels in the analysis areas. The segmentation process for three example patients is shown in Figs. 1, 2, 3.
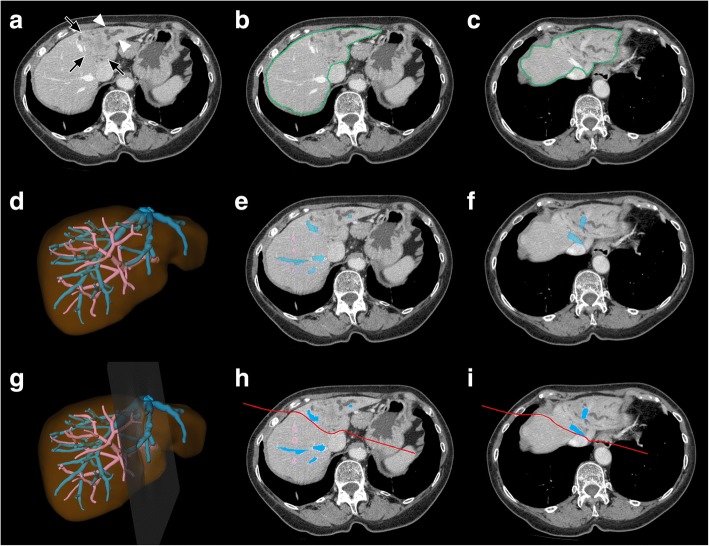
Fig. 1.
Segmentation process in a patient planned for left hemihepatectomy with resection of the MHV due to intrahepatic CC a) Plain axial CT image at the level of the maximum tumor extension shows the hypoattenuated tumor in the atrophic left liver lobe (black arrows) with upstream cholestasis (white arrowheads). b) and c) Detection of the liver outline. d) – f) Detection of the intrahepatic PV (in pink) and HV (in blue). Note occlusion of the left PV and of branches of the MHV and left HV due to tumor invasion. g) – h) Definition of the transection plane (in red)
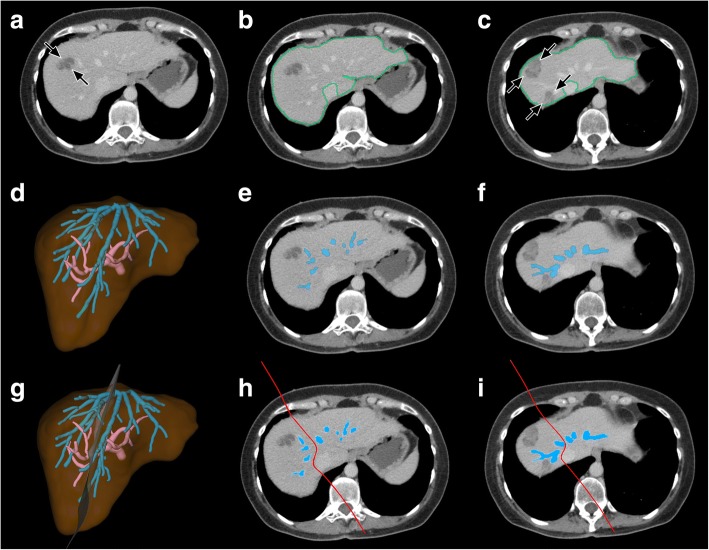
Fig. 2.
Segmentation process in a patient planned for right hemihepatectomy without resection of the MHV due to metastases of GCT a) Plain axial CT image shows a hypoattenuated metastasis in liver segment 8 (black arrows). b) and c) Detection of the liver outline. Further metastases are depicted at the level of the proximal MHV in c) (black arrows). d) – f) Detection of the intrahepatic PV (in pink) and HV (in blue). g) – h) Definition of the transection plane (in red)
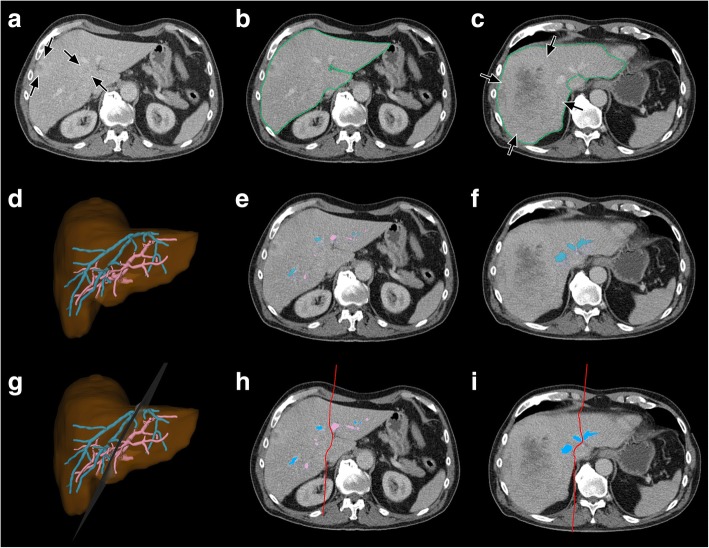
Fig. 3.
Segmentation process in a patient planned for extended right hemihepatectomy due to metastases of TC a) Plain axial CT image shows small peripherally hyperattenuated metastases (black arrows). b) and c) Detection of the liver outline. Another large metastasis is depicted at the level of the proximal MHV in c) (blue arrows). d) – f) Detection of the intrahepatic PV (in blue) and HV (in pink). g) – h) Definition of the transection plane (in red)
Intraoperative volumes and weights
The mean intraoperative volumes were 950 cm3 overall (range: 125–2010 cm3, n = 24), 253 cm3 in left hemihepatectomy specimens (range: 125–515 cm3, n = 6), 918 cm3 in right hemihepatectomy specimens (range: 475–1310 cm3, n = 11), and 1598 cm3 in extended right hemihepatectomy specimens (range: 1018–2010 cm3, n = 7).
The mean intraoperative weights were 988 g overall (range: 162–2112 g, n = 24), 282 g in left hemihepatectomy specimens (range: 162–553 g, n = 6), 956 g in right hemihepatectomy specimens (range: 516–1306 g, n = 11), and 1642 g in extended right hemihepatectomy specimens (range: 1070–2112 g, n = 7).
Comparative data analysis
The maximum discrepancies between the preoperatively defined transection planes and the actual transection planes according to the resection border visible on postoperative CT images (taking into account the course of the PV and HV braches close to the resection border) were rated as ≤1.0 cm in 11 specimens, as 1.1 to 2.0 cm in 7 specimens, and as > 2.0 cm in 0 specimens.
CT volumes determined by S showed strong significant correlations with intraoperative volumes and weights overall (r = 0.992, p < 0.001, and r = 0.987, p < 0.001, n = 24), in left hemihepatectomy specimens (r = 0.989, p < 0.001, and r = 0.995, p < 0.001, n = 6), in right hemihepatectomy specimens (r = 0.995, p < 0.001, and r = 0.991, p < 0.001, n = 11), and in extended right hemihepatectomy specimens (r = 0.968, p < 0.001, and r = 0.941, p = 0.002, n = 7).
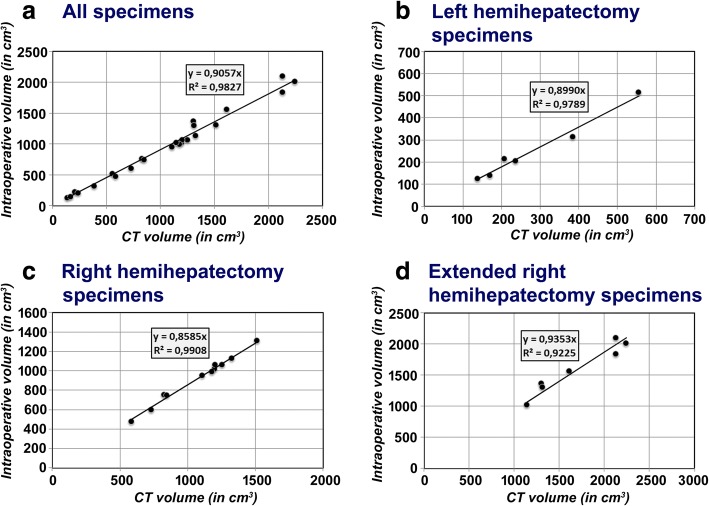
Correlation coefficients and conversion factors calculated by linear regression analyses using the method of least squares are shown in Table 2. Scatter plots with the results of this regression analyses are shown in Fig. 4.
Table 2.
Correlation coefficients and conversion factors
| Correlation coefficient with CT volume | Conversion factor from CT volume (in cm3) | ||
|---|---|---|---|
| All patients (n = 24) | Intraoperative volume (in cm3) | 0.992* | 0.907 |
| Intraoperative weight (in g) | 0.987* | 0.931 | |
| Patients with left hemiheptectomy (n = 6) | Intraoperative volume (in cm3) | 0.989* | 0.899 |
| Intraoperative weight (in g) | 0.995* | 0.995 | |
| Patients with right hemihepatectomy (n = 11) | Intraoperative volume (in cm3) | 0.995* | 0.860 |
| Intraoperative weight (in g) | 0.991* | 0.892 | |
| Patients with extended right hemihepatectomy (n = 7) | Intraoperative volume (in cm3) | 0.968* | 0.935 |
| Intraoperative weight (in g) | 0.941* | 0.954 |
Correlation coefficients that are statistically significant are marked with*
Fig. 4.
Results of the regression analyses of intraoperative volumes and CT volumes The linear regression analysis with the method of least-squares was performed for a) all specimens, and for b) – d) each specific type of operation performed
Average intraoperative volumes and weights (in cm3 and g) were smaller than CT volumes (in cm3), except for intraoperative weights (in g) compared to CT volumes (in cm3) in left hemihepatectomy specimens. Differences between mean CT volumes and mean intraoperative volumes were 106 cm3 overall (n = 24), 29 cm3 in left hemihepatectomy specimens (n = 6), 152 cm3 in right hemihepatectomy specimens (n = 11), and 99 cm3 in extended right hemihepatectomy specimens (n = 7).
Differences between mean CT volumes and mean intraoperative volumes as well as mean absolute and relative disagreements between CT volumes (with and without conversion factors) and intraoperative volumes are shown in Table 3. CT volume was bigger than intraoperative volume in all right hemihepatectomy specimens. Thus, the absolute disagreement was identical to the difference between mean CT volumes and mean intraoperative volumes in this specimen group. However, CT volumes were smaller than intraoperative volumes in one extended right hemihepatectomy specimen and in one left hemihepatectomy specimen. Therefore, absolute disagreements and differences between mean CT volumes and mean intraoperative volumes were not identical in these specimen groups.
Table 3.
Differences between mean CT volumes and mean intraoperative volumes as well as mean absolute and relative disagreements between CT volumes and intraoperative volumes
| Differences between mean CT volumes and mean intraoperative volumes | Mean absolute and relative disagreements of CT volumes (with and without conversion factors) and intraoperative volumes | |||
|---|---|---|---|---|
| without conversion factor | with correction by general conversion factor | with correction by operation-specific conversion factor | ||
| All patients (n = 24) |
106 cm3* | 112 cm3/13.1% | 57 cm3/6.3% | 41 cm3/4.6% |
| Patients with left hemihept-ectomy (n = 6) | 29 cm3 | 32 cm3/13.2% | 16 cm3/6.6% | 16 cm3/6.5% |
| Patients with right hemihepat-ectomy (n = 11) | 152 cm3* | 152 cm3/16.9% | 52 cm3/6.0% | 19 cm3/2.5% |
| Patients with extended right hemihepat-ectomy (n = 7) | 99 cm3 | 116 cm3/7.2% | 99 cm3/6.3% | 95 cm3/6.1% |
Differences between CT volumes and intraoperative volumes that are statistically significant (using a two-tailed Wilcoxon signed-rank test) are marked with*
Using a two-tailed Wilcoxon signed-rank test, differences between CT volumes and intraoperative volumes were statistically significant when calculated for all specimens (p < 0.001, n = 24) and for right hemihepatectomy specimens (p = 0.001, n = 11), but not statistically significant when calculated for left hemihepatectomy specimens (p = 0.063, n = 6) or for extended right hemihepatectomy specimens (p = 0.109, n = 7). CT volume overestimated the hemihepatectomy specimens’ volume with an average of 11.1% when compared to intraoperative volume in all specimens (n = 24).
Using a two-tailed Mann-Whitney U test, mean relative disagreements (with and without conversion factors) were not statistically different between a) groups with slice thicknesses ≤3 mm (n = 17) and > 3 mm (n = 7), b) between groups with resected (n = 11) and non-resected MHV (n = 13), as well as c) between groups with (n = 10) and without evidence of steatosis, siderosis, fibrosis, and/or inflammatory infiltration of the resected non-neoplastic liver parenchyma (n = 14). The exact p values are summarized in Table 4.
Table 4.
Comparisons of relative disagreements of CT volumes and mean intraoperative volumes in different patient groups
| Without conversion factor | With general conversion factor coverall | With operation-specific conversion factor cspecific | |
|---|---|---|---|
| Slice thickness ≤ 3 mm (n = 17) vs. > 3 mm (n = 7) | p = 0.569 | p = 0.897 | p = 0.631 |
| With (n = 11) vs. without resection of the MHV (n = 13) | p = 0.374 | p = 0.865 | p = 0.082 |
| With (n = 10) vs. without without evidence of steatosis, siderosis, fibrosis, and/or inflammatory infiltration of the resected non-neoplastic liver parenchyma (n = 14) | p = 0.772 | p = 0.219 | p = 0.466 |
Using a two-tailed Mann-Whitney U test, relative disagreements are not statistically different between groups with different slice thicknesses, between groups with vs. without resection of the middle hepatic vein (MHV), or between groups with vs. without evidence of parenchymal damage
Discussion
Over the past years, liver surgery has become more aggressive in oncological patients with the objective to achieve microscopically radical resection margins and, hence, better survival [6]. One of the major factors which influences procedural success is the size of the future liver remnant (FLR) [7]. The FLR is used as a surrogate for the risk of postoperative liver failure [14]. A smaller FLR is associated with an increased risk of postoperative infection and severe hepatic dysfunction [5]. Imaging-based volumetry is being utilised in clinical practice with increasing frequency to predict the size of the FLR [7]. CT volumetry (CTV) is often considered the current gold standard [6]. However, several studies have reported a significant inaccuracy rate of preoperatively measured liver volumes by CTV [9], which makes it difficult to preoperatively estimate the risk of perioperative morbidity and mortality in patients who are planned for major hepatic resections.
In this context, the objective of our study was to evaluate the accuracy of hemi-hepatectomy resectate volumes, determined by CTV using a semi-automated segmentation software (S) when compared with intraoperative volumes and weights in patients undergoing hemihepatectomy for primary or secondary hepatic malignancy. 24 hemihepatectomy specimens were evaluated prospectively. Similarly to most previous studies in the field of CTV of the liver, the volumes of the intrahepatic vessels of the hemihepatectomy specimens were included in, and the volumes of major extrahepatic vessels were excluded from, the CT volumes and intraoperative volumes and weights, respectively [11, 15, 16].
We have shown a good correlation between CT volumes based on venous CT-phase on the one side, and intraoperative volumes and weights on the other side. However, CT volume overestimated the hemihepatectomy specimens’ volume by an average of 11.1% when compared to intraoperative volume in all specimens when no conversion factor was applied. Average intraoperative weights (in g) were also smaller than CT volumes (in cm3), except in left hemihepatectomy specimens. This exception could be attributable to measuring inaccuracies in the comparatively small left hemihepatectomy specimens. Mean relative disagreements of CT volumes (with and without conversion factors) and intraoperative volumes were not statistically significant in patients with or without evidence of steatosis, portal fibrosis with formation of septa and/or parenchymal necrosis of the resected non-neoplastic liver parenchyma.
The overestimation of liver volumes by CTV is in line with the results of several previous studies [11, 15–17]. A majority of these studies focused on CTV in the field of LRLT [7]. In a study by Schroeder et al., a manual method of CTV overestimated graft-volumes in 10 of 13 donors in LRLT [17]. But, it is noteworthy that graft-volume was determined by actual weighing and assuming a 1:1 conversion factor from grams to cm3 [17]. Radtke et al. reported a 20.9% mean overestimation error for graft-volumes by CTV, based on venous CT-phase using a semi-automatic modified live-wire algorithm in 43 adult donors in LRLT [16]. Preoperative CT volumes of liver specimens were reported to be 14.0% larger on average than intraoperatively measured volumes in a study by Karlo et al. of patients undergoing partial liver resection [9]. According to the work of Hwang et al. and Niehues et al., blood perfusion is the most relevant factor that is accountable for the systematic differences between in vivo CTV and ex vivo water displacement volumetry [15, 18]. Hwang et al. demonstrated a much smaller difference between blood-filled graft volumes and volumetric graft volumes than between blood-free graft weight and volumetric graft volumes in 12 right lobe grafts in LRLT, and they proposed a conversion factor of 1.220 between blood-free graft weight and blood-filled graft volume [18]. Niehues et al. concluded that blood perfusion was the only relevant factor leading to their 13.0% systematic difference between whole liver volumes determined by CTV and liver volumes measured by water displacement volumetry in their pig animal model [15]. Our results support the use of conversion factors to predict the actual weight of liver specimens more reliably, on the basis of CTV and we approve that blood filling of the specimens seems to account for much of the differences between CT volumes and intraoperatively measured volumes.
In our study, a conversion factor of c = 0.906 most precisely predicted intraoperative volumes of exsanguinated hemihepatectomy specimens from CT volumes in all patients with mean absolute and relative disagreements between CT volumes and intraoperative volumes of 57 ml and 6.3%. The use of operation-specific conversion factors, i.e. specific for left hemihepatectomy, right hemihepatectomy, or extended right hemihepatectomy specimens, yielded even better results. The different disagreements regarding CT volumes and intraoperative volumes in the different operation-groups could be due to differing degrees of exsanguination of the specimens.
A number of other factors are known to influence CTV of liver specimens, among them being technical factors of the examination technique and image reconstruction (e.g. contrast agent phase in multiphasic CT imaging, the slice thickness), the method of segmentation (manual, semi-automated, automated) and patient-related factors (nutrition, circadian variations, physical activity) [16, 19, 20].
The method of CTV influences accuracy, precision and rapidity [7]. The manual method of CTV of the liver was initially described by Heymsfield et al. as manually tracing the liver contour in each CT slice image and then multiplying the area by slice thickness [21]. The biggest benefit of semi-automated and automated methods of CTV is an increase of rapidity when comparing automated methods to semi-automated methods, and when comparing semi-automated methods to manual techniques [7, 22]. However, to date automated methods of CTV often provide suboptimal results in CT images that have missing edges due to similar density of adjacent tissues or organs, and in most cases semi-automated methods of CTV are reported to outperform automated methods [7]. Our study could show a good performance of the semi-automated software S. Ling et al. reported that, when compared to other semi-automated and automated liver segmentation approaches, the precision of S is at the upper end of the scale [12]. However, we have not compared the performance of S directly to other methods of CTV.
Radtke et al. investigated the impact of the contrast agent phase in multiphasic CT imaging in the field of CTV [16]. They found that the native phase provided mean graft-volumes that were smaller compared to mean graft-volumes measured by CTV with venous phase, and that CTV with native phase overestimated actual graft-volumes to a lesser degree [16]. They hypothesized that the difference might be attributable to osmotic effects which increase hepatic intravascular water content following the intravenous infusion of the contrast agent [16], although we consider partial volume effects to be more likely. Similar to our approach with CTV, the volumes of intrahepatic vessels were included in their graft-volumes determined by CTV, and major extrahepatic vessels were excluded [16]. However, the venous phase is typically preferred for CTV by most authors because it delineates the vascular anatomy better than unenhanced CT [7]. Since exact volume estimation by CTV relies on precise segmentation it is intuitive that one can expect more accurate results utilizing CT images with smaller slice thickness [7]. It was reported that liver volumes determined by CTV increase with smaller slice thickness [19]. This was attributed to reduced errors from partial volume effects with thinner CT image slices [19]. The disadvantage of using thinner CT image slices is the increased expenditure of time, especially for manual methods of CTV [23]. Hori et al. concluded that 5-mm-thick CT image slices are sufficient for CTV of liver grafts in LRLT if a maximum error of 5.0% in the calculated volume is acceptable [19]. In a study by Reiner et al., MDCT images of the abdomen were reconstructed using the slice thicknesses 2, 4, 6, and 8 mm, and total liver volumes were measured using a semi-automated method of CTV [23]. A statistical difference was seen only between volumes based on 2-mm versus 8-mm slices [23]. However, the semi-automated segmentation module S had been tested on a highly heterogenous dataset containing 75 volumes of 6 different organs and was reported to be robust against variations of slice thickness, scanning protocols and contrast agent phase [12]. In line with this, the accuracy of CT volumes in datasets with low slice thickness (≤ 3 mm) was not statistically different from the accuracy of CT volumes in datasets with high slice thickness (> 3 mm) when compared to intraoperative volumes.
The discrepancies between the preoperatively defined transection planes and the actual transection planes according to the resection border visible on postoperative CT images were comparatively low. An explanation for this could be that the transection planes on the preoperative CT scans were defined in concordance with the liver surgeon who also operated on the patients.
There were limitations to our study. First, we quite certain cannot exclude that the heterogeneity regarding CT scanners, reconstruction kernels and slice thickness of reconstructed images represent confounders in our study, but S was shown to be robust in a highly heterogeneous dataset. Second, the absolute time to perform CTV using S was not recorded systematically and the performance of S was not compared directly to a manual method of CTV. Third, we did not include data on patient nutrition, physical activity, and times of day in our statistical analysis, although liver volume was reported to be dependent on these variables [20].
Conclusion
CTV performed with S yielded good measurements of CT volumes of hemihepatectomy specimens when compared to actual specimens’ volumes and weights. The difference can be explained by the fact that blood filling of the specimens was included in the CT volumes performed with S, but it was excluded in the intraoperatively measured volumes. Therefore, we propose the use of conversion factors which allow to predict intraoperative volume and weights from CT scans of hemihepatectomy specimens more precisely. This allows to precisely estimate the volume of a future liver remnant and thus minimize the risk of PHLF in patients undergoing major hepatic resections.
Acknowledgements
We thank Christoph Panknin for his technical support.
Funding
This study was supported financially by Siemens Healthcare, Germany. The funding body contributed to the design of the study. Christoph Panknin, an employee of Siemens Healthcare, helped with the installation of the analysis software and gave an introduction and tutorial to its range of functions and clinical use. The funding body didn’t have any influence on the collection, analysis, and interpretation of data or on writing the manuscript.
Availability of data and materials
The numerical data sets analysed during the current study are available from the corresponding author on reasonable request. The DICOM files cannot be made freely available because of privacy restrictions.
Abbreviations
- c
Conversion factor
- CC
Cholangiocarcinoma
- coverall
General conversion factor for all specimens
- cspecific
Operation-specific conversion factor
- CT
Computed tomography
- CTV
Computed tomography volumetry
- f
Female
- FLR
Future liver remnant
- FLRV
Future liver remnant volume
- GCT
Granulosa cell tumor
- HV
Hepatic vein(s)
- LRLT
Living-related liver transplantation
- m
Male
- MHV
Middle hepatic vein
- MM
Malignant melanoma
- MRI
Magnetic resonance imaging
- n.e.
No evidence of steatosis, portal fibrosis with formation of septa or parenchymal necrosis
- PHLF
Post-hepatectomy liver failure
- PV
Portal vein
- S
Syngo.CT Liver Analysis VA30
- TC
Thyroid cancer
Authors’ contributions
The study was concepted by MK, CS, and HK. MG and PS performed the measurements of intraoperative volumes and weights. MG, MK, and PS performed the computed tomography volumetry. NW conducted the histopathological analysis of resected hemihepatectomy specimens. PM and TM performed the analysis and interpretation of the data. The manuscript was drafted by PM. All authors revised the manuscript critically. All authors gave final approval of the manuscript version to be published. All authors agreed to be accountable for all aspects of the work.
Ethics approval and consent to participate
The study was approved by the Institutional Review Board (Ethikkommission der Medizinischen Fakultät Heidelberg, Heidelberg, Germany, approval number: S-192/2013). All procedures performed were in accordance with the declaration of Helsinki. Written informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
This study was supported financially by Siemens Healthcare, Germany.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Philipp Mayer, Phone: 004962215637345, Email: philipp.mayer@med.uni-heidelberg.de.
Martin Grözinger, Email: martin.groezinger@gmx.de.
Theresa Mokry, Email: theresa.mokry@med.uni-heidelberg.de.
Peter Schemmer, Email: peter.schemmer@medunigraz.at.
Nina Waldburger, Email: nina.waldburger@web.de.
Hans-Ulrich Kauczor, Email: hans-ulrich.kauczor@med.uni-heidelberg.de.
Miriam Klauss, Email: miriam.klauss@med.uni-heidelberg.de.
Christof-Matthias Sommer, Email: christof.sommer@med.uni-heidelberg.de.
References
- 1.Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the international study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Shoup M, Gonen M, D’Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325–330. doi: 10.1016/S1091-255X(02)00370-0. [DOI] [PubMed] [Google Scholar]
- 3.Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. How much remnant is enough in liver resection? Dig Surg. 2012;29:6–17. doi: 10.1159/000335713. [DOI] [PubMed] [Google Scholar]
- 4.Pulitano C, Crawford M, Joseph D, Aldrighetti L, Sandroussi C. Preoperative assessment of postoperative liver function: the importance of residual liver volume. J Surg Oncol. 2014;110:445–450. doi: 10.1002/jso.23671. [DOI] [PubMed] [Google Scholar]
- 5.Schindl MJ, Redhead DN, Fearon KCH, Garden OJ, Wigmore SJ. Edinburgh liver surgery and transplantation experimental research group (eLISTER). The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289–296. doi: 10.1136/gut.2004.046524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cieslak KP, Runge JH, Heger M, Stoker J, Bennink RJ, van Gulik TM. New perspectives in the assessment of future remnant liver. Dig Surg. 2014;31:255–268. doi: 10.1159/000364836. [DOI] [PubMed] [Google Scholar]
- 7.Lim MC, Tan CH, Cai J, Zheng J, Kow AWC. CT volumetry of the liver: where does it stand in clinical practice? Clin Radiol. 2014;69:887–895. doi: 10.1016/j.crad.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 8.Abdalla EK, Denys A, Chevalier P, Nemr RA, Vauthey J-N. Total and segmental liver volume variations: implications for liver surgery. Surgery. 2004;135:404–410. doi: 10.1016/j.surg.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Karlo C, Reiner CS, Stolzmann P, Breitenstein S, Marincek B, Weishaupt D, et al. CT- and MRI-based volumetry of resected liver specimen: comparison to intraoperative volume and weight measurements and calculation of conversion factors. Eur J Radiol. 2010;75:e107–e111. doi: 10.1016/j.ejrad.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Sakamoto S, Uemoto S, Uryuhara K, Kim ID, Kiuchi T, Egawa H, et al. Graft size assessment and analysis of donors for living donor liver transplantation using right lobe. Transplantation. 2001;71:1407–1413. doi: 10.1097/00007890-200105270-00009. [DOI] [PubMed] [Google Scholar]
- 11.Lemke A-J, Brinkmann MJ, Pascher A, Steinmüller T, Settmacher U, Neuhaus P, et al. Accuracy of the CT-estimated weight of the right hepatic lobe prior to living related liver donation (LRLD) for predicting the intraoperatively measured weight of the graft. Rofo. 2003;175:1232–1238. doi: 10.1055/s-2003-41938. [DOI] [PubMed] [Google Scholar]
- 12.Ling H, Zhou SK, Zheng Y, Georgescu B, Suehling M, Comaniciu D. Hierarchical, learning-based automatic liver segmentation. IEEE; 2008. p. 1–8. Available from: http://ieeexplore.ieee.org/document/4587393. Accessed 08 Sept 2017.
- 13.Mokry T, Bellemann N, Müller D, Lorenzo Bermejo J, Klauß M, Stampfl U, et al. Accuracy of estimation of graft size for living-related liver transplantation: first results of a semi-automated interactive software for CT-volumetry. PLoS One. 2014;9:e110201. doi: 10.1371/journal.pone.0110201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Onofrio M, De Robertis R, Demozzi E, Crosara S, Canestrini S, Pozzi Mucelli R. Liver volumetry: is imaging reliable? Personal experience and review of the literature. World J Radiol. 2014;6:62–71. doi: 10.4329/wjr.v6.i4.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niehues SM, Unger JK, Malinowski M, Neymeyer J, Hamm B, Stockmann M. Liver volume measurement: reason of the difference between in vivo CT-volumetry and intraoperative ex vivo determination and how to cope it. Eur J Med Res. 2010;15:345–350. doi: 10.1186/2047-783X-15-8-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radtke A, Sotiropoulos GC, Nadalin S, Molmenti EP, Schroeder T, Lang H, et al. Preoperative volume prediction in adult living donor liver transplantation: how much can we rely on it? Am J Transplant. 2007;7:672–679. doi: 10.1111/j.1600-6143.2006.01656.x. [DOI] [PubMed] [Google Scholar]
- 17.Schroeder T, Malagó M, Debatin JF, Goyen M, Nadalin S, Ruehm SG. “All-in-one” imaging protocols for the evaluation of potential living liver donors: comparison of magnetic resonance imaging and multidetector computed tomography. Liver Transpl. 2005;11:776–787. doi: 10.1002/lt.20429. [DOI] [PubMed] [Google Scholar]
- 18.Hwang S, Lee SG, Kim KH, Park KM, Ahn CS, Moon DB, et al. Correlation of blood-free graft weight and volumetric graft volume by an analysis of blood content in living donor liver grafts. Transplant Proc. 2002;34:3293–3294. doi: 10.1016/S0041-1345(02)03603-5. [DOI] [PubMed] [Google Scholar]
- 19.Hori M, Suzuki K, Epstein ML, Baron RL. Computed tomography liver volumetry using 3-dimensional image data in living donor liver transplantation: effects of the slice thickness on the volume calculation. Liver Transpl. 2011;17:1427–1436. doi: 10.1002/lt.22419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung NW, Farrant P, Peters TJ. Liver volume measurement by ultrasonography in normal subjects and alcoholic patients. J Hepatol. 1986;2:157–164. doi: 10.1016/S0168-8278(86)80074-5. [DOI] [PubMed] [Google Scholar]
- 21.Heymsfield SB, Fulenwider T, Nordlinger B, Barlow R, Sones P, Kutner M. Accurate measurement of liver, kidney, and spleen volume and mass by computerized axial tomography. Ann Intern Med. 1979;90:185–187. doi: 10.7326/0003-4819-90-2-185. [DOI] [PubMed] [Google Scholar]
- 22.Nakayama Y, Li Q, Katsuragawa S, Ikeda R, Hiai Y, Awai K, et al. Automated hepatic volumetry for living related liver transplantation at multisection CT. Radiology. 2006;240:743–748. doi: 10.1148/radiol.2403050850. [DOI] [PubMed] [Google Scholar]
- 23.Reiner CS, Karlo C, Petrowsky H, Marincek B, Weishaupt D, Frauenfelder T. Preoperative liver volumetry: how does the slice thickness influence the multidetector computed tomography- and magnetic resonance-liver volume measurements? J Comput Assist Tomogr. 2009;33:390–397. doi: 10.1097/RCT.0b013e3181806c29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The numerical data sets analysed during the current study are available from the corresponding author on reasonable request. The DICOM files cannot be made freely available because of privacy restrictions.