Abstract
Background: In 2012, updated cervical cancer screening recommendations were released with consensus on Papanicolaou (Pap) testing every 3 years for women age 21–65 years or Pap–human papillomavirus (HPV) cotesting at 5-year intervals for women age 30–65 years. Primary study aims: Assess current use of Pap-HPV cotesting and describe local population trends over time in Pap and Pap-HPV cotesting. Secondary aim: Assess sociodemographic factors correlating with screening.
Methods: We assessed Rochester Epidemiology Project data for Pap and Pap-HPV cotesting among women age 16 years and older living in Olmsted County, Minnesota, yearly from 2005 (study population n = 47,203) through 2016 (study population n = 49,510). We calculated 3-year (Pap) and 5-year (Pap-HPV) moving prevalence rates of screening as proportion of eligible population. Multivariable logistic regression was used to assess factors potentially associated with screening.
Results: In 2016, 64.6% of 27,418 eligible 30- to 65-year-old women were up to date with cervical cancer screening; 60.8% had received Pap-HPV cotest screening. Significant declines in Pap completion rates over time were observed in all age groups, including an unexpected decline in 21- to 29-year-old women. Coincident with decreasing Pap screening rates, Pap-HPV cotesting significantly increased among women age 30–65 years, from 10.0% in 2007 to 60.8% in 2016.
Conclusions: This suggests increasing adoption of 2012 screening recommendations in the 30- to 65-year-old population. However, decline in Pap screening among 21- to 29-year-old women is concerning. Disparities by race, ethnicity, smoking status, and comorbidity level were observed. Results suggest need for multilevel patient and clinician interventions to increase cervical cancer screening adherence.
Keywords: adolescent health, gynecologic cancer, health disparities, sexually transmitted infections
Introduction
In 2018, an estimated 13,240 cases of invasive cervical cancer will be diagnosed and an estimated 4,170 cervical cancer-related deaths will occur in the United States.1 Although Papanicolaou (Pap) screening has reduced incidence of cervical cancer and death by more than 60% since being introduced in the 1950 s,2 underscreened and especially never-screened women continue to be at particularly high risk.3–5 Multiple organizations provide recommendations for cervical cancer screening, including the U.S. Preventive Services Task Force,6 the American Cancer Society (ACS) in conjunction with the American Society for Colposcopy and Cervical Pathology (ASCCP) and the American Society for Clinical Pathology,7 and the American College of Obstetricians and Gynecologists (ACOG).8 Previously, concordance did not exist between the recommendations of these groups. However, with updates published in 2012, consensus has developed among the organizations, advising Pap testing every 3 years for women age 21–65 years or Pap–human papillomavirus (HPV) cotesting at 5-year intervals for women age 30–65 years. ACOG, ACS, and ASCCP have recommended Pap-HPV cotesting as the preferred screening method for women age 30–65 years.
Reported population adherence to recommended cervical cancer screening tests is generally high. Data from the 2015 National Health Interview Survey (NHIS) indicate that 81.1% of women age 21–65 years report having had a Pap test within the past 3 years.9 However, this rate is below the Healthy People 2020 goal of 93%,10 and declines have been noted in Pap testing among women age 21–65 years between 2000 and 2015.9 Among women 30–65 years of age, Pap-HPV cotesting has a lower false-negative rate than Pap testing alone because use of the HPV cotest reduces the likelihood of missing an abnormality. Use of HPV cotesting also may improve detection of glandular cell abnormalities compared with the use of Pap testing alone.11 Despite these benefits, national self-reported rates of Pap-HPV cotesting are much lower than self-reported Pap test rates, with 41.0% of women age 30–39 years; 29.8%, age 40–49; and 20.3%, age 50–65 years reporting Pap-HPV cotesting within 3 years.9 However, a recent analysis of claims data indicated an increase in rates of Pap-HPV cotesting among women age 30–65 years from 2005 to 2014.12
The primary aims of our study were to assess current rates of Pap-HPV cotesting in Olmsted County, Minnesota, and to describe trends over time in both Pap testing and Pap-HPV cotesting in our local population in the context of the 2012 national screening recommendations. Our secondary aim was to evaluate sociodemographic correlates of being up to date with cervical cancer screening in the 2016 population.
Materials and Methods
Our study used the research infrastructure of the Rochester Epidemiology Project (REP) to assess completion of Pap test and Pap-HPV cotest among adolescent girls and women age 16 years and older living in Olmsted County, Minnesota, each year from 2005 to 2016. REP is a data linkage research infrastructure that captures virtually all health care in Olmsted County.13–16 It links health care visit dates to address information; this information is used to define residency at a given point in time (REP Census). Population coverage for Olmsted County is nearly complete.15
We identified women residing in Olmsted County on January 1 each year from 2005 to 2016 and included those who gave authorization to use their medical records for research (96% of the eligible population; Minnesota State privacy law Statute 144.335, 1997). We electronically searched the diagnostic indexes of REP to extract International Classification of Diseases, Ninth Revision, and International Classification of Diseases, Tenth Revision, and Current Procedural Terminology17 code sets for screening Pap (CPT codes) and HPV testing (CPT and laboratory codes) from 2003 to 2016 (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/jwh). Persons with the following characteristics were excluded: cervical dysplasia or carcinoma in situ; cervical cancer; prior hysterectomy or cervicectomy; HIV diagnosis; history of in utero exposure to diethylstilbestrol; or solid organ transplant. They were excluded because these characteristics would alter the recommended screening interval.
Demographic variables, tobacco use, and comorbid diagnoses were obtained electronically from the linked health care information available from REP. All study procedures were approved by Mayo Clinic and Olmsted Medical Center Institutional Review Boards.
Statistical analyses
Demographic information describing the 2005 and 2016 study populations (yearly population minus exclusions) were calculated. Three-year (Pap testing) and 5-year (Pap-HPV cotesting) moving prevalence rates of screening were calculated as a proportion of the study population. The rate of Pap testing was defined as at least 1 Pap test in the current year or the previous 2 years. The rate of Pap-HPV cotesting was defined as at least 1 cotest (defined as Pap and HPV test done within the same week) in the current year or the previous 4 years. We were not able to distinguish between reflex HPV testing and HPV testing performed as part of a cotest, although HPV reflex testing represents a low proportion of the total HPV testing performed in our cytopathology laboratory (95% cotest vs. 5% reflex HPV). Pap test and HPV data were available from 2003 to 2016; therefore, rates of Pap testing were calculated for the 2005–2016 period and rates of Pap-HPV cotesting calculated for 2007–2016. A statistical trend analysis was performed with logistic regression models, used to test for temporal trends in Pap testing and Pap-HPV cotesting by age group. Generalized estimating equations accounted for repeated measurements among individuals. Prevalence rates of Pap testing and Pap-HPV cotesting by age group were summarized graphically. Multinomial logistic regression was used to assess factors that might be associated with completion of Pap or Pap-HPV cotesting in the 2016 population compared with no Pap testing or no Pap-HPV cotesting. Variables assessed included age, race, ethnicity, smoking status, and Charlson Comorbidity Index. Results are reported as odds ratios (ORs) and 95% confidence intervals (CIs). p values <0.05 were considered statistically significant. All analyses were performed with SAS version 9.4 (SAS Institute, Inc.).
Results
The yearly population of women in the REP increased from 56,848 in 2005 to 62,092 in 2016. The percentage that was excluded was consistent and ranged from 17.0% to 20.3% over the 12 years. The distribution of the reasons for exclusion was as follows: prior hysterectomy, 36.5%; cervical dysplasia or carcinoma in situ, 17.0%; cervical cancer, 5.5%; HIV, 4%; in utero diethylstilbestrol exposure, 1.5%; solid organ transplant 0.5%; and 2 or more of the above exclusion criteria, 35%. The final study populations ranged from 47,203 to 49,510 across the time frame. Sociodemographic characteristics of the 2005 and the 2016 eligible populations are summarized in Table 1. Most women in our population in both 2005 and 2016 were age 30–65 years, white, and non-Hispanic.
Table 1.
Descriptive Characteristics of Study Population
| 2005 | 2016 | |
|---|---|---|
| Characteristics | Patients, No. (%) | Patients, No. (%) |
| Age, years | ||
| 16 to <21 | 5,186 (11.0) | 4,411 (8.9) |
| 21 to <30 | 9,524 (20.2) | 9,705 (19.6) |
| 30–65 | 25,667 (54.4) | 27,418 (55.4) |
| >65 | 6,826 (14.5) | 7,976 (16.1) |
| Race | ||
| White | 39,632 (84.0) | 40,366 (81.5) |
| Black | 1,910 (4.1) | 2,932 (5.9) |
| Asian | 2,155 (4.6) | 3,110 (6.3) |
| Other | 1,899 (4.0) | 2,328 (4.7) |
| Unknown | 1,607 (3.4) | 774 (1.6) |
| Ethnicity | ||
| Hispanic | 1,796 (3.8) | 2,577 (5.2) |
| Non-Hispanic | 43,900 (93.0) | 46,266 (93.5) |
| Unknown | 1,507 (3.2) | 667 (1.4) |
| Smoking | ||
| Never | 28,317 (60.0) | 21,637 (43.7) |
| Past | 16,874 (35.8) | 25,520 (51.6) |
| Current | 2,012 (4.3) | 2,353 (4.8) |
| Charlson Comorbidity Index | ||
| 0 | 39,424 (83.5) | 40,423 (81.7) |
| 1–2 | 5,247 (11.1) | 5,685 (11.5) |
| 3–4 | 1,506 (3.2) | 1,835 (3.7) |
| ≥5 | 1,026 (2.2) | 1,567 (3.2) |
In the 2016 population, 64.6% of study-eligible women, age 30–65 years, were up to date with cervical cancer screening; 60.8% were screened with Pap-HPV cotest within 5 years and 3.9% were screened with Pap test within 3 years. Among screening-eligible women age 21–29 years, 53.8% were adherent with screening, with the majority appropriately screened with Pap tests (47.3%) rather than Pap-HPV cotests (6.5%). Total Pap and Pap-HPV cotest screening rates in 2016 for adolescent girls and women age 16 to less than 21 years and more than 65 years were appropriately low, 2.1% and 8.8%, respectively, and were consistent with recommendations against screening women in those age groups.
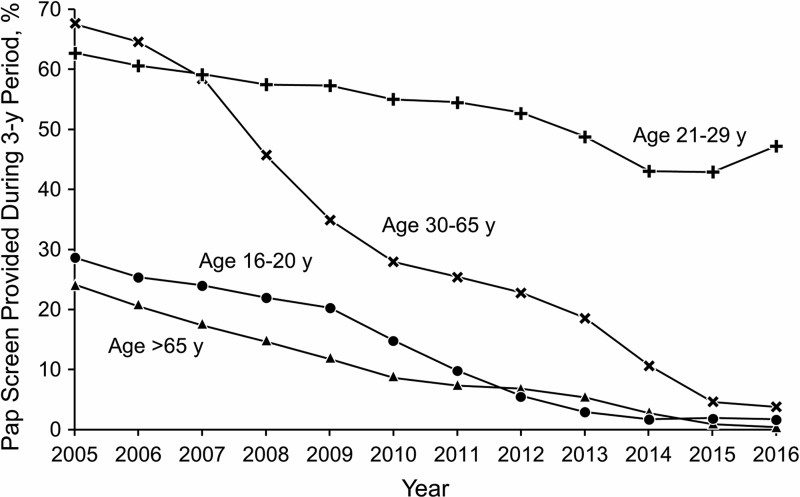
Figure 1 illustrates trends over time in Pap testing every 3 years by age group. Significant declines in Pap test rates were observed from 2005 to 2016 in every age group (p < 0.01). Among adolescent girls and women age 16 to less than 21 years, Pap screening rates that were 28.8% in 2005 decreased to 1.8% in 2016. Pap test rates among women 21–29 years of age decreased from 62.8% in 2005 to 47.3% in 2016. For women 30–65 years of age, Pap test rates decreased from 67.5% in 2005 to 3.9% in 2016. Pap test screening for women older than 65 years decreased from 24.2% to 0.6%.
FIG. 1.
Percentage of Adolescent Girls and Women Age 16 Years and Older Who Had ≥1 Pap Test Every 3 Years, 2005–2016. Pap, Papanicolaou.
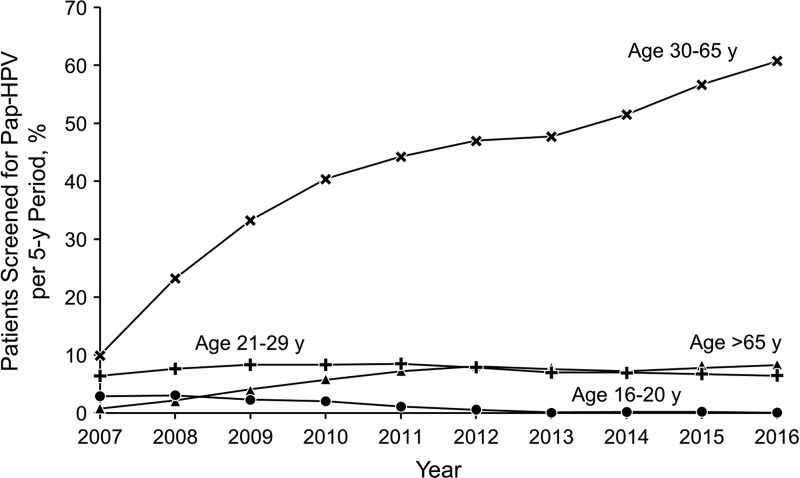
Trends in Pap-HPV cotesting every 5 years (Fig. 2) varied by age group, with a small but significant decline among adolescent girls and women age 16–21 years from 3.0% in 2007 to 0.2% in 2016 (p < 0.01). For women age 21–29 years, the underlying trend for Pap-HPV cotesting varied significantly over time (p < 0.01). However, the observed changes were nonlinear, where rates of Pap-HPV cotesting increased between 2007 and 2010, from 6.4% to 8.5%, and returned to 6.5% in 2016. A significant increase in Pap-HPV cotesting was observed for women age 30–65 years, from 10.0% in 2007 to 60.8% in 2016 (p < 0.01). This change correlates with the decrease in Pap test rate from 2005 to 2016 in that age group. Pap-HPV cotest rate increased in women older than 65 years from 2007 (0.9%) to 2016 (8.3%), but overall, the rates in this age group were low (p < 0.01).
FIG. 2.
Percentage of Adolescent Girls and Women Age 16 Years and Older With ≥1 Pap-HPV Cotest Every 5 Years, 2007–2016. Pap-HPV, Papanicolaou-Human papillomavirus.
Sociodemographic predictors of screening in the 2016 population are presented in Table 2. Compared with white women, Pap testing and Pap-HPV cotesting were less likely among black women (Pap-adjusted OR [95% CI], 0.56 [0.49–0.64]; cotest-adjusted OR [95% CI], 0.50 [0.45–0.55]) and Asian women (Pap-adjusted OR [95% CI], 0.74 [0.64–0.85]; cotest-adjusted OR [95% CI], 0.69 [0.63–0.76]). Hispanic women were less likely to have had Pap-HPV cotesting than non-Hispanic women (adjusted OR [95% CI], 0.84 [0.74–0.94]). Compared with never smokers, prior smokers (Pap-adjusted OR [95% CI], 2.48 [2.31–2.67]; cotest-adjusted OR [95% CI], 3.08 [2.92–3.24]) and current smokers (Pap-adjusted OR [95% CI], 1.68 [1.44–1.94]; cotest-adjusted OR [95% CI], 1.39 [1.24–1.56]) were more likely to have been screened. Greater multimorbidity as measured by Charlson Comorbidity Index was associated with a lower likelihood of screening (Pap-adjusted OR [95% CI], 0.94 [0.90–0.99]; cotest-adjusted OR [95% CI], 0.96 [0.94–0.98]).
Table 2.
Sociodemographic Predictors of Screening
| Predictor | Pap (Every 3 years), OR (95% CI) | Pap-HPV (Every 5 years), OR (95% CI) |
|---|---|---|
| Age, years | ||
| 21 to <30 | Ref | Ref |
| 30–65 | 0.11 (0.10–0.12) | 12.97 (11.86–14.18) |
| Race | ||
| White | Ref | Ref |
| Black | 0.56 (0.49–0.64) | 0.50 (0.45–0.55) |
| Asian | 0.74 (0.64–0.85) | 0.69 (0.63–0.76) |
| Other/unknown | 0.80 (0.68–0.94) | 0.67 (0.59–0.76) |
| Ethnicity | ||
| Non-Hispanic | Ref | Ref |
| Hispanic | 1.05 (0.89–1.23) | 0.84 (0.74–0.94) |
| Smoking | ||
| Never | Ref | Ref |
| Past | 2.48 (2.31–2.67) | 3.08 (2.92–3.24) |
| Current | 1.68 (1.44–1.94) | 1.39 (1.24–1.56) |
| Charlson Comorbidity Index | 0.94 (0.90–0.99) | 0.96 (0.94–0.98) |
CI, confidence interval; HPV, human papillomavirus; OR, odds ratio; Pap, Papanicolaou; Ref, reference.
Discussion
The primary aims of our study were to assess current use of Pap-HPV cotesting and to describe trends over time in Pap testing and Pap-HPV cotesting in the Olmsted County population. In concordance with updated national screening recommendations and a recent analysis of claims data,12 a significant increase in Pap-HPV cotesting was observed for women age 30–65 years from 2007 to 2016. The rate observed in our 2016 data exceeds the cotest uptake reported in the 2015 NHIS data, although underreporting likely occurred in the NHIS survey because women may not know whether an HPV test was performed along with their Pap test.9 Rates of HPV cotesting in our population were higher than those observed in an analysis of claims data of privately insured women across the United States in 2014, the most recent comparable year of claims data reported. The women were primarily enrolled in fee-for-service plans (71.6%), with the largest percentage residing in southern states.12 In a large academic medical center with greater racial diversity than our population, comparable increases over time in use of Pap-HPV cotesting for screening were reported for 27,035 women age 30–65 years.18 The investigators reported Pap-HPV cotesting rates as a proportion of all screening tests performed and results were confirmed through a pathology data system, with an observed increase from 8.9% in 2006 to 78.4% in 2013.
Significant declines in cervical cancer screening among adolescent girls and women age 16 to almost 21 and older than 65 years from 2005 to 2016 observed in our population align with the 2012 recommendations. These recommendations are to initiate screening at age 21 years regardless of sexual history, rather than to potentially start screening earlier, and to discontinue screening in average-risk women with adequate past screening after age 65 years. However, results from a recent retrospective cross-sectional chart review of 3,920 Pap tests from women in a single metropolitan health system showed that, among women identified as screening ineligible based on 2012 recommendations, 40% of Pap tests for women older than 65 years and 51% of Pap tests in women younger than 21 years were not indicated.19 These results illustrate the ongoing need to address overutilization of cervical cancer screening tests. An especially concerning observation in our study was the decline in Pap test screening among women age 21–29 years, for whom the test is recommended. The downward trend is consistent with 2015 NHIS data that showed a decline in self-reported historical screening in this age group, from 86.8% in 2000 to 77.6% in 2015.9 However, the screening rates in our population were much lower, with declines in screening from 62.8% in 2005 to 47.3% in 2016; our observed trends were consistent with those reported by Watson et al.12 in their analysis of claims data from 2005 to 2014.
The secondary aim of our study was to assess sociodemographic factors that may affect screening. In Olmsted County, Pap and Pap-HPV cotest completion was more likely among white women than among black women or Asian women. Non-Hispanic women were more likely to have had Pap-HPV cotesting than Hispanic women. These findings are consistent with past studies highlighting racial disparities in cervical cancer screening, particularly among black and Hispanic women.20,21 In a report of 2015 NHIS data, cervical cancer screening was lowest among Asian women (75.8%).22 Non-Hispanic women (83.5%) recounted higher overall Pap or Pap-HPV cotest completion at intervals consistent with the 2012 recommendations than Hispanic women (78.6%).
Research results are mixed on correlation between smoking status and cervical cancer screening compliance. Several studies have reported lower likelihood of cervical cancer screening compliance in smokers compared with nonsmokers.23,24 However, in a cross-sectional analysis of Behavioral Risk Factor Surveillance System data, MacLaughlan et al.25 distinguished between former, current, and never smokers in reporting cervical cancer screening rates. They found that screening compliance was highest among former smokers (86.7%) compared with never smokers (83.7%) and current smokers (81.7%; p < 0.001). In a report from the Joint Canada/United States Survey of Health, current smokers in the United States and current or former smokers in Canada were more likely to be up to date with cervical cancer screening than never smokers.26 Comparably, in the Olmsted County population, the past or current smokers were more likely to have completed screening Pap testing and Pap-HPV cotesting than never smokers.
Greater multimorbidity, as measured by the Charlson Comorbidity Index, was associated with a slightly lower likelihood of screening in our study population. This finding is consistent with earlier literature showing that increased comorbidity27 and increased severity of disability28 are associated with less cervical cancer screening compliance. It suggests that efforts are still needed to improve screening in this vulnerable population.
Study limitations and strengths
The Olmsted County population is less ethnically diverse than the U.S. population, which represents a limitation in applicability of our findings in other regions, although it reflects the demographic characteristics of Minnesota and the Upper Midwest.29 Overcounting of persons 20–29 years of age is a recognized limitation of the REP data.15 We expect that many of those persons are not full-time residents of the community, but are able to continue coverage under their parent's insurance through age 25 years. If they receive health care while in Olmsted County and have a local billing address, they are counted as residents of the REP community. However, if they obtain health care outside this community (e.g., reproductive health care services from student health services), this information is missed. An additional limitation of our study is that we did not assess for overscreening. We defined the rate of Pap testing as at least 1 Pap test in 3 years and the rate of Pap-HPV cotesting as at least 1 Pap-HPV cotest in 5 years. It is possible that some women had more than 1 test during those time periods.
Strengths of the study include validated and longitudinal Pap test and Pap-HPV cotest completion data in a defined population. Past research has shown that self-reported cervical cancer screening frequency is often overestimated.30–32 Overreporting may help explain the discrepancy between the much lower screening rates observed in Olmsted County compared with the 2015 NHIS data that were collected through patient interviews and were dependent upon patient recollection of screening type and date. For NHIS data, there is no medical record review to confirm that testing was completed, and therefore the data may be inaccurate.9 In contrast, diagnosis and billing codes were used in the present study to confirm Pap test and Pap-HPV cotest screening, not patient self-reports.
Conclusion
We observed a significant upward trend in the uptake of Pap-HPV cotesting as a screening method for women age 30–65 years, along with an overall decline in screening of adolescent girls and women 16 up to 21 years of age and greater than 65 years. These findings suggest increasing adoption of the 2012 national screening guidelines in the clinical practices captured in REP. However, the observed significant declines in Pap test screening among women age 21–29 years is concerning. Furthermore, cervical cancer screening rates for all eligible women in our 2016 population are well below the Healthy People 2020 goals, and disparities were observed for race, ethnicity, smoking status, and comorbidity levels. The results support the need for interventions and can inform multilevel patient- and clinician-facing interventions to increase cervical cancer screening adherence.
Supplementary Material
Acknowledgments
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676, and by the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery in Rochester, Minnesota. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The funding sources had no involvement in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article.
Author Disclosure Statement
R. M. Jacobson serves on external safety review committees overseeing postlicensure safety studies of the HPV vaccines (Gardasil in male patients and Gardasil 9 in female patients) funded by Merck & Co. He also serves on an external data monitoring committee overseeing a series of prelicensure trials of 15-valent pneumococcal vaccines in adults and infants funded by Merck & Co. All other authors report no financial conflicts of interest related to this work.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30 [DOI] [PubMed] [Google Scholar]
- 2. National Institutes of Health. NIH Fact Sheets: Cervical Cancer. Internet]. 2010. Available at: https://report.nih.gov/nihfactsheets/viewfactsheet.aspx?csid=76 Accessed May30, 2018
- 3. Miller JW, Royalty J, Henley J, White A, Richardson LC. Breast and cervical cancers diagnosed and stage at diagnosis among women served through the National Breast and Cervical Cancer Early Detection Program. Cancer Causes Control 2015;26:741–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mubiayi N, Bogaert E, Boman F, et al. . [Cytological history of 148 women presenting with invasive cervical cancer]. Gynecol Obstet Fertil 2002;30:210–217 [DOI] [PubMed] [Google Scholar]
- 5. Janerich DT, Hadjimichael O, Schwartz PE, et al. . The screening histories of women with invasive cervical cancer, Connecticut. Am J Public Health 1995;85:791–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moyer VA, Force USPST. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;156:880–891, W312 [DOI] [PubMed] [Google Scholar]
- 7. Saslow D, Solomon D, Lawson HW, et al. . American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. J Low Genit Tract Dis 2012;16:175–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Committee on Practice B-G. ACOG Practice Bulletin Number 131: Screening for cervical cancer. Obstet Gynecol 2012;120:1222–1238 [DOI] [PubMed] [Google Scholar]
- 9. Watson M, Benard V, King J, Crawford A, Saraiya M. National assessment of HPV and Pap tests: Changes in cervical cancer screening, National Health Interview Survey. Prev Med 2017;100:243–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Office of Disease Prevention and Health Promotion. Healthy People 2020: Cancer Objectives. Internet]. 2018. Available at: www.healthypeople.gov/2020/topics-objectives/topic/cancer/objectives Accessed May30, 2018
- 11. National Cancer Institute. Pap and HPV Testing. Internet]. 2014. Available at: www.cancer.gov/types/cervical/pap-hpv-testing-fact-sheet Accessed May30, 2018
- 12. Watson M, Benard V, Flagg EW. Assessment of trends in cervical cancer screening rates using healthcare claims data: United States, 2003–2014. Prev Med Rep 2018;9:124–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kurland LT, Molgaard CA. The patient record in epidemiology. Sci Am 1981;245:54–63 [DOI] [PubMed] [Google Scholar]
- 14. Melton LJ, 3rd., History of the Rochester Epidemiology Project. Mayo Clin Proc 1996;71:266–274 [DOI] [PubMed] [Google Scholar]
- 15. St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: The Rochester epidemiology project. Am J Epidemiol 2011;173:1059–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ, 3rd., History of the Rochester Epidemiology Project: Half a century of medical records linkage in a US population. Mayo Clin Proc 2012;87:1202–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. American Medical Association. Current Procedural Terminology: CPT. Chicago, IL: American Medical Association, 2016 [Google Scholar]
- 18. Silver MI, Rositch AF, Phelan-Emrick DF, Gravitt PE. Uptake of HPV testing and extended cervical cancer screening intervals following cytology alone and Pap/HPV cotesting in women aged 30–65 years. Cancer Causes Control 2018;29:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Teoh D, Isaksson Vogel R, Hultman G, et al. . Single Health System Adherence to 2012 Cervical Cancer Screening Guidelines at Extremes of Age and Posthysterectomy. Obstet Gynecol 2017;129:448–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bazargan M, Bazargan SH, Farooq M, Baker RS. Correlates of cervical cancer screening among underserved Hispanic and African-American women. Prev Med 2004;39:465–473 [DOI] [PubMed] [Google Scholar]
- 21. Musselwhite LW, Oliveira CM, Kwaramba T, et al. . Racial/Ethnic Disparities in Cervical Cancer Screening and Outcomes. Acta Cytol 2016;60:518–526 [DOI] [PubMed] [Google Scholar]
- 22. White A, Thompson TD, White MC, et al. . Cancer Screening Test Use - United States, 2015. MMWR Morb Mortal Wkly Rep 2017;66:201–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nelson W, Moser RP, Gaffey A, Waldron W. Adherence to cervical cancer screening guidelines for U.S. women aged 25–64: Data from the 2005 Health Information National Trends Survey (HINTS). J Womens Health (Larchmt) 2009;18:1759–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Byrne MM, Davila EP, Zhao W, et al. . Cancer screening behaviors among smokers and non-smokers. Cancer Epidemiol 2010;34:611–617 [DOI] [PubMed] [Google Scholar]
- 25. MacLaughlan SD, Lachance JA, Gjelsvik A. Correlation between smoking status and cervical cancer screening: A cross-sectional study. J Low Genit Tract Dis 2011;15:114–119 [DOI] [PubMed] [Google Scholar]
- 26. Blackwell DL, Martinez ME, Gentleman JF. Women's compliance with public health guidelines for mammograms and pap tests in Canada and the United States: An analysis of data from the Joint Canada/United States Survey of Health. Womens Health Issues 2008;18:85–99 [DOI] [PubMed] [Google Scholar]
- 27. Kiefe CI, Funkhouser E, Fouad MN, May DS. Chronic disease as a barrier to breast and cervical cancer screening. J Gen Intern Med 1998;13:357–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Horner-Johnson W, Dobbertin K, Andresen EM, Iezzoni LI. Breast and cervical cancer screening disparities associated with disability severity. Womens Health Issues 2014;24:e147–e153 [DOI] [PubMed] [Google Scholar]
- 29. St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: An illustration from the Rochester Epidemiology Project. Mayo Clin Proc 2012;87:151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McPhee SJ, Nguyen TT, Shema SJ, et al. . Validation of recall of breast and cervical cancer screening by women in an ethnically diverse population. Prev Med 2002;35:463–473 [DOI] [PubMed] [Google Scholar]
- 31. Rauscher GH, Johnson TP, Cho YI, Walk JA. Accuracy of self-reported cancer-screening histories: A meta-analysis. Cancer Epidemiol Biomarkers Prev 2008;17:748–757 [DOI] [PubMed] [Google Scholar]
- 32. Howard M, Agarwal G, Lytwyn A. Accuracy of self-reports of Pap and mammography screening compared to medical record: A meta-analysis. Cancer Causes Control 2009;20:1–13 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.