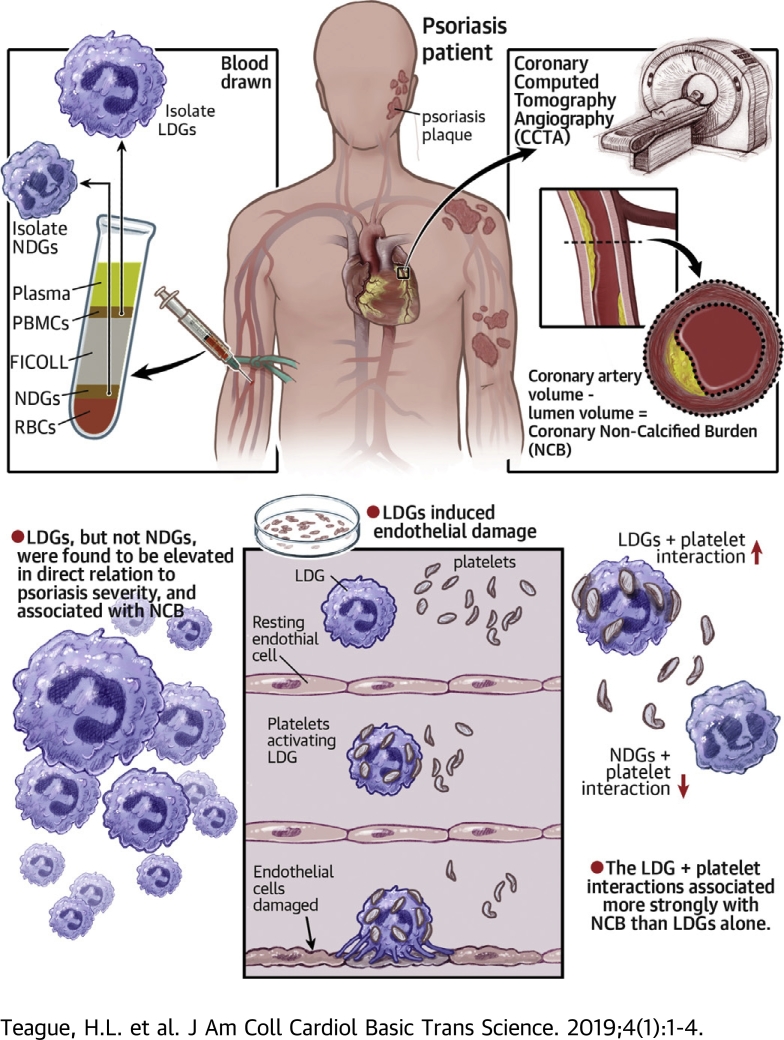
Visual Abstract
Key Words: cardiovascular disease, low-density granulocytes, neutrophils, platelets, psoriasis
Abbreviations and Acronyms: CCTA, coronary computed tomography angiography; CVD, cardiovascular disease; FDR, false discovery rate; HAoEC, human aortic endothelial cell; LDG, low-density granulocyte; MI, myocardial infarction; NCB, noncalcified coronary plaque burden; NDG, normal-density granulocyte; NET, neutrophil extracellular trap; PASI, psoriasis area severity index; SLE, systemic lupus erythematosus; TB, total coronary plaque burden
Highlights
-
•
LDGs are a subset of neutrophils that were elevated in psoriasis and associated with the severity of disease.
-
•
In psoriasis, LDGs associated with noncalcified coronary plaque burden beyond cardiovascular risk factors and in vitro, induced endothelial cell damage.
-
•
Compared to normal-density granulocyte neutrophils, platelet-associated biological pathways were upregulated in LDGs, suggesting enhanced platelet adherence to the LDG surface.
-
•
LDGs co-localized with platelets in circulation, and the LDG-platelet interaction associated more strongly with non-calcified coronary burden by coronary CTA compared to LDGs alone.
Summary
Psoriasis is an inflammatory skin disease associated with increased cardiovascular risk and serves as a reliable model to study inflammatory atherogenesis. Because neutrophils are implicated in atherosclerosis development, this study reports that the interaction among low-density granulocytes, a subset of neutrophils, and platelets is associated with a noncalcified coronary plaque burden assessed by coronary computed tomography angiography. Because early atherosclerotic noncalcified burden can lead to fatal myocardial infarction, the low-density granulocyte−platelet interaction may play a crucial target for clinical intervention.
Psoriasis is a chronic inflammatory, immune-mediated skin disease that affects 2% to 3% of the adult U.S. population 1, 2, 3. Psoriasis is associated with detrimental effects beyond the skin; it significantly reduces the quality of life through emotional and physical complications (4). Most concerning, multiple studies have demonstrated that psoriasis patients have increased susceptibility to early-onset atherosclerosis and its ensuing complications, including myocardial infarction (MI), stroke, and cardiovascular mortality beyond traditional cardiovascular disease (CVD) risk factors 1, 2, 5, 6. CVD is the leading cause of mortality in psoriasis, especially in patients with severe psoriasis 7, 8.
The immune response plays a pivotal role in the development of atherosclerosis, with neutrophils playing an important role in plaque progression 9, 10, 11. Circulating neutrophil frequency is reported to be a potential biomarker of CVD (12), and in inflammatory diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis, and HIV, neutrophils are associated with accelerated atherogenesis 13, 14, 15. Circulating neutrophils in psoriasis exhibit an activated phenotype, and the inflammatory neutrophil protein calprotectin (S100A8/A9) is elevated in psoriasis (16). Moreover, S100A8/A9 is related to vascular disease. Neutrophils are the foremost immune cells to infiltrate the papillary layer and subepidermal zone of the skin before psoriatic lesion formation, which suggests they may be a potential link between early-onset CVD and psoriasis (17). The distinct subset of neutrophils termed low-density granulocytes (LDGs) are of particular interest. LDGs are neutrophils purified from the less dense peripheral blood mononuclear cell (PBMC) fraction after density gradient centrifugation 18, 19, 20 and are associated with CVD in chronic inflammatory disease states 19, 21. LDGs have an enhanced capacity to spontaneously form neutrophil extracellular traps (NETs), a cell death process termed NETosis, which is characterized by the extracellular release of chromatin material bound to proteins present in neutrophil granules 22, 23, 24. However, the stimulus that activates the spontaneous NETosis mechanism in LDGs in inflammatory diseases remains unclear.
Activated platelets have been described to play a role among the various stimuli known to induce NETs 25, 26, 27. Platelet activation characterized by the expression of platelet activation molecules (e.g., CD36) is associated with atherosclerosis and other inflammatory conditions 25, 26. Although platelets are involved in NET formation, only a few studies have investigated this in nonchronic inflammatory states 25, 26. Furthermore, when spontaneous NETosis occurred at a higher frequency in a small preliminary study, it was not studied, but the reason may be related, in part, to unexplored neutrophil−platelet interactions (28).
In the present study, we aimed to characterized LDGs and normal-density granulocytes (NDGs) in psoriasis. Our goal was to understand the potential relationship between neutrophil subsets and the presence of early coronary artery disease in humans with psoriasis. We hypothesized that LDGs would be associated with psoriasis skin disease severity and early noncalcified coronary plaque burden (NCB) as assessed by coronary computed tomography angiography (CCTA). Subsequently, we identified the interaction between LDGs and platelets as a prospective mechanism that stimulated increased LDG NETosis, which resulted in endothelial damage.
Methods
Study population
Study approval for the cohort study was obtained from the Institutional Review Board of the National Heart, Lung, and Blood Institute in accordance with the principles of Declaration of Helsinki. This study reported the baseline visits of patients recruited longitudinally and consecutively into 2 ongoing protocols from January 2013 to May 2017 (Supplemental Figure 1). To be included in the study, psoriasis patients were required to have a formal diagnosis of psoriasis confirmed by a health care provider. All patients underwent CCTA to assess coronary plaque burdens, as described previously (29). Psoriasis skin disease severity was assessed with the psoriasis area and severity index (PASI) score and was measured as published (30). The PASI score combines the severity of lesions and the area affected into a single score, considering erythema, induration, and desquamation within each lesion. A combination of isolation and flow cytometry was used to determine the frequencies of LDGs and NDGs for each patient. Exclusion criteria for healthy control subjects included a history of systemic inflammatory or vascular disease, active infectious disease, uncontrolled hypertension, and overweight to obese individuals (body mass index >30 kg/m2). In total, 81 psoriasis patients and 36 healthy control subjects were enrolled with comprehensive CCTA data (Supplemental Figure 1). Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were followed for reporting the findings of our observational study (31).
Acquisition of CCTA
All patients underwent CCTA on the same day as the blood draw, using the same computed tomography scanner (320-detector row Aquilion ONE ViSION, Toshiba, Japan).
Analysis of CCTA
A single, blinded reader (blinded to treatment and time of scan) evaluated coronary plaque characteristics across each of the main coronary arteries at >2 mm using dedicated software (QAngio CT, Medis Medical Imaging Systems, Leiden, the Netherlands) 32, 33. Results of the automated contouring were also reviewed on transverse reconstructed cross sections of the artery on a section-by-section basis at 0.5-mm increments. Lumen attenuation was adaptively corrected on an individual scan basis using gradient filters and intensity values within the artery.
Laboratory procedures
For detailed methods see the Supplemental Methods section.
Whole blood processing and immunophenotyping
Briefly, lysed whole blood cells or ficoll-separated PBMCs were incubated for 30 min in a 10-color antibody cocktail (Supplemental Table 1) and acquired on a BD Biosciences LSRII flow cytometer using DIVA 6.1.2 software (BD Bioscience, San Jose, California). We determined the frequency of LDGs by quantitating the percentage of CD14loCD15hiCD10hi cells in the PBMC fraction by flow cytometry and used the complete blood count to determine the frequency of LDGs per microliter.
RNA sequencing analysis
Paired NDGs or LDGs (n = 50,000) were isolated from 7 psoriasis patients. We performed quantile normalization and used limma (34) for differential expression analysis to identify genes that were dysregulated between the NDG and LDG subsets, controlling for the individual and batch effects. The false discovery rate (FDR) was used for multiple testing, and significant differentially expressed genes had a FDR ≤0.1 and |log2(fold change)| ≥ 1.5. We then identified functions or gene ontologies that were enriched among differentially expressed genes, and FDR ≤0.1 was used to declare significance. All graphical illustrations and RNA-seq analyses were conducted using custom scripts and libraries implemented in R (R Foundation, Vienna, Austria).
Statistical analysis
Summary statistics were presented as mean ± SD for normally distributed variables, medians and interquartile range were used for non-normally distributed continuous variables, and frequencies were used for categorical variables. Normality was assessed by skewness and kurtosis. Parametric variables were compared between groups using Student’s t-test, whereas the Mann-Whitney U test was performed for nonparametric variables. Dichotomous variable comparisons were done using Pearson’s chi-square test. Unadjusted regression analyses were performed to evaluate for potential relationships between LDG frequency and coronary plaque burden, and regression results were represented as standardized beta-coefficients with p values. We conducted multivariable linear regression analyses to evaluate the association of coronary plaque burden with LDG and NDG frequency. These analyses were adjusted for traditional CVD risk as assessed by the Framingham 10-year risk, body mass index, type 2 diabetes, treatment with statins, and treatment with systemics. Results were presented with 95% confidence intervals, where applicable, and p values <0.05 were considered statistically significant. Statistical analyses were performed with STATA version 12.0 (StataCorp, College Station, Texas).
Results
Clinical characteristics of study participants
We summarized the characteristics of our study population in Table 1. The study cohort consisted of 81 consecutively recruited psoriasis patients and 36 healthy control subjects for LDG and NDG frequency comparisons (Table 1, Supplemental Figure 1). The psoriasis cohort was middle aged (49.1 ± 12.9 years), with a slight male predominance (64%), and a low CV risk as assessed by Framingham 10-year risk (median: 2; interquartile range: 1 to 4). The median PASI score was 7.4 (interquartile range: 3.4 to 11.8), which was consistent with moderate psoriasis skin disease severity (Table 1).
Table 1.
Baseline Characteristics of Psoriasis Patients and Healthy Control Subjects
| Psoriasis (n = 81) | Healthy Control Subjects (n = 36) | p Value | |
|---|---|---|---|
| Demographic and clinical characteristics | |||
| Age, yrs | 49.1 ± 12.9 | 33.6 ± 12.6 | <0.001‡ |
| Males | 52 (64) | 22 (61) | 0.75 |
| Hypertension | 18 (22) | 3 (8) | 0.07 |
| Hyperlipidemia | 25 (31) | 5 (14) | 0.05 |
| Type 2 diabetes | 7 (9) | 1 (3) | 0.25 |
| Body mass index, kg/m2 | 28.5 ± 5.2 | 24.1 ± 3.1 | <0.001‡ |
| Current smoker | 6 (7) | 2 (6) | 0.71 |
| Lipid treatment | 18 (22) | 1 (3) | 0.008† |
| Clinical and laboratory values | |||
| Total cholesterol, mg/dl | 185.3 ± 37.9 | 170.8 ± 31.3 | 0.02∗ |
| High-density lipoprotein, mg/dl | 56.6 ± 19.8 | 61.3 ± 16.2 | 0.11 |
| Low-density lipoprotein, mg/dl | 105.7 ± 29.1 | 91.2 ± 25.9 | 0.006† |
| Triglycerides, mg/dl | 101.0 (79.0–142.0) | 83.5 (72.0–97.5) | 0.02∗ |
| C-reactive protein | 2.2 (0.9–4.1) | 0.7 (0.5–1.6) | <0.001‡ |
| Framingham risk score | 2.0 (1.0–4.0) | 1.0 (1.0–1.0) | <0.001‡ |
| Absolute neutrophil count, K/μl | 3.9 ± 1.2 | 3.1 ± 1.2 | <0.001‡ |
| Psoriasis characteristics | |||
| Psoriasis area severity index score | 7.4 (3.4–11.8) | ||
| Systemic treatment | 8 (10) | ||
| Cytokines characterization | |||
| Tumor necrosis factor-α | 1.30 (0.85–1.85) | 1.00 (0.65–1.36) | 0.045∗ |
| Interleukin-6 | 1.32 (0.74–2.13) | 0.70 (0.41–1.07) | 0.006† |
| Interleukin-1β | 0.13 (0.08–0.16) | 0.10 (0.04–0.14) | 0.08∗ |
| Interleukin-18 | 390 (307–543) | 300 (220–449) | 0.01∗ |
| Interleukin-17A | 1.60 (0.88–2.85) | 0.73 (0.30–1.03) | <0.001‡ |
| Coronary CT angiography | |||
| Total burden, mm2 (×100) | 1.12 ± 0.43 | 0.93 ± 0.27 | <0.001‡ |
| Noncalcified burden, mm2 (×100) | 1.10 ± 0.43 | 0.91 ± 0.27 | <0.001‡ |
| Dense-calcified burden, mm2 (×100) | 0.006 (0.002–0.023) | 0.009 (0.004–0.017) | 0.31 |
Values are mean ± SD, n (%), or median (interquartile range).
The p values were calculated by using an unpaired Student’s t-test or Mann-Whitney U test for continuous variables and Pearson’s chi-square test for categorical variables. Significance set at
CT = computed tomography.
p < 0.05.
p < 0.01, and
p < 0.001.
Circulating LDG counts in psoriasis are associated with psoriasis skin disease severity
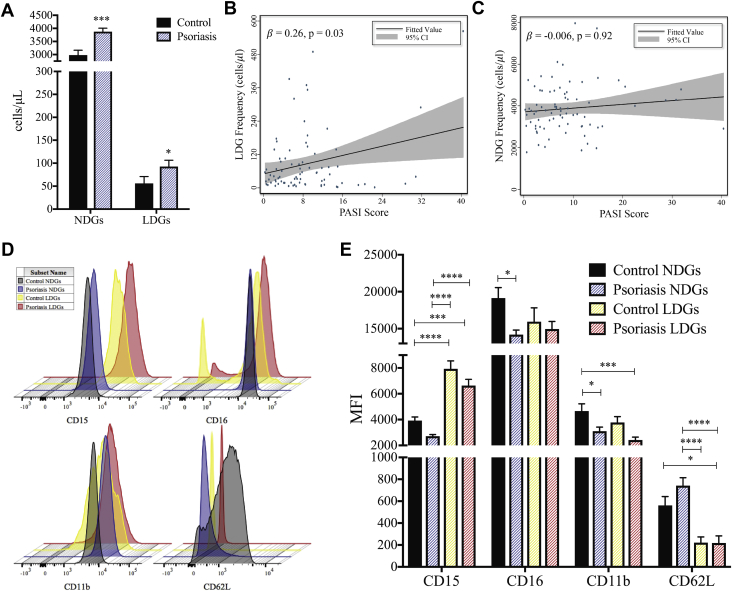
Both LDG and NDG subsets were elevated in psoriasis patients compared with healthy control subjects (1.3- and 2.0-fold, respectively) (Figure 1A). The frequency of circulating LDGs was associated with psoriasis severity (PASI: β = 0.28; p = 0.01), which remained significant after adjustment for body mass index, psoriasis treatment, and absolute neutrophil count (β = 0.26; p = 0.03) (Figure 1B). However, an association between NDG frequency and psoriasis skin disease severity was not detected (β = −0.006; p = 0.92) (Figure 1C). We then compared the surface markers of LDGs and NDGs in psoriasis to LDGs and NDGs from healthy control subjects (Figure 1D) and observed a significant elevation in CD15 in healthy control and psoriasis LDGs compared with both healthy control and psoriasis NDGs. This was concomitant with a reduction in CD11b on psoriasis LDGs and NDGs compared with healthy control NDGs (Figure 1E). CD62L was significantly downregulated on psoriasis LDGs compared with both psoriasis and healthy control NDGs (Figure 1E). Increased shedding of CD62L might indicate that psoriasis LDGs were in a higher state of activation compared with NDGs. No change in the surface expression of CD15, CD16, CD11b, or CD62L was observed when comparing LDGs from psoriasis patients with LDGs from healthy control subjects (Figure 1E).
Figure 1.
LDGs Are Elevated in Psoriasis Patients and Are Associated With Psoriasis Severity
(A) Normal-density granulocyte (NDG) and low-density granulocyte (LDG) frequencies were determined by flow cytometry and are elevated in psoriasis patients (n = 81) compared with healthy control subjects (n = 36). Data are represented as mean ± SEM. The Mann-Whitney test was performed, and significance was set at *p < 0.05* and ***p < 0.001. Regression analyses between (B) LDGs but not (C) NDGs are associated with the psoriasis area severity index (PASI) score for the psoriasis cohort (n = 81). (D) Surface marker expression of NDGs and LDGs was analyzed by flow cytometry and (E) showed a significant elevation in CD15 on psoriasis LDGs compared with healthy control and psoriasis NDGs, as well as lower CD11b and CD62L expression on psoriasis LDGs compared with healthy control LDGs. Data are represented as mean ± SEM. Significance was established by 1-way analysis of variance (ANOVA) and a Tukey’s multiple comparisons test set at *p < 0.05, ****p < 0.001, and ****p < 0.0001. MFI = median fluorescence intensity.
NCB in psoriasis associates with LDG counts
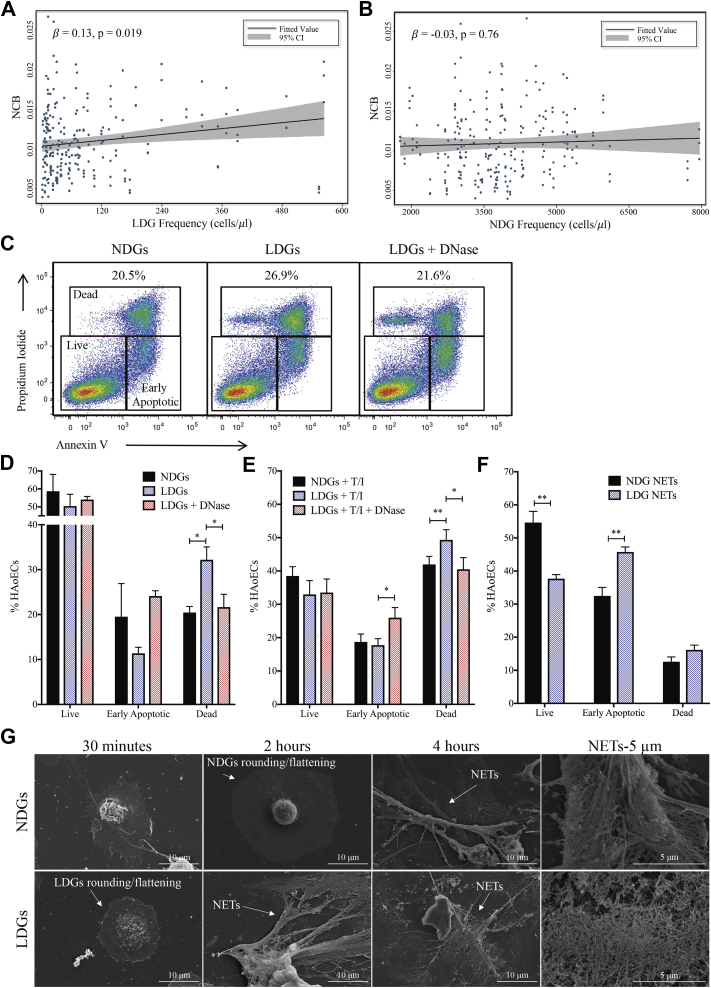
Evidence of early coronary atherosclerosis in psoriasis patients is driven by an increase in NCB (Table 1). Moreover, total coronary plaque burden (TB) and NCB within 3 major epicardial coronary arteries were positively associated with LDG frequency (β = 0.18; p = 0.005), which persisted beyond adjustment for traditional CVD risk factors and lipid treatment (TB: β = 0.13; p = 0.026) (NCB: β = 0.13; p = 0.019) (Figure 2A). Furthermore, no association was observed between NDG frequency and TB, as well as NCB, even when adjusted for traditional risk factors (TB: β = −0.003; p = 0.98; NCB: β = −0.03; p = 0.76) (Figure 2B).
Figure 2.
LDGs and Their NETs Induce Endothelial Cell Damage
(A) Psoriasis LDGs but not (B) psoriasis NDGs are associated with noncalcified coronary plaque burden (NCB) in psoriasis (n = 81). (C) Representative flow cytometry plots from the cytotoxicity assay show (D) psoriasis LDGs (n = 7) increase apoptosis of human aortic endothelial cells (HAoECs) compared with psoriasis NDGs (n = 5), an effect abrogated by DNase treatment (n = 4). Data are represented as mean ± SEM. Significance established by a 1-way ANOVA and a Tukey’s multiple comparisons test set at *p < 0.05, **p < 0.01, and ***p < 0.0001. (E) Cytotoxicity of HAoECs pre-treated with tumor necrosis factor-α and interferon-γ is further increased by LDGs. Data are represented as means ± SEM. Significance established by 1-way ANOVA and a Tukey’s multiple comparisons test and set at *p < 0.05 and **p < 0.01. (F) HAoECs were incubated for 18 h with NDG neutrophil extracellular trap (NET) associated (n =5) or LDG-NET associated proteins (n =5), and apoptosis was quantified using flow cytometry. Data are represented as mean ± SEM. Significance established by unpaired 2-tailed Student’s t-test and set at **p < 0.01. (G) Scanning electron microscopy images of the formation of NETs from NDGs and LDGs over time subsequent to purification. CI = confidence interval; T/I = tumor necrosis factor alpha/interferon gamma; other abbreviations as in Figure 1.
LDGs induce apoptosis in human aortic endothelial cells
Because psoriasis LDGs were associated with NCB compared with psoriasis NDGs, we hypothesized that LDGs from psoriasis and their NETs would exert enhanced cytotoxic effects on human aortic endothelial cells (HAoECs) compared with psoriasis NDGs. To normalize activation between LDGs and NDGs from psoriasis due to the isolation process, we sorted both LDGs and NDGs following an identical gating strategy (Supplemental Figure 2). We then measured the cytotoxic potential of psoriasis LDGs compared with psoriasis NDGs by quantifying the percentage of apoptotic CD146+ HAoECs via flow cytometry in a co-culture system (Figure 2C). LDGs (2:1, LDGs-to-HAoECs) led to an increase in the percentage of apoptotic HAoECs by 1.6-fold compared with the same number of NDGs (Figure 2D). To further support our hypothesis that HAoEC death might have been due be to LDG-derived NET formation, HAoECs were simultaneously treated with DNase and LDGs. DNase-treated co-cultures led to a 1.5-fold decrease in the percentage of HAoEC deaths comparable with NDGs, which resulted in increased HAoECs in the early apoptotic stage compared with LDGs alone (Figure 2D). To further mimic the psoriasis-like inflammatory state, HAoECs were pre-treated with tumor necrosis factor-α and interferon-γ, followed by psoriasis LDGs or NDGs (35). LDGs further increased the percentage of HAoEC death upon activation (Figure 2E).
We next measured the cytotoxic potential of NETs harvested from psoriasis LDGs and NDGs, which contain NET-associated proteins and fragmented DNA, on HAoECs. Upon treatment of HAoECs with LDG or NDG NETs, normalized to 50 μg of either LDG or NDG NET-associated proteins, we determined that HAoECs treated with LDG NETs showed a 32% reduction in live HAoECs concomitant with a 1.4-fold increase in early apoptotic cells compared with NDG NET-associated proteins (Figure 2F). No difference was detected in the percentage of dead HAoECs subsequent to LDG and NDG NET treatments (Figure 2F). This suggested that the mechanism by which LDGs exerted cytotoxic effects might, in part, rely on the cellular interaction between LDGs and HAoECs or intact DNA. To visualize the NETosis process between psoriasis LDGs and NDGs, we acquired scanning electron microscopy images of NET formation over time. Nonstimulated LDGs formed NETs by the 2-h timepoint compared with psoriasis NDGs stimulated with phorbol 12-myristate 13-acetate (PMA), which is an inducer of NETosis. NETs were not observed until 4 h (Figure 2G). We observed the initial stages of NETosis, characterized by rounding and flattening at 30 min for LDGs and 2 h for NDGs (24).
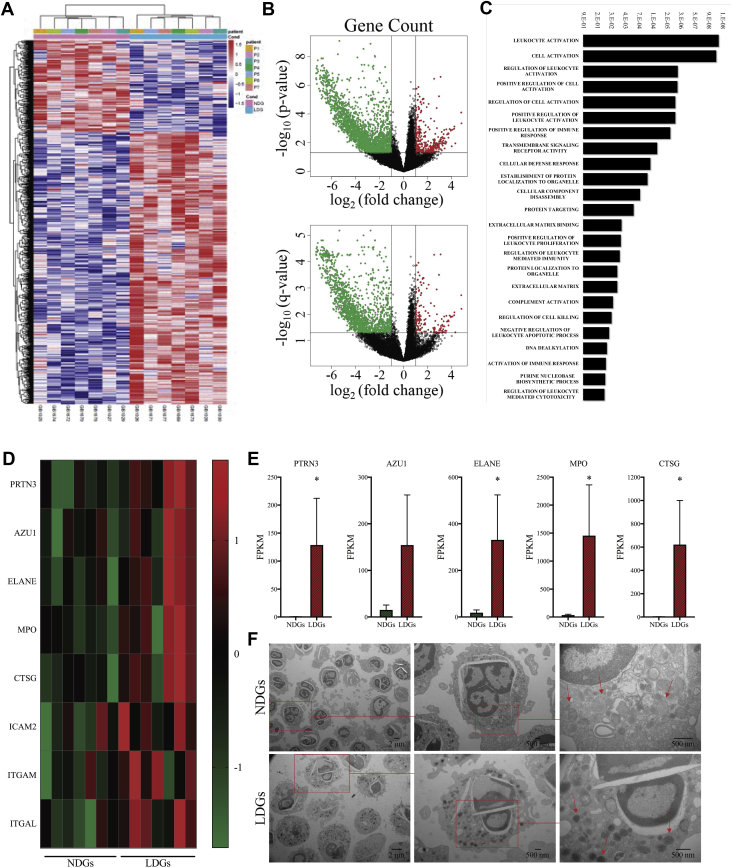
RNA sequencing of LDGs compared with NDGs reveals upregulation of granule proteins and adhesion molecules
To understand the relationship between psoriasis LDGs and NCB, which is a relationship not observed with psoriasis NDGs, we studied RNA expression between these neutrophil subsets derived from 7 patients with active psoriasis. By comparing the transcriptomes between LDGs and NDGs, we determined that 1,076 (Supplemental Table 1) were differentially expressed (Figure 3A). The volcano plots showed a separation of genes corresponding to NDGs relative to LDGs (Figure 3B). Functional analyses revealed that gene pathways that were differentially expressed between LDGs and NDGs were clustered in leukocyte activation (p = 1 × 10−10; FDR = 3 × 10−8) and cell activation (p = 2 × 10−10; FDR = 4 × 10−8) (Figure 3C). In addition, the granule proteins were upregulated at the gene level in LDGs (Figure 3D and 3E), which is a mechanism that typically stops before release from the bone marrow (36). Transmission electron microscopy further confirmed this finding, demonstrating LDGs had more electron-dense granules that corresponded with primary granules (Figure 3F) (37). Concomitant with increased granule proteins, we observed that the adhesion molecules intercellular adhesion molecule-2, integrin subunit alpha M (ITGAM), and integrin alpha subunit L (ITGAL) were upregulated in LDGs (Figure 3D).
Figure 3.
Granule Proteins and Adhesion Molecules Are Upregulated in LDGs Compared With NDGs at the Gene Level
RNA sequencing analysis was completed on 50,000 LDGs relative to NDGs from psoriasis patients. (A) Differentially expressed genes (n = 1,076) were identified between NDGs and LDGs from psoriasis patients (n = 7). NDGs are the reference, as are upregulated genes in LDGs. (B) The volcano plot shows clear separation between NDGs and LDGs. The upregulated (red) and downregulated (green) transcriptomes are NDGs, as LDGs are the reference sample. (C) The gene ontology biological process analysis highlighted biological processes that were differentially expressed between NDGs and LDGs. Significance was established by false discovery rate (FDR). (D) Granule proteins are upregulated in all patients (n = 7) and when normalized and by (E) FPKM values (n = 7). Data are represented as means ± SEM. Significance was established by the unpaired Mann-Whitney Student’s t-test and set at *p < 0.05. (F) Transmission electron microscopy images of LDGs and NDGs show LDGs had more electron dense granules. AZU1 = azurocidin 1; CTSG = cathepsin G; ELANE = neutrophil elastase; FPKM = fragments per kilobase of transcripts per million; ICAM2 = intercellular adhesion molecule 2; ITGAL = integrin subunit alpha L; ITGAM = integrin subunit alpha M; MPO = myeloperoxidase; P = patient; PRTN3 = proteinase 3; other abbreviations as Figures 1 and 2.
Co-localization of LDGs with platelets correlate with NCB
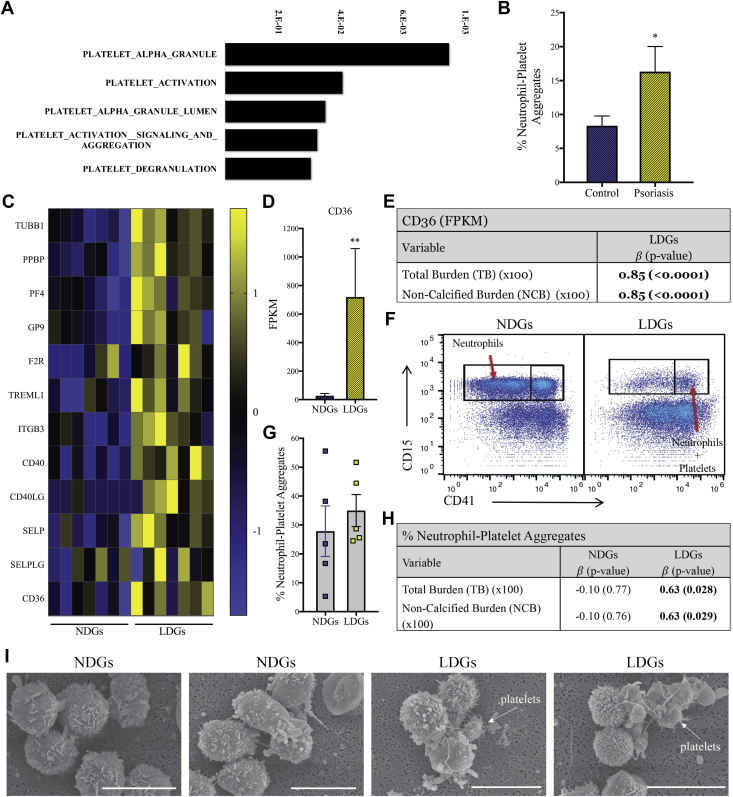
A stark difference in RNA sequencing between LDG and NDG data was the presence of platelet-associated biological pathways upregulated in the LDG samples compared with NDGs (Figure 4A). Therefore, we investigated the relationship of platelets and LDGs as a potential link to the positive association between LDGs and NCB. From the transcriptome data, we observed a clear upregulation of platelet-specific biological processes in the LDG sample, clustered in pathways that included platelet alpha granules (p = 4 × 10−5; FDR = 2 × 10−3), platelet activation (p = 2 × 10−3; FDR = 3 × 10−2), platelet alpha granule lumen (p = 3 × 10−3; FDR = 6 × 10−2), platelet activation signaling and aggregation (p = 4 × 10−3; FDR = 7 × 10−2), and platelet degranulation (p = 6 × 10−3; FDR = 9 × 10−2) (Figure 4A). These were selected pathways because they were not the most significant from the biological pathways list. To understand neutrophil−platelet interactions, we tested the frequency of neutrophil platelet aggregates and found an upregulation of neutrophil platelet aggregates in psoriasis (Figure 4B). We then focused on a subset of those genes and determined that CD40 and SELP (platelet-specific receptors that bind to CD40LG and SELPLG on neutrophils), as well as CD40LG and SELPLG were upregulated in LDGs, which suggested increased adhesion between platelets and LDGs (Figure 4C). We measured CD36 expression because CD36 promoted thrombosis, and we determined that CD36 expression was upregulated on LDGs compared with NDGs (Figures 4C and 4D) and were also associated with NCB (Figure 4E). After measuring the percentage of LDGs or NDGs that aggregated with platelets in psoriasis using flow cytometry (Figure 4F), we determined that the NDG platelet aggregation was highly variable, and there was no difference in the percentage of aggregates compared with LDGs when considering the mean (Figure 4G). However, the percentage of LDG platelet aggregates had a positive linear association with NCB, and this association was specific to LDGs (Figure 4H). Scanning electron microscopy images confirmed the presence of platelets that adhered to LDGs, a finding that was not observed in the NDG samples (Figure 4I).
Figure 4.
Upregulated Genes in LDGs Show Increased Binding to Platelets
RNA sequencing analysis shows an (A) upregulation of platelet-specific biological pathways in psoriasis LDGs versus psoriasis NDGs. Significance was established by FDR. (B) Neutrophil platelet aggregates were increased in psoriasis patients (n = 12) compared with matched control subjects (n = 10). Data are represented as mean ± SEM. Significance was established by the unpaired Mann-Whitney Student’s t-test and set at *p < 0.05. (C) The platelet-specific transcriptomes were upregulated in LDGs compared with NDGs (n = 7). (D) The platelet receptor, CD36, was highly upregulated in LDGs, and the FPKM values were associated with (E) NCB (n = 7). (F) Flow cytometry plots show (G) aggregates of NDGs or LDGs with platelets and the percentages of platelets LDG aggregates are (H) highly associated with NCB. (I) Scanning electron microscopy images of NDGs and LDGs demonstrate platelet LDG aggregates. Scale bar: 10 μm. CD40LG = CD40 ligand; F2R = coagulation factor II thrombin receptor; GP9 = glycoprotein IX platelet; ITGB3 = integrin subunit beta 3; PF4 = platelet factor 4; PPBP = pro-platelet basic protein; SELP = selectin P; SELPLG = selectin P ligand; TREML1 = triggering receptor expressed on myeloid cells like 1; TUBB1 = tubulin beta 1 class VI; other abbreviations as in Figures 1, 2, and 3.
Spontaneous NETosis of LDGs is associated with platelet frequency
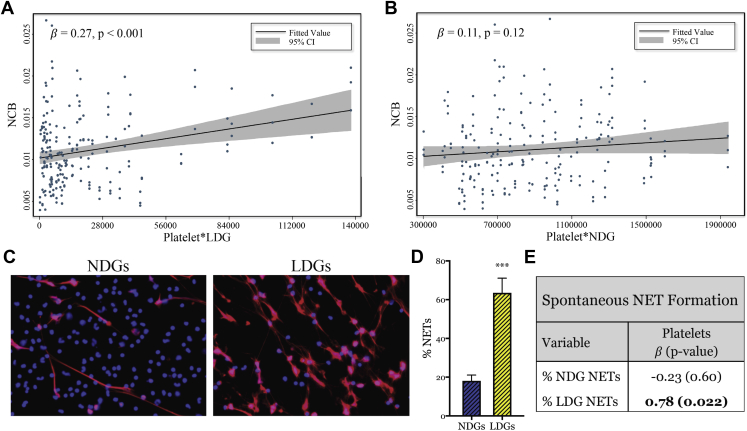
In addition to our previously described findings, the product of LDGs and platelets in circulation were also correlated with NCB beyond traditional risk factors (β = 0.27; p < 0.001) (Figure 5A). This finding was similar to the association between LDGs and NCB (Figure 2A); however, the association between LDG platelet aggregates and NCB was more robust. Consistent with previous data, this association was attenuated and not significant with the product of NDGs and platelets (β = 0.11; p = 0.12) (Figure 5B). Lastly, we confirmed that LDGs had increased spontaneous NETosis (Figures 5C and 5D) and determined that the percentage of LDGs to spontaneously form NETs was associated with the frequency of circulating platelets (β = 0.78; p = 0.022) (Figure 5E).
Figure 5.
Percentage of LDGs to Spontaneously Form NETs Is Associated With Circulating Platelet Counts
(A) The product of platelets and LDGs in circulation is associated with NCB; however, (B) this relationship is absent with the product of platelets and NDGs. (C) LDGs spontaneously form NETs at a (D) higher frequency than NDGs (n = 9). Data are represented as mean ± SEM. Significance was established by an unpaired Mann-Whitney Student’s t-test and set at ***p < 0.001. (E) The percentage of LDGs (n = 8) to undergo spontaneous NETosis is associated with the frequency of circulating platelets. Abbreviation as in Figures 1, 2, and 3.
Discussion
We demonstrated the following: 1) an increase in LDG frequency was associated with psoriasis severity and TB, specifically NCB, in psoriasis beyond in vitro traditional risk factors for CVD; 2) the frequency of NDGs, the dominant neutrophil subset, was not associated with TB or NCB; 3) psoriasis LDGs might exert a cytotoxic effect on endothelial cells compared with NDGs, similar to SLE; and 4) the amount of CD36 gene expression, a platelet gene, in LDGs and the percentage of circulating platelet LDG aggregates were associated with early atherosclerotic NCB. These findings suggested that in an inflammatory environment platelets might potentially interact with LDGs and promote vascular damage. These observations suggested that the adherence of LDG to platelets might be an important link between psoriasis skin disease severity and early atherogenesis, as well as represented a potential target for treatment of both diseases in the future.
Neutrophils are critical to the development of psoriasis, and a reduction in circulating neutrophils is accompanied by regression of psoriatic plaque development (38). Our previous study reported an increased circulating frequency of activated neutrophils, as defined by lower CD62L and CD16 surface expression, in psoriasis patients compared with healthy control subjects (16). When we focused on biologic-naïve psoriasis patients and the different neutrophil subsets in this study, we determined that CD62L expression on LDGs was significantly lower compared with both healthy control and psoriasis NDGs. We found CD62L expression to be decreased on LDGs compared with NDGs. Although both CD16 and CD62L mark a heightened activation state, only CD62L was reduced on LDGs compared with NDGs in psoriasis. This might, in part, be a result of the formation of LDG platelet aggregates. Neutrophil platelet aggregation in resting neutrophils reduces CD62L expression, and primes neutrophils for adhesion (39). This might explain why only the LDGs were associated with psoriasis severity and psoriatic comorbidities. In addition, this positive relationship between LDG frequency and psoriasis severity suggested that LDGs might potentially be a clinical target for treating psoriasis.
Neutrophils are increasingly being recognized as significant contributors to the pathogenesis of CVD. Neutrophil frequency was a predictor of coronary events (40), and more recently, this frequency was involved in early atherosclerotic plaque development (41). To understand if the development of in vivo atherogenesis is related to neutrophils, we leveraged CCTA as a reliable, noninvasive imaging technique to detect atherosclerotic plaque composition in coronary arteries. Comprehensive plaque characterization permitted us to directly assess and correlate TB and NCB with the frequencies of both neutrophil subsets in circulation. Similar to psoriasis severity, we found a positive linear relationship between LDGs and TB in the major coronary arteries, primarily driven by NCB. Furthermore, these activated neutrophils might, in part, be responsible for early damage to both the epidermis and endothelium.
We hypothesized that early plaque formation evidenced by the increase in NCB in psoriasis was potentially related to the cytotoxic effects of LDGs by 2 factors. First, LDG NETs themselves are more cytotoxic than NDG NETs, and second, LDGs form NETs spontaneously; therefore, the amount of circulating NETs in psoriasis was increased due to increased LDGs. NETs are known to play a role in atherosclerotic plaque development independently of autoimmunity; NETs are released from neutrophils in response to cholesterol-crystal priming, and within the atherosclerotic lesion, NETs are localized to cholesterol-rich areas (42). Impairments in DNA clearance by DNase I were described in autoimmunity and might lead to an enhanced half-life of immunogenic material present in NETs, further exacerbating endothelial damage. When endothelial cells were treated with isolated NETs from LDGs or NDGs, we did not observe an increase in endothelial cell apoptosis from LDG NETs. However, the early stage of apoptosis was elevated by LDG-NET treatment compared with NDG NETs, and there was a significant reduction in live HAoECs. Endothelial cell apoptosis required a cell−cell interaction. Notably, DNase I treatment abrogated the cytotoxic effect of LDGs, which suggested that endothelial cell death was not induced by LDGs independent of NET formation. Studies focused on the effects of NETs showed multiple outcomes. At lower concentrations of NETs normalized to DNA content, NETs that contained fragmented DNA were not as potent at activating human pulmonary artery endothelial cells (43). However, at significantly higher DNA concentrations, fragmented DNA induced endothelial cell apoptosis to the same extent as intact DNA (44). In the present study, the NDG and LDG NETs were composed of NET-associated proteins and fragmented DNA. In addition, we normalized the HAoEC treatments to protein content as opposed to DNA concentrations. This might explain the lack of apoptotic HAoECs subsequent to LDG NET treatment.
To understand the enhanced cytotoxicity of LDGs compared with NDGs, we completed RNA sequencing of paired LDGs and NDGs derived from biologic-naïve psoriasis patients with severe, active skin disease and identified a potential mechanistic target driving the spontaneous NETosis of LDGs. First, RNA sequencing data showed that LDGs were activated, which was demonstrated by the differential biological pathways corresponding to leukocyte activation, cell activation, regulation of leukocyte activation, positive regulation of cell activation, and regulation of cell activation. These data are in agreement with the decrease in surface expression of CD62L observed on the LDG surface compared with both healthy and psoriasis NDGs. Second, we determined that P-selectin and P-selectin ligand transcriptomes were upregulated in LDGs compared with NDGs. P-selectin is a platelet-specific receptor that is upregulated on activated platelets and binds to the P-selectin ligand on neutrophils. Concomitantly, CD40 and CD40 ligand were upregulated in the LDG sample. This is of interest because activated platelets are reported to induce NET formation from neutrophils in acute lung injury and sepsis 25, 26, 27. Furthermore, for the neutrophil inflammatory response to fully ensue, stimulation through the P-selectin ligand is required and drives neutrophil migration (45). Blockade of P-selectin ligand signaling altered neutrophil migration and protected mice against thromboinflammatory injury (45). Although it was clear that the interaction of platelets and neutrophils through P-selectin and P-selectin ligand is essential, it was shown that high-mobility group box-1 on platelets directs neutrophils to undergo NETosis (27). High mobility group box-1 expression increases on the platelet surface upon activation and elicits NET formation through a receptor for advanced glycation end products, a process that is independent of toll-like receptor-4 (27).
We determined that the frequency of LDGs co-localized with platelets had a linear association with early NCB. This observation was further strengthened by a significant association between the fragments per kilobase of transcript per million reads of CD36 in the LDG sample and NCB. The CD36 reads were most likely contributed by platelets co-localized with LDGs because our samples were immunophenotyped by flow cytometry to exclude other cell populations that might express CD36. Combined, our data highlighted a potential role for the LDG−platelet interactions in early atherogenesis. It was possible that the association between the percentage of LDGs to spontaneously form NETs and platelet counts was driven by an increase in activated platelets in psoriasis. Further investigations are required to validate this hypothesis and decipher a biological mechanism by which platelets contribute to NET formation in psoriasis. Recent studies provided insight into which LDGs undergo spontaneous NETosis 46, 47. Spontaneous NETosis in isolated SLE LDGs occurs within 50 min. This was reported to occur by a mitochondrial reactive oxygen species−dependent mechanism (47). A similar NETosis timeframe was observed when neutrophils were treated with platelet activating factor. Because the adherence of platelets to LDGs might stimulate the release of platelet activating factor, and the NETosis timeframe seen in our studies was similar, we proposed that platelet activating factor was involved in the mechanism of LDG-dependent NETosis.
This study could be extended to other autoinflammatory pathologies such as SLE. In SLE patients, activated platelets enhance the interferon response, and platelet depletion in an SLE murine model significantly improved disease measures and survival (48).
Study limitations
There were important limitations to our study. This was an observational study; therefore, it was subjected to potential for confounders and needs experimental follow-up. We also acknowledged that our control group was not adequately matched to our psoriasis group, which was a limitation. Thus, our results should be interpreted with caution. Our plaque characterization and quantification by CCTA was used as a surrogate marker for atherosclerosis, although intravascular ultrasound would be the gold standard to prove these findings (49). In vitro characterization studies are lacking and will be conducted to determine potential drivers of neutrophil platelet aggregation. The RNA sequencing should be followed up with validation studies of protein content and include control samples to determine if LDGs from healthy control subjects have a similar RNA signature compared with psoriasis LDGs. In addition, future studies using single cell RNA sequencing to better characterize our findings and validation studies should be conducted.
Conclusions
We demonstrated that LDG frequency is elevated in psoriasis and is related to skin disease severity and NCB. Our in vitro studies showed that psoriasis LDGs were cytotoxic to the endothelium following direct contact. This study identified the interactions between LDGs and platelets as a mechanistic focus of future studies to determine how spontaneous NETosis of LDGs might be partly dependent upon platelets. Furthermore, this LDG−platelet interaction might provide a potential therapeutic target in the future to reduce atherosclerosis in psoriasis.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Neutrophils have been shown to play an important role in the development early-onset atherogenesis, especially in inflammatory disease states. In addition, a distinct subset of neutrophils termed LDGs are shown to be associated with cardiovascular disease in chronic inflammatory diseases. Finally, the inter-relationship of LDG, platelets, and CCTA-derived early NCB highlights the potential role of LDG−platelet interaction as a driver in chronic inflammatory disease−associated atherosclerosis.
TRANSLATIONAL OUTLOOK 1: Studies in vitro should focus on the effect of antiplatelet therapy on neutrophil−platelet aggregate interactions.
TRANSLATIONAL OUTLOOK 2: Studies in vivo should deplete platelets in pre-clinical models to understand if neutrophil platelet aggregates and atherosclerosis are reduced.
Acknowledgments
The authors would also like to thank the National Heart, Lung, and Blood Institute Electron Microscopy Core for their contribution to the scanning electron microscopy images.
Appendix
References
- 1.Prodanovich S., Kirsner R.S., Kravetz J.D., Ma F., Martinez L., Federman D.G. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700–703. doi: 10.1001/archdermatol.2009.94. [DOI] [PubMed] [Google Scholar]
- 2.Mehta N.N., Azfar R.S., Shin D.B., Neimann A.L., Troxel A.B., Gelfand J.M. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000–1006. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rachakonda T.D., Schupp C.W., Armstrong A.W. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512–516. doi: 10.1016/j.jaad.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Meyer N., Paul C., Feneron D. Psoriasis: an epidemiological evaluation of disease burden in 590 patients. J Eur Acad Dermatol Venereol. 2010;24:1075–1082. doi: 10.1111/j.1468-3083.2010.03600.x. [DOI] [PubMed] [Google Scholar]
- 5.Brauchli Y.B., Jick S.S., Miret M., Meier C.R. Psoriasis and risk of incident myocardial infarction, stroke or transient ischaemic attack: an inception cohort study with a nested case-control analysis. Br J Dermatol. 2009;160:1048–1056. doi: 10.1111/j.1365-2133.2008.09020.x. [DOI] [PubMed] [Google Scholar]
- 6.Gelfand J.M., Neimann A.L., Shin D.B., Wang X., Margolis D.J., Troxel A.B. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 7.Yeung H., Takeshita J., Mehta N.N. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149:1173–1179. doi: 10.1001/jamadermatol.2013.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abuabara K., Azfar R.S., Shin D.B., Neimann A.L., Troxel A.B., Gelfand J.M. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the U.K. Br J Dermatol. 2010;163:586–592. doi: 10.1111/j.1365-2133.2010.09941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansson G.K. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 10.Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circ Res. 2012;110:875–888. doi: 10.1161/CIRCRESAHA.111.257535. [DOI] [PubMed] [Google Scholar]
- 11.Naruko T., Ueda M., Haze K. Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation. 2002;106:2894–2900. doi: 10.1161/01.cir.0000042674.89762.20. [DOI] [PubMed] [Google Scholar]
- 12.Arbel Y., Finkelstein A., Halkin A. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography. Atherosclerosis. 2012;225:456–460. doi: 10.1016/j.atherosclerosis.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan R.C., Kingsley L.A., Sharrett A.R. Ten-year predicted coronary heart disease risk in HIV-infected men and women. Clin Infect Dis. 2007;45:1074–1081. doi: 10.1086/521935. [DOI] [PubMed] [Google Scholar]
- 14.Kim C.H., Al-Kindi S.G., Jandali B., Askari A.D., Zacharias M., Oliveira G.H. Incidence and risk of heart failure in systemic lupus erythematosus. Heart. 2017;103:227–233. doi: 10.1136/heartjnl-2016-309561. [DOI] [PubMed] [Google Scholar]
- 15.Giles J.T., Post W.S., Blumenthal R.S. Longitudinal predictors of progression of carotid atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2011;63:3216–3225. doi: 10.1002/art.30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naik H.B., Natarajan B., Stansky E. Severity of psoriasis associates with aortic vascular inflammation detected by FDG PET/CT and neutrophil activation in a prospective observational study. Arterioscler Thromb Vasc Biol. 2015;35:2667–2676. doi: 10.1161/ATVBAHA.115.306460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chowaniec O., Jablonska S., Beutner E.H., Proniewska M., Jarzabek-Chorzelska M., Rzesa G. Earliest clinical and histological changes in psoriasis. Dermatologica. 1981;163:42–51. doi: 10.1159/000250139. [DOI] [PubMed] [Google Scholar]
- 18.Hacbarth E., Kajdacsy-Balla A. Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthritis Rheum. 1986;29:1334–1342. doi: 10.1002/art.1780291105. [DOI] [PubMed] [Google Scholar]
- 19.Carmona-Rivera C., Kaplan M.J. Low-density granulocytes: a distinct class of neutrophils in systemic autoimmunity. Semin Immunopathol. 2013;35:455–463. doi: 10.1007/s00281-013-0375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denny M.F., Yalavarthi S., Zhao W. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol. 2010;184:3284–3297. doi: 10.4049/jimmunol.0902199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakou M., Knowlton N., Frank M.B. Gene expression in systemic lupus erythematosus: bone marrow analysis differentiates active from inactive disease and reveals apoptosis and granulopoiesis signatures. Arthritis Rheum. 2008;58:3541–3549. doi: 10.1002/art.23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brinkmann V., Reichard U., Goosmann C. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 23.Urban C.F., Ermert D., Schmid M. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuchs T.A., Abed U., Goosmann C. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald B., Urrutia R., Yipp B.G., Jenne C.N., Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe. 2012;12:324–333. doi: 10.1016/j.chom.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Caudrillier A., Kessenbrock K., Gilliss B.M. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122:2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maugeri N., Campana L., Gavina M. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J Thromb Haemost. 2014;12:2074–2088. doi: 10.1111/jth.12710. [DOI] [PubMed] [Google Scholar]
- 28.Lin A.M., Rubin C.J., Khandpur R. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol. 2011;187:490–500. doi: 10.4049/jimmunol.1100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salahuddin T., Natarajan B., Playford M.P. Cholesterol efflux capacity in humans with psoriasis is inversely related to non-calcified burden of coronary atherosclerosis. Eur Heart J. 2015;36:2662–2665. doi: 10.1093/eurheartj/ehv339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langley R.G., Ellis C.N. Evaluating psoriasis with psoriasis area and severity index, psoriasis global assessment, and lattice system physician's global assessment. J Am Acad Dermatol. 2004;51:563–569. doi: 10.1016/j.jaad.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 31.von Elm E., Altman D.G., Egger M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lerman J.B., Joshi A.A., Chaturvedi A. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263–276. doi: 10.1161/CIRCULATIONAHA.116.026859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwan A.C., May H.T., Cater G. Coronary artery plaque volume and obesity in patients with diabetes: the Factor-64 study. Radiology. 2014;272:690–699. doi: 10.1148/radiol.14140611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ritchie M.E., Phipson B., Wu D. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta N.N., Teague H.L., Swindell W.R. IFN-gamma and TNF-alpha synergism may provide a link between psoriasis and inflammatory atherogenesis. Sci Rep. 2017;7:13831. doi: 10.1038/s41598-017-14365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Theilgaard-Monch K., Jacobsen L.C., Borup R. The transcriptional program of terminal granulocytic differentiation. Blood. 2005;105:1785–1796. doi: 10.1182/blood-2004-08-3346. [DOI] [PubMed] [Google Scholar]
- 37.Bainton D.F., Farquhar M.G. Origin of granules in polymorphonuclear leukocytes. Two types derived from opposite faces of the Golgi complex in developing granulocytes. J Cell Biol. 1966;28:277–301. doi: 10.1083/jcb.28.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toichi E., Tachibana T., Furukawa F. Rapid improvement of psoriasis vulgaris during drug-induced agranulocytosis. J Am Acad Dermatol. 2000;43:391–395. doi: 10.1067/mjd.2000.103264. [DOI] [PubMed] [Google Scholar]
- 39.Peters M.J., Dixon G., Kotowicz K.T., Hatch D.J., Heyderman R.S., Klein N.J. Circulating platelet-neutrophil complexes represent a subpopulation of activated neutrophils primed for adhesion, phagocytosis and intracellular killing. Br J Haematol. 1999;106:391–399. doi: 10.1046/j.1365-2141.1999.01553.x. [DOI] [PubMed] [Google Scholar]
- 40.Horne B.D., Anderson J.L., John J.M. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45:1638–1643. doi: 10.1016/j.jacc.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 41.Quillard T., Araujo H.A., Franck G., Shvartz E., Sukhova G., Libby P. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion. Eur Heart J. 2015;36:1394–1404. doi: 10.1093/eurheartj/ehv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warnatsch A., Ioannou M., Wang Q., Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349:316–320. doi: 10.1126/science.aaa8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aldabbous L., Abdul-Salam V., McKinnon T. Neutrophil extracellular traps promote angiogenesis: evidence from vascular pathology in pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2016;36:2078–2087. doi: 10.1161/ATVBAHA.116.307634. [DOI] [PubMed] [Google Scholar]
- 44.Saffarzadeh M., Juenemann C., Queisser M.A. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7:e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sreeramkumar V., Adrover J.M., Ballesteros I. Neutrophils scan for activated platelets to initiate inflammation. Science. 2014;346:1234–1238. doi: 10.1126/science.1256478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta S., Chan D.W., Zaal K.J., Kaplan M.J. A High-throughput real-time imaging technique to quantify NETosis and distinguish mechanisms of cell death in human neutrophils. J Immunol. 2018;200:869–879. doi: 10.4049/jimmunol.1700905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lood C., Blanco L.P., Purmalek M.M. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med. 2016;22:146–153. doi: 10.1038/nm.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duffau P., Seneschal J., Nicco C. Platelet CD154 potentiates interferon-alpha secretion by plasmacytoid dendritic cells in systemic lupus erythematosus. Sci Transl Med. 2010;2:47ra63. doi: 10.1126/scitranslmed.3001001. [DOI] [PubMed] [Google Scholar]
- 49.Park H.B., Lee B.K., Shin S. Clinical feasibility of 3D automated coronary atherosclerotic plaque quantification algorithm on coronary computed tomography angiography: comparison with intravascular ultrasound. Eur Radiol. 2015;25:3073–3083. doi: 10.1007/s00330-015-3698-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.