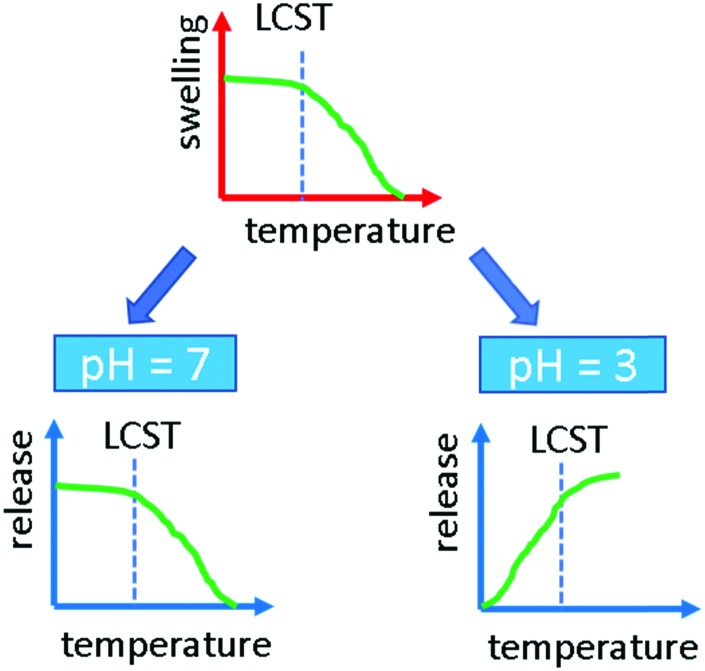
 Temperature-controlled release and study on the effects of the drug–polymer interaction and pH.
Temperature-controlled release and study on the effects of the drug–polymer interaction and pH.
Abstract
Control over drug delivery may be interestingly achieved by using temperature responsive encapsulants, which change their thickness and mesh size with temperature. The prototype N-isopropylacrylamide hydrogel cross-linked with di(ethylene glycol) divinyl ether p(NIPAAm-co-DEGDVE) swells at low temperature and collapses above the lower critical solution temperature (LCST), ∼29 °C in a buffer. It might be expected that drug release from such encapsulation is always favored below the LCST, due to the larger free volume present in the swollen polymer film. Recent results show contradicting behavior where some cases behave as expected and others release much less when the polymer layer is swollen. In this study, layers of the drugs phenytoin, clotrimazole and indomethacin were drop cast on glass and p(NIPAAM-co-DEGDVE) layers were then synthesized directly on top of these drug layers via initiated chemical vapor deposition (iCVD), a solvent-free and gentle polymerization technique. Dissolution experiments were then performed, in which the drug release through the hindrance of the hydrogel was measured at different pH values. The results show that not only the swelling but also the permeate (drug in this case)–polymer interaction plays an important role in the release.
Introduction
For treatment of a medical condition, different drug administration routes (e.g., by injection, inhalation, transdermal patches or by classic oral administration) are available in modern medicine. Choosing the appropriate administration route will strongly influence how easily a drug reaches the intended target site, affecting drug dosage and dosage regimen in turn. It can thus be highly desirable to control the release behavior of the drug delivery platform, allowing for example for sustained medication or for targeted, site-specific drug liberation. The application of a smart polymer coating is one possible way to obtain control over the release behavior. In such systems, the polymeric coating can serve multiple purposes at once. For instance, stabilization of the solid state of a drug may be achieved by polymeric encapsulation, which limits the environmental exposure.1 At the same time, these coatings can be beneficial for several therapies as they enable controlled drug release and minimize severe side effects due to burst release.2
Stimuli-responsive polymers are currently receiving a lot of attention because of their intriguing property of giving a strong response to a small external stimulus. These stimuli can be manifold, such as temperature,3 pH4 or light.5,6 The material response might be a change in thickness, wettability and/or color. For instance, a thermo-responsive hydrogel is a polymer that exhibits a temperature-dependent thickness change in a liquid environment. Such polymers are also researched for biomedical applications because they can exhibit good biocompatibility and little to no toxicity within the body.7
Various approaches for polymeric encapsulation exist. For instance, solution-processing by drop casting, spraying or ink-jet printing, amongst others, can be employed. The drawback of these techniques, however, is that solvent choice might be restricted by the drug/regulatory or might lead to unwanted interactions (e.g., (re)crystallization of the drug). However, there are also various solvent-free techniques available, which do not succumb to these limitations. Amongst them, initiated chemical vapor deposition (iCVD) is one of the most potent techniques, allowing for the synthesis of thin polymer films directly on various types of substrates via a surface polymerization reaction.8 This solvent free technique allows the application of a coating after drug loading, without harming the drug layer. The iCVD technique allows the deposition of conformal coatings with nanometer thickness control, in a completely solvent-free environment. Moreover, the chemical composition of the polymer layer can easily be controlled9 with great fidelity on the functional groups of the monomers.10 This is of great importance when a high percentage of functional groups in the polymer is desired, e.g., in the case of stimuli-responsive hydrogels.
Using a thermo-responsive coating such as a copolymer of N-isopropylacrylamide (NIPAAm), cross-linked with di(ethylene glycol) divinyl ether (DEGDVE), p(NIPAAm-co-DEGDVE), McInnes et al. demonstrated temperature-dependent drug delivery.11 This is only possible because the polymer of NIPAAm, p(NIPAAm), undergoes a reversible coil-to-globule transition at a lower critical solution temperature (LCST) of about 32 °C, i.e. near the human body temperature.12 In particular, the release of the drug camptothecin was demonstrated to be accelerated above the LCST. Other studies demonstrated instead that pharmaceuticals such as albumin13 and indomethacin14 give a faster release behavior below the LCST. With some studies reporting faster deliveries below the LCST and others above, it seems that there is not a unique mechanism in which p(NIPAAm) liberates a pharmaceutical. Thus, the present work aims to further the understanding of which factors influence the delivery. For this purpose, thin films of different model drugs were encapsulated in a p(NIPAAm-co-DEGDVE) hydrogel layer by iCVD. The drug release experiments from the different model drug delivery systems were performed at different temperatures and at different pH values. These data are complemented by a study of the pristine drug layers and the polymer coatings, with a particular focus on the temperature-dependent thickness change of the p(NIPAAm-co-DEGDVE) hydrogel layer in a liquid environment.
Experimental
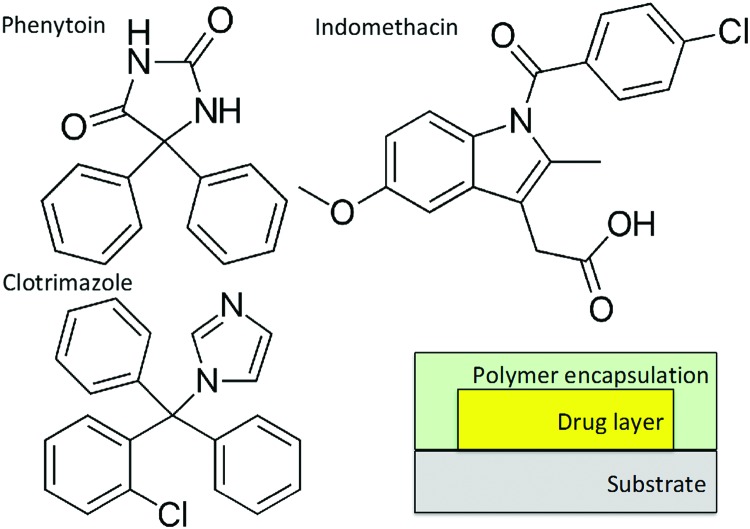
Conventional glass slides were used as substrates, cut into 2.5 × 2.5 cm2 pieces. Prior to usage, the substrates were cleaned in an acetone ultrasonic bath for 15 min and rinsed with deionized water. Three different active pharmaceutical ingredients (APIs) were used as model systems in the dissolution tests: clotrimazole (Gatt-Koller GmbH, Austria), indomethacin and phenytoin (both Sigma Aldrich, Germany). Their chemical structures are depicted in Scheme 1 and all chemicals were of pharmaceutical grade and used without any further purification. API layers were deposited on the substrates by drop casting, from tetrahydrofuran (THF, Merck, Germany) solutions at the following concentrations: 2.5 mg mL–1 for phenytoin, 28.6 mg mL–1 for clotrimazole and 0.286 mg mL–1 for indomethacin. 350 μL of these solutions were drop cast on the glass. While phenytoin crystallized within a few minutes into the bulk phase,15 the other drugs remained amorphous over the course of the experiments or longer (up to days).
Scheme 1. Chemical formulae of the different model pharmaceuticals tested within this study and the sample arrangement.
After ambient storage for 24 hours to evaporate any eventual leftover solvent, a polymer coating was deposited on the samples by iCVD to encapsulate the drug layer (see Scheme 1). A custom build reactor setup was used as described in ref. 16. The monomers NIPAAm and DEGDVE were heated to 85 °C and 70 °C, respectively, and fed into the reactor through a heated mixing line. The flow rates were controlled by needle valves. The initiator tert-butylperoxide (TBPO) was kept at room temperature and fed into the reactor through a mass flow controller. The flow rates were 0.25 ± 0.05 sccm for NIPAAm, 0.80 ± 0.15 sccm for DEGDVE and 0.50 ± 0.05 sccm for TBPO. The depositions were performed at a constant working pressure of 200 mTorr, with the substrate and filament temperatures at 30 °C and 250 °C, respectively. The polymer layers were grown up to a thickness of 200 nm, monitored in situ by laser interferometry.
To test the amount of drug release from the surface either without or with the hydrogel encapsulations, the samples were immersed into 50 mL of a buffer solution of NaH2PO4 at different pH values, to simulate either stomach (pH 1.5–3.5) or neutral environments. Furthermore, the temperatures were kept at either 37 °C or 25 °C to be above and below the LCST. During the entire experiment the samples were shaken using a standard shaking device minimizing diffusion problems due to convection absence. The dissolution medium was sampled every 10 min by withdrawing 500 μL of the solution, thus resulting in a small reduction of dissolution volume over time, which was accounted for in the concentration determination using high performance liquid chromatography (HPLC).
For HPCL measurements, internal standards (ISTD) were added to the analyte, to evaluate potential experimental errors. 5 μg mL–1 of phenytoin in acetonitrile was used for indomethacin evaluation and 100 μg mL–1 indomethacin in acetonitrile was used for phenytoin and clotrimazole evaluation. Prior to injection of the sample into the HPLC, the solvents were evaporated at 95 °C under a nitrogen atmosphere (TECHNE Sample Concentrator). 100 μL of acetonitrile (ACN) re-dissolved the drug and the internal standard, which was then measured using UV-vis absorption. Each experiment was repeated three times and the error is given as standard deviation.
The thickness of the polymer thin films and the temperature-dependent swelling were evaluated on bare silicon substrates without the drug layer, by spectroscopic ellipsometry (J.A. Woollam M-2000). Data acquisition was performed in air at three angles (65, 70 and 75°) in the wavelength range from 370 to 1000 nm. Data were evaluated using simulations with an optical model comprised of three layers; the silicon substrate, the native silicon dioxide (1.7 nm) and a Cauchy layer representing the polymer. The hydrogel swelling behavior in both buffer solution (pH = 3) and buffer plus API (at 15 μg mL–1) was also explored by in situ ellipsometry at a fixed angle of 75°, utilizing a heated liquid cell (Woollam, USA). The temperature was ramped from room temperature to 50 °C at a rate of 1 °C min–1. The effective medium approximation (EMA) was used to model the composite consisting of polymer and water. The model mixes the optical constants of water with those of the dry Cauchy layer (i.e. polymer) according to their relative fraction (which is the fitting parameter).
Results and discussion
The deposition of the p(NIPAAM-co-DEGDVE) using iCVD resulted in a homogeneous coating on top of the silicon substrate. The thickness of this layer was chosen to be about 200 nm since this represents a good trade-off between deposition time and sample performance, as demonstrated earlier.9 The surface roughness was about 1 nm. The chemical characterization was shown previously, demonstrating the retention of the functional groups.17
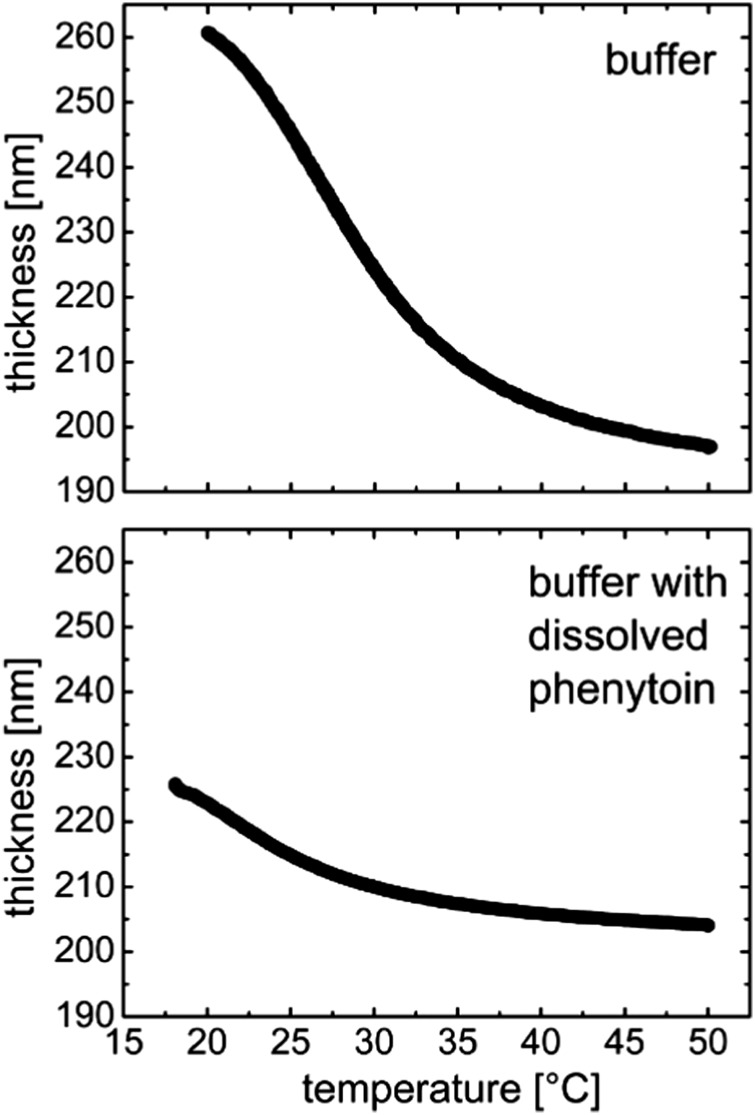
The temperature-dependent swelling of the p(NIPAAM-co-DEGDVE) film for two different environments is shown in Fig. 1. Upon immersion of the layer into a NaH2PO4 buffer solution at pH = 3, the polymer is already in its equilibrium swollen state at 20 °C, exhibiting a 30% thickness increase from the dry state (from 200 nm to 261 nm) (Fig. 1a). As the temperature increases, the layer thickness decreases steadily with the greatest rate of change (slope) at 29 °C. This temperature was identified as the LCST of the p(NIPAAM-co-DEGDVE) layer we synthesized. Below this temperature, the hydrogel is predominantly in its highly swollen state, while it gradually collapses at elevated temperatures. When the temperature reaches 50 °C, the layer shrinks to almost its initial dry thickness of 200 nm, suggesting that most of the free water is pushed out of the polymer.
Fig. 1. Temperature-induced swelling profiles of the p(NIPAAM-co-DEGDVE) hydrogel when exposed to a NaH2PO4 buffer at pH = 3 (top) or exposed to a solution containing phenytoin dissolved in the same buffer. The two curves share a common abscissa.
The experiment was repeated with phenytoin as a model drug being added to the buffer solution to identify any potential interaction of the drug with the polymeric layer and its swelling behavior. Such temperature-dependent swelling profile is shown in Fig. 1b for the case of dissolved phenytoin. The transition from the swollen to the shrunken state still occurred but was less pronounced overall. The equilibrium thickness at 20 °C was just 225 nm. Again, heating led to a thickness reduction and at 50 °C the layer was collapsed into its initial, dry thickness. As phenytoin is poorly soluble in the buffer solution, the solvation ability of the buffer towards the polymer coils is reduced when phenytoin is present (similar to the salting-out of macromolecules). Thus, the poorer solvent quality results in less expansion as solvent contact is unfavorable. In addition, when phenytoin is present in the medium, the transition from swollen to shrunken occurs at a lower LCST: 22 °C instead of 29 °C. This means that the hydrogel needs less energy to remove the solution from within its meshes, hinting that the solvation forces are strongly altered.
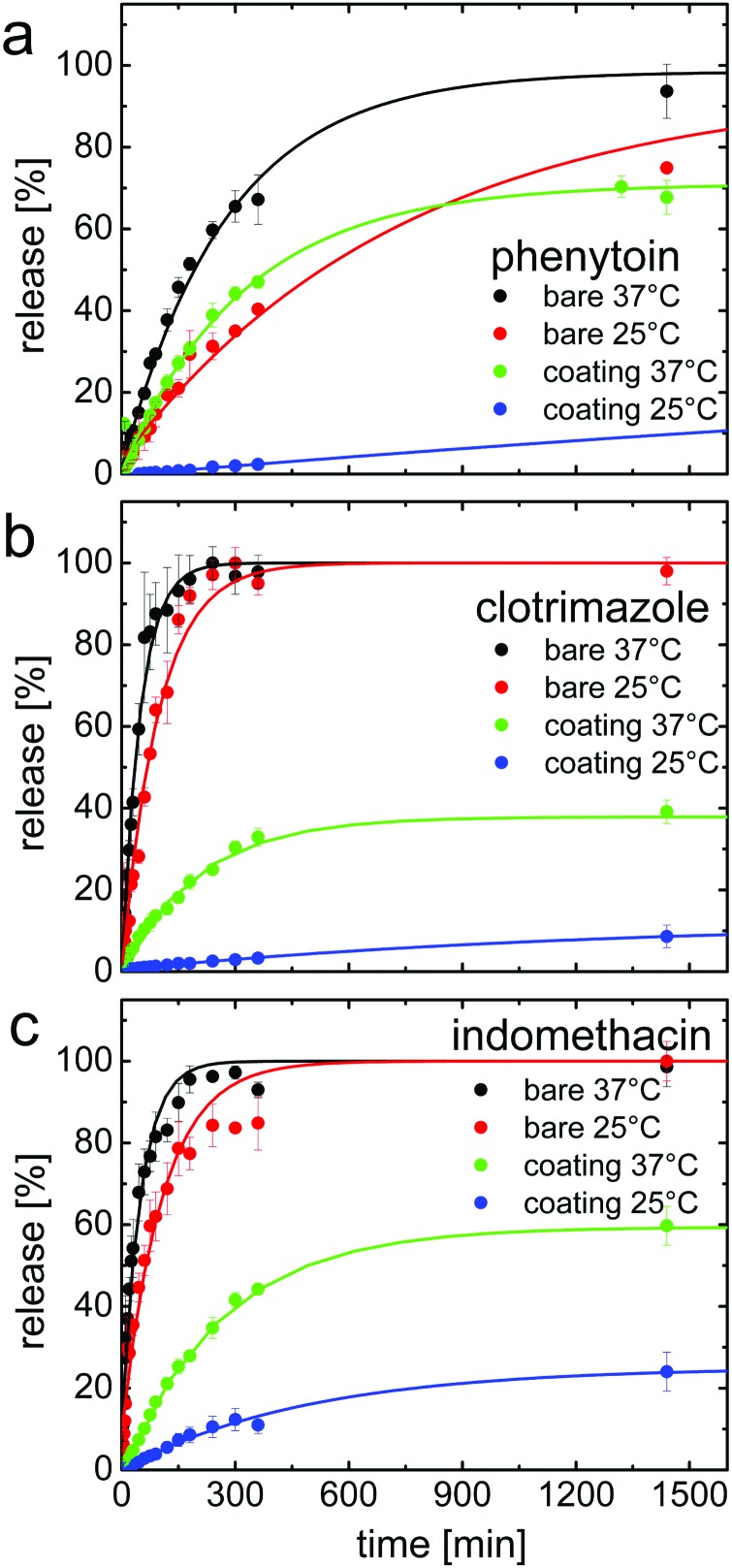
Commonly, hydrogels release solutes following the free volume theory: the solutes can permeate the expanded polymer network much faster as the increased free volume in the matrix provides more diffusion paths through the system. To test this assumption, we performed dissolution experiments at two different temperatures in NaH2PO4 buffer, adjusted to pH = 3. In Fig. 2, the results for phenytoin, clotrimazole and indomethacin are summarized. The acidic dissolution media and the temperature of 37 °C were chosen to mimic the human digestive tract. The second dissolution temperature, 25 °C, was chosen to be below the lower critical solution temperature (LCST) of our hydrogel coating (i.e. the polymer is in the highly swollen state). Even though this temperature is not physiological, it gives a good insight into the thermo-responsive behavior of the coating and how it affects the drug release.
Fig. 2. Drug release profiles of phenytoin (a), clotrimazole (b) and indomethacin (c) all measured either at 25 °C and 37 °C in NaH2PO4 buffer (pH = 3). For each drug, data of bare and of coated samples (200 nm) of p(NIPAAm-co-DEGDVE) are shown. Data points represent experimental data with standard deviation. Full lines represent fits with the model in eqn (1). The different graphs share a common x-axis.
For the bare phenytoin samples, i.e. without coating, a steady release behavior is noted for the thin films (see Fig. 2a, black and red curves), with the release rate gradually declining after about 60% of phenytoin has been released from the surface. For both temperatures tested (25 °C and 37 °C), about 20% of the drug load was dissolved within the first 60 minutes. After this initial stage, the release at 37 °C proceeded with a steeper slope. After 400 min, about 50% was released at 25 °C and more than 60% at the elevated temperatures. Measurements taken after 1400 minutes show that full release was achieved at both temperatures. It should be noted, that the release profile can be changed when the morphology18 or the polymorph is changed,15,19 but to identify the impact of the coating, it is important to generate reproducible drug films of identical properties, which was the focus of this study.
The phenytoin encapsulated by a p(NIPAAm-co-DEGDVE) layer instead was released with a strong delay. In particular, at 25 °C only negligible drug release occurred within the first 180 minutes and after 360 minutes, less than five percent had been released. After 1400 minutes, about a quarter of the total mass had been liberated. This is surprising as ellipsometric measurements tell that the layer is in its swollen state at this temperature while the layer collapses at 37 °C. At increased temperatures a much faster release behavior is noted, similar to that of bare phenytoin at 25 °C. Within 1400 minutes, 70% had been released. At this stage little release occurs, meaning that some phenytoin will likely remain entrapped under or within the polymer network.
A similar behavior was displayed by the drugs clotrimazole and indomethacin but with differences in the time scales. This is in part attributed to the solid state of these drugs. During sample preparation, both clotrimazole and indomethacin remained amorphous while phenytoin rapidly crystallized. The dissolution of an amorphous layer is in general faster since there is less energy required for molecules to transit in the solvated state from the solid–liquid interface.9 The impact of the crystalline state on the indomethacine release is shown in ref. 20. This along with differences in the intrinsic solubilities causes a significantly faster release for both pharmaceuticals when compared to phenytoin under the same experimental conditions. Further, drug–substrate interactions as well as the wettability of the different systems might vary.
The encapsulation resulted also in a retarded release behavior of clotrimazole and indomethacin below the LCST of the hydrogels. For clotrimazole, 55% of the drug was released at 37 °C and only 10% at 25 °C. The indomethacin, instead was released with a higher slope at 37 °C but the total amount released after 1400 minutes was the same as at 25 °C, differently from the case of the other two drugs investigated.
To evaluate the release curves in more detail, mathematical models are fitted to the experimental data. Two different models are utilized which provide some insight into the differences of the samples investigated. For many of the more sophisticated models described in the literature, a detailed parametrization of the polymer system is required (e.g. determination of the molecular weight). Such detailed analysis of iCVD polymers is often difficult or outright impossible because of the small quantity of polymer thin films produced. In addition, these models are mostly aimed at standard dissolution experiments, i.e. using standard dosage forms such as tablets or capsules, and not drug thin films as in this case. Therefore instead of a too elaborated data analysis, simple models such as the one by Korsmeyer and Peppas were used to obtain an idea about differences in the transport mechanism. The Korsmeyer and Peppas model well describes the release behavior up to a fractional release of about 60%. Along with the Korsmeyer–Peppas model, we use a simplified exponential model, which is based on analytic description of the release behavior of the slab geometry present in the current study. This model describes the time-dependence of the fractional release Mt/M∞ by
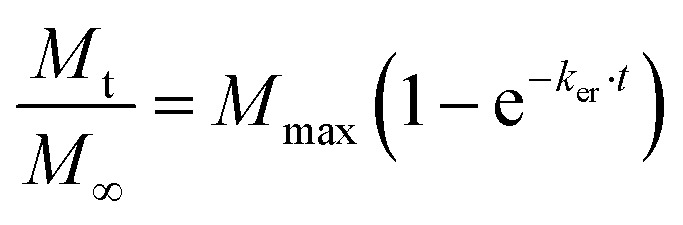 |
1 |
Here, ker is an effective release constant and Mmax estimates the maximum amount of drug release from the system under investigation. Fits to the experimental data from Fig. 2 are summarized in Table 1. The fits and our experimental data are in reasonable agreement with the coefficient of determination R2, being above 0.97. The Mmax values vary according to the maximum amount being released, as evident from the curves in Fig. 2. This suggests that in the presence of the polymer encapsulation, part of the drug remains entrapped and cannot be released from the system, or at least it would take much longer.
Table 1. Summary of the dissolution data from the various samples and the modeling according to eqn (1) and (2) with Mmax being the estimated maximum release, ker being the effective release constant, R2 being the coefficient of determination for the fit with model 1 and n being the exponent of the fits using eqn (2).
| Material | Coating | T [°C] | M max [%/100] | k er [103 min–1] | R 2 | n (Kors. Peppa) |
| Phen | – | 25 | 1.00 | 1.37 | 0.97 | 0.877 |
| – | 37 | 1.00 | 3.64 | 0.99 | 0.948 | |
| + | 25 | 0.43 | 0.18 | 0.99 | 1.348 | |
| + | 37 | 0.71 | 3.09 | 0.97 | 1.063 | |
| Clot | – | 25 | 1.00 | 10.42 | 0.98 | 0.828 |
| – | 37 | 1.00 | 20.41 | 0.99 | 0.950 | |
| + | 25 | 0.12 | 0.79 | 1.00 | 0.471 | |
| + | 37 | 0.38 | 4.88 | 1.00 | 1.386 | |
| Indo | – | 25 | 1.00 | 9.35 | 0.97 | 0.870 |
| – | 37 | 1.00 | 20.43 | 0.98 | 0.853 | |
| + | 25 | 0.25 | 2.07 | 1.00 | 1.067 | |
| + | 37 | 0.59 | 3.79 | 1.00 | 1.108 | |
| Indo pH 7 | – | 25 | Burst release | |||
| – | 37 | Burst release | ||||
| + | 25 | 0.73 | 24.40 | 0.99 | 0.685 | |
| + | 37 | 0.28 | 99.05 | 0.97 | 0.717 | |
The release constants show that the amorphous drug films (i.e. clotrimazole and indomethacin) released almost twice as fast at 37 °C than at 25 °C. For the crystalline sample, i.e. phenytoin, the temperature dependence is even more pronounced, with the ratio k37°C/k25°C being 2.65.
According to Korsmeyer and Peppas, the drug release from a polymeric system can be described by the simple formula:
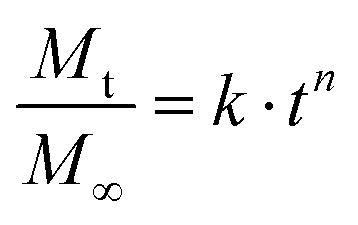 |
2 |
where k is another release constant and n is the release exponent. The exponent n allows for an estimate of the transport mechanism with which liberation from the sample into the surrounding dissolution medium occurs. The meaning of the exponent depends on the shape of the system.21 For a thin film, n equal to 0.5 is attributed to Fickian diffusion, while 0.5 < n < 1 occurs for non-Fickian diffusion. An exponent of 1 corresponds to case II transport or zero order release and for values greater than 1, super case II transport is present. As the exact release mechanism is unknown, here we also fit the release exponent to obtain some idea about the release mechanism. The exponent values n are reported in Table 1. For the bare drug layers, this exponent varies between 0.85 and 1. This value is well within the anomalous transport i.e. within the release process the diffusion is non-linear with time, differently from typical conventional diffusion. Similarly, the evaluation of the coated samples shows that the exponent increased to 1 and above. Such exponential values are a strong indication that a zero order release is involved, whereby a zero-order release describes the capability to deliver drug at a constant rate, thus providing a predictable bioavailability status over a long time.22 In comparison, the clotrimazole sample at 25 °C shows a much-reduced exponent compared to all others, therefore this is the only sample behaving according to Fickian diffusion.
Considering that in a swollen layer there is more free volume for drug diffusion, one would have expected to obtain faster release at lower temperatures for our encapsulated layers. The results unexpectedly show, however, that the release of all drugs is faster at higher temperatures when measured at pH = 3. One possible explanation could be related to the coil-to-globule transition: below the LCST, the polar amide groups are exposed towards the interphase with the environment, leading to the formation of H-bonding with water molecules and swelling. Above the LCST, the configuration changes and the apolar isopropyl groups are exposed at the interphase with the environment instead. Since all molecules have polar hydrophilic sites, it is likely that also the drug molecules form H-bonds with the hydrogel and remain entrapped in the network without dissolving into the free dissolution medium. Furthermore, the increase in temperature means that the drug release from the bare film is at least two times faster, since diffusion increases with temperature. Both effects might very well overcompensate for a poorer permeability through a collapsed hydrogel.
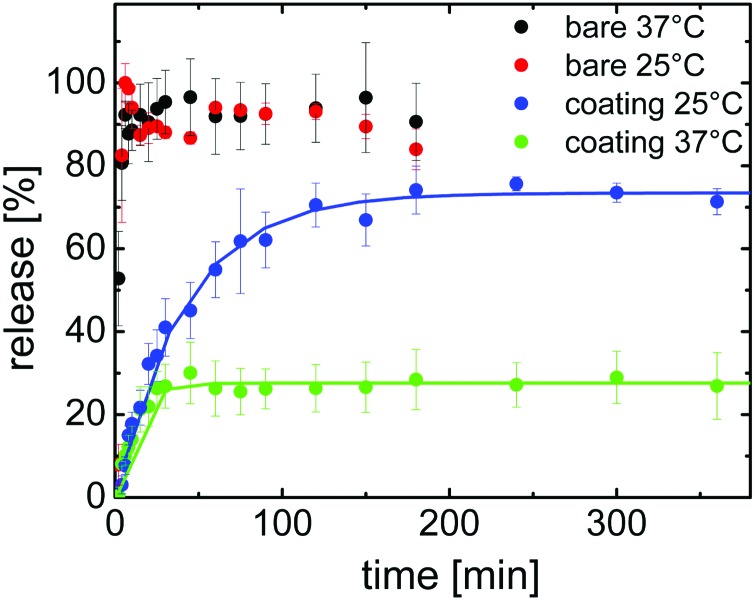
It is worth noticing that among the three model drugs, indomethacin is the one with the lowest logarithmic acid dissociation constant, pKa = 4.5. Therefore, in our acidic environment the indomethacin has the carboxylic group in its undissociated state. At pH = 7, instead, the indomethacin is in its dissociated form which is more soluble in water. Therefore we studied also the release at neutral pH, and the results are shown in Fig. 3. The dissolution studies of the bare indomethacin at pH = 7 show a very rapid release so that 100% of the drug is burst into the surrounding media within the first 5 minutes. A change in the temperature did not change the release behavior of the bare drug significantly, at least within the limit of the used time resolution for these experiments (2 minutes).
Fig. 3. Drug release at different temperatures for indomethacin bare and encapsulated within 200 nm of p(NIPAAm-co-DEGDVE). The dissolution medium was NaH2PO4 at pH = 7.
When the indomethacin film is encapsulated into the p(NIPAAM-co-DEGDVE), the temperature-dependence of the release changes drastically at pH = 7, especially when compared to the behavior at pH = 3 (cf. 2 and 3, but please mind the different time scaling). Hereby, at 37 °C, the dissolution of the drug seems to be very quick with a corresponding release exponent k = 99.05 × 103 min–1. Surprisingly, the maximum amount of indomethacin released at pH = 7 from under the coating is just 28% and is reached already after 30 minutes. At 37 °C the layer is collapsed, which means the free volume for molecular diffusion is small.
At 25 °C the polymer coating layer is swollen and the release is a bit quicker in terms of mass transfer over time compared to that at 37 °C. The maximum amount able to escape the dosage form is 73% which is about 2.6 more compared to indomethacin samples at 37 °C. It should be kept in mind that the difference in pH is not expected to influence the secondary amino group of NIPAAm, since both pH values in the experiments (pH = 3 and 7) are below the pKb typical of this functional group and also swelling experiments did not show any significant differences (data not shown). So the much faster release can be explained by the larger free volume formed in the swollen state.
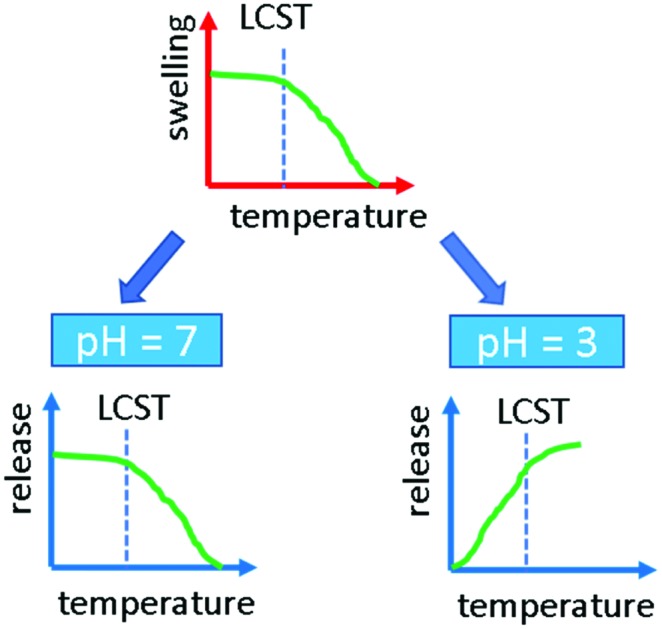
The question arises, why at pH = 7 the larger free volume actually provides better molecular transport into the dissolution medium while at pH = 3 the situation seems to be reversed (see Fig. 4). First of all, the solubility of indomethacin changes drastically meaning that dissolution at the boundary layer is strongly enhanced. Higher solubility also means that the diffusion behavior changes as molecule–molecule interactions are less problematic. This can be followed by the change of the exponent using the Korsmeyer–Peppa model, which decreases well below 1 for pH = 7 while it is above 1 at pH = 3. The deprotonation of indomethacin at pH = 7 might also alter the interaction with the NIPAAm so that the effect of vacant volume sites created upon swelling, i.e. at low temperature, is now more dominant than the temperature and H-bonding effects on diffusion. At high temperatures and neutral pH, upon chains collapsing, the number of free paths towards the dissolution medium reduces and more drug material remains entrapped.
Fig. 4. Scheme of the behavior of indomethacin as a function of temperature and pH.
Conclusions
The objective of this work was to demonstrate which conditions (pH, drug solubility) affect the temperature-dependent release of different drugs encapsulated by a hydrogel layer of p(NIPAAM-co-DEGDVE) deposited by iCVD. This polymer undergoes a fast coil-to-globule transition at the low critical solution temperature, which was estimated around 29 °C or 22 °C in the presence of a drug. Above these temperatures, the hydrogel is in its shrunken, collapsed state in which the apolar isopropyl groups are exposed towards the interface with the environment. Below the LCST, the conformation of the polymer changes, the polar groups are exposed towards the interface and the polymer can adsorb water in its meshes.
At low pH, all model drugs were released faster above the LCST, when the polymer is in its collapsed state. This was ascribed to the formation of H-bonds between the polymer and the drugs. Such strong interaction is enhanced when the polar groups are exposed towards the interphase, e.g. below the LCST. The bonding between the rather hydrophobic drug molecules chosen and the polymer trapped the drugs, hindering their release. This is also reflected by the reduction of swelling capabilities, when there is some phenytoin present in the buffer solution. At neutral pH, the release behavior for indomethacin was reversed: a faster release was recorded below the LCST, i.e. when the polymer is in its swollen state. At this pH the indomethacin is in its deprotonated form and therefore less prone to form H-bonding with the polymer. In this case, thus, the release was faster when more free volume was available, i.e. in the shrunken state, below the LCST.
The findings in here show, that the molecular species as well as the polymer itself have a great impact on the dissolution behavior. This provides clarity to the somehow contradicting literature results previously observed from various research groups dealing with p(NIPAAM). In fact, it is not only the LCST but also the polymer–drug interaction that governs the drug release, which might also be used to gain further control over responsive behavior.
Conflicts of interest
There are no conflicts to declare.
Acknowledgments
The authors want to thank the BioTechMed Graz. Part of the research was supported by funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement 715403).
References
- Perrotta A., Werzer O., Coclite A. M. Adv. Eng. Mater. 2017;20:1700639. [Google Scholar]
- Mura S., Nicolas J., Couvreur P. Nat. Mater. 2013;12:991. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- Alf M. E., Hatton T. A., Gleason K. K. Polymer. 2011;52:4429. doi: 10.1021/la201935r. [DOI] [PubMed] [Google Scholar]
- Karaman M., Çabuk N. Thin Solid Films. 2012;520:6484. [Google Scholar]
- Unger K., Salzmann P., Masciullo C., Cecchini M., Koller G., Coclite A. M. ACS Appl. Mater. Interfaces. 2017;9:17408. doi: 10.1021/acsami.7b01527. [DOI] [PubMed] [Google Scholar]
- Stuart M. A., Huck W. T., Genzer J., Muller M., Ober C., Stamm M., Sukhorukov G. B., Szleifer I., Tsukruk V. V., Urban M., Winnik F., Zauscher S., Luzinov I., Minko S. Nat. Mater. 2010;9:101. doi: 10.1038/nmat2614. [DOI] [PubMed] [Google Scholar]
- Alvarez-Lorenzo C., Concheiro A. Chem. Commun. 2014;50:7743. doi: 10.1039/c4cc01429d. [DOI] [PubMed] [Google Scholar]
- Coclite A. M., Howden R. M., Borrelli D. C., Petruczok C. D., Yang R., Yague J. L., Ugur A., Chen N., Lee S., Jo W. J., Liu A., Wang X., Gleason K. K. Adv. Mater. 2013;25:5392. doi: 10.1002/adma.201301878. [DOI] [PubMed] [Google Scholar]
- Christian P., Ehmann H. M., Coclite A. M., Werzer O. ACS Appl. Mater. Interfaces. 2016;8:21177. doi: 10.1021/acsami.6b06015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason K. K., CVD Polymers: Fabrication of Organic Surfaces and Devices, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2015. [Google Scholar]
- McInnes S. J., Szili E. J., Al-Bataineh S. A., Vasani R. B., Xu J., Alf M. E., Gleason K. K., Short R. D., Voelcker N. H. Langmuir. 2016;32:301. doi: 10.1021/acs.langmuir.5b03794. [DOI] [PubMed] [Google Scholar]
- Alf M. E., Hatton T. A., Gleason K. K. Langmuir. 2011;27:10691. doi: 10.1021/la201935r. [DOI] [PubMed] [Google Scholar]
- Zhang X. Z., Wu D. Q., Chu C. C. Biomaterials. 2004;25:3793. doi: 10.1016/j.biomaterials.2003.10.065. [DOI] [PubMed] [Google Scholar]
- Bae Y. H., Okano T., Hsu R., Kim S. W. Die Makromol. Chemie. 1987;8:481. [Google Scholar]
- Reischl D., Röthel C., Christian P., Roblegg E., Ehmann H. M. A., Salzmann I., Werzer O. Cryst. Growth Des. 2015;15:4687. doi: 10.1021/acs.cgd.5b01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranacher C., Resel R., Moni P., Cermenek B., Hacker V., Coclite A. M. Macromolecules. 2015;48:6177. [Google Scholar]
- Salzmann P., Perrotta A., Coclite A. M. ACS Appl. Mater. Interfaces. 2018;10:6636. doi: 10.1021/acsami.7b18878. [DOI] [PubMed] [Google Scholar]
- Ehmann H. M. A., Baumgartner R., Reischl D., Roblegg E., Zimmer A., Resel R., Werzer O. Cryst. Growth Des. 2015;15:326. doi: 10.1021/cg501391j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werzer O., Baumgartner R., Zawodzki M., Roblegg E. Mol. Pharmacol. 2014;11:610. doi: 10.1021/mp4006479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian P., Tumphart S., Ehmann H. M. A., Riegler H., Coclite A. M., Werzer O. Sci. Rep. 2018;8:7134. doi: 10.1038/s41598-018-24238-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbinatto F. M., de Castro A. D., Evangelista R. C., Cury B. S. F. Asian J. Pharm. Sci. 2014;9:27. [Google Scholar]
- Ofokansi K. C., Kenechukwu F. C. ISRN Pharm. 2013;2013:1. doi: 10.1155/2013/838403. [DOI] [PMC free article] [PubMed] [Google Scholar]