Abstract
The prevalence and incidence of nontuberculous mycobacterial (NTM) infections increased in South Korea from 2007 to 2016. Annual prevalence of NTM infection increased to 39.6 cases/100,000 population in 2016 and annual incidence to 19.0 cases/100,000 population. Overall prevalence for the study period was higher in the elderly, in females, and in cities.
Keywords: nontuberculous mycobacteria, epidemiology, bacteria, mycobacteria, South Korea, tuberculosis and other mycobacteria
The prevalence and incidence of nontuberculous mycobacterial (NTM) infection are increasing worldwide (1), and it is important to characterize the distribution of NTM infection by demographics or ecologic region to optimize disease control. However, data vary widely by study, and the epidemiology of NTM infection differs among countries. Although the number of patients with NTM infection has increased in South Korea (2), representative nationwide population-based epidemiologic data on the extent and distribution of NTM infection are lacking. We evaluated the prevalence, incidence, and spatial distribution of NTM infection in South Korea over a 10-year period, using national health insurance data.
The Study
We based our study on the Health Insurance Review and Assessment Service database, which includes universal health insurance claim data in South Korea. Approximately 97% of the population (≈52 million persons) is included in the system (3). We obtained demographic and medical data from the database for 2007–2016. We defined NTM infection as the presence of a diagnostic code associated with NTM infection (International Classification of Diseases, 10th ed., code A31) as either a primary or secondary diagnosis (4) and claims data for acid-fast bacilli smears or mycobacterial culture.
We defined annual prevalence as the number of all patients with NTM infection alive during each year and annual incidence as the number of newly diagnosed NTM infections occurring each year after excluding patients who were diagnosed in a previous study period (e.g., patients diagnosed during 2007–2015 were excluded from the estimate of the 2016 incidence rate). We first calculated the crude prevalence rate and incidence rate using each year’s total population as the denominator. To adjust for different population structures between years, we calculated the age- and sex-standardized annual prevalence rate and incidence rate of NTM disease using the 2005 population as a standard population, based on the Statistics Korea method (http://kostat.go.kr/portal/eng/index.action). First, we calculated the prevalence and incidence rates for age and sex groups by stratifying all NTM cases into the groups and dividing by the group-specific populations in each year. Then, we multiplied each year’s group-specific rates and the group-specific populations in 2005, combined the multiplied values, and divided by the 2005 total population. We calculated standardized overall period prevalence of NTM infection by age, sex, and administrative divisions using the population in the middle of the study period. We conducted statistical analyses using SAS version 9.4 (SAS Institute, http://www.sas.com). Because we used deidentified data in the study, institutional review board approval and patient consent were not required.
A total of 33,974 cases of NTM infection were identified during 2007–2016. The age- and sex-adjusted annual prevalence of NTM infection increased by more than 5-fold, from 6.7 cases/100,000 population in 2007 to 39.6 cases/100,000 population in 2016 (Figure 1). The age- and sex-adjusted annual incidence of NTM infection more than tripled, from 6.0 cases/100,000 population/year in 2008 to 19.0 cases/100,000 population/year in 2016. The overall period prevalence of NTM infection increased with patient age (Figure 2, panel A): prevalence was lowest at <19 years of age (1.7 cases/100,000 population), increased dramatically after 50 years of age, and peaked in those >70 years of age (223.0 cases/100,000 population). The overall period prevalence of NTM infection was higher among females in all age groups, except for those >70 years of age (Figure 2, panel B).
Figure 1.
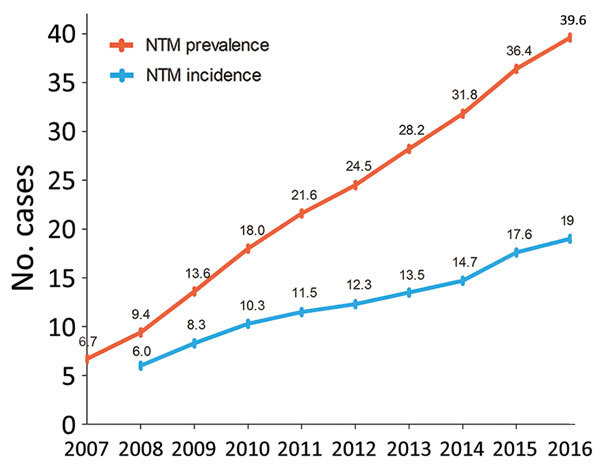
Annual prevalence (total no. cases/100,000 population) and incidence (no. new cases/100,000/year) of nontuberculous mycobacterial infection, adjusted for age and sex, South Korea, 2007–2016.
Figure 2.
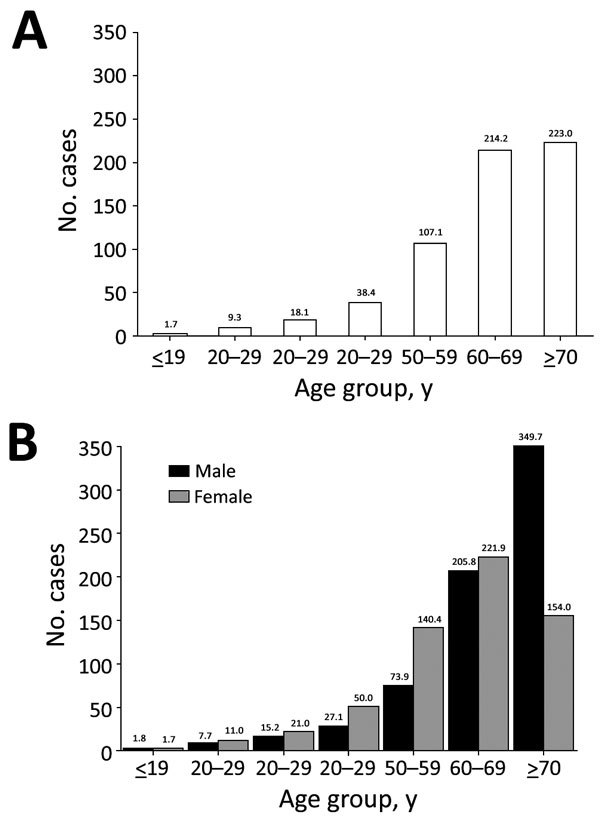
Overall period prevalence (total no. cases/100,000 population) of nontuberculous mycobacterial infection, by age group (A) and by age group and sex (B), South Korea, 2007–2016.
In South Korea, the overall period prevalence of NTM infection tended to be higher in metropolitan cities, which had greater population densities and higher income levels, than in the provinces (Table 1). The overall period prevalence exceeded 100 cases/100,000 population in most metropolitan cities (5/7, 71.4%) but did not exceed that rate in most provinces (7/9, 77.8%). The prevalence was highest in Gwangju and in Seoul, the capital of South Korea, both of which had >250 cases/100,000 population. Prevalence was lowest in Cheju, the largest and southernmost island (35.8 cases/100,000 population) (Appendix Figure).
Table 1. Overall prevalence of nontuberculous mycobacterial infections by administrative division, adjusted for age and sex, South Korea, 2007–2016.
| District | No. infections | No. cases/100,000 population |
|---|---|---|
| Metropolitan city | ||
| Seoul | 13,671 | 254.5 |
| Busan | 2,055 | 102.6 |
| Daejeon | 1,384 | 194.4 |
| Gwangju | 2,121 | 302.0 |
| Daegu | 1,642 | 119.9 |
| Incheon | 1,169 | 86.3 |
| Ulsan |
401 |
81.5 |
| Province | ||
| Gyeonggi-do | 6,195 | 110.2 |
| Gangwon-do | 1,107 | 108.9 |
| Jeollabuk-do | 1,213 | 98.4 |
| Jeollanam-do | 470 | 35.1 |
| Gyeongsangbuk-do | 497 | 26.8 |
| Gyeongsangnam-do | 1,201 | 66.0 |
| Chungcheongbuk-do | 285 | 30.6 |
| Chungcheongnam-do | 444 | 35.2 |
| Cheju-do (island) | 119 | 35.8 |
Analysis of age and underlying conditions indicated that most case-patients (83.4%) were >50 years of age (Table 2). The most frequent underlying respiratory condition was asthma (53.0%), followed by bronchiectasis (43.6%) and chronic obstructive pulmonary disease (COPD) (32.3%). Lung cancer was the most common malignancy (6.3%). Depressive disorder was noted in 23.1% of case-patients.
Table 2. Sex, age, and underlying conditions among patients with nontuberculous mycobacterial infection, South Korea, 2007–2016*.
| Category | No. (%) infections, n = 33,974 |
|---|---|
| Sex | |
| F | 18,555 (54.6) |
| M |
15,419 (45.4) |
| Age group, y | |
| <19 | 197 (0.6) |
| 20–29 | 622 (1.8) |
| 30–39 | 1,479 (4.4) |
| 40–49 | 3,341 (9.8) |
| 50–59 | 7,880 (23.2) |
| 60–69 | 8,961 (26.4) |
|
>70 |
11,494 (33.8) |
| Underlying condition | |
| Asthma | 18,003 (53.0) |
| Bronchiectasis | 14,815 (43.6) |
| COPD | 10,969 (32.3) |
| Interstitial pulmonary diseases | 1,832 (5.4) |
| Myocardial infarction | 1,016 (3.0) |
| Congestive heart failure | 3,581 (10.5) |
| Cerebrovascular disease | 7,789 (22.9) |
| Cancer | 7,808 (23.0) |
| Lung cancer | 2,124 (6.3) |
| Stomach cancer | 1,336 (3.9) |
| Colon cancer | 975 (2.9) |
| Liver cancer | 661 (2.0) |
| Prostate cancer | 606 (1.8) |
| Breast cancer | 577 (1.7) |
| Uterine cervical cancer | 126 (0.4) |
| Thyroid cancer | 673 (2.0) |
| Liver disease | 15,218 (44.8) |
| Renal disease | 1,391 (4.1) |
| Diabetes mellitus | 11,010 (32.4) |
| Depressive disorder | 7,857 (23.1) |
*Underlying conditions are listed based on codes from the International Classification of Diseases, 10th edition. COPD, chronic obstructive pulmonary disease.
Conclusions
We identified a remarkable increase in the prevalence and incidence of NTM infection in South Korea from 2007 to 2016. The overall period prevalence increased with patient age and was higher in female patients in most age groups. These trends are consistent with previous studies from Europe (4), the United States (5,6), and Japan (7,8). A representative US study that analyzed Medicare beneficiaries during 1997–2007 revealed that the prevalence of NTM disease increased from 20 to 47 cases/100,000 population and the prevalence was higher among women (4). A study conducted in Germany reported an increasing prevalence of NTM disease, from 2.3 cases/100,000 population in 2009 to 3.3 cases/100,000 population in 2014 (4). Studies in Japan, which is geographically close to South Korea, showed similar trends, but the rates were higher than those in the United States or Europe. The estimated annual prevalence of NTM disease in Japan in 2005 was 33–65 cases/100,000 population (7), and the incidence in 2015 was 14.7 cases/100,000 population (8), which were similar to our data. These results suggest that NTM infection is increasing globally, especially in East Asia, and that epidemiologic distribution can be affected by region or ethnicity. In particular, some studies have indicated that people in East Asia are vulnerable to NTM infection (9,10).
Another notable finding in our study was the difference in NTM prevalence by administrative division, which indicates that NTM infection is related to population density and the degree of urbanization. Supporting our data, other studies have attributed NTM infection to environmental exposure (9), and recent studies in the United States revealed that areas with high risk for NTM infection had higher population densities or higher education and income levels (9,11). In South Korea, the population density is highest in metropolitan cities. These data suggest the importance of epidemiologic surveillance in understanding NTM infection.
We frequently observed airway diseases in NTM infection in our study. A US study also reported COPD in 41% and bronchiectasis in 37% of NTM patients (10), whereas a study in Germany noted COPD or emphysema in 62%–79% of patients (4). One study reported mental disorders as concurrent NTM-related conditions (12). In our study, depression was also identified in NTM, which might be explained by the burden of COPD (13). These data suggest that an assessment of underlying disease is needed in managing NTM infection.
Our study had some limitations. First, because we used diagnosis codes, the distinction between pulmonary and other diseases is unclear, and we could not confirm whether the patients completely met the diagnostic criteria of NTM infection. Second, because we only have data for 2007–2016, we could not exclude patients who had received diagnoses before 2007 when estimating the annual incidence rate. Finally, our definition of NTM infection on the basis of the International Classification of Diseases code may have underestimated the true disease prevalence (14,15) because prevalence could be affected by loss to follow-up or death during the study period.
In conclusion, we report a substantial increasing trend in the prevalence and incidence of NTM infection in South Korea during 2007–2016 and evaluated regional variations. These data facilitate a better understanding of the epidemiologic trends of NTM infection globally.
Additional information about nontuberculous mycobacterial infection in South Korea.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Ministry of Science and ICT (Information and Communication Technologies), South Korea (grant no. NRF2018R1C1B6001708; W. Myung), and by the Basic Science Research Program through the NRF of Korea funded by the Ministry of Education, South Korea (grant no. NRF-2018R1D1A1B07049034; H. Lee).
Dr. Lee is a research assistant professor at the Institute of Health and Environment at Seoul National University, South Korea. Her work focuses on health impact assessment of environmental risk factors, including air pollutants and climatic factors.
Footnotes
Suggested citation for this article: Lee H, Myung W, Koh W-J, Moon SM, Jhun BW. Epidemiology of nontuberculous mycobacterial infection, South Korea, 2007–2016. Emerg Infect Dis. 2019 Mar [date cited]. https://doi.org/10.3201/eid2503.181597
These authors contributed equally to this article.
References
- 1.Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med. 2015;36:13–34. 10.1016/j.ccm.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ko RE, Moon SM, Ahn S, Jhun BW, Jeon K, Kwon OJ, et al. Changing epidemiology of nontuberculous mycobacterial lung diseases in a tertiary referral hospital in Korea between 2001 and 2015. J Korean Med Sci. 2018;33:e65. 10.3346/jkms.2018.33.e65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheol Seong S, Kim YY, Khang YH, Heon Park J, Kang HJ, Lee H, et al. Data Resource Profile: The National Health Information Database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017;46:799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ringshausen FC, Wagner D, de Roux A, Diel R, Hohmann D, Hickstein L, et al. Prevalence of nontuberculous mycobacterial pulmonary disease, Germany, 2009–2014. Emerg Infect Dis. 2016;22:1102–5. 10.3201/eid2206.151642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185:881–6. 10.1164/rccm.201111-2016OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henkle E, Hedberg K, Schafer S, Novosad S, Winthrop KL. Population-based incidence of pulmonary nontuberculous mycobacterial disease in Oregon 2007 to 2012. Ann Am Thorac Soc. 2015;12:642–7. 10.1513/AnnalsATS.201412-559OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morimoto K, Iwai K, Uchimura K, Okumura M, Yoshiyama T, Yoshimori K, et al. A steady increase in nontuberculous mycobacteriosis mortality and estimated prevalence in Japan. Ann Am Thorac Soc. 2014;11:1–8. 10.1513/AnnalsATS.201303-067OC [DOI] [PubMed] [Google Scholar]
- 8.Namkoong H, Kurashima A, Morimoto K, Hoshino Y, Hasegawa N, Ato M, et al. Epidemiology of pulmonary nontuberculous mycobacterial disease, Japan. Emerg Infect Dis. 2016;22:1116–7. 10.3201/eid2206.151086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adjemian J, Olivier KN, Seitz AE, Falkinham JO III, Holland SM, Prevots DR. Spatial clusters of nontuberculous mycobacterial lung disease in the United States. Am J Respir Crit Care Med. 2012;186:553–8. 10.1164/rccm.201205-0913OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adjemian J, Frankland TB, Daida YG, Honda JR, Olivier KN, Zelazny A, et al. Epidemiology of nontuberculous mycobacterial lung disease and tuberculosis, Hawaii, USA. Emerg Infect Dis. 2017;23:439–47. 10.3201/eid2303.161827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olivier KN, Weber DJ, Wallace RJ Jr, Faiz AR, Lee JH, Zhang Y, et al. ; Nontuberculous Mycobacteria in Cystic Fibrosis Study Group. Nontuberculous mycobacteria. I: multicenter prevalence study in cystic fibrosis. Am J Respir Crit Care Med. 2003;167:828–34. 10.1164/rccm.200207-678OC [DOI] [PubMed] [Google Scholar]
- 12.Mirsaeidi M, Machado RF, Garcia JG, Schraufnagel DE. Nontuberculous mycobacterial disease mortality in the United States, 1999-2010: a population-based comparative study. PLoS One. 2014;9:e91879. 10.1371/journal.pone.0091879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev. 2014;23:345–9. 10.1183/09059180.00007813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182:970–6. 10.1164/rccm.201002-0310OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winthrop KL, Baxter R, Liu L, Varley CD, Curtis JR, Baddley JW, et al. Mycobacterial diseases and antitumour necrosis factor therapy in USA. Ann Rheum Dis. 2013;72:37–42. 10.1136/annrheumdis-2011-200690 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information about nontuberculous mycobacterial infection in South Korea.


