Abstract
Background: The incidence of pregnancy-associated cancer (PAC) is expected to increase as more women delay childbearing until later ages. However, information on frequency and incidence of PAC is scarce in the United States.
Methods: We identified pregnancies among women aged 10–54 years during 2001–2013 from five U.S. health plans participating in the Cancer Research Network (CRN) and the Medication Exposure in Pregnancy Risk Evaluation Program (MEPREP). We extracted information from the health plans' administrative claims and electronic health record databases, tumor registries, and infants' birth certificate files to estimate the frequency and incidence of PAC, defined as cancer diagnosed during pregnancy and up to 1 year postpartum.
Results: We identified 846 PAC events among 775,709 pregnancies from 2001 to 2013. The overall incidence estimate was 109.1 (95% confidence interval [CI] = 101.8–116.7) per 100,000 pregnancies. There was an increase in the incidence between 2002 and 2012 (the period during which complete data were available), from 75.0 (95% CI = 54.9–100.0) per 100,000 pregnancies in 2002 to 138.5 (95% CI = 109.1–173.3) per 100,000 pregnancies in 2012. The most common invasive cancers diagnosed were breast (n = 208, 24.6%), thyroid (n = 168, 19.9%), melanoma (n = 93, 11.0%), hematologic (n = 87, 10.3%), and cervix/uterus (n = 74, 8.7%).
Conclusions: Our study provides contemporary incidence estimates of PAC from a population-based cohort of U.S. women. These estimates provide the data needed to help develop clinical and public health policies aimed at diagnosing PAC at an early stage and initiating appropriate therapeutic interventions in a timely manner.
Keywords: : cancer, pregnancy, pregnancy-associated cancer
Introduction
As more women are delaying childbearing until later ages, the incidence of cancer coinciding with pregnancy is expected to increase. Although it is generally recognized that pregnancy-associated cancer (PAC) is a growing public health problem,1–3 information on its frequency and incidence is scarce and outdated. The published incidence estimates of PAC vary from 17 to 137 per 100,000 pregnancies4–10 and include only one population-based study from the United States, a study of women who gave birth in California.7 More contemporary incidence estimates are needed to help guide the development of clinical and public health policies aimed at diagnosing cancers at an early stage and initiating appropriate therapeutic interventions in a timely manner. In this study, we estimated the frequency and incidence of PAC, defined as cancer diagnosed during pregnancy and up to 1 year postpartum,5,7,9,10 during 2001–2013 within a large population-based cohort of pregnant women in the United States.
Materials and Methods
Data source and study population
This study used data from five health plans participating in both the Cancer Research Network (CRN)11 and the Medication Exposure in Pregnancy Risk Evaluation Program (MEPREP).12 The CRN is a National Cancer Institute-funded initiative to support and facilitate cancer research based on 14 U.S. not-for-profit health plans and healthcare delivery systems. MEPREP is a collaborative research program between the U.S. Food and Drug Administration and 11 health plan-affiliated research institutions that enables the conduct of multicenter studies of medication use and outcomes in pregnancy. The five participating health plans in this study were Fallon Community Health Plan, Harvard Pilgrim Health Care, HealthPartners, Kaiser Permanente Colorado, and Kaiser Permanente Northern California. Together, these health plans provided care to over 6 million current enrollees within eight states.
To conduct population-based cancer research, each CRN site employs a distributed, standardized data model known as the Virtual Data Warehouse (VDW).13,14 Briefly, the VDW is populated with patient-level data extracted from administrative claims and electronic health record databases. The VDW contains tumor registry, outpatient pharmacy dispensing, and procedure and diagnosis data associated with inpatient and outpatient encounters. The tumor registry file contains data consistent with the North American Association of Central Cancer Registries standards.15 Data are obtained from manual reviews of cancer patients' medical charts by trained abstractors and include coded clinical data associated with inpatient and outpatient events. MEPREP employs a common data model that is almost identical to the one used by CRN and includes two additional files—a mother–baby linkage file and a birth certificate file—created specifically for pregnancy research. Infant birth certificates provide information on sociodemographic, medical, and reproductive factors. Approximately 450,000 pregnancies that ended in live birth were linked to infant birth certificates from 2001 to 2008 in the five participating health plans. Linked birth certificate files were not available for pregnancies that occurred during 2009–2013.
For this study, we linked the VDW data from CRN sites with the mother–baby linkage and birth certificate files from MEPREP. We obtained information on maternal age, calendar year of pregnancy, and pregnancy outcome (live birth, stillbirth, spontaneous abortion, induced abortion, and ectopic pregnancy) (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/jwh) from the health plans' administrative claims data; information on maternal race/ethnicity, education level, marital status, plurality, gravidity, gestational age, and birth weight from the birth certificate file (whenever available); and information on cancer diagnosis and tumor site from the tumor registry file. The source population for the current study included all women with a clinically recognized pregnancy between 2001 and 2013. To be eligible, the women had to be 10–54 years of age at the end of pregnancy and continuously enrolled in the health plan 90 days before and during pregnancy and up to 1 year postpartum.
Identification of pregnancy and trimesters of pregnancy
We identified live-born deliveries, stillbirths, spontaneous abortions, induced abortions, and ectopic pregnancies in the health plan data using previously validated algorithms.16,17 Briefly, the algorithms searched for pregnancies using diagnosis and procedure codes within the health plan data and then assigned a pregnancy outcome type and an end date of pregnancy.
For live-born deliveries for which the last menstrual period (LMP)–based gestational age was available in the birth certificate file, we used the first day of the LMP as the beginning of the first trimester. Trimesters were classified as the first (days 0–89), second (days 90–179), and third (days 180 through the end of pregnancy). If the LMP was missing or had an invalid value, the start date of pregnancy was defined as the delivery date minus the gestational age based on clinical or obstetrical estimates.18
When gestational age information was missing from the birth certificates or if there was not a linked birth certificate, we applied a previously validated claims-based algorithm that used the delivery date and specific diagnosis and procedure codes recorded in the health plan claims data to estimate the trimesters.19,20 The algorithm assumed a start date of pregnancy as delivery date minus 270 days if there was no International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code for preterm birth; as delivery date minus 245 days if there was a code for preterm birth of unspecific gestational age; and as delivery date minus the upper limit of the gestational age range if there was a code for preterm birth with a specified range, for example, delivery date minus 238 days for deliveries with an ICD-9-CM code 765.27 (“33–34 weeks of gestation”).
For all pregnancy episodes other than live births (stillbirths, spontaneous abortions, induced abortions, and ectopic pregnancies), we calculated gestational age based on published estimates and expert consultation.16,17 The start date of pregnancy was the end of pregnancy date minus 210 days for stillbirths, 70 days for spontaneous abortions, 70 days for induced abortions, and 56 days for ectopic pregnancies.
Identification of PAC
We identified all incident invasive cancer cases (except nonmelanoma skin cancer) in the tumor registry file. Topography and morphology for each cancer were coded according to the third edition of the International Classification of Diseases for Oncology (ICD-O-3) (Supplementary Table S2). We defined PAC as cancer diagnosed during pregnancy and up to 1 year postpartum.5,7,9,10 A cancer episode associated with two consecutive pregnancies was counted as a PAC event in both pregnancies (e.g., a cancer diagnosed during the first trimester of one pregnancy also fell within the 1-year postpartum period of a previous pregnancy); however, this was rare (n = 35).
Statistical analyses
We estimated the incidence of PAC per 100,000 pregnancies and the exact 95% confidence intervals (CIs), overall and separately for pregnancies that ended in live birth and non-live birth. We also stratified the analysis by year of pregnancy, maternal age, timing of diagnosis, and cancer site. Twins were counted as one pregnancy episode. We used the Cochran–Armitage test of trends to test for linear trends in the incidence of PAC by year of pregnancy. We excluded years 2001 and 2013 from the trend analysis because complete data before pregnancy, during pregnancy, and up to 1 year postpartum were not available. We assessed the association between maternal age and year of pregnancy using an ANOVA test. We performed all analyses using SAS 9.2 (SAS Institute, Inc., Cary, NC). The study was approved by the institutional review boards at the participating sites and where applicable, the state departments of public health from which we requested tumor or birth certificate files.
Results
We identified 775,709 pregnancies from 2001 to 2013. The highest number of pregnancies occurred in the age group 30–34 years (30%), followed by the age group 25–29 years (24%) (Table 1). There were 846 PAC events among the 775,709 pregnancies. The mean maternal age at the time of PAC diagnosis was 34.1 years and did not change significantly during the study period (p = 0.24). PAC in the age group 30–34 years accounted for 32% of all events, followed by the age group 35–39 years with 26%. Approximately 65% of all cancer-associated pregnancies ended in live birth, a proportion similar to the proportion observed in pregnancies not associated with cancer (66%).
Table 1.
Characteristics of Pregnancies Associated with Cancer Compared with Pregnancies Not Associated with Cancer Among U.S. Women Aged 10–54 Years Between 2001 and 2013
| Characteristic | Pregnancies associated with cancer | Pregnancies not associated with cancer | Total pregnancies | ||
|---|---|---|---|---|---|
| All | Live births | Non-live births | |||
| N (%)a | N (%)a | N (%)a | N (%)a | N (%)a | |
| Total | 846 (100.0) | 549 (100.0) | 297 (100.0) | 774,863 (100.0) | 775,709 (100.0) |
| Maternal age (years) | |||||
| <20 | 16 (1.9) | 5 (0.9) | 11 (3.7) | 50,005 (6.5) | 50,021 (6.4) |
| 20–24 | 48 (5.7) | 34 (6.2) | 14 (4.7) | 104,296 (13.5) | 104,344 (13.5) |
| 25–29 | 127 (15.0) | 98 (17.9) | 29 (9.8) | 186,916 (24.1) | 187,043 (24.1) |
| 30–34 | 272 (32.2) | 205 (37.3) | 67 (22.6) | 234,657 (30.3) | 234,929 (30.3) |
| 35–39 | 223 (26.4) | 150 (27.3) | 73 (24.6) | 147,280 (19.0) | 147,503 (19.0) |
| 40–44 | 118 (13.9) | 53 (9.7) | 65 (21.9) | 45,782 (5.9) | 45,900 (5.9) |
| 45–54 | 42 (5.0) | 4 (0.7) | 38 (12.8) | 5927 (0.8) | 5969 (0.8) |
| Year of pregnancy | |||||
| 2001 | 64 (7.6) | 35 (6.4) | 29 (9.8) | 49,199 (6.3) | 49,263 (6.3) |
| 2002 | 46 (5.4) | 32 (5.8) | 14 (4.7) | 61,319 (7.9) | 61,365 (7.9) |
| 2003 | 70 (8.3) | 58 (10.6) | 12 (4.0) | 62,772 (8.1) | 62,842 (8.1) |
| 2004 | 58 (6.9) | 43 (7.8) | 15 (5.0) | 61,526 (7.9) | 61,584 (7.9) |
| 2005 | 61 (7.2) | 40 (7.3) | 21 (7.0) | 61,896 (8.0) | 61,957 (8.0) |
| 2006 | 66 (7.8) | 41 (7.5) | 25 (8.4) | 63,419 (8.2) | 63,485 (8.2) |
| 2007 | 57 (6.7) | 37 (6.7) | 20 (6.7) | 64,280 (8.3) | 64,337 (8.3) |
| 2008 | 76 (9.0) | 59 (10.7) | 17 (5.7) | 63,557 (8.2) | 63,633 (8.2) |
| 2009 | 83 (9.8) | 50 (9.1) | 33 (11.1) | 62,768 (8.1) | 62,851 (8.1) |
| 2010 | 63 (7.4) | 39 (7.1) | 24 (8.1) | 62,353 (8.1) | 62,416 (8.1) |
| 2011 | 71 (8.4) | 39 (7.1) | 32 (10.8) | 53,142 (6.9) | 53,213 (6.9) |
| 2012 | 76 (9.0) | 44 (8.0) | 32 (10.8) | 54,816 (7.1) | 54,892 (7.1) |
| 2013 | 55 (6.5) | 32 (5.8) | 23 (7.7) | 53,816 (6.9) | 53,871 (6.9) |
| Pregnancy type | |||||
| Live birth | 549 (64.9) | 549 (100.0) | 0 (0.0) | 508,247 (65.6) | 508,796 (65.6) |
| Induced abortion | 98 (11.6) | 0 (0.0) | 98 (33.0) | 123,635 (16.0) | 123,733 (16.0) |
| Spontaneous abortion | 124 (14.7) | 0 (0.0) | 124 (41.8) | 124,697 (16.1) | 124,821 (16.1) |
| Ectopic pregnancy | 69 (8.2) | 0 (0.0) | 69 (23.2) | 14,999 (1.9) | 15,068 (1.9) |
| Stillbirth | 6 (0.7) | 0 (0.0) | 6 (2.0) | 3285 (0.4) | 3291 (0.4) |
| Timing of cancer diagnosis | |||||
| First trimester | 127 (15.0) | 33 (6.0) | 94 (31.7) | — | — |
| Second trimester | 58 (6.9) | 57 (10.4) | 1 (0.3) | — | — |
| Third trimester | 58 (6.9) | 57 (10.4) | 1 (0.3) | — | — |
| 0–6 Months after pregnancy | 339 (40.1) | 216 (39.3) | 123 (41.4) | — | — |
| 7–12 Months after pregnancy | 264 (31.2) | 186 (33.9) | 78 (26.3) | — | — |
| Cancer site | |||||
| Thyroid | 168 (19.9) | 139 (25.3) | 29 (9.8) | — | — |
| Breast | 208 (24.6) | 120 (21.9) | 88 (29.6) | — | — |
| Melanoma | 93 (11.0) | 79 (14.4) | 14 (4.7) | — | — |
| Hematologic | 87 (10.3) | 63 (11.5) | 24 (8.1) | — | — |
| Cervix/uterus | 74 (8.7) | 40 (7.3) | 34 (11.4) | — | — |
| Ovary | 44 (5.2) | 13 (2.4) | 31 (10.4) | — | — |
| Other | 172 (20.3) | 95 (17.3) | 77 (25.9) | — | — |
Within each characteristic, proportions are calculated using the number of each cell as the numerator and the total number of each column as the denominator.
Incidence by year of pregnancy and maternal age
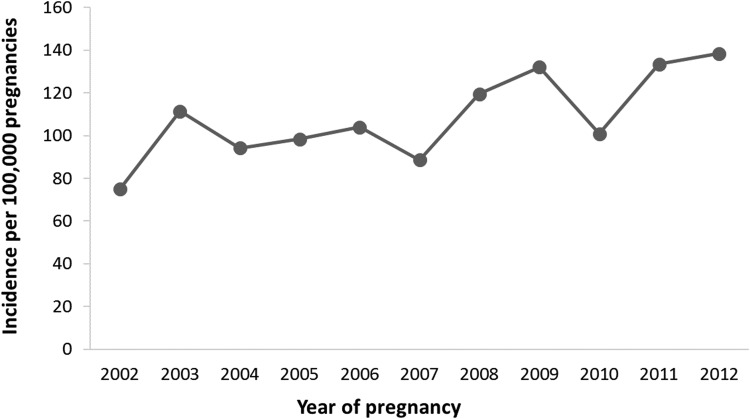
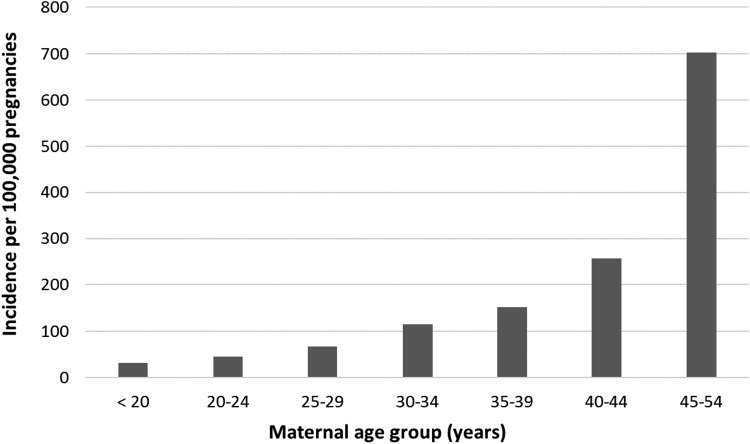
The overall incidence estimate of PAC was 109.1 (95% CI = 101.8–116.7) per 100,000 pregnancies. There was an increase in incidence between 2002 and 2012 (the period during which complete data were available), from 75.0 (95% CI = 54.9–100.0) in 2002 to 138.5 (95% CI = 109.1–173.3) per 100,000 pregnancies in 2012 (ptrend < 0.001) (Fig. 1). The incidence of PAC per 100,000 pregnancies also increased with maternal age, from 32.0 (95% CI = 18.3–51.9) among women younger than 20 years to 703.6 (95% CI = 507.6–949.3) among women aged 45–54 years (Fig. 2).
FIG. 1.
Incidence of pregnancy-associated cancer, by year of pregnancy, among U.S. women aged 10–54 years between 2002 and 2012. Years 2001 and 2013 were excluded from the trend analysis because complete data before pregnancy, during pregnancy, and up to 1 year postpartum were not available.
FIG. 2.
Incidence of pregnancy-associated cancer, by age group, among U.S. women between 2001 and 2013.
Incidence by timing of diagnosis and cancer site
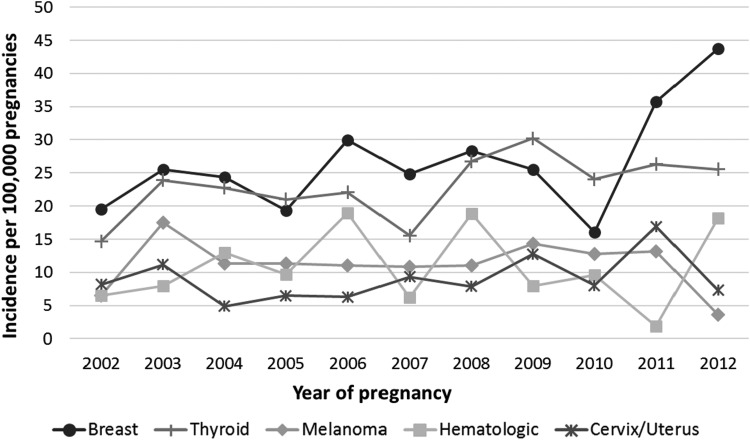
Of the 846 PAC events, 243 (29%) were diagnosed during pregnancy and 603 (71%) during the 1-year postpartum period (Tables 1 and 2). Over 50% (n = 127) of the cancer events identified during pregnancy were diagnosed during the first trimester. Of these 127 PAC events, 33 (26%) had an induced abortion. These induced abortions were most commonly associated with breast (21%), hematologic (21%), and cervical/uterine cancer (15%). Of the 603 cancer events diagnosed postpartum, 56% (n = 339) were diagnosed within 6 months after delivery. The most common cancer sites were breast (n = 208, 25%), thyroid (n = 168, 20%), melanoma (n = 93, 11%), hematologic (included lymphoma, leukemia, and myeloma) (n = 87, 10%), and cervix/uterus (n = 74, 9%). Breast cancer was the only site-specific PAC to increase during the study period (Fig. 3). However, the incidence of the remaining PACs, taken as a group, also increased from 2002 to 2012.
Table 2.
Number and Proportion of Pregnancy-Associated Cancer, by Timing of Diagnosis and Cancer Site, Among U.S. Women Aged 10–54 Years Between 2001 and 2013
| Cancer site | Pregnancy | Postpartum | Total | |||
|---|---|---|---|---|---|---|
| First trimester | Second trimester | Third trimester | 1–6 Months | 7–12 Months | ||
| N (%)a | N (%)a | N (%)a | N (%)a | N (%)a | N (%)b | |
| All sites | 127 (15.0) | 58 (6.9) | 58 (6.9) | 339 (40.1) | 264 (31.2) | 846 (100.0) |
| Breast | 26 (12.5) | 9 (4.3) | 21 (10.1) | 81 (38.9) | 71 (34.1) | 208 (24.6) |
| Thyroid | 11 (6.5) | 19 (11.3) | 3 (1.8) | 67 (39.9) | 68 (40.5) | 168 (19.9) |
| Melanoma | 5 (5.4) | 7 (7.5) | 10 (10.8) | 45 (48.4) | 26 (28.0) | 93 (11.0) |
| Hematologic | 12 (13.8) | 7 (8.0) | 9 (10.3) | 28 (32.2) | 31 (35.6) | 87 (10.3) |
| Cervix/uterus | 16 (21.6) | 3 (4.1) | 6 (8.1) | 34 (45.9) | 15 (20.3) | 74 (8.7) |
| Ovary | 26 (59.1) | 3 (6.8) | 0 (0.0) | 12 (27.3) | 3 (6.8) | 44 (5.2) |
| Other | 31 (18.0) | 10 (5.8) | 9 (5.2) | 72 (41.9) | 50 (29.1) | 172 (20.3) |
Proportions are calculated using the number of each cell as the numerator and the total number of each row as the denominator.
Proportions are calculated using the number of each cell as the numerator and the total number of the column as the denominator.
FIG. 3.
Incidence of site-specific pregnancy-associated cancer, by year of pregnancy, among U.S. women aged 10–54 years between 2002 and 2012. Years 2001 and 2013 were excluded from the trend analysis because complete data before pregnancy, during pregnancy, and up to 1 year postpartum were not available.
Incidence by live birth status and cancer site
The incidence of PAC was slightly higher in pregnancies that ended in non-live birth than in pregnancies that ended in live birth (111.3 vs. 107.9 per 100,000 pregnancies) (Table 3). The most common cancer sites among the 297 PAC diagnosed in pregnancies that ended in non-live birth were breast (n = 88, 30%), cervix/uterus (n = 34, 11%), ovary (n = 31, 10%), and thyroid (n = 29, 10%) (Table 1). PAC diagnosed in pregnancies that ended in non-live birth had a higher proportion of events diagnosed during the first trimester (32%) compared with PAC diagnosed in pregnancies that ended in live birth (6%). They also occurred more frequently in older women.
Table 3.
Incidence of Pregnancy-Associated Cancer, by Live Birth Status and Cancer Site, Among U.S. Women Aged 10–54 Years Between 2001 and 2013
| Cancer siteaand pregnancy type | N | Incidence per 100,000 pregnancies (95% confidence interval) |
|---|---|---|
| All sites | ||
| All pregnancies | 846 | 109.1 (101.8–116.7) |
| Live births | 549 | 107.9 (99.1–117.3) |
| Non-live births | 297 | 111.3 (99.0–124.7) |
| Breast | ||
| All pregnancies | 208 | 26.8 (23.3–30.7) |
| Live births | 120 | 23.6 (19.6–28.2) |
| Non-live births | 88 | 33.0 (26.4–40.6) |
| Thyroid | ||
| All pregnancies | 168 | 21.7 (18.5–25.2) |
| Live births | 139 | 27.3 (23.0–32.3) |
| Non-live births | 29 | 10.9 (7.3–15.6) |
| Melanoma | ||
| All pregnancies | 93 | 12.0 (9.7–14.7) |
| Live births | 79 | 15.5 (12.3–19.4) |
| Non-live births | 14 | 5.2 (2.9–8.8) |
| Hematologic | ||
| All pregnancies | 87 | 11.2 (9.0–13.8) |
| Live births | 63 | 12.4 (9.5–15.8) |
| Non-live births | 24 | 9.0 (5.8–13.4) |
| Cervix/uterus | ||
| All pregnancies | 74 | 9.5 (7.5–12.0) |
| Live births | 40 | 7.9 (5.6–10.7) |
| Non-live births | 34 | 12.7 (8.8–17.8) |
| Ovary | ||
| All pregnancies | 44 | 5.7 (4.1–7.6) |
| Live births | 13 | 2.6 (1.4–4.4) |
| Non-live births | 31 | 11.6 (7.9–16.5) |
Includes only the six most common cancer sites.
Compared with the incidence in pregnancies that ended in live birth, the incidence was lower for thyroid, melanoma, and hematologic cancer and higher for breast, cervical/uterine, and ovarian cancer in pregnancies that ended in non-live birth. For example, the incidence of breast cancer per 100,000 pregnancies was 26.8 for all pregnancies, 23.6 for pregnancies that ended in live birth, and 33.0 for pregnancies that ended in non-live birth (Table 3). The incidence of thyroid cancer per 100,000 pregnancies was 21.7 for all pregnancies, 27.3 for pregnancies that ended in live birth, and 10.9 for pregnancies that ended in non-live birth.
Discussion
In this study, we identified a total of 846 PAC events among 775,709 pregnancies for an overall incidence of 109.1 per 100,000 pregnancies between 2001 and 2013. This crude incidence is similar to some previous studies,7,10 higher than others,4,5,8 and lower than one Australian study that found an incidence of 137.3 per 100,000 live births.9 The higher incidence in the Australian study was influenced by a very high proportion of melanoma, for which Australia has the highest incidence in the world.21 The studies that had a lower incidence of cancer coinciding with pregnancy, compared with our study, may reflect the fact that they were from earlier calendar periods or populations who had children at younger ages.
The incidence of PAC increased during our study period. This finding is consistent with the results from other studies.6,9,22 We also found a higher incidence with increasing maternal age. Some studies have shown that an increased incidence of PAC over time persisted, although lessened after controlling for age.9,10 We did not control for age when estimating the incidence of PAC by year of pregnancy. However, there was no noticeable increase in the mean maternal age at diagnosis during our study period. In addition to postponing childbirth, other factors that may contribute to the observed temporal trend include improved diagnostic techniques in more recent years, and an increase in the frequency of interactions with healthcare personnel that provided additional opportunities to diagnose cancer.7,9
Only 29% of the PAC events found in our study were diagnosed during pregnancy, the remaining 71% were diagnosed in the 1-year postpartum period. This is in agreement with other studies.7,9,10,22 Physiological changes seen with pregnancy may mask the signs and symptoms of cancer delaying the diagnoses.7 It is also plausible that potentially harmful diagnostic procedures, including mammography and surgical biopsies, may be delayed until the postpartum period. Finally, the development, promotion, and growth of breast cancer may be influenced by the physiological changes that occur during pregnancy and the postpartum period.23
We found that more than 50% of cancer events diagnosed during pregnancy were diagnosed in the first trimester. This finding is different than results from other studies that reported cancer diagnoses steadily increasing during pregnancy.7,9 One potential explanation for the disparate findings is that prior studies did not include all pregnancies, so cancer events diagnosed during pregnancies that were ultimately terminated were not considered. Not only were cancer events likely underestimated in those studies, the proportion of cancer events identified during the first trimester would, by design, be less than what we observed in our study. Importantly, lending to the robustness of our analysis, the most common PAC sites seen in our study were breast, thyroid, melanoma, hematologic, and cervix/uterus, similar to the most common cancer sites seen among non-pregnant U.S. women of reproductive age.24
We found an overall incidence of 26.8 per 100,000 pregnancies for breast cancer, similar to estimates reported by others.7,9,10 However, our incidence of thyroid cancer was higher than other studies.5,7,9 This may reflect the high incidence of thyroid cancer in the United States compared with most of the world.25 We observed an incidence of 9.5 per 100,000 pregnancies for cervical/uterine cancer, much lower than reported by a Danish study10 but similar to other studies.5,7,9 Our incidence estimates of breast, cervical/uterine, and ovarian cancer were considerably higher in pregnancies that ended in non-live birth than in pregnancies that ended in live birth. This is not surprising as cervix/uterus and breast were two of the most commonly diagnosed cancer sites associated with induced abortions in the first trimester in our study.
Our study has several strengths. First, it is a large U.S. population-based study that included geographically and demographically diverse populations. Second, study information was collected from data obtained during routine healthcare delivery and payment and thus was not susceptible to recall bias. Third, unlike the majority of the previous studies, our study included pregnancies that not only ended in live birth but also medically attended stillbirths, spontaneous abortions, induced abortions, and ectopic pregnancies. Fourth, our study contains validated information on tumor characteristics and reproductive factors from tumor registries and birth certificate files.
As with any retrospective observational study, however, our study has limitations. We likely did not fully capture certain pregnancy episodes. Specifically, our databases did not have early pregnancy losses that were not medically recognized or attended, or pregnancies that occurred outside the participating health plans (e.g., some induced abortions, certain home births). However, live births, which occurred with the majority of pregnancies, were well captured and many had also been linked to the infant birth certificate file. Also, it is unlikely that we missed substantial numbers of pregnancy episodes given the similarity of our frequencies of pregnancy outcomes to those of national statistics. In the United States, 65% of all pregnancies ended in live birth, 18% in induced abortions, and 17% in fetal loss in 2008.26 In our study, among women aged 10–54 years during 2001–2013, 66% of pregnancies ended in live birth, 16% in induced abortions, and 18% in fetal loss. Our study population was older and mostly privately insured, which may account for the small differences.
We did not have gestational age information on pregnancies that ended in non-live birth and live-born deliveries without linked infant birth certificate files. However, the gestational age algorithms that we used in these pregnancies were adapted from previously validated algorithms.16,17,19,20 We did not have reasonably complete postpartum data for pregnancies identified in 2013. We also did not require women to be continuously enrolled in the health plan for the entire 1-year postpartum period to avoid potential selection bias that might arise from restricting the analysis to women who had insurance coverage and survived during the entire postpartum year. This might have led to an under-ascertainment of cancer events during the postpartum period. However, only approximately 8% of women in our study left the health plan during the year after delivery, any under-ascertainment of cancer event was likely not substantial.
We did not examine factors (e.g., maternal comorbidity, breastfeeding) that might be predictive of PAC risk. This is a topic of future research. Finally, our population included only women with private health insurance, who are more likely to be employed, have higher incomes, and have more interactions with healthcare providers compared with those on Medicaid or uninsured.27 Higher socioeconomic status has been shown to be associated with a higher incidence of PAC, possibly due in part to more interactions with healthcare providers and opportunities for diagnosis.9 Therefore, our results may not be generalizable to all women of childbearing age.
Conclusions
Our study provides contemporary incidence estimates of PAC from a large population-based cohort of U.S. women. These estimates provide the data needed to create clinical and public health policies aimed at diagnosing PAC at an early stage and initiating appropriate therapeutic interventions in a timely manner.
Supplementary Material
Acknowledgments
The study was supported by the National Cancer Institute at the National Institutes of Health through a pilot project grant from the Cancer Research Network (grant number U24 CA171524 to S.T.; overall PI: Kushi). The authors thank Beth Syat for her project management support.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Amant F, Loibl S, Neven P, Van Calsteren K. Breast cancer in pregnancy. Lancet 2012;379:570–579 [DOI] [PubMed] [Google Scholar]
- 2. Brenner B, Avivi I, Lishner M. Haematological cancers in pregnancy. Lancet 2012;379:580–587 [DOI] [PubMed] [Google Scholar]
- 3. Morice P, Uzan C, Gouy S, Verschraegen C, Haie-Meder C. Gynaecological cancers in pregnancy. Lancet 2012;379:558–569 [DOI] [PubMed] [Google Scholar]
- 4. Haas JF. Pregnancy in association with a newly diagnosed cancer: A population-based epidemiologic assessment. Int J Cancer 1984;34:229–235 [DOI] [PubMed] [Google Scholar]
- 5. Lambe M, Ekbom A. Cancers coinciding with childbearing: Delayed diagnosis during pregnancy? BMJ 1995;311:1607–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith LH, Dalrymple JL, Leiserowitz GS, Danielsen B, Gilbert WM. Obstetrical deliveries associated with maternal malignancy in California, 1992 through 1997. Am J Obstet Gynecol 2001;184:1504–1512; discussion 1512–1503 [DOI] [PubMed] [Google Scholar]
- 7. Smith LH, Danielsen B, Allen ME, Cress R. Cancer associated with obstetric delivery: Results of linkage with the California cancer registry. Am J Obstet Gynecol 2003;189:1128–1135 [DOI] [PubMed] [Google Scholar]
- 8. Stensheim H, Moller B, van Dijk T, Fossa SD. Cause-specific survival for women diagnosed with cancer during pregnancy or lactation: A registry-based cohort study. J Clin Oncol 2009;27:45–51 [DOI] [PubMed] [Google Scholar]
- 9. Lee YY, Roberts CL, Dobbins T, et al. . Incidence and outcomes of in Australia, 1994–2008: A population-based linkage study. BJOG 2012;119:1572–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eibye S, Kjaer SK, Mellemkjaer L. Incidence of pregnancy-associated cancer in Denmark, 1977–2006. Obstet Gynecol 2013;122:608–617 [DOI] [PubMed] [Google Scholar]
- 11. Wagner EH, Greene SM, Hart G, et al. . Building a research consortium of large health systems: The Cancer Research Network. J Natl Cancer Inst Monogr 2005:3–11 [DOI] [PubMed] [Google Scholar]
- 12. Andrade SE, Davis RL, Cheetham TC, et al. . Medication exposure in pregnancy risk evaluation program. Matern Child Health J 2012;16:1349–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hornbrook MC, Hart G, Ellis JL, et al. . Building a virtual cancer research organization. J Natl Cancer Inst Monogr 2005;35:12–25 [DOI] [PubMed] [Google Scholar]
- 14. Ross TR, Ng D, Brown JS, et al. . The HMO Research Network Virtual Data Warehouse: A Public Data Model to Support Collaboration. EGEMS (Wash DC) 2014;2:1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. North American Association of Central Cancer Registries. NAACCR Strategic Management Plan. Available at: www.naaccr.org 2012 Accessed May15, 2018
- 16. Hornbrook MC, Whitlock EP, Berg CJ, et al. . Development of an algorithm to identify pregnancy episodes in an integrated health care delivery system. Health Serv Res 2007;42:908–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Naleway AL, Gold R, Kurosky S, et al. . Identifying pregnancy episodes, outcomes, and mother-infant pairs in the Vaccine Safety Datalink. Vaccine 2013;31:2898–2903 [DOI] [PubMed] [Google Scholar]
- 18. Toh S, Li Q, Cheetham TC, et al. . Prevalence and trends in the use of antipsychotic medications during pregnancy in the U.S., 2001–2007; a population-based study of 585,615 deliveries. Arch Womens Ment Health 2013;16:149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Q, Andrade SE, Cooper WO, et al. . Validation of an algorithm to estimate gestational age in electronic health plan databases. Pharmacoepidemiol Drug Saf 2013;22:524–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raebel MA Ellis JL, Andrade SE. Evaluation of gestational age and admission date assumptions used to determine prenatal drug exposure from administrative data. Pharmacoepidemiol Drug Saf 2005;14:829–836 [DOI] [PubMed] [Google Scholar]
- 21. Ferlay J, Soerjomataram I, Dikshit R, et al. . Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–E386 [DOI] [PubMed] [Google Scholar]
- 22. Andersson TM, Johansson AL, Fredriksson I, Lambe M. Cancer during pregnancy and the postpartum period: A population-based study. Cancer 2015;121:2072–2077 [DOI] [PubMed] [Google Scholar]
- 23. Lyons TR, Schedin PJ, Borges VF. Pregnancy and breast cancer: When they collide. J Mammary Gland Biol Neoplasia 2009;14:87–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. National Cancer Institute. Adolescents and young adults with cancer. Available at: www.cancer.gov/types/aya Accessed May15, 2018
- 25. Kilfoy BA, Zheng T, Holford TR, et al. . International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control 2009;20:525–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ventura SJ, Curtin SC, Abma JC, Henshaw SK. Estimated pregnancy rates for the United States, 1990–2008: An update. Natl Vital Stat Rep 2012;60:1–20 [PubMed] [Google Scholar]
- 27. Paul Fronstin. Sources of health insurance and characteristics of the uninsured: Analysis of the March 2012 Current Population Survey. EBRI Issue Brief, no. 376. Available at: www.ebri.org/pdf/briefspdf/EBRI_IB_09-2011_No362_Uninsured1.pdf Accessed May15, 2018 [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.