The daily rhythmicity, or circadian pattern, of cluster headache is a fascinating part of the disease. Take for example a cluster headache patient from our clinic, who was being interviewed about his headache attacks. He stopped and said, quite confidently, “I get a headache every day at 11am and right now it’s 10:45. If you wait 15 minutes, I’ll show you what my headache looks like.” He had an attack at 11:38am; after further discussion with the assistance of his headache diary, it was clear that he could routinely predict his attacks within the hour. On a larger scale, in a survey of 1134 cluster headache patients, 82% stated that they had headaches “more or less the same time each day”1. In that same survey, patients with episodic cluster headache were more likely to have headaches at the same time every year (usually in the spring or fall). The circadian system clearly appears to be involved in cluster headache. Here we present several preliminary lines of evidence that the circadian system may be abnormal in cluster headache. Further investigation into these circadian abnormalities may be important in elucidating the full etiology and in developing treatments for the disease.
The basic element of the circadian system is the single cell, which contains transcriptional-translational feedback loops that turn each other on and off over 24 hours (Fig 1). This molecular machinery is present in most if not all cells, but these cells are not completely independent. The cells of each organ generally cycle together in a unit called a peripheral clock. Daily variations in blood pressure, alertness, and gluconeogenesis are just a few examples of the activity of these peripheral clocks. The peripheral clocks are regulated by the central clock, otherwise known as the suprachiasmatic nucleus (SCN), in the anterior hypothalamus. The SCN is special in its ability to be calibrated, or “entrained,” by light and in turn synchronize the peripheral clocks throughout the body. This entrainment is what keeps us on a precise 24 hour schedule and what allows us to adapt to new time zones. In addition to light, a wide variety of stimuli can entrain the clock network, including food, exercise, temperature, melatonin, corticosteroids, and numerous other molecules. There is also a yearly or “circannual” pattern, which uses information including the length of daytime light and melatonin.
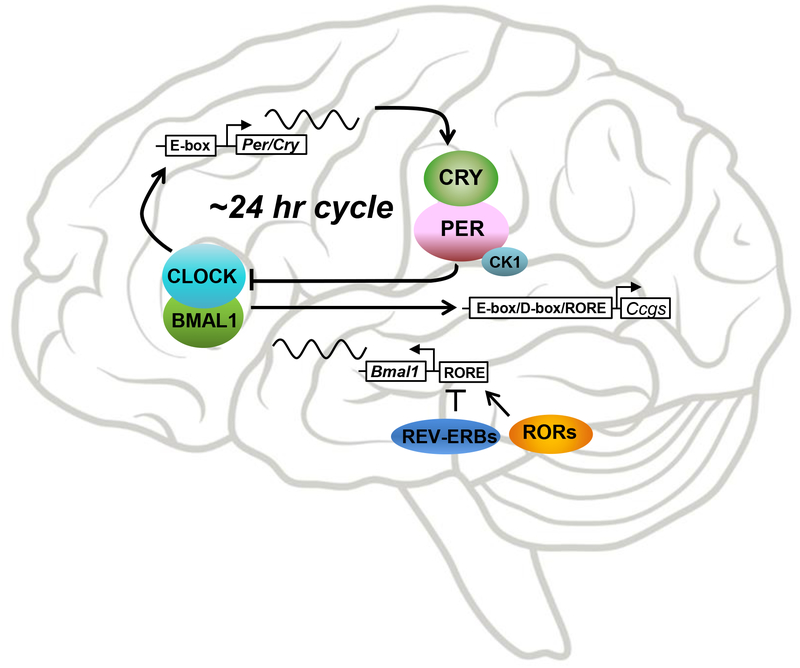
Figure 1:
The core circadian transcriptional-translational feedback loops. The circadian cell-autonomous oscillator is characterized by an intracellular interaction of a handful of core clock components that activate and inhibit each other over approximately 24 hours. These feedback loops are present in virtually all cells of the body and are synchronized by the central pacemakers, the suprachiasmatic nuclei. They are transcriptional-translational because the feedback occurs partly through protein-protein interactions (shown as circles) and partly through activation of gene transcription via E-box, D-box, and RORE promoter elements (shown as rectangles). Gene names are italicized. Bmal1: Brain and Muscle ARNTL-Like 1 (ARNTL, Aryl Hydrocardon Receptor Nuclear Translocator Like). CLOCK: Circadian locomotor output cycles kaput. Cry: Cryptochrome. Per: Period. CK1: Casein kinase 1. Rev-ERBs: Reverse strand of Erb nuclear receptor subfamily. RORs: RAR-related orphan receptors (RAR, retinoid acid receptor).
Anatomically, the SCN consists of a core area of vasoactive intestinal peptide (VIP)-positive neurons and a shell of arginine vasopressin (AVP)-positive neurons. It projects to other areas including the pineal gland (the site of melatonin release), the lateral hypothalamus (the site of orexinergic neurons), the periventricular nucleus and nucleus of the vagus (which connect to the autonomic system), other hypothalamic areas, and the pituitary gland2.
In cluster headache, studies have shown abnormalities in multiple aspects of circadian biology. Melatonin, widely considered a biomarker of the circadian system, is decreased in cluster headache subjects compared to controls3. Alterations have been shown in cluster headache subjects in the levels of VIP, AVP, orexin, and pituitary hormones4. Specific mRNA changes have also been found in the circadian transcriptional-translational feedback loops, notably in the expression of Rev-ERB-alpha but not Clock5,6. As is expected in an uncommon disease like cluster headache, most of these studies have been small, and the connections from the SCN to other hypothalamic areas are not fully understood. But in a disease with a remarkable clock-like regularity, the wealth of changes in molecules tied closely to the circadian system warrants further investigation. Ultimately it may be possible to harness the circadian system as a therapeutic target for cluster headache. A total of 11 preventive medications receive a recommendation of effective, probably effective, or possibly effective from either the American Headache Society or the European Federation of Neurological Societies7,8. At least four alter the circadian transcriptional-translational feedback loops, including melatonin, corticosteroids, lithium, and valproic acid9. Melatonin and corticosteroids, as above, reset the body clock. Lithium inhibits glycogen synthase kinase 3β, a protein that may be involved in stabilization of several core circadian genes including Period2 and Rev-ERB-alpha (see Fig 1). Valproic acid shifts the timing of Period2, possibly via histone deacetylation inhibition that affects core circadian gene expression. Additional circadian-altering compounds are emerging using cellular assays10, and new compounds that strongly or precisely alter the circadian transcriptional-translational feedback loops might provide effective prevention of cluster headache attacks.
Cluster headache has many connections to circadian biology, including the exquisite timing of the attacks, the anatomic connections from the suprachiasmatic nucleus to the autonomic system, several altered biomarkers, and changes in the expression of core circadian genes. Moreover, at least one-third of the preventive medications are known circadian modulators. Therefore we propose that the clock system should be considered a novel drug target in cluster headache. For current medications, chronotherapy and the timing of medications may be important. For new drug discovery, a medication that is safe, has minimal side effects, and shares circadian properties with melatonin, corticosteroids, lithium, and valproic acid should be considered a candidate for clinical trials as a cluster headache preventive.
Acknowledgements
Dr. Burish receives funds from the American Headache Society, National Headache Foundation, and Will Erwin Headache Research Foundation. Dr. Chen receives funds from the Welch Foundation (AU-1731) and National Institute on Aging (R01AG045828). Dr. Yoo receives funds from National Institute of General Medical Sciences (R01GM114424). The authors declare no conflicts of interest.
References
- 1.Rozen TD, Fishman RS. Cluster headache in the United States of America: demographics, clinical characteristics, triggers, suicidality, and personal burden. Headache. 2012;52(1):99–113. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht U Timing to Perfection: The Biology of Central and Peripheral Circadian Clocks. Neuron. 2012;74(2):246–260. [DOI] [PubMed] [Google Scholar]
- 3.Peres M Melatonin, the pineal gland and their implications for headache disorders. Cephalalgia. 2005;25(6):403–411. [DOI] [PubMed] [Google Scholar]
- 4.Waldenlind E, Sjostrand C. Pathophysiology of cluster headache and other trigeminal autonomic cephalalgias In: Nappi G, Moskowitz M, eds. Handbook of Clinical Neurology. Vol. 97 Elsevier B.V.; 2010:389–411. [DOI] [PubMed] [Google Scholar]
- 5.Fourier C, Ran C, Zinnegger M, et al. A genetic CLOCK variant associated with cluster headache causing increased mRNA levels. Cephalalgia. March 2017:33310241769870. [DOI] [PubMed] [Google Scholar]
- 6.Costa M, Squassina A, Piras IS, et al. Preliminary Transcriptome Analysis in Lymphoblasts from Cluster Headache and Bipolar Disorder Patients Implicates Dysregulation of Circadian and Serotonergic Genes. J Mol Neurosci. 2015;56(3):688–695. [DOI] [PubMed] [Google Scholar]
- 7.Robbins MS, Starling AJ, Pringsheim TM, Becker WJ, Schwedt TJ. Treatment of Cluster Headache: The American Headache Society Evidence-Based Guidelines. Headache J Head Face Pain. 2016;56(7):1093–1106. [DOI] [PubMed] [Google Scholar]
- 8.May A, Leone M, Afra J, et al. EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias. Eur J Neurol. 2006;13(10):1066–1077. [DOI] [PubMed] [Google Scholar]
- 9.Gloston GF, Yoo S-H, Chen Z. Clock-Enhancing Small Molecules and Potential Applications in Chronic Diseases and Aging. Front Neurol. 2017;8(March):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z, Yoo S-H, Park Y-S, et al. Identification of diverse modulators of central and peripheral circadian clocks by high-throughput chemical screening. Proc Natl Acad Sci U S A. 2012;109(1):101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]