Summary
Type I interferon (IFN) production within the tumor microenvironment is important in shaping the immune response to the tumor. In this issue of Immunity, Marcus et al (2018) reveal that tumor cells produce 2’3’-cGAMP, which activates the STING pathway in non-tumor cells leading to type I IFN production and the priming of natural killer cells for tumor rejection.
Preview
The cGAS-STING pathway has emerged as a major regulator of the innate immune response to cytosolic DNA derived from viruses and other microbial pathogens, as well as cytosolic DNA that aberrantly accumulates in tumor cells. Cyclic GMP-AMP synthase (cGAS) binds double-stranded DNA in the cytosol and generates 2’, 3’-cGAMP, a soluble second messenger that binds the ER-resident STING, promoting its re-localization to perinuclear Golgi vesicles and activation of the kinase TBK1 and its downstream substrate, the transcription factor IRF3. This culminates in the production of type I interferons (IFN) and the triggering of a STAT1-driven antiviral response. This pathway is also relevant in cancer. The very mechanisms by which cancers circumvent the DNA damage response and undergo unchecked proliferation have been shown to trigger the release of dsDNA from the nucleus, which activates cGAS-STING signaling in tumor cells as well as antigen presenting cells (APCs) (Ng et al, 2018). Engagement of cGAS-STING thus promotes tumor innate immune recognition and can mediate potent rejection (Woo et al, 2014). Indeed, epigenetic silencing of cGAS and/or STING in tumor cells has recently been identified as a mechanism of immune escape (Xia et al, 2016). Interestingly, 2’, 3’-cGAMP is not only limited to binding intracellular STING, but can also be extruded from cells via extracellular vesicles or transferred intercellularly via gap junctions and confer STING agonism in neighboring cells (Bridgeman et al, 2015; Chen et al, 2016). In this issue of Immunity, Marcus et al (2018) reveal a paracrine impact of this second messenger on the tumor immune microenvironment, and in the regulation of antitumor immunity.
Marcus et al (2018) examined the role of host STING expression on tumor growth of several well-established models, including poorly immunogenic B16-BL melanoma, TAP2-deficient RMA-S lymphoma, and MC38 colon carcinoma. Tumor growth following subcutaneous transplantation into STING-deficient (Stinggt/gt) mice was significantly accelerated, and independent of T cells since the same effect was observed in a Rag2−/− Stinggt/gt background. Because this observation suggested a potential role for NK cells, the authors specifically depleted NK cells, or utilized an NK-resistant variant of RMA based upon MHCI expression. In these settings tumors could instead grow equally well in wild-type (WT) and Stinggt/gt backgrounds. Moreover, transduction of MHCI+ RMA lymphoma with RAE-1e, a ligand known to increase the expression of NK cell activating receptors via STING-dependent signaling (Lam et al, 2014), restored tumor rejection downstream of STING. Together these findings implicated NK cells as primary effectors of STING-mediated tumor rejection in these models. Importantly, Stinggt/gt mice contained functional NK cells, and could reject MHCI-deficient bone marrow grafts, ruling out the possibility of an NK cell defect in the absence of STING, and pointing to the importance of the tumor microenvironment context.
The authors generated cGas−/− mice using CRISPR/Cas9 technology and examined B16-BL6 or RMA-S tumor growth in this context. Whereas cGas−/− mice were just as susceptible to viral infection as Stinggt/gt mice, they retained the ability to reject these tumors. Since the transplanted tumor cells contained intact cGAS and could still produce 2’,3’-cGAMP in this model, these data suggested that tumor-derived 2’,3’-cGAMP might somehow be activating NK cells extrinsically (Figure 1). Indeed, intraperitoneal injection of 2’,3’-cGAMP was sufficient to activate NK cells. This effect was observed in WT mice, but not Stinggt/gt or Ifnar−/− mice, revealing the importance of STING-induced type I IFN production in promoting NK cell activation. Using Golgi transport inhibitors after injection of 2’,3’-cGAMP directly into tumors, followed by intracellular IFN-β staining of different immune populations, the authors identified CD11b+ cells as the dominant intermediary immune cell type in the tumor immune microenvironment. They also demonstrated that other cell types such as B-lymphocytes can respond to 2’,3’-cGAMP when injected intraperitoneally. To directly examine the requirement for tumor-derived 2’,3’-cGAMP, Marcus et al. (2018) genetically deleted Cgas in tumor cells using CRISPR/Cas9 technology. cGAS-deficient B16-BL6 cells not only failed to respond to transfected DNA in vitro, but also lacked basal expression of genes induced by endogenous cGAS-STING signaling in B16-BL6 tumor cells, such as CCL5, which is involved in chemoattraction. These results are consistent with the notion that tumors frequently contain higher levels of cytosolic DNA than normal, which is also exacerbated by chemotherapy treatment and the further accumulation of damaged DNA (Ng et al, 2018). Most notably, the catalytic activity of cGAS in the tumor was critical for triggering the therapeutic host STING-mediated immune response – cGAS-deficient B16-BL6 cells rescued with a catalytically inactive form of cGAS were not rejected by mice, whereas cells rescued with WT cGAS were rejected in a STING-dependent fashion. This finding confirms that it is 2’, 3’-cGAMP generated in the tumor cell, rather than transfer of tumor DNA itself into host cells, that activates the STING-mediated antitumor response driven by NK cells.
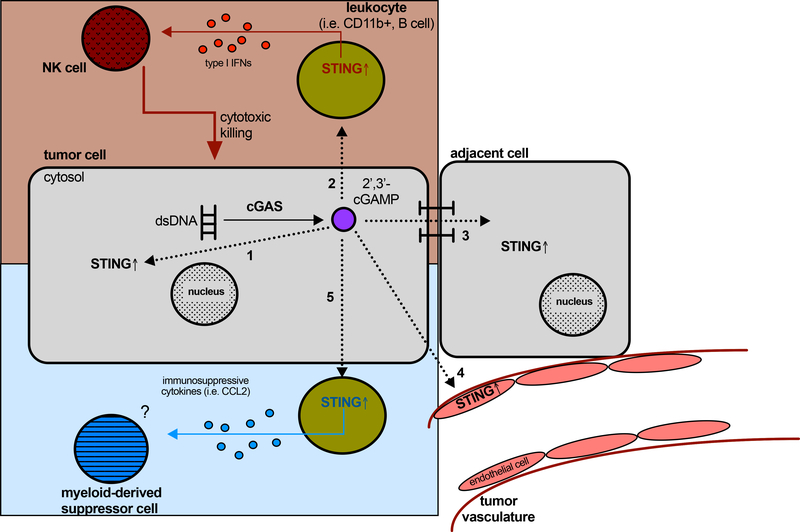
Figure 1. Potential paracrine roles of 2’, 3’-cGAMP in the tumor microenvironment.
In addition to tumor cell intrinsic signaling (1), tumor-derived 2’, 3’-cGAMP can be recognized by leukocytes such as CD11b+ and B cells and subsequently activate STING, inducing type I interferon production by the leukocytes to prime NK cells for cytotoxic killing of tumor cells (2). Intercellular gap junctions enable transfer of this second messenger in adjacent cells, which has been linked to metastasis (3). It is also well established that the tumor vasculature responds to cyclic dinucleotides, though how this impacts the tumor microenvironment is poorly understood (4). Finally, STING activation in leukocytes or other cells by 2’, 3’-cGAMP could also promote production of immunosuppressive cytokines such as CCL2, which can recruit myeloid-derived suppressor cells (MDSCs) (5). Red = anti-tumorigenic effects and blue = potential pro-tumorigenic effects of STING agonism in the tumor microenvironment.
The results of Marcus et al (2018) provide intriguing insights into the role of innate immunity in regulating the NK cell response to tumors. They uncover a direct connection between cytosolic accumulation of DNA in cancer cells, paracrine second messenger signaling via 2’,3’-cGAMP, and activation of NK cells via STING-dependent type I IFN production from local immune cell populations. Notably, cGAS signaling has also been implicated in cellular senescence (Glück et al, 2014), where NK cells are known to monitor and clear damaged cells. Thus, it is possible the authors have uncovered a more fundamental signaling mechanism that organisms utilize to detect and clear cells that have gone awry. In this regard, tumor cell silencing of cGAS (Woo et al, 2014; Xia et al, 2016), or production of immune suppressive cytokines/chemokines, may represent a way of escaping or inhibiting this NK cell mediated detection.
These results may have important implications for tumors that resist PD-1 blockade. Loss of B2M or JAK inactivation, which results in a loss of interferon-inducible proteins associated with antigen presentation such as MHC-I and TAP1, have been identified as a mediators of acquired resistance to PD-1 immunotherapy in melanoma (Zaretsky et al, 2017; Sade-Feldman et al, 2017). Importantly, B16-BL6 melanoma and the TAP2-deficient RMA lymphoma were susceptible to STING-induced NK cell activity in the absence of MHC-1 mediated CD8+ T cell activation. Furthermore, expression of activating NK cell ligands on tumors was shown to facilitate the STING-dependent NK cell response and suppress tumor growth irrespective of MHC-1 status. Cytosolic DNA sensing by STING induces expression of these NK-activating receptors (Lam et al, 2014), and consistent with this, Marcus et al (2018) show that increased levels of cGAS correlated positively with levels of NK-cell receptor expression and survival in melanoma. Together, these findings provide evidence of a potential therapeutic role of NK cells in STING-driven tumor immunity, even in tumors with defective antigen presentation that resist PD-1 blockade.
However, it is important to note that the observed immune response to transplanted tumors in murine models do not always translate directly to the human immune response in vivo. While in a syngeneic model, the immune response to transplanted tumors with de novo mutations does not necessarily recapitulate the interactions between a chronically exhausted immune system and a human tumor that has undergone immunoediting to subvert the immune system and persist. For example, as above, it is likely that tumors with high levels of cytosolic DNA have adapted to and escaped this mechanism by silencing cGAS and/or STING or altering the immune microenvironment in such a fashion that paracrine 2’,3’-cGAMP is unable to prime an IFN effector response to activate NK cells. To illustrate, in addition to type I IFN, leukocytes activated by tumor-derived 2,’3-cGAMP could also produce immunosuppressive cytokines like CCL2 via STING-mediated NF-κB activation, possibly recruiting myeloid-derived suppressor cells that could impair the adaptive immune response (Figure 1). Moreover, 2’,3’-cGAMP could act on other cells, such as the tumor vasculature, and promote paracrine signals in stromal cells such as astrocytes which favor metastasis (Chen et al, 2016) (Figure 1). Thus, how extracellular 2’,3’-cGAMP impacts the complicated milieu of established human tumors remains to be determined. Nonetheless, although responses in murine models may not directly correlate well with the more complex biology observed in patients, these findings lend support to an anti-tumorigenic effect of the cGAS-STING pathway and provide a greater understanding of the relationship between tumor cell cytosolic DNA and 2’,3’-cGAMP production and sensing within the tumor immune microenvironment.
Ongoing efforts by pharmaceutical companies to develop effective STING agonists that can induce immune cell infiltration of the tumor microenvironment underscore the importance of elucidating the detailed mechanism by which 2’,3’-cGAMP mounts the antitumor immune response. The data from Marcus et al. (2018) suggest that targeting cGAMP to effector populations that facilitate a robust type I IFN response capable of activating NK cells would be most effective in priming tumor clearance. As opposed to direct tumor injection, it is possible that more sophisticated approaches to retain cGAMP in the tumor microenvironment and target it to certain cell populations will be more effective. In addition, this work also suggests that therapeutic strategies that naturally engage cGAMP-STING signaling, such as treatment with certain DNA damaging agents, are likely to prime innate immune responses, which could be tailored in combination therapies to maximize NK cell activation. Regardless, these findings provide a rationale to further our understanding of the mechanisms of 2’,3’-cGAMP transport and modulation of cGAS activity, in order to maximally augment tumor immunogenicity and broaden the therapeutic utility of cancer immunotherapy.
References
- 1.Ng KW, Marshall EA, Bell JC & Lam WL cGAS–STING and Cancer: Dichotomous Roles in Tumor Immunity and Development. Trends Immunol. 39, 44–54 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, Duggan R, Wang Y, Barber GN, Fitzgerald KA, Alegre ML, Gajewski TF. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 41, 830–842 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xia T, Konno H & Barber GN Recurrent loss of STING signaling in melanoma correlates with susceptibility to viral oncolysis. Cancer Res. 76, 6747–6759 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez-Soto A, Jacob L, Patwa R, Shah H, Xu K, Cross JR, Massagué J. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature (2016). doi: 10.1038/nature18268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bridgeman A, Maelfait J, Davenne T, Partridge T, Peng Y, Mayer A, Dong T, Kaever V, Borrow P, Rehwinkel J.Viruses transfer the antiviral second messenger cGAMP between cells. Science 349, 1228–1232 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcus Assaf, Mao Amy Jiamei, Lensink-Vasan Monisha, Wang LeeAnn, V. RE and R. DH cGAMP production by the DNA sensor cGAS in tumor cells triggers a STING-mediated interferon response that activates the anti-tumor response of NK cells. Immunity (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam AR, Bert NL, Ho SS, Shen YJ, Tang LF, Xiong GM, Croxford JL, Koo CX, Ishii KJ, Akira S, Raulet DH, Gasser S RAE1 ligands for the NKG2D receptor are regulated by STING-dependent DNA sensor pathways in lymphoma. Cancer Res. 74, 2193–2203 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glück S, Guey B, Gulen MF, Wolter K, Kang TW, Schmacke NA, Bridgeman A, Rehwinkel J, Zender L, Ablasser A. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat. Cell Biol 19, 1061–1070 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, Saco J, Homet Moreno B, Mezzadra R, Chmielowski B, Ruchalski K, Shintaku IP, Sanchez PJ, Puig-Saus C, Cherry G, Seja E, Kong X, Pang J, Berent-Maoz B, Comin-Anduix B, Graeber TG, Tumeh PC, Schumacher TN, Lo RS, Ribas A. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N. Engl. J. Med 375, 819–829 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sade-Feldman M, Jiao YJ, Chen JH, Rooney MS, Barzily-Rokni M, Eliane JP, Bjorgaard SL, Hammond MR, Vitzthum H, Blackmon SM, Frederick DT, Hazar-Rethinam M, Nadres BA, Van Seventer EE, Shukla SA, Yizhak K, Ray JP, Rosebrock D, Livitz D, Adalsteinsson V, Getz G, Duncan LM, Li B, Corcoran RB, Lawrence DP, Stemmer-Rachamimov A, Boland GM, Landau DA, Flaherty KT, Sullivan RJ, Hacohen N Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun 8, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]